Wiskott-Aldrich syndrome (WAS) is caused by defects in the WAS protein (WASP) gene on the X chromosome. We previously reported that flow cytometric analysis of intracellular WASP expression (FCM-WASP) was useful in the diagnosis of WAS in patients and carriers. In this study, we applied FCM-WASP to evaluate the mixed chimera (MC) status of 12 WAS patients who underwent hematopoietic stem cell transplantation (HST). After HST, donor- and recipient-derived peripheral blood mononuclear cells (PBMCs) could be distinguished easily with this method, since the donor cells were WASPbright, whereas the defective recipient cells were WASPdim. Furthermore, with use of 2-color FCM-WASP, the MC status could be characterized by cell lineage. Six of the 12 patients with WAS were found to have MC status after HST, whereas others had complete chimera status. MC status was observed in every cell lineage examined. However, among PBMCs, recipient cells were most commonly observed in the monocyte population. Finally, to investigate the naive/memory status of donor and recipient T cells in these patients, 3-color FCM-WASP using anti-CD45RA or CD45RO was performed. We found that, in contrast to WASPbright T cells, most WASPdim T cells remained naive (CD45RA+/RO−) more than a year after HST. No imbalance in the ratio of naive to memory T cells was observed in WAS patients before HST. We conclude that FCM-WASP is a potentially useful method for clinical follow-up of WAS patients who have undergone HST. Our findings may also have important implications for the role of WASP during hematopoietic development.
Introduction
Wiskott-Aldrich syndrome (WAS) is an X-linked recessive disorder characterized by the triad of thrombocytopenia, eczema, and immune deficiency, as well as a proclivity toward lymphoid malignant disease.1-3 The gene responsible for WAS has been identified and termed WASP, for WAS protein.4 The WASP gene is expressed mainly in all nonerythroid hematopoietic cells,5 and its product, WASP, seems to play a role in transducing signals related to cell growth.6 In addition, WASP specifically associates with the activated form of Cdc42, suggesting that WASP is involved in regulating cytoskeletal architecture through actin polymerization.7 Indeed, lymphocytes from patients with WAS have activation and cytoskeletal-structure defects.8-11 However, the mechanisms by which defectiveWASP gene expression lead to most of the clinical features observed in these patients have not been fully elucidated.
Without successful hematopoietic stem cell transplantation (HST), patients with WAS have a poor prognosis. The median survival time for these patients is about 15 years, and the usual causes of death are infection, bleeding, and malignant disease.3 The results of a multivariate outcome analysis of HST in WAS patients, however, encourage physicians to use this treatment, especially in patients under 5 years of age.12
We previously established a method for flow cytometric analysis of intracellular WASP expression (FCM-WASP) and reported that it was useful in the diagnosis of WAS in patients13 and carriers.14 Furthermore, during the course of WAS screening using FCM-WASP, we observed a patient with WAS who had a small population of lymphocytes in which a spontaneous reversion of the inherited WASP gene mutation had occurred.15 It was shown that the WASP gene–corrected cells had growth advantages over other cells. We concluded that the case could be a good example for showing that WASP is involved in cell growth.
In this study, we used FCM-WASP to evaluate the mixed chimera (MC) status of 12 patients with WAS who had undergone HST. Peripheral blood mononuclear cells (PBMCs) in WAS patients show little or no expression of intracellular WASP (WASPdim).13 14 No exceptional WAS patients have been found. Therefore, using FCM-WASP, we could distinguish between donor cells (WASPbright) and recipient cells (WASPdim), which allows analysis of MC status without difficulty in WAS patients who have received HST. Furthermore, with 2-color FCM-WASP, MC status could be characterized in different cell lineages. We conclude that FCM-WASP is a useful follow-up method in patients with WAS who have undergone HST. WASP may also have a role in the development of hematopoietic cells in vivo.
Patients, materials, and methods
Patients
The study included 12 patients with a clinical diagnosis of WAS confirmed by genetic studies. The WAS mutations in these patients, some of which were previously reported,14 16 are listed in Table 1. All patients underwent either cord blood stem cell transplantation (CBT) or bone marrow transplantation (BMT). Transplantation procedures were performed between July 1995 and June 2001 at 6 medical facilities in Japan. The median age of the patients at transplantation was 1.7 years (range, 0.3-4.6 years; Table 1). Five of the 12 patients underwent CBT with cells from an unrelated donor; the rest underwent BMT. The BMT donors were 4 unrelated donors, 2 phenotype-identical mothers (one carrier and one noncarrier), and an identical sister (noncarrier). Four of the 12 patients had a one-antigen–mismatched donor. Preparative regimens and prophylaxis for graft-versus-host disease (GVHD) are shown in Table 1.
Characteristics of all patients
| MC status/ patient no. . | Mutation . | Year of HST . | Age at HST . | Nucleated cells transplanted, no. . | Donor/ transplant type* . | HLA compatibility . | Conditioning regimen . | GVHD prophylaxis . | Acute GVHD grade . | Chronic GVHD . |
|---|---|---|---|---|---|---|---|---|---|---|
| With MC status after HST | ||||||||||
| 1 | 1 base deletion exon 9† | 2001 | 4 mo | 7.5 × 108 | U/CBT | 5/6 matched | TBI, BuCy, ATG | CsA, Mtx | 0 | No |
| 2 | Missense exon 4 (Ala134-Val) | 2000 | 1 y 2 mo | 1.42 × 108 | U/CBT | 5/6 matched | TBI, Cy, ATG | Mtx, FK506 | 1 | No |
| 3 | 1 base deletion exon/intron 11; abnormal splice skip exon 11 | 1999 | 1 y 2 mo | 8.9 × 108 | R/BMT | 6/6 matched | BuCy, ATG | CsA, Mtx | 0 | No |
| 4 | 1 base deletion exon 10† | 2001 | 2 y | 2.45 × 108 | U/BMT | 6/6 matched | TBI, BuCy, ATG | Mtx, FK506 | 0 | No |
| 5 | Large deletion | 1999 | 1 y 7 mo | 4.8 × 108 | U/BMT | 5/6 matched | TBI, Cy, ATG | Mtx, FK506 | 0 | Yes |
| 6 | Nonsense exon 7 (Arg211-stop)‡ | 1998 | 1 y 1 mo | 3.34 × 109 | U/BMT | 6/6 matched | BuCy, ATG | CsA, Mtx | 0 | No |
| Without MC status after HST | ||||||||||
| 7 | 2 bases deletion exon 1 | 2000 | 9 mo | 8.8 × 108 | U/CBT | 5/6 matched | TBI, BuCy, ATG | CsA, PDN | 1 | No |
| 8 | Missense exon 2 (Ile85-Thr)‡ | 2000 | 4 y 7 mo | 4.2 × 108 | U/BMT | 6/6 matched | TBI, BuCy, ATG | Mtx, FK506 | 3 | Yes |
| 9 | Missense exon 1 (Glu31-Lys)‡ | 1998 | 1 y 3 mo | 7.0 × 108 | U/CBT | 6/6 matched | BuCy | CsA, Mtx | 1 | Yes |
| 10 | Missense exon 1 (Glu31-Lys)‡ | 1996 | 1 y 7 mo | 8.9 × 108 | R/BMT | Compatible | BuCy | CsA, Mtx | 0 | No |
| 11 | 1 base deletion exon 1‡ | 1995 | 4 y 3 mo | 8.5 × 109 | R/BMT | 6/6 matched | BuCy, ATG | CsA, Mtx | 0 | No |
| 12 | 2 bases deletion exon 1 | 2001 | 7 mo | 2.9 × 108 | U/CBT | 6/6 matched | BuCy, ATG | CsA, PDN | 0 | No |
| MC status/ patient no. . | Mutation . | Year of HST . | Age at HST . | Nucleated cells transplanted, no. . | Donor/ transplant type* . | HLA compatibility . | Conditioning regimen . | GVHD prophylaxis . | Acute GVHD grade . | Chronic GVHD . |
|---|---|---|---|---|---|---|---|---|---|---|
| With MC status after HST | ||||||||||
| 1 | 1 base deletion exon 9† | 2001 | 4 mo | 7.5 × 108 | U/CBT | 5/6 matched | TBI, BuCy, ATG | CsA, Mtx | 0 | No |
| 2 | Missense exon 4 (Ala134-Val) | 2000 | 1 y 2 mo | 1.42 × 108 | U/CBT | 5/6 matched | TBI, Cy, ATG | Mtx, FK506 | 1 | No |
| 3 | 1 base deletion exon/intron 11; abnormal splice skip exon 11 | 1999 | 1 y 2 mo | 8.9 × 108 | R/BMT | 6/6 matched | BuCy, ATG | CsA, Mtx | 0 | No |
| 4 | 1 base deletion exon 10† | 2001 | 2 y | 2.45 × 108 | U/BMT | 6/6 matched | TBI, BuCy, ATG | Mtx, FK506 | 0 | No |
| 5 | Large deletion | 1999 | 1 y 7 mo | 4.8 × 108 | U/BMT | 5/6 matched | TBI, Cy, ATG | Mtx, FK506 | 0 | Yes |
| 6 | Nonsense exon 7 (Arg211-stop)‡ | 1998 | 1 y 1 mo | 3.34 × 109 | U/BMT | 6/6 matched | BuCy, ATG | CsA, Mtx | 0 | No |
| Without MC status after HST | ||||||||||
| 7 | 2 bases deletion exon 1 | 2000 | 9 mo | 8.8 × 108 | U/CBT | 5/6 matched | TBI, BuCy, ATG | CsA, PDN | 1 | No |
| 8 | Missense exon 2 (Ile85-Thr)‡ | 2000 | 4 y 7 mo | 4.2 × 108 | U/BMT | 6/6 matched | TBI, BuCy, ATG | Mtx, FK506 | 3 | Yes |
| 9 | Missense exon 1 (Glu31-Lys)‡ | 1998 | 1 y 3 mo | 7.0 × 108 | U/CBT | 6/6 matched | BuCy | CsA, Mtx | 1 | Yes |
| 10 | Missense exon 1 (Glu31-Lys)‡ | 1996 | 1 y 7 mo | 8.9 × 108 | R/BMT | Compatible | BuCy | CsA, Mtx | 0 | No |
| 11 | 1 base deletion exon 1‡ | 1995 | 4 y 3 mo | 8.5 × 109 | R/BMT | 6/6 matched | BuCy, ATG | CsA, Mtx | 0 | No |
| 12 | 2 bases deletion exon 1 | 2001 | 7 mo | 2.9 × 108 | U/CBT | 6/6 matched | BuCy, ATG | CsA, PDN | 0 | No |
U indicates unrelated; TBI, total-body irradiation; Bu, busulfan; Cy, cyclophosphamide; ATG, antithymocyte globulin; CsA, cyclosporin A; Mtx, methotrexate; FK506, tacrolimus hydrate; R, related; and PDN, prednisone.
Related donors were noncarrier mother for patient 3, noncarrier sister for patient 10, and carrier mother for patient 11.
Novel mutation.
Mutation previously reported by us.
Mutation analysis
Genomic DNA was purified from the patients' PBMCs or granulocytes by using Sepa Gene (Sanko Junyaku, Tokyo, Japan). The primers and polymerase chain reaction (PCR) conditions used were described previously.16 PCR was performed with a GeneAmp PCR system (9700; PE Applied Biosystems, Foster City, CA). Each PCR-amplified fragment was purified from agarose gel and was directly sequenced. Sequencing was performed with an ABI Prism Dye Terminator Cycle Sequencing Ready Reaction kit (PE Applied Biosystems) and an automated ABI 373A DNA analyzer (PE Applied Biosystems).
FCM-WASP
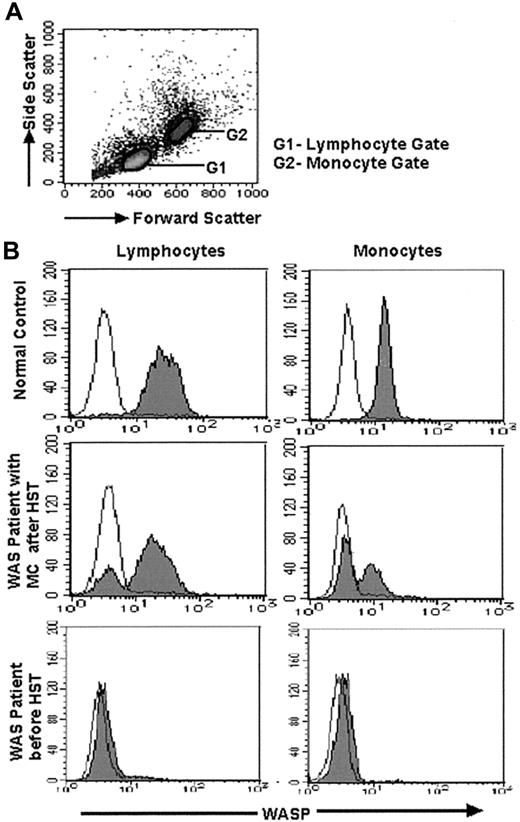
The methods used for FCM-WASP and 2-color FCM-WASP were reported previously.15 We developed 3-color FCM-WASP for further analysis. Briefly, PBMCs were stained for cell-surface antigens by using the following monoclonal antibodies (mAbs): phycoerythrin (PE)–conjugated anti-CD4, anti-CD8, and anti-CD56 (Southern Biotechnology Associates, Birmingham, AL); PE-conjugated anti-CD20 (Beckman-Coulter, Fullerton, CA); and PE-Cy5–conjugated anti-CD45RA and anti-CD45RO (eBioscience, San Diego, CA). After being washed twice, cells were treated with Cytofix/Cytoperm solution from a CytoStain kit (PharMingen, San Diego, CA) at 4°C for 60 minutes. Cells were then washed twice and incubated with 1:100 diluted anti-WASP (3F3-A5) mAb17 or purified mouse IgG1 (Becton Dickinson, Mountain View, CA) at 4°C for 30 minutes. The cells were then washed twice and allowed to react with 1:100 diluted fluorescein isothiocyanate–labeled goat anti–mouse IgG1 antibody (Southern Biotechnology Associates) at 4°C for 30 minutes. Stained cells were analyzed with a FACScan flow cytometer using CellQuest software (Becton Dickinson). WASP expression in lymphocytes and monocytes was determined after gating the respective distribution patterns by forward and side scatter (Figure1A). Because anti-WASP mAb belongs to the mouse IgG1 subclass, all antibodies for staining were of either the mouse IgG2 or IgG3 subclass to avoid a cross-reaction.
Results of FCM-WASP of lymphocytes and monocytes.
(A) Density plot shows forward- and side-scatter data from PBMCs. Lymphocytes (G1 gate) and monocytes (G2 gate) are gated according to the values of these variables. (B) FCM-WASP with a 3-decade log scale. The x-axis represents WASP expression; the y-axis represents cell numbers. The open histogram indicates isotype-control staining; the solid histogram indicates specific staining of WASP. Shown is WASP expression in lymphocytes and monocytes from a healthy control (top), a WAS patient with MC status after HST (middle; data from patient 2 on day 360 after HST), and a WAS patient (patient 12) before HST (bottom).
Results of FCM-WASP of lymphocytes and monocytes.
(A) Density plot shows forward- and side-scatter data from PBMCs. Lymphocytes (G1 gate) and monocytes (G2 gate) are gated according to the values of these variables. (B) FCM-WASP with a 3-decade log scale. The x-axis represents WASP expression; the y-axis represents cell numbers. The open histogram indicates isotype-control staining; the solid histogram indicates specific staining of WASP. Shown is WASP expression in lymphocytes and monocytes from a healthy control (top), a WAS patient with MC status after HST (middle; data from patient 2 on day 360 after HST), and a WAS patient (patient 12) before HST (bottom).
Results
Analysis of WAS mutations
Among our 12 patients with WAS, we identified 10 differentWASP mutations (Table 1). Patient 7 and patient 12 had the same mutation, as did patient 9 and patient 10, although none of these patients' families were related. We reported 5 of these mutations previously14 16; 2 mutations (in patients 1 and 4) were novel.
Evaluation of MC status by FCM-WASP
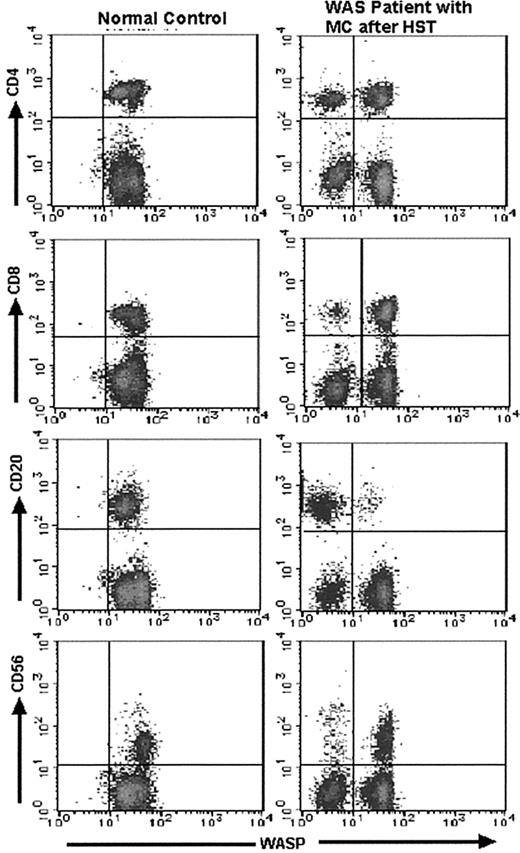
The lymphocyte and monocyte MC status of the WAS patients before and after HST was evaluated by FCM-WASP (Figure 1). Together with WASPbright donor cells, 6 of the 12 patients had WASPdim recipient monocytes and, of these, 5 had WASPdim recipient lymphocytes. In these patients, MC status persisted for at least 0.5 to 2.5 years after HST. In the other patients, MC status was not observed or observed only transiently. We also performed 2-color FCM-WASP using antibodies to CD4, CD8, CD20, and CD56 (Figure 2). With this method, we were able to characterize in more detail the MC status of these patients according to cell lineage.
Results of 2-color FCM-WASP.
Density plots are from 2-color FCM-WASP using CD4, CD8, CD20, and CD56 antibodies. All cells in the lymphocyte gate were analyzed. The x-axis represents WASP intensity; the y-axis represents the intensity of the respective CD marker. Shown are the results for patient 3 on day 720 after HST.
Results of 2-color FCM-WASP.
Density plots are from 2-color FCM-WASP using CD4, CD8, CD20, and CD56 antibodies. All cells in the lymphocyte gate were analyzed. The x-axis represents WASP intensity; the y-axis represents the intensity of the respective CD marker. Shown are the results for patient 3 on day 720 after HST.
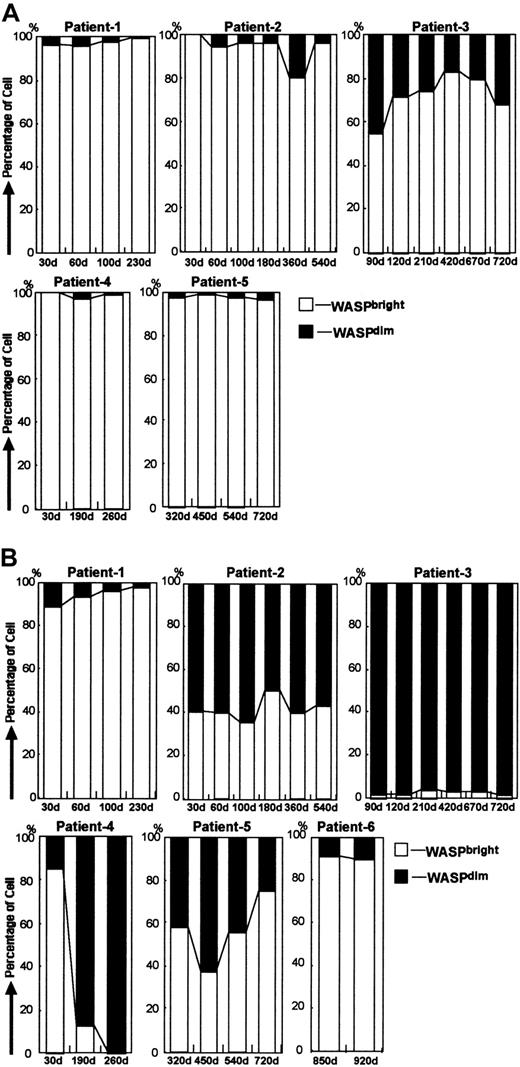
Follow-up of patients with MC status in PBMCs
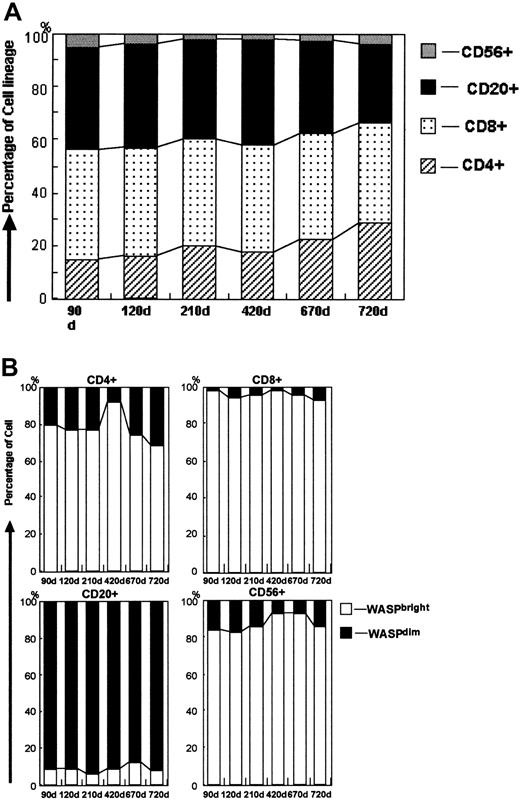
We used 2-color FCM-WASP to follow the 6 patients with MC status for 0.5 to 2.5 years after transplantation (Figure3). The MC patterns observed varied among the patients, although WASPdim recipient cells were found most frequently in the monocyte population (Table2). The number of WASPdimrecipient lymphocytes and monocytes in patient 1 appeared to diminish gradually, indicating that all hematopoietic cells would eventually be replaced by donor cells. In patients 2 and 5, we observed stable MC status with regard to monocytes, of which approximately half were found to be recipient cells. In contrast, most of the lymphocytes were WASPbright donor cells. Patient 2 had a transient increase in CD4+ WASPdim recipient cells at day 360 after HST, which was correlated with a WASPbright donor CD4+ increment (data not shown). The same phenomenon was observed on a different day after HST in patient 1. In patients 3 and 4, the monocytes were mostly WASPdim, whereas most of their lymphocytes were WASPbright, except for the CD20+ cells in patient 3. The precise MC status in patient 3, who consistently had WASPdim lymphocytes, is shown in Figure 4. It was noteworthy that most of his CD20+ cells were WASPdim. In patient 4, we observed a surprising conversion of the dominant proportion of monocytes from WASPbright to WASPdim 30 to 190 days after HST. Patient 6, who underwent HST more than 900 days before testing, had no recipient cells of any lymphocyte lineage, whereas approximately 10% of his monocytes remained WASPdim.
Follow-up studies in patients with MC status after HST.
(A) Bar charts show changes in MC status in lymphocytes obtained from 5 WAS patients after HST. Open bars and solid bars depict the proportion of lymphocytes with WASPbright donors and WASPdim recipients, respectively. (B) Bar charts show changes in MC status in monocytes obtained from 6 WAS patients after HST. Open bars and solid bars depict the proportion of monocytes with WASPbright donors and WASPdim recipients, respectively.
Follow-up studies in patients with MC status after HST.
(A) Bar charts show changes in MC status in lymphocytes obtained from 5 WAS patients after HST. Open bars and solid bars depict the proportion of lymphocytes with WASPbright donors and WASPdim recipients, respectively. (B) Bar charts show changes in MC status in monocytes obtained from 6 WAS patients after HST. Open bars and solid bars depict the proportion of monocytes with WASPbright donors and WASPdim recipients, respectively.
Findings in patients with MC status
| Recipient cells, %* . | Results of sequencing DNA from granulocytes . | Recipient cells in BM, % . | Platelet no., × 109/L/platelet vol, fL . | |||
|---|---|---|---|---|---|---|
| Patient no. . | Lymphocytes . | Monocytes . | Before HST . | After HST . | ||
| 1 | 1 (230) | 2 (230) | Normal ≫ mutation (30) | ND | 2.1/5.3 | 35.9/8.8 (104) |
| 2 | 4 (540) | 60 (540) | Mutation = normal (230) | 68 (260) | 1.4/5.4 | 10.8/7.3 (671) |
| 3 | 20 (720) | 99 (720) | Mutation ≫ normal (720) | 90 (730) | 1.1/5.2 | 2.8/ND (720) |
| 4 | 0.6 (260) | 99 (260) | Mutation ≫ normal (260) | 85 (170) | 4.0/5.2 | 2.2/4.4 (279) |
| 5 | 3.5 (720) | 25 (720) | NA | 32 (310) | 3.1/6.4 | 11.0/(ND) (655) |
| 6 | 0 (920) | 10 (920) | ND | ND | 4.3/5.9 | 25.0/8.5 (859) |
| Recipient cells, %* . | Results of sequencing DNA from granulocytes . | Recipient cells in BM, % . | Platelet no., × 109/L/platelet vol, fL . | |||
|---|---|---|---|---|---|---|
| Patient no. . | Lymphocytes . | Monocytes . | Before HST . | After HST . | ||
| 1 | 1 (230) | 2 (230) | Normal ≫ mutation (30) | ND | 2.1/5.3 | 35.9/8.8 (104) |
| 2 | 4 (540) | 60 (540) | Mutation = normal (230) | 68 (260) | 1.4/5.4 | 10.8/7.3 (671) |
| 3 | 20 (720) | 99 (720) | Mutation ≫ normal (720) | 90 (730) | 1.1/5.2 | 2.8/ND (720) |
| 4 | 0.6 (260) | 99 (260) | Mutation ≫ normal (260) | 85 (170) | 4.0/5.2 | 2.2/4.4 (279) |
| 5 | 3.5 (720) | 25 (720) | NA | 32 (310) | 3.1/6.4 | 11.0/(ND) (655) |
| 6 | 0 (920) | 10 (920) | ND | ND | 4.3/5.9 | 25.0/8.5 (859) |
Values in parentheses are the number of days after HST.
BM indicates bone marrow; ND, not determined; and NA, not available because of a large deletion mutation.
WASPdim on FCM-WASP.
Evaluation of the precise MC status in patient 3.
(A) Change in lymphocyte subsets (combined WASPbright and WASPdim cells) in patient 3 after HST. The 4 patterns in the bar show the proportions of CD4+, CD8+, CD20+, and CD56+ cells, respectively. (B) Change in MC status in each lymphocyte subset of CD4+, CD8+, CD20+, and CD56+ cells. Shown is the proportion of WASPbright donors (open bars) and WASPdim recipients (solid bars) in each subset.
Evaluation of the precise MC status in patient 3.
(A) Change in lymphocyte subsets (combined WASPbright and WASPdim cells) in patient 3 after HST. The 4 patterns in the bar show the proportions of CD4+, CD8+, CD20+, and CD56+ cells, respectively. (B) Change in MC status in each lymphocyte subset of CD4+, CD8+, CD20+, and CD56+ cells. Shown is the proportion of WASPbright donors (open bars) and WASPdim recipients (solid bars) in each subset.
Evaluation of MC status of granulocytes and platelets
Because of unknown technical reasons, FCM-WASP could not be used to detect WASP in myeloid cells. Instead, we tried to evaluate the MC status of the patients' granulocytes by directly sequencing DNA from these cells. This method could be used for every patient except patient 5, whose mutation was a large deletion of the WASP gene. However, donor type (sex and carrier status) must be taken into account for this evaluation; a cell from a male donor has a wild-type WASP allele, a normal cell from a female donor has 2 wild-type WASP alleles, and a cell from a female carrier has a mutant and a wild-type WASP allele. The MC status of the patients' granulocytes as evaluated by this method correlated roughly with that of his monocytes on FCM-WASP (Table 2). After HST, most patients showed an increase in the number of platelets with a normal mean volume. However, patients 3 and 4, most of whose monocytes and granulocytes were WASPdim, had persistent thrombocytopenia (Table 2).
MC status in bone marrow
Results of evaluations of MC status in bone marrow by fluorescence in situ hybridization (FISH) or polymorphic analysis with short tandem repeats are summarized in Table 2. Use of this method revealed that in patients 3 and 4, 84% to 90% of the bone marrow cells were of recipient origin. These patients are now being scheduled to receive a second HST.
Immunologic evaluation of patients who underwent HST
Serum IgM and IgE levels in patients before and after HST are shown in Table 3. After HST, most patients, including those with MC status, had an increase in serum IgM levels and a decrease in serum IgE levels. In patients 3, 4, and 8, however, serum IgE levels did not normalize after HST.
Immunologic results in patients with WAS before and after HST
| Patient no. . | IgM (g/L) . | IgE (kU/L) . | ||
|---|---|---|---|---|
| Before HST . | After HST . | Before HST . | After HST . | |
| 1 | 0.48 | 1.44 (377) | 2200 | 58 (391) |
| 2 | 0.18 | 2.46 (666) | 100 | < 5 (505) |
| 3 | 0.37 | 1.58 (832) | 1100 | 930 (882) |
| 4 | 0.24 | 1.45 (188) | 1800 | 500 (286) |
| 5 | 0.79 | 0.65 (652) | 230 | 69 (634) |
| 6 | 0.63 | 1.53 (859) | 6652 | 45 (859) |
| 7 | < 0.18 | 1.14 (579) | 115 | 47 (148) |
| 8 | 0.24 | 0.80 (476) | 36 | 1100 (559) |
| 9 | 0.22 | 0.93 (1187) | 732 | < 2 (1187) |
| 10 | 0.46 | 0.89 (1963) | 3372 | 47 (1963) |
| 11 | 0.31 | 0.58 (2229) | 600 | 56 (2229) |
| 12 | 0.22 | 0.70 (117) | 732 | 6 (117) |
| Patient no. . | IgM (g/L) . | IgE (kU/L) . | ||
|---|---|---|---|---|
| Before HST . | After HST . | Before HST . | After HST . | |
| 1 | 0.48 | 1.44 (377) | 2200 | 58 (391) |
| 2 | 0.18 | 2.46 (666) | 100 | < 5 (505) |
| 3 | 0.37 | 1.58 (832) | 1100 | 930 (882) |
| 4 | 0.24 | 1.45 (188) | 1800 | 500 (286) |
| 5 | 0.79 | 0.65 (652) | 230 | 69 (634) |
| 6 | 0.63 | 1.53 (859) | 6652 | 45 (859) |
| 7 | < 0.18 | 1.14 (579) | 115 | 47 (148) |
| 8 | 0.24 | 0.80 (476) | 36 | 1100 (559) |
| 9 | 0.22 | 0.93 (1187) | 732 | < 2 (1187) |
| 10 | 0.46 | 0.89 (1963) | 3372 | 47 (1963) |
| 11 | 0.31 | 0.58 (2229) | 600 | 56 (2229) |
| 12 | 0.22 | 0.70 (117) | 732 | 6 (117) |
Values in parentheses are the number of days after HST.
Naive/memory T cells of patients after HST
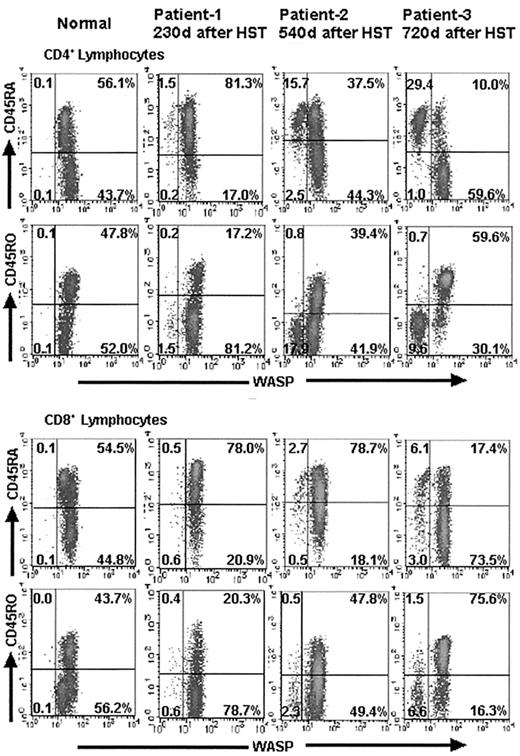
To determine the naive/memory profile of the donor and recipient T cells in the patients after HST, we performed 3-color FCM-WASP with anti-CD45RA or anti-CD45RO antibodies for each population of CD4+ and CD8+ T cells. We found that WASPbright donor T cells developed into CD45RA−/CD45RO+ memory cells during the first year after HST (Figure 5). In contrast, most of the WASPdim recipient T cells (both CD4+ and CD8+) remained CD45RA+/CD45RO− (naive) cells, even after more than a year. The results in patients 1, 2, and 3 at 230, 540, and 720 days, respectively, after HST are shown in Figure 5. These findings were more striking in CD4+ cells than in CD8+ cells.
Evaluation of the naive and memory profile of T cells in WAS patients after HST.
Density plots from 2-color immunofluorescence depict the results of 3-color FCM-WASP using anti-CD45RA or anti-CD45RO for each population of CD4+ and CD8+ T cells. All cells pictured were gated with a forward- and side-scatter lymphocyte gate combined with a CD4+ or CD8+ gate. The x-axis represents WASP staining intensity; the y-axis represents CD45RA or CD45RO expression. Numbers within the density plots represent the percentage of cells in each quadrant. It is noteworthy that in contrast to the WASPbright donor CD4+ and CD8+cells, most WASPdim recipient cells in patients 1, 2, and 3 remained CD45RA+/CD45RO− (naı̈ve) at the indicated days after HST.
Evaluation of the naive and memory profile of T cells in WAS patients after HST.
Density plots from 2-color immunofluorescence depict the results of 3-color FCM-WASP using anti-CD45RA or anti-CD45RO for each population of CD4+ and CD8+ T cells. All cells pictured were gated with a forward- and side-scatter lymphocyte gate combined with a CD4+ or CD8+ gate. The x-axis represents WASP staining intensity; the y-axis represents CD45RA or CD45RO expression. Numbers within the density plots represent the percentage of cells in each quadrant. It is noteworthy that in contrast to the WASPbright donor CD4+ and CD8+cells, most WASPdim recipient cells in patients 1, 2, and 3 remained CD45RA+/CD45RO− (naı̈ve) at the indicated days after HST.
Discussion
We here report an evaluation of MC status in 12 patients with WAS who underwent HST. With FCM-WASP, donor and recipient PBMCs could be distinguished easily. Moreover, the MC status of these patients according to cell lineage was characterized by using 2-color FCM-WASP. Although several follow-up studies in WAS patients who have undergone HST have been reported,12,18 19 this is the first study in which MC status of these patients was evaluated precisely. We found that 6 of the 12 patients with WAS evaluated (50%) had MC status lasting for at least 7 months (maximum, 2.5 years).
Ozsahin et al18 studied the outcome of BMT in patients with WAS and reported that 7 of 23 patients (30.4%) showed MC status, although these patients were not characterized in detail. Several factors likely contribute to the rate of MC status after BMT. The higher rate of MC status in our study, however, may be due to use of the FCM-WASP procedure, which seems to be more sensitive in detecting recipient cells than the method used in previous studies.
Findings in the WAS patients we studied who were positive for MC status are summarized in Table 2. The residual recipient cells were detected most consistently in the monocyte population. These results are consistent with those of our previous studies, which concluded that WAS carriers have a mixed population of WASPdim and WASPbright monocytes, whereas most carriers have no detectable WASPdim cells in the lymphocyte compartment.13 14 We speculate that there are in vivo differences among the hematologic cell lineages in their dependence on WASP during development and that monocytes are somehow less dependent on WASP than are other PBMCs. It is noteworthy that the dominant population among CD20+ cells in patient 3 was WASPdim. This may help pinpoint the position of B cells in the hierarchy of WASP dependency among the hematopoietic cells (monocytes have less WASP dependency than B cells, which have less WASP dependency than other lymphocytes).
We retrospectively surveyed our patients in an attempt to find other factors that could be related to MC status after HST. No significant differences were detected in type of mutation, type of donor, number of cells transplanted, regimen used for conditioning, or grade of GVHD (Table 1). We found a difference in the proportion of cells of the WASPbright donor cell lineage in the early phase after HST. Most patients without MC status or with transient MC status had a high number of CD56+ cells among their WASPbrightdonor cell populations (more than 50% earlier than day 90 after HST; data not shown). In contrast, in all patients with MC status except patient 1, CD56+ cells comprised less than 10% of the WASPbright donor population at that time (data not shown). It was previously reported that CD56+ cells recovered earlier than cells of other lineages (such as CD8+, CD4+, and CD20+ cells) after unrelated CBT.20 We do not know the mechanism involved, but these cells may help to provide favorable conditions for engraftment of donor cells after HST. On the other hand, the initial development of CD56+ cells may be an indicator of earlier engraftment and growth of donor hematologic stem cells of that lineage than of other lineages. It is possible that these cells are actually mature donor T cells that are mixed in during transplantation.21
We were not able to measure WASP expression in granulocytes by FCM-WASP for unknown reasons. There is controversy regarding whether mature granulocytes express WASP in their cytoplasm.5 However, the recent finding of X-linked neutropenia with WASP mutation (L270P) may indicate that WASP is at least expressed in myeloid precursor cells.22 To evaluate the MC status of granulocytes, we sequenced DNA from purified granulocytes from some of our patients with WAS. By comparing nucleotide signals of the wild-type and mutant at the mutation site and taking donor type into consideration, we could estimate roughly the proportion of donor and recipient cells in this cell population.15 The granulocyte proportion of donor to recipient cells seemed similar to that of the monocyte proportion (Table 2). We also evaluated platelet status after HST by assessing platelet numbers and mean volume. Although WASP expression in platelets was not studied, the origin of platelets after HST looked the same as that of monocytes. The number of platelets correlated roughly with the proportion of WASPbright in monocytes. After HST, persistent thrombocytopenia was observed in patients 3 and 4, and patients 2 and 5 had slightly low platelet counts (Table 2). We think that these results together indicate that the precursors of both granulocytes and platelets, similar to those of monocytes, are less WASP dependent during their development than are lymphocyte precursors.
The MC status of the bone marrow of some patients (2, 3, 4, and 5) was studied by using FISH or short tandem repeat analysis (Table 2). It is noteworthy that the proportion of recipient cells in bone marrow was approximately equal to that of monocytes studied by using FCM-WASP. Therefore, the peripheral blood monocyte profile may accurately represent MC status of the bone marrow in WAS patients after HST. As previously reported,14 patient 11 received hematopoietic stem cells from his carrier mother, and 10% to 20% of his monocytes were subsequently found to be WASPdim. However, karyotype analysis showed that these cells were actually derived from donor hematopoietic stem cells. The same WASPdim proportion was observed in monocytes from the patient's mother. Thus, in cases in which the donor is a WAS carrier, this problem must be taken into account when determining MC status. In patients 3 and 4, most of the donor cells appeared to be rejected, yet the lymphocytes in these patients were found to be predominantly WASPbright cells of donor origin (except the CD20+ cells in patient 3), suggesting again that WAS protein provides lymphocytes with a strong growth or survival advantage during development or circulation.
Using 3-color FCM-WASP, we evaluated the naive (CD45RA+/RO−) and memory/activated (CD45RA−/RO+) subsets of the patients' recipient and donor peripheral CD4+ and CD8+cells. Patients who underwent HST had naive donor T cells in their peripheral blood after 6 months. These cells were found to develop into memory/activated T cells during the next 6 months. In contrast, most recipient T cells remained CD45RA+/RO− (naive) a full year after HST; these findings were more striking in CD4+ cells than in CD8+cells. Patients with WAS were previously found to have a marked number of CD45RA−/RO+ T cells in their peripheral blood before HST.22 We also observed this in 2 of the patients we examined before HST, whose ratio of CD45RA+/RO− to CD45RA−/RO+ cells was comparable to that in age-matched controls (data not shown). Thus, the results obtained with 3-color FCM-WASP in this study may represent the outcome under a condition of competition between WASPbright and WASPdim memory T cells. The same phenomenon was observed during a study of somatic mosaicism induced by genetic reversion in a patient with WAS.23 Although the patient's CD45RA+ T cells consisted of an even number of WASPbright and WASPdim cells, most of the CD45RO+ T cells (> 90%) were WASPbright. Together, these results suggest that WASPbright T cells have a growth advantage over WASPdim T cells after stimulation with antigens, although a shortened lifespan of WASPdim memory T cells as a result of spontaneous apoptosis could also be responsible.24 25
Finally, we examined serum IgM and IgE levels in our patients before and after HST to evaluate immunologic status. Most patients, including those with MC status, had an increase in serum IgM and a decrease in serum IgE levels after HST. It is interesting that patient 3 also had a marked increase in IgM. Because most of his B cells and monocytes, but not his T cells, were WASPdim, this finding may indicate that WASPbright T cells, especially memory T cells, play a key role in normal IgM production in WAS patients after HST. Contrary to our expectations, however, patients 3 and 4 regained approximately the same IgE levels, and patient 8 had an increase in IgE after HST. A larger number of cases are required to assess the use of IgE levels for immunologic evaluations after HST, because regulation of IgE seems to be complicated and WASP is not the only molecule involved in this process.
We conclude that FCM-WASP is a useful method for follow-up of patients with WAS who have undergone HST. Our findings may also have important implications regarding the in vivo role of WASP during the development of hematopoietic cells.
We thank Professor K. Kobayashi for critical comments on this article and the medical staff for excellent patient care.
Prepublished online as Blood First Edition Paper, April 30, 2002; DOI 10.1182/blood-2002-01-0211.
Supported by grant H12 genome 003 from the Ministry of Health, Labour and Welfare, Japan, and grant 13670776 from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Tadashi Ariga, Research Group of Human Gene Therapy, Hokkaido University, Graduate School of Medicine, N-14, W-7, Kita-ku, Sapporo, Japan, 060-8638.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal