Tumor necrosis factor (TNF) has marked effects on permeability and procoagulant activity on tumor-associated neovasculature when used in isolation perfusion, the latter effect primarily mediated via induction of cell surface expression of tissue factor (TF) on endothelial tissue. However, the cellular events that result in rapid alterations in endothelial cell (EC) permeability after intravascular TNF administration in isolation perfusion are not well characterized. We demonstrate that short exposure intervals to TNF induces TF expression on ECs but has no effect on permeability as assessed by flux of Evans blue–bound albumin across confluent EC monolayers using a 2-compartment model under basal culture conditions. However, a rapid and significant increase in EC permeability occurred with TNF in the presence of factor VIII–deficient plasma. Permeability was induced only with luminal versus abluminal TNF exposure and was blocked by antithrombin III, TF pathway inhibitor, or anti-TF antibody cotreatment. These data indicate that EC surface expression of TF and extrinsic clotting factors are critical in augmenting capillary leak following intravascular TNF administration. Alterations in permeability were associated with intercellular gap formation at sites of down-regulation of vascular endothelial (VE)–cadherin expression, the primary endothelial intercellular adhesion molecule, and intracellular contraction and alignment of F-actin cytoskeletal elements. Rapid induction of TF by TNF may be the primary EC response that results in alterations in permeability and procoagulant activity observed following intravascular TNF administration in isolation perfusion.
Introduction
Tumor necrosis factor (TNF) and melphalan administered via isolated organ perfusion of the limb (isolated limb perfusion, ILP) or liver (isolated hepatic perfusion) are under active clinical evaluation. Complete response rates between 60% and 80% for patients with in-transit extremity melanoma,1,2 limb salvage rates greater than 80% for patients with unresectable high-grade extremity sarcoma,3-5 and partial response rates of up to 74% for patients with advanced unresectable primary or metastatic cancers confined to liver6 7 have been reported following isolated organ perfusion using TNF and melphalan. ILP with TNF and melphalan has been approved in Europe for patients with high-grade unresectable extremity sarcoma.
The characteristics of tumor regression following isolated organ perfusion with TNF and melphalan are quite rapid and distinct compared with the characteristics of response following melphalan alone.8,9 After isolated perfusion with TNF, there is immediate tumor softening and edema presumably due to local augmentation of capillary leak in the tumor bed8,10followed by rapid necrosis and eschar formation of superficial tumors and cystic degeneration of large deep-seated tumors associated with obliteration of tumor neovasculature.11-13 Tumor response appears to correlate with the completeness of ablation of tumor neovasculature in patients with extremity sarcoma treated with ILP using TNF and melphalan.14 These data coupled with the known wide spectrum of tumor histologies sensitive to TNF and melphalan in isolation perfusion are consistent with the prevailing noesis that TNF exerts its effects primarily on the tumor-associated neovasculature. However, TNF can result in significant regional toxicity after isolated organ perfusion and, if there is a perfusate leak of only 5% or less during treatment, systemic toxicity can also be severe.15-17 To that end, insights into the mechanisms responsible for the augmentation of capillary permeability and procoagulant activity induced by TNF during isolated perfusion will help in the refinement of its clinical use.
There are laboratory data characterizing the procoagulant activity18-20 and changes in vascular permeability21-23 on endothelial cells (ECs) following exposure to TNF, which can vary depending on the experimental conditions used.24,25 These 2 actions are thought to represent distinct cellular responses to TNF. The former is due to the induction of cell surface expression of tissue factor (TF) via activation of the nuclear factor–κB and the AP-1 transcription factor family26 and is responsible for the activation of the extrinsic coagulation cascade. The latter has been ascribed to down-regulation of αVβ3 integrin expression on ECs following exposure to TNF.27 However, the alterations in αVβ3 expression associated with endothelial permeability after TNF may not be a reflection of the mechanisms responsible for augmentation of vascular permeability in clinical isolation perfusion. First, decreased αVβ3 integrin expression with associated loss of EC adhesion by TNF is observed only in the presence of interferon, which has no established role in mediating clinical antitumor effects and the use of which has been abandoned in isolation perfusion.1 2Second, the effects on cell adhesion are only apparent after long exposure intervals (24 hours) to TNF that are not consistent with the effects observed in clinical use.
The current experiments were conducted to characterize the effects of TNF on ECs under conditions comparable to its use in isolation perfusion. Our data show that a brief (90 minutes) exposure to TNF has minimal activity on the permeability of EC monolayers under basal culture conditions but that a rapid (within 30 minutes) increase in permeability occurs in the presence of plasma deficient in factor VIII. The permeability effects are blocked by antithrombin III (AT III), tissue factor pathway inhibitor (TFPI), and anti-TF antibody cotreatment, implicating an essential role for EC surface expression of TF and the extrinsic clotting cascade in augmenting capillary leak following TNF exposure. The changes in permeability occur in association with a down-regulation of the primary endothelial intercellular adhesion molecule, vascular endothelial (VE)–cadherin, and with a marked intracellular reorganization and contraction of F-actin cytoskeletal elements. Together, these data provide a novel and essential role for TF and the extrinsic clotting cascade in augmenting capillary permeability across endothelial monolayers immediately following TNF exposure under conditions achieved following intravascular TNF in clinical isolation perfusion.
Materials and methods
Reagents
Thrombin, tetramethyl rhodamine-isothiocyanate–phalloidin, and ethylenediaminetetraacetic acid were obtained from Sigma (St Louis, MO). AT III was obtained from Biogen (Cambridge, MA), TNF was obtained from Knoll Pharmaceuticals (Whippany, NJ), and TFPI and a monoclonal antihuman tissue factor antibody (anti-TF antibody) were obtained from American Diagnostica (Greenwich, CT). Anti–VE-cadherin (polyclonal goat immunoglobulin G [IgG] sc-6458), blocking peptide (sc-6458), and goat ant–irabbit IgG were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). For immunofluorescence studies, secondary antibodies were purchased from Molecular Probes (Eugene, OR). Recombinant human TF was purchased from American Diagnostica.
Cell culture
Human umbilical vein endothelial cells (HUVECs) (Clonetics, Walkersville, MD) were maintained at 37°C in a 5% CO2incubator in EGM-2 media (basal endothelial cell media [EBM-2] enriched with hydrocortisone, fibroblast growth factor, insulinlike growth factor–1, ascorbic acid, epidermal growth factor, GA-1000 [gentamicin/amphotericin], and heparin). They were passaged for no more than 5 generations in the experiments described. Cells were plated at a cell density of 100 000/cm2 either on fibronectin-coated glass cover slides (22 × 22 mm, Becton-Dickinson, Bedford, MA) in 6-well plates for use in immunfluorescence studies or on Transwell polycarbonate membranes (1-μm pore size, 23.1-mm diameter) in triplets for permeability assays. Cells were cultured for 3 days in EGM-2 medium. At day 2, nonadherent cells were removed and medium was replaced with fresh EGM-2.
TF induction assay
HUVECs were plated at a cell density of 100 000/cm2in a 6-well multiplate (Corning Costar Corporation, Cambridge, MA). After 72 hours (at day 2 nonadherent cells were removed and EGM-2 medium was added), cells were washed twice with phosphate-buffered saline (PBS) and treated in triplicate with TNF. At the experimental time points HUVECs were washed twice with sterile PBS. One milliliter of lysis buffer (25 mM Tris, pH 8) was added per well and incubated for 10 minutes at 37°C followed by freezing at −80°C for 3 hours. Cells were then thawed at 37°C, harvested using mechanical scrapers, and centrifuged at 2000g for 5 minutes. The cell pellet was then resuspended in 120 μL of 50 nM Tris/100 nM NaCl/0.1% bovine serum albumin (BSA) and stored at −80°C until assayed.
A 1-stage coagulation assay was performed by adding 100 μL factor VIII–deficient human plasma (George-King Biomedical, Overland Park, KS) to 100 μL EC suspension and incubating for 2 minutes at 37°C. The reaction was catalyzed by the addition of 100 μL of 30 nM CaCl2: Clotting time was measured using an Amelung microcoagulation analyzer (Sigma). A standard curve of TF activity was generated by reconstituting 50 ng lipidated human recombinant TF (American Diagnostica) in 100 μL filtered deionized water according to the manufacturer's specifications. Serial dilutions of stock solution were used to generate a standard curve with upper and lower detection limits of 25 and 0.395 ng/mL (470 to 7 pM/mL).
Permeability of HUVECs cultured on Transwell filters was assayed using Evans blue bound to BSA; 2 mg Evans blue (FLUKA, Milwaukee, WI) was added per 100 mL Dulbecco PBS with calcium and magnesium and 0.1% BSA (Life Technologies/GIBCO, Rockville, MD).
At day 3, EGM-2 media were replaced with EBM-2 (EBM-2/2% fetal bovine serum [FBS]) containing 2% FBS supplemented with TNF (1 μg/mL) into the luminal or abluminal chamber as indicated and incubated for 3 hours at 37°C in a 5% CO2 incubator. In experiments with abluminal TNF stimulation, EBM-2 supplemented with 2% FBS was added luminally. TNF/ EBM-2/2% FBS was aspirated luminally or abluminally, and cells were briefly washed with PBS with Ca++ and Mg++. Fresh EBM-2 was added to the luminal chamber supplemented with 1% factor VIII–deficient plasma (George-King Biomedical) and incubated for the designated times. In experiments evaluating inhibitors of thrombin, 50 μL AT III (5 μM) was added 2 hours after TNF and cells incubated for 1 additional hour at 37°C. In experiments evaluating the effects of TF inhibition, 2 μL TFPI (100 ng/μL) or 10 μL anti-TF antibody (1 mg/mL) was added to inserts 2 hours after TNF and incubated for an additional hour at 37°C. The inserts were then washed and media replaced with EBM-2 containing the appropriate inhibitors prior to the addition of 1% factor VIII–deficient plasma and incubated for the indicated times. Then, inserts were washed with PBS with Ca++ and Mg++, and 1.5 mL Evans blue bound to 0.1% BSA in PBS with Ca++ and Mg++ was added to the luminal chamber; 2 mL PBS with Ca++ and Mg++ was added to the abluminal (lower) chamber. After 1 hour, 100 μL from the abluminal chamber was removed, and the absorbance was determined with a spectrophotometer at 620 nm (DU 530; Beckman, Fullerton, CA). Thrombin (2 U/mL) and ethylenediaminetetraacetic acid (50 mM) were used as positive controls. The experiments were repeated no fewer than 3 times. Data shown are from representative experiments.
Immunofluorescence
HUVECs were grown on glass cover slides and treated in the same manner as described for the permeability assay except that samples were treated for 30 minutes and 90 minutes. Samples were fixed with 3.7% paraformaldehyde for 10 minutes at room temperature. Slides were washed 5 times with PBS with Ca++ and Mg++ and permeabilized with 0.5% Triton X for 20 minutes. Primary antibody against VE-cadherin (polyclonal goat IgG from Santa Cruz Biotechnology; VE-cadherin: sc-6458) was used at a dilution of 1:200 in PBS with 1% BSA and incubated for 6 hours at 4°C. To confirm the specificity of the anti–VE-cadherin antibody, the immunizing peptide (sc-6458P) was preincubated with primary antibody for 30 minutes at room temperature. Cells were washed in PBS and incubated with Alexa Fluor 488 donkey anti–goat IgG (Molecular Probes) for 1 hour at room temperature. Cells were washed again with PBS with Ca++ and Mg++and subsequently stained with tetramethyl rhodamine-isothiocyanate–phalloidin at a concentration of 5 μg/mL for 40 minutes at room temperature for visualizing actin filaments. Cells were washed again with PBS and mounted with SlowFade Light (Molecular Probes) and sealed. Photomicrographs were taken with a Zeiss Axiophot (Zeiss, Oberkochen, Germany) at × 400.
Statistical analysis
Data are given as mean ± SD and analyzed using the Student t test (2-sided).
Results
TNF induction of TF expression on ECs
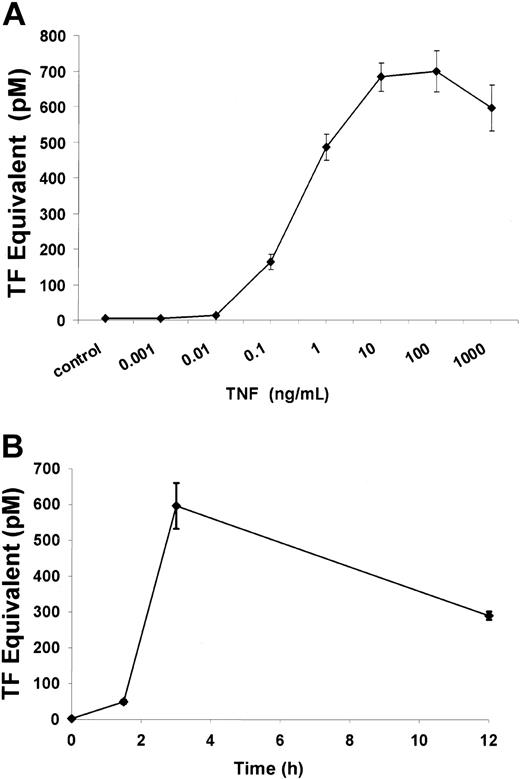
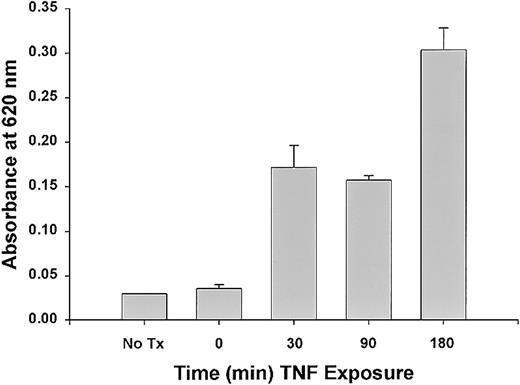
To assess whether TNF is able to induce tissue (TF) on ECs under the conditions being tested, HUVECs were exposed to TNF added to culture media at increasing concentrations. As seen in Figure1, TNF induced TF expression in HUVECs in a time- and dose-dependent manner; significant increases in TF expression were achieved with TNF concentrations above 0.1 ng/mL and at 3 hours of TNF exposure. After 12 hours of TNF exposure at 1 μg/mL, TF expression was still significantly elevated compared with untreated controls (289.6 ± 11.3 pM vs 1.8 pM; P < .005).
TF expression in HUVECs induced by TNF.
TNF induces TF activity in a dose-dependent manner (A). TF was quantitated by a 1-stage clotting assay as described in “Materials and methods.” Incubation of ECs with increasing concentrations of TNF from 1 pg/mL to 1 μg/mL for 3 hours demonstrated increased TF activity with doses above 1 ng/mL. Significant TF activity was observed after a 3-hour exposure to 1 μg/mL TNF (B).
TF expression in HUVECs induced by TNF.
TNF induces TF activity in a dose-dependent manner (A). TF was quantitated by a 1-stage clotting assay as described in “Materials and methods.” Incubation of ECs with increasing concentrations of TNF from 1 pg/mL to 1 μg/mL for 3 hours demonstrated increased TF activity with doses above 1 ng/mL. Significant TF activity was observed after a 3-hour exposure to 1 μg/mL TNF (B).
Requirement for factor VIII–deficient plasma for TNF permeability on ECs
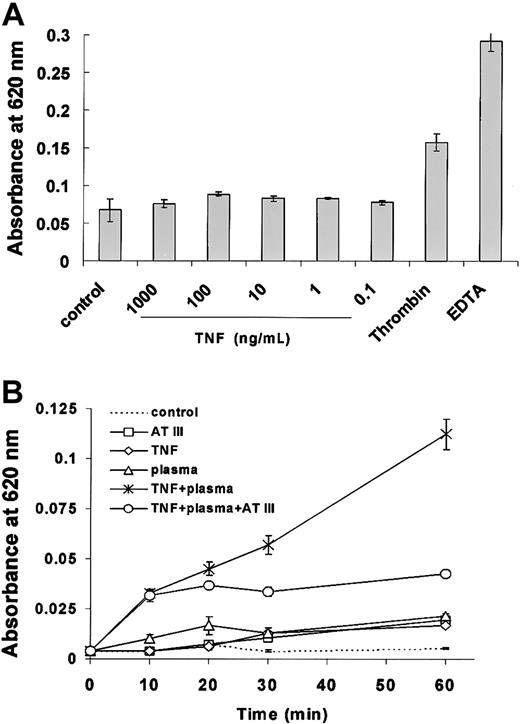
To determine effects of TNF permeability across a functional EC monolayer, HUVECs were exposed to various concentrations of the protein. After exposure to TNF alone for 90 minutes (Figure2) or 3 hours (data not shown), there was no effect on EC permeability at any doses tested. However, when factor VIII–deficient plasma was added to cells following exposure to TNF, the barrier function of ECs changed rapidly and significantly. As seen in Figure 2, EC monolayer permeability increased within minutes following addition of factor VIII–deficient plasma to HUVECs that were previously treated for 90 minutes with TNF. Average absorbances at 620 nm were 0.0327 at 10 minutes, 0.045 at 20 minutes, 0.057 at 30 minutes, and 0.112 at 60 minutes and significantly higher compared with untreated HUVECs (P < .005 at 30 and 60 minutes). The effect of factor VIII–deficient plasma on TNF-pretreated EC monolayers was largely abrogated by AT III, implicating a central role for the activated extrinsic clotting cascade in mediating TNF permeability under these conditions.
TNF-induced permeability across EC monolayers is dependent on factor VIII–deficient plasma.
Permeability across functional EC monolayers was determined by measuring absorbance at 620 nm 1 hour following TNF treatment for 90 minutes as described in “Materials and methods.” TNF in the range of 1 μg/mL to 100 pg/mL did not result in any increase in permeability under basal culture conditions (A). However, TNF induced a rapid and significant increase in permeabiliity in the presence of factor VIII–deficient plasma and was partially blocked by AT III (B). Permeability was unaffected by factor VIII–deficient plasma, AT III, or TNF alone. Each value is the mean ± SD of triplicates in one representative experiment.
TNF-induced permeability across EC monolayers is dependent on factor VIII–deficient plasma.
Permeability across functional EC monolayers was determined by measuring absorbance at 620 nm 1 hour following TNF treatment for 90 minutes as described in “Materials and methods.” TNF in the range of 1 μg/mL to 100 pg/mL did not result in any increase in permeability under basal culture conditions (A). However, TNF induced a rapid and significant increase in permeabiliity in the presence of factor VIII–deficient plasma and was partially blocked by AT III (B). Permeability was unaffected by factor VIII–deficient plasma, AT III, or TNF alone. Each value is the mean ± SD of triplicates in one representative experiment.
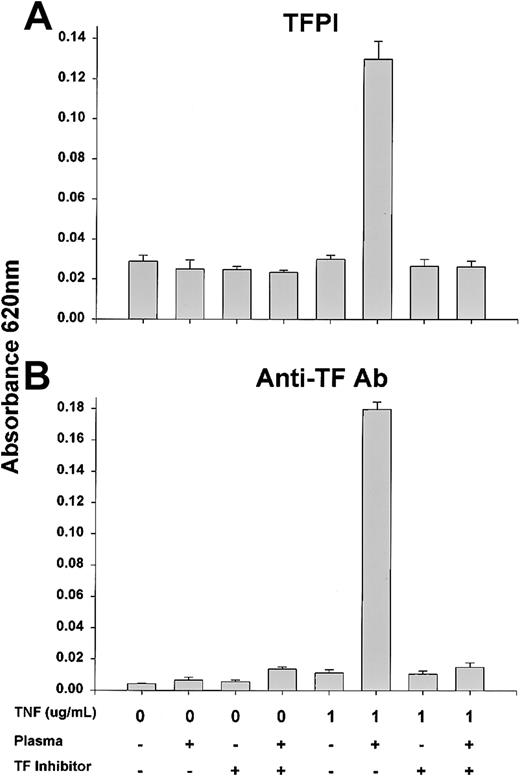
To assess whether TF was directly responsible for the permeability effects observed after exposure to TNF and plasma, EC monolayers were exposed to 1 μg/mL TNF with or without factor VIII–deficient plasma and specific inhibitors of TF activity. The addition of recombinant TFPI or a neutralizing anti-TF antibody as described in “Materials and methods” completely abrogated the permeability effects of TNF and plasma (Figure 3).
TNF-induced permeability across EC monolayers is abrogated by TFPI or anti-TF antibody.
Permeability across functional EC monolayers was determined by measuring absorbance at 620 nm 1 hour following TNF treatment for 90 minutes with or without TFPI (A) or anti-TF antibody (B) as described in “Materials and methods.” There was a significant increase in permeability in the presence of TNF and factor VIII–deficient plasma that was largely abrogated by TF inhibition.
TNF-induced permeability across EC monolayers is abrogated by TFPI or anti-TF antibody.
Permeability across functional EC monolayers was determined by measuring absorbance at 620 nm 1 hour following TNF treatment for 90 minutes with or without TFPI (A) or anti-TF antibody (B) as described in “Materials and methods.” There was a significant increase in permeability in the presence of TNF and factor VIII–deficient plasma that was largely abrogated by TF inhibition.
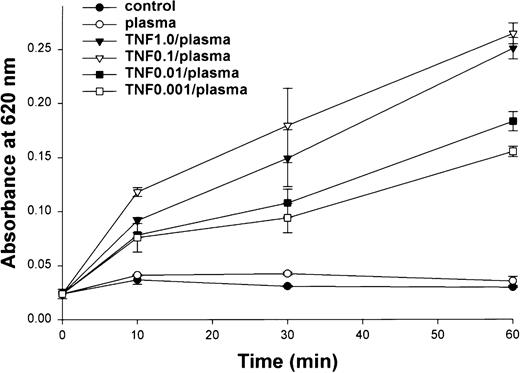
Permeability induced by TNF in the presence of factor VIII–deficient plasma across EC monolayers was somewhat time- and dose-dependent. There was a prompt and significant increase in permeability with plasma following a 90-minute exposure to TNF at doses between 0.001 and 1 μg/mL (Figure 4). Sixty minutes after the addition of plasma, the effects of TNF on permeability were greatest with 1 and 0.1 μg/mL TNF. After ECs were exposed to 1 μg/mL TNF for increasing intervals and permeability assessed 60 minutes after addition of plasma, the greatest increase in permeability was observed after a 3-hour TNF pretreatment (Figure5).
Dose-dependent effects of TNF on EC monolayer permeability.
Permeability across functional EC monolayers was determined by measuring absorbance at 620 nm after a 90-minute exposure to TNF at various concentrations. TNF doses of 1.0 and 0.1 μg/mL resulted in a significantly greater effect on permeability after 60 minutes than lower doses.
Dose-dependent effects of TNF on EC monolayer permeability.
Permeability across functional EC monolayers was determined by measuring absorbance at 620 nm after a 90-minute exposure to TNF at various concentrations. TNF doses of 1.0 and 0.1 μg/mL resulted in a significantly greater effect on permeability after 60 minutes than lower doses.
Time-dependent effects of TNF on EC monolayer permeability.
Significant changes in EC monolayer permeability 60 minutes after addition of plasma were observed after a 30-minute treatment with 1 μg/mL TNF and were greatest after a 3-hour treatment.
Time-dependent effects of TNF on EC monolayer permeability.
Significant changes in EC monolayer permeability 60 minutes after addition of plasma were observed after a 30-minute treatment with 1 μg/mL TNF and were greatest after a 3-hour treatment.
Permeability across endothelial monolayers is dependent on luminal exposure to TNF
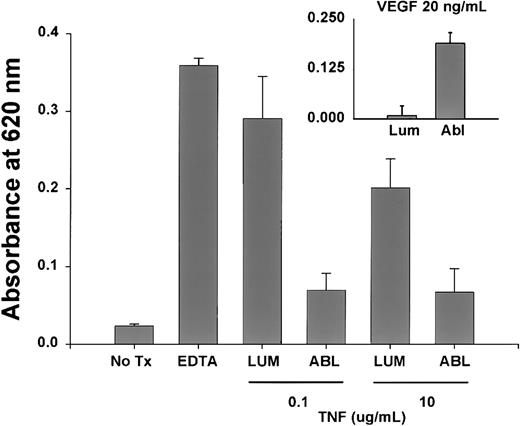
To determine if change in permeability secondary to TNF was dependent on the site of TNF exposure, functional EC monolayers were treated with 0.1 or 10 μg/mL TNF placed in the upper well (luminal) or lower well (abluminal). Although EC monolayers treated with abluminal TNF had a modest increase in permeability compared with control EC monolayers, there was a significantly greater effect when the same TNF dose was administered to the luminal EC surface (Figure6). Interestingly, vascular endothelial growth factor induced EC permeability in this model only with abluminal exposure, indicating a site-specific response to luminal versus abluminal TNF (Figure 6, inset).
Site-dependent effect of TNF on EC monlayer permeability.
Significant increases in EC permeability were observed only with a 90-minute luminal (lum) versus abluminal (abl) exposure to 0.1 and 10 μg/mL TNF as described. Under identical conditions, vascular endothelial growth factor caused a significant increase in permeability with abluminal exposure, demonstrating a true specificity for the luminal TNF response.
Site-dependent effect of TNF on EC monlayer permeability.
Significant increases in EC permeability were observed only with a 90-minute luminal (lum) versus abluminal (abl) exposure to 0.1 and 10 μg/mL TNF as described. Under identical conditions, vascular endothelial growth factor caused a significant increase in permeability with abluminal exposure, demonstrating a true specificity for the luminal TNF response.
TNF and activation of the extrinsic clotting pathway leads to a disassembly of endothelial adherent junctions via alteration of VE-cadherin
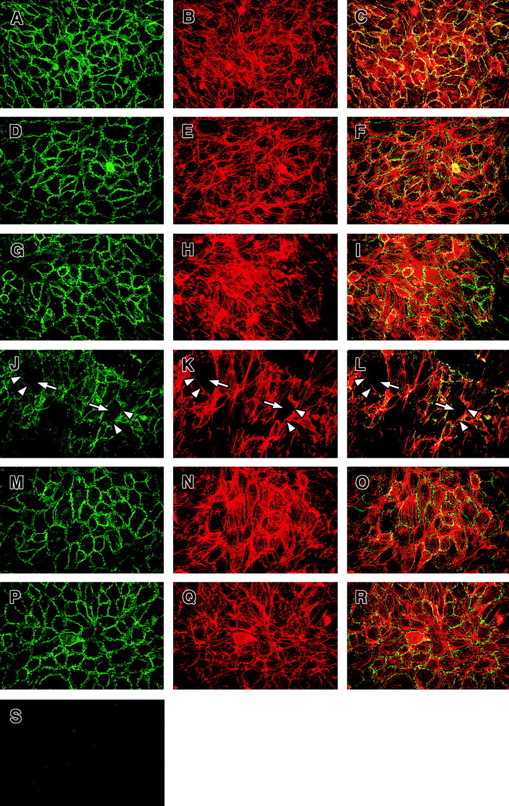
The VE-cadherin complex anchors to F-actin on ECs, which modifies the function and localization of VE-cadherin, the main complex responsible for maintaining intercellular tight junctions in endothelium.28 To explore whether the activation of the extrinsic pathway of the clotting system leads to a change in the function of VE-cadherin and the F-actin distribution in ECs, we performed immunofluorescence staining of EC monolayers exposed to factor VIII–deficient plasma following TNF treatment. As seen in Figure 7, in quiescent confluent EC monolayers there was homogeneous staining and distribution of VE-cadherin along cellular junctions. In untreated ECs forming an intact and functional confluent monolayer, the staining pattern of F-actin showed a dense circumferential belt and colocalization with VE-cadherin resulting in a diffuse yellow staining pattern in these areas.
Effect of factor VIII–deficient plasma on VE-cadherin distribution on ECs.
HUVECs were grown on fibronectin-coated glass cover slides, stained for VE-cadherin and F-actin, and visualized by double immunofluorescence (original magnification × 40) as described in “Materials and methods.” Overlays show F-actin in red and VE-cadherin in green; colocalization appears in yellow. (A-C) Staining for VE-cadherin and F-actin in untreated cells. VE-cadherin and F-actin staining of ECs exposed to factor VIII–deficient plasma without TNF (D-F), TNF alone (G-I), factor VIII–deficient plasma on ECs pretreated with TNF (J-L), factor VIII–deficient plasma pretreated with TNF in the presence of AT III (M-O), and AT III alone (P-R). Slides from a 90-minute time point are shown. Note the gaps between the adjacent ECs (white arrows) and the lack of VE-cadherin (white arrowheads) only on ECs exposed to factor VIII–deficient plasma pretreated with TNF (J-L). Preabsorbtion of anti–VE-cadherin antibody with an appropriate peptide abolished the VE-cadherin staining (S).
Effect of factor VIII–deficient plasma on VE-cadherin distribution on ECs.
HUVECs were grown on fibronectin-coated glass cover slides, stained for VE-cadherin and F-actin, and visualized by double immunofluorescence (original magnification × 40) as described in “Materials and methods.” Overlays show F-actin in red and VE-cadherin in green; colocalization appears in yellow. (A-C) Staining for VE-cadherin and F-actin in untreated cells. VE-cadherin and F-actin staining of ECs exposed to factor VIII–deficient plasma without TNF (D-F), TNF alone (G-I), factor VIII–deficient plasma on ECs pretreated with TNF (J-L), factor VIII–deficient plasma pretreated with TNF in the presence of AT III (M-O), and AT III alone (P-R). Slides from a 90-minute time point are shown. Note the gaps between the adjacent ECs (white arrows) and the lack of VE-cadherin (white arrowheads) only on ECs exposed to factor VIII–deficient plasma pretreated with TNF (J-L). Preabsorbtion of anti–VE-cadherin antibody with an appropriate peptide abolished the VE-cadherin staining (S).
When pretreated with TNF to induce cell surface expression of TF, EC monolayers exposed to factor VIII–deficient plasma resulted in a marked change in the staining for both VE-cadherin and F-actin over time. As shown in Figure 7A-C, untreated EC monolayers had uniform cell membrane–associated staining for VE-cadherin (green) and cytosolic F-actin (red) with colocalization of these elements appearing as yellow. Ninety minutes after adding factor VIII–deficient plasma to TNF-treated ECs, there was loss of cell-to-cell contact with intercellular gap formation (Figure 7, arrows in panels J-L) and loss of VE-cadherin staining specifically in areas where intercellular gaps had formed. F-actin staining became irregular with clustering and alignment of F-actin stress fibers at sites distinct from the cell membrane. In ECs treated with factor VIII–deficient plasma alone (Figure 7D-F), TNF alone (Figure 7G-I), or AT III alone (Figure 7P-R), there was a slight increase in intracellular alignment of F-actin fibers (Figure 7E,H,Q, respectively) and less colocalization with VE-cadherin (more green staining in Figure 7F,I,R, respectively) compared with untreated cells. Changes in VE-cadherin and F-actin staining patterns were also observed in ECs treated with TNF and factor VIII–deficient plasma in the presence of AT III (Figure 7M-O). The mild changes in VE-cadherin and F-actin staining observed in the various control groups are consistent with the small but measurable increases in EC permeability observed under these conditions (Figure2). Alterations in VE-cadherin and F-actin staining were observable within 30 minutes after adding factor VIII–deficient plasma (not shown).
Discussion
These data show that rapid augmentation of permeability by TNF across EC monolayers requires the presence of plasma containing the factors responsible for the extrinsic clotting cascade and luminal versus abluminal exposure to the cytokine. Because plasma alone had no significant effect on permeability and the effects of TNF and plasma were blocked by AT III, TFPI, and anti-TF antibody cotreatment, the primary mechanism of TNF-induced permeability appears to be the induction of EC surface expression of TF. The rapid changes in permeability following exposure to luminal TNF are consistent with its effects following intravascular administration in clinical isolation perfusion.16 These data suggest that cell surface expression of TF may be the common initiating event through which the initial changes in permeability and subsequent procoagulant effects of TNF are mediated in vivo and, in particular, during isolation perfusion. In mice bearing a meth A fibrosarcoma, there is evidence of rapid intravascular fibrin deposition and erythrostasis followed by thrombosis within hours of intravenous TNF administration.19 In clinical isolation perfusion there is gross and histologic evidence of immediate tumor softening and localized edema8-10 within hours of treatment followed by obliteration of tumor neovasculature.11-13,29 In fact, the complete ablation of neovasculature following TNF and melphalan ILP has been shown to predict response in patients with extremity sarcoma.14 However, it is important to also recognize that TNF alone has no clinically meaningful antitumor activity in isolation perfusion, suggesting that the vascular effects may be important but not sufficient to cause tumor regression.29,30 Together, these data suggest that TNF may act as a “gatekeeper” that initially augments delivery of melphalan into tumor tissue by increasing vascular permeability and subsequently causing selective retention of melphalan in the tumor interstitium via obliteration of tumor-associated vasculature. Although there are considerable data to support this hypothesis, it is quite possible that other factors such as hyperthermia or other unknown perfusion factors may be contributing significantly to the vascular or antitumor effects seen in isolation perfusion.31
The central role of TF in mediating not only the procoagulant but the permeability effects of TNF suggests a single mechanism for these 2 distinct endothelial responses. Previous data have shown that down-regulation of αVβ3, the cell surface integrin primarily responsible for anchoring endothelial tissue to basement membrane,27 might be responsible for the disruption of vasculature from TNF. However, the conditions under which TNF caused loss of cell adhesion to basement membrane were not consistent with the rapid effects observed in tumor vasculature by TNF in preclinical and clinical studies.11 19 Our data show that within hours after exposure to TNF that intercellular gap formation occurs in association with decreased VE-cadherin expression and reorganization of the F-actin cytoskeletal elements in endothelial tissue.
Finally, in a broader perspective these data provide supporting evidence for the role of TF in the initiation of tumor-associated angiogenesis. Alteration in vascular permeability is one of the earliest events in neovessel formation and allows extravasation of fibrin into the interstitium to serve as a provisional matrix for subsequent EC migration and neoangiogenesis.32 Tumor cell lines that have high cell surface expression of TF have increased growth and metastatic potential compared with low-expressing lines.33 34 Our data would suggest that tumor cells expressing high levels of TF that are entrapped in microvessels or in the interstitial space might provide an alternate source of TF that promotes localized vascular permeability and interstitial fibrin matrix deposition.
J.F. is the recipient of a grant from the Max Kade Foundation, New York, NY.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
H. Richard Alexander, Head, Surgical Metabolism Section, Division of Clinical Sciences, NCI, Building 10, Room 2B07, Bethesda, MD 20892; e-mail: richard_alexander@nih.gov.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal