This study is focused on the functional significance of neutrophil lactosylceramide (LacCer)–enriched microdomains, which are involved in the initiation of a signal transduction pathway leading to superoxide generation. Treatment of neutrophils with anti-LacCer antibody, T5A7 or Huly-m13, induced superoxide generation from the cells, which was blocked by PP1, a Src kinase inhibitor; wortmannin, a phosphatidylinositol-3 kinase inhibitor; SB203580, a p38 mitogen-activated protein kinase (MAPK) inhibitor; and H7, an inhibitor for protein kinase C. When promyelocytic leukemia HL-60 cells were differentiated into neutrophilic lineage by dimethyl sulfoxide (DMSO) treatment, they acquired superoxide-generating activity but did not respond to anti-LacCer antibodies. Density gradient centrifugation revealed that LacCer and Lyn were recovered in detergent-insoluble membrane (DIM) of neutrophils and DMSO-treated HL-60 cells. However, immunoprecipitation experiments indicated that LacCer was associated with Lyn in neutrophils but not in DMSO-treated HL-60 cells. Interestingly, T5A7 induced the phosphorylation of Lyn in neutrophils but not in DMSO-treated HL-60 cells. Moreover, T5A7 induced the phosphorylation of p38 MAPK in neutrophils. T5A7-induced Lyn phosphorylation in neutrophil DIM fraction was significantly enhanced by cholesterol depletion or sequestration with methyl-β-cyclodextrin or nystatin. Collectively, these data suggest that neutrophils are characterized by the presence of cell surface LacCer-enriched glycosphingolipid signaling domain coupled with Lyn and that the ligand binding to LacCer induces the activation of Lyn, which may be suppressibly regulated by cholesterol, leading to superoxide generation through the phosphatidylinositol-3 kinase–, p38 MAPK–, and protein kinase C–dependent signal transduction pathway.
Introduction
Human neutrophils play the first line of defense against invading microorganisms and an important role in acute inflammatory reaction.1 They exert their bactericidal activities through the mechanisms that lead to the destruction of microorganisms, involving the generation of toxic oxygen derivatives by reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and release of microbicidal molecules from the granules.
Glycosphingolipids (GSLs) are membrane components consisting of hydrophobic ceramide lipid and hydrophilic sugar moieties.2 They are characterized by clustering to form microdomains in plasma membrane and, also, in liposome phospholipid bilayers even in the absence of cholesterol. GSL clusters are essentially insoluble in detergent and constitute “detergent-insoluble membrane” (DIM; also termed DRM [detergent-resistant membrane domains] or GEM [glycolipid-enriched membrane]), which is separable from soluble components by density gradient centrifugation.3 A number of studies have shown that DIM contains several transducer molecules such as Src family kinases4 and that microdomains form functional units, termed lipid rafts or caveolae, which mediate signal transduction and cell functions.5 Recent studies on gangliosides6-9 have proposed the concepts that GSL clusters form GSL signaling domains (GSDs), and extracellular stimuli are transmitted via GSL clusters to signal transducer molecules, leading to cellular functions.2
It has been demonstrated that lactosylceramide (LacCer, CDw17; Galβ4Glcβ1Cer) activates NADPH oxidase to modulate the intercellular adhesion molecule-1 expression on human umbilical vein endothelial cells10 and to induce the proliferation of human aortic smooth muscle cells.11 Therefore, it is possible that LacCer activates NADPH oxidase, thereby affecting the functions of superoxide-producing cells. In mature neutrophils, more than 70% of GSLs are LacCer.12 LacCer is not expressed on the surface of myeloblasts and promyelocytes but is aberrantly expressed at high levels on mature neutrophils.13Moreover, it has been demonstrated that anti-LacCer antibodies induced superoxide production by neutrophils14 and that LacCer itself up-regulates CD11/CD18 on neutrophils.15Interestingly, Gram-negative bacteria, Gram-positive bacteria, and fungi can bind to LacCer.16 Therefore, LacCer is likely involved in neutrophil functions such as superoxide generation. However, the mechanisms by which LacCer activates NADPH oxidase in neutrophils have not yet been well characterized. To elucidate the mechanisms involved, we have isolated and characterized the LacCer-enriched DIM from neutrophils. Here we provide the evidence that LacCer forms GSDs coupled with Src family kinase Lyn in neutrophils and that LacCer-mediated Lyn activation causes the activation of phosphatidylinositol-3 kinase (PI-3K), p38 mitogen-activated protein kinase (MAPK), and protein kinase C, leading to NADPH oxidase activation.
Materials and methods
Materials and antibodies
Mouse anti-LacCer monoclonal immunoglobulin M (IgM) T5A7 and anti-GM3 monoclonal IgG DH2 established as described previously17,18 were a generous gift from Dr Sen-itiroh Hakomori (Department of Pathobiology, University of Washington, Seattle). Rabbit anti-p22phox and anti-gp91phox polyclonal antibodies were prepared as described before.19 Mouse anti-LacCer monoclonal IgM Huly-m13 (Ancell, Bayport, MN); goat antimouse IgG F(ab′)2(ICN Pharma, Costa Mesa, CA); rabbit antihuman focal adhesion kinase IgG (Upstate Biotechnology, Lake Placid, NY); rat antimouse-IgM IgG R6-60.2 and phycoerythrin-labeled mouse anti-CD11b monoclonal IgG ICRF44 (BD PharMingen, San Diego, CA); and mouse anti–Ha-ras monoclonal IgG Ab-1 (Oncogene Research Products, Boston, MA) were used. Mouse anti-Lyn monoclonal IgG, mouse anti–Tyr-182 phosphorylated p38 MAPK monoclonal IgM D-8, and other antibodies used here were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). LacCer (bovine) was from Matreya (Pleasant Gap, PA). Phosphatidylethanolamine, phosphatidylserine, phosphatidylcholine, phosphatidylinositol, cholesterol, diisopropyl fluorophosphonate (DFP), phenylmethylsulfonyl fluoride (PMSF), dimethyl sulfoxide (DMSO), N-formylmethionine-leucine-phenylalanine (fMLP), nystatin, and methyl β-cyclodextin were purchased from Sigma Chemical (St Louis, MO). PP1, a pharmacological inhibitor of Src-family tyrosine kinase, was from Biomol Research Laboratories (Plymouth Meeting, PA). Wortmannin, SB203580, and PD98059 were obtained from Merck Japan (Tokyo, Japan).
Cells
Human neutrophils were isolated from heparinized peripheral blood of healthy volunteers by Polymorphprep (Nycomed Pharma, Oslo, Norway) centrifugation as described previously20 and suspended at 107 cells per milliliter in Dulbecco phosphate-buffered saline (PBS) containing 1 mM MgSO4 and 1 mM CaCl2. Differential cell counts with Wright-Giemsa stain showed a purity above 95%.
Myeloid leukemia cell line HL-60 cells were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum. For differentiation into neutrophilic lineage cells, HL-60 cells were cultured with 1.3% DMSO for 5 days. Differentiation was confirmed by CD11b expression on DMSO-treated HL-60 cells using flow cytometry as described previously.21
Preparation of low-density DIM and LacCer-enriched DIM
Cells (2 × 107/mL) were pretreated with 5 mM DFP in PBS for 10 minutes on ice. After centrifugation at 270gfor 10 minutes, cell pellet (2 × 108 cells) was lysed in the 1 mL of lysis buffer A containing 1% Triton X-100, 10 mM Tris-HCl (pH 7.5), 150 mM NaCl, 5 mM ethylenediaminetetraacetic acid (EDTA), 1 mM DFP, and 1/20 (vol/vol) Complete (Roche Diagnostics, Mannheim, Germany) at 4°C and followed by sucrose density gradient centrifugation according to the method described previously.9 A white light–scattering band located at about 5% to 7% sucrose was collected and termed “DIM fraction.” A series of fractions were taken starting from the top, and 100 μL or 20 μL aliquots were subjected to high-performance thin-layer chromatography (HPTLC) or sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE)/immunoblotting analysis, respectively. Protein content of each fraction was determined using a MicroBCA kit (Pierce Chemical, Rockford, IL).
LacCer-enriched DIM fraction was isolated from DIM fractions of neutrophils and HL-60 cells using the combination of anti-LacCer monoclonal antibody (mAb) Huly-m13 and rat antimouse IgM mAb-bound protein G–Sepharose beads (Amersham Pharmacia Biotech, Tokyo, Japan) as described previously.22
Lipid components of DIM fractions were extracted with chloroform/methanol (2/1 [vol/vol]) solution and isolated using Bond Elut-C18 packed columns (Analytichem International, Harbor City, CA) and then analyzed by 2-dimensional thin-layer chromatography as described previously.22
Immunoblot analysis
Immunoblot analysis was performed as previously described.20 Briefly, proteins separated on 7.5% SDS-PAGE under reducing conditions were electroblotted onto polyvinylidene fluoride (PVDF) membranes (Immobilon-P; Millipore, Bedford, MA). The blots were blocked with BlockAce (Dainippon Pharmacy, Osaka, Japan), probed with primary antibodies, and then incubated with horseradish peroxidase–conjugated secondary antibodies. The reacted proteins were detected by SuperSignal chemiluminescent substrate (Pierce).
In some experiments, the LacCer-enriched DIM fraction was subjected to immunoslot blot analysis, and LacCer was detected using anti-LacCer mAb Huly-m13 as described previously.22 Under these conditions, Huly-m13 reacted with only LacCer standard but not other GSL standards (data not shown).
Measurement of superoxide generation
A total of 100 μL of rat antimouse IgM mAb solution (10 μg/mL in PBS) was incubated in 96-well culture plates (Iwaki Glass, Tokyo, Japan) overnight at 4°C. After washing with PBS, 100 μL mouse anti-LacCer IgM T5A7, Huly-m13, or mouse normal IgM (10 μg/mL in PBS) was added to the wells and then incubated overnight at 4°C. Alternatively, 96-well culture plates were first coated with goat antimouse IgG F(ab′)2 solution and then incubated with mouse anti-GM3 IgG DH2 or normal mouse IgG. After washing, the secondary antibody-coated wells were treated with 100 μL of 0.1% heat-inactivated bovine serum albumin in PBS for 1 hour at room temperature. After 3 washes, cells (2.5 × 105/100 μL) in PBS containing 1 mM MgSO4, 1 mM CaCl2, and 60 μM cytochrome c with or without 50 μg/mL superoxide dismutase were added to 96-well plates and incubated for 30 minutes at 37°C. In some experiments, cells (2 × 107/mL) were preincubated with 1 μg/mL LacCer for 30 minutes at 4°C or 10 mM methyl-β-cyclodextrin at 25°C for 30 minutes and washed with PBS, and then superoxide generation was analyzed. In case of fMLP and LacCer stimulation, cells were incubated with 0.1 μM fMLP, 1 μg/mL LacCer, or 0.5% DMSO (as a solvent control) in bovine serum albumin–treated noncoated wells for 30 minutes at 37°C. The reaction was terminated by placing on ice, followed by centrifugation at 190g for 8 minutes. The absorbance at 550 nm of the supernatants was measured with a Beckman DU640 spectrophotometer (Beckman Instruments, Fullerton, CA), and the value of cytochrome c reduction was calculated using the formula E550 (molecular extinction coefficient at 550 nm) = 2.1 × 104M−1cm−1.23
In some experiments, neutrophils were preincubated for 30 minutes at 4°C in the presence of 0.0005 to 200 μM Src family kinase–specific inhibitor PP1, PI-3K–specific inhibitor wortmannin, p38 MAPK–specific inhibitor SB203580, protein kinase C inhibitor H7, MAPK-1 inhibitor PD98059, or 0.5% DMSO as a solvent control and then analyzed for the superoxide generation.
Measurement of NADPH oxidase activity in a cell-free system was performed using neutrophil DIM (fraction 5) and the high-density (fractions 9-12) fractions per second at final concentrations of 2 × 107 equivalent cells per milliliter as described previously.23
Assay for the kinase activity in neutrophils and DMSO-treated HL-60 cells
Culture dishes (10 cm) were coated with 5 mL of 10 μg/mL anti-LacCer mAb T5A7 or normal IgM solutions as described above; 5 mL of 2.5 × 106 cells per milliliter in PBS containing 1 mM MgSO4 and 1 mM CaCl2 were added to the coated dishes and incubated for 2, 5, or 10 minutes at 37°C. The reaction was terminated by placing on ice, and cells were harvested using PBS containing 5 mM EDTA, followed by treatment with 5 mM DFP for 10 minutes on ice. Cells were washed and then lysed in the lysis buffer B (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 2 mM NaF, 1 mM EDTA, 1 mM ethyleneglycotetraacetic acid, 1 mM PMSF, 1/20 (vol/vol) Complete, and 1% Triton X-100) and sonicated for 10 seconds with 10% duty cycle at output 2.5 using an ultrasonic disruptor UD-201 (Tominaga Works, Tokyo, Japan). After centrifugation at 440g for 5 minutes, the supernatant was recovered and precleared by incubating with 50 μL protein G–Sepharose. Then the supernatant was incubated with mouse anti-Lyn mAb H2 or rabbit anti–p38 MAPK IgG (1 μg IgG per milliliter) overnight at 4°C, followed by incubation with 50 μL protein G–Sepharose beads for 2 hours.
To measure the Lyn activation, the immunoprecipitated beads were washed 3 times with lysis buffer B and twice with kinase buffer (30 mM HEPES [pH 7.5], 10 mM MgCl2, 2 mM MnCl2, 1 mM CaCl2). The immunoprecipitants were incubated at 37°C for 5 minutes with 10 μM adenosine triphosphate (ATP) and 5 μCi [γ-32P]-ATP (18.5 KBq; 3000 Ci/mM, NEN Life Science Products, Boston, MA). Samples were placed on ice, centrifuged at 440g for 5 minutes, and then the Sepharose beads were boiled with SDS sample buffer containing 5% 2-mercaptoethanol. The samples were run on 7.5% SDS-PAGE and blotted on PVDF membranes. Autoradiography was carried out by exposing the electroblotted membranes to Fuji x-ray film (Tokyo, Japan) at −80°C with intensifying screens (Dupont Lightning Plus, Wilmington, DE). After autoradiography, the blotted membranes were probed with rabbit anti-Lyn IgG, and Lyn was detected using SuperSignal reagent. Detected bands were scanned, and the intensities (optical density) of the autoradiograms (phosphorylated Lyn) and chemiluminescence signals (Lyn protein) were quantified using Scion image (Scion, Frederick, MD) for calculation of Lyn activities. The activity of Lyn was expressed as fold increase compared with that in the cells kept on ice.
To measure p38 MAPK activation, anti–p38 MAPK IgG-precipitated beads were run on 7.5% SDS-PAGE and blotted on PVDF membranes. The blots were probed with mouse anti–-pp38 MAPK mAb D-8, and phosphorylated p38 MAPK was detected using SuperSignal reagent. To determine the amount of p38 MAPK in each band, the membranes were reprobed with rabbit anti–p38 MAPK IgG. Detected bands were scanned, and the intensities of the chemiluminescence signals were quantified for calculation of p38 MAPK activities.
Assay for the Lyn and p38 MAPK activation in DIM
Activation of Lyn and p38 MAPK in neutrophil DIM fraction was assayed according to the method described previously.22 In brief, 5 mL of the diluted neutrophil DIM fraction (2 μg/mL) was added to the anti-LacCer mAb T5A7–coated 10-cm dishes, followed by incubation for 15 hours on ice. An aliquot of 50 μCi (1.85 MBq) [γ32P]-ATP was added to each dish, and the mixtures were incubated at 37°C for 5 minutes. The reaction was terminated by placing on ice and addition of 5 mL ice-cold 2 × concentrated stop buffer (15 mM HEPES [pH 7.5], 150 mM NaCl, 5 mM EDTA, 1 mM Na3VO4, 1% Triton X-100). The reaction mixtures were corrected and precipitated with 10% trichloroacetic acid. Then the pellets were dissolved in 1.0 mL lysis buffer B or radioimmunoprecipitation assay (RIPA) buffer (30 mM HEPES [pH 7.4], 150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, 5 mM EDTA, 1 mM Na3VO4, 50 mM NaF, 1 mM PMSF, 1/20 [vol/vol] Complete). The solubilized materials were added with 1 μg/mL anti-Lyn IgG or antiphospho p38 MAPK IgM and incubated at 4°C overnight, followed by incubation with 20 μL protein G–Sepharose or rat antimouse IgM-bound protein G–Sepharose beads at 4°C for 2 hours. After washing, the bound materials on the Sepharose beads were run on 7.5% SDS-PAGE under reducing conditions and blotted on polyvinylidene difluoride (PVDF) membranes, followed by autoradiography. To evaluate the effect of PP1 on T5A7-induced Lyn and p38 MAPK activation, 100 nM PP1 was added to the antibody-coated dishes and incubated with DIM for 30 minutes at 4°C before kinase assay.
In some experiments, the diluted DIM fraction was treated with 60 μg/mL nystatin, 10 mM methyl-β-cyclodextrin, or 0.5% DMSO, as a solvent control and incubated for 30 minutes at 25°C. After treatment, the mixtures were centrifuged at 400 000g for 1 hour, and the resultant DIM pellets were resuspended in 5 mL of the kinase buffer and used for activation of Lyn.
Subcellular fractionation
Subcellular fractionation of neutrophils was performed by Percoll centrifugation techniques as described previously,24 and the 4 visible bands obtained after centrifugation were designated from the bottom as the α band, containing azurophil granules (azurophil granule fraction); the β1 band, containing specific granules (specific granule fraction); the β2 band, containing gelatinase granules (gelatinase granule fraction); and the γ band, containing plasma membrane and secretory vesicles (plasma membrane fraction). The resultant supernatant above the γ band was termed the cytosol fraction. The recovery of marker enzymes were analyzed as described previously.24
Results
Anti-LacCer antibody induces superoxide generation by neutrophils but not DMSO-treated HL-60 cells
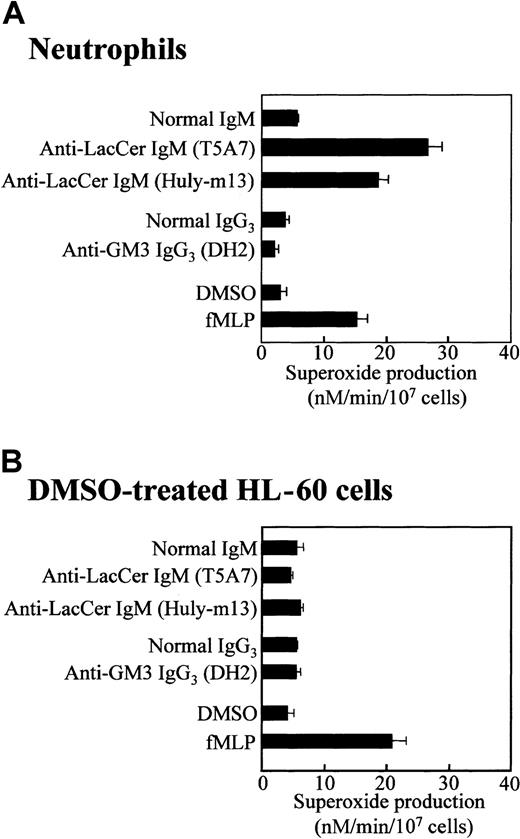
Neutrophils express several kinds of GSLs, such as LacCer and GM3,12 and anti-LacCer mAbs have been demonstrated to activate neutrophils.14 As shown in Figure1A, anti-LacCer mAbs T5A7 and Huly-m13 induced superoxide generation from neutrophils (26.6 ± 2.2 and 18.1 ± 2.7 nM/min/107 cells, respectively, mean ± SD of 4 experiments), whereas anti-GM3 mAb DH2 did not induce the superoxide generation. These results confirm that neutrophils generate superoxide anion via the LacCer-mediated signaling.14
Anti-LacCer antibody induced superoxide generation by neutrophils but not DMSO-treated HL-60 cells.
Neutrophils and DMSO-treated HL-60 cells were incubated at 37°C for 30 minutes in 96-well plates coated with anti-LacCer IgM T5A7, anti-LacCer IgM Huly-m13, normal IgM, anti-GM3 IgG3 DH2, or normal IgG3. As a positive control of superoxide generation, cells were incubated with 10−7 M fMLP or 0.5% DMSO (as a solvent control) in bovine serum albumin–coated wells at 37°C for 30 minutes. Superoxide production was calculated by measurement of the superoxide dismutase–inhibitable reduction of cytochrome c at 550 nm. Each bar shows the mean ± SD of 4 independent experiments.
Anti-LacCer antibody induced superoxide generation by neutrophils but not DMSO-treated HL-60 cells.
Neutrophils and DMSO-treated HL-60 cells were incubated at 37°C for 30 minutes in 96-well plates coated with anti-LacCer IgM T5A7, anti-LacCer IgM Huly-m13, normal IgM, anti-GM3 IgG3 DH2, or normal IgG3. As a positive control of superoxide generation, cells were incubated with 10−7 M fMLP or 0.5% DMSO (as a solvent control) in bovine serum albumin–coated wells at 37°C for 30 minutes. Superoxide production was calculated by measurement of the superoxide dismutase–inhibitable reduction of cytochrome c at 550 nm. Each bar shows the mean ± SD of 4 independent experiments.
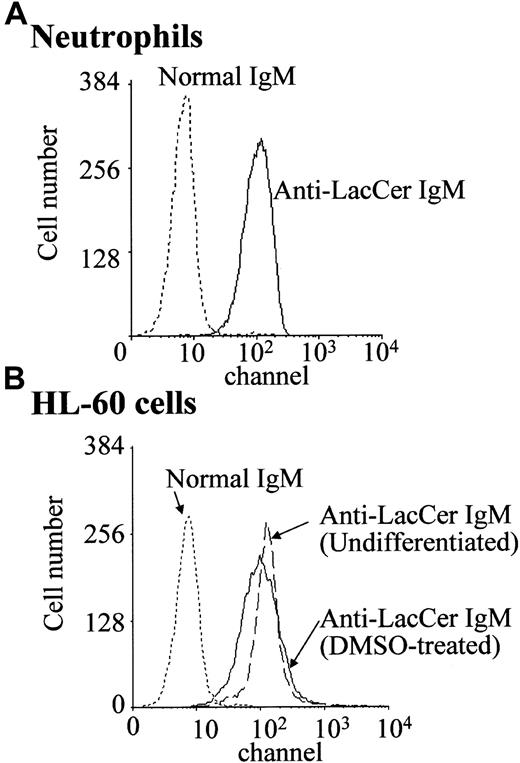
In the presence of DMSO, a leukemic promyelocytic cell line HL-60 cell differentiates into neutrophilic lineage cell.25 DMSO-treated HL-60 cells express fMLP receptors and NADPH oxidase components.19 25 Flow cytometric analysis revealed that, regardless of DMSO treatment, HL-60 cells expressed almost the same amount of LacCer on plasma membrane as that of neutrophils (Figure 2). Unexpectedly, neither LacCer mAb T5A7 nor Huly-m13 induced superoxide generation by the DMSO-treated HL-60 cells, although these cells could produce superoxide in response to fMLP (Figure 1B). Thus, our findings indicate that DMSO-treated HL-60 cells expressed LacCer but could not respond to anti-LacCer antibodies.
Expression of LacCer on neutrophils and HL-60 cells.
Neutrophils and undifferentiated and DMSO-treated HL-60 cells were incubated with anti-LacCer IgM T5A7 or normal IgM for 30 minutes at 4°C. After washing with PBS, cells were stained with fluorescein isothiocyanate–labeled rabbit antimouse IgM antibody and analyzed by flow cytometry.
Expression of LacCer on neutrophils and HL-60 cells.
Neutrophils and undifferentiated and DMSO-treated HL-60 cells were incubated with anti-LacCer IgM T5A7 or normal IgM for 30 minutes at 4°C. After washing with PBS, cells were stained with fluorescein isothiocyanate–labeled rabbit antimouse IgM antibody and analyzed by flow cytometry.
LacCer has been reported to enhance superoxide generation from neutrophils.15 Thus, we examined the effect of exogenously added LacCer on superoxide generation from DMSO-treated HL-60 cells. LacCer induced superoxide generation by 2 × 106 cells per milliliter neutrophils (3.0 ± 0.2 [solvent] vs 6.3 ± 0.6 [LacCer] nM/min/107 cells, n = 4), whereas LacCer did not induce superoxide generation from DMSO-treated HL-60 cells (4.3 ± 0.5 [solvent] vs 3.8 ± 0.4 [LacCer] nM/min/107 cells). Moreover, LacCer loading hardly affected anti-LacCer antibody-induced superoxide generation (4.5 ± 0.7 [solvent] vs 4.2 ± 0.2 [LacCer loading] nM/min/107cells, n = 4) from DMSO-treated HL-60 cells, although LacCer loading slightly enhanced superoxide generation by neutrophils from 3.0 ± 0.1 to 4.7 ± 0.3 nM/min/107 cells. These results suggest that exogenously added LacCer is unlikely involved in the signal transduction in DMSO-treated HL-60 cells.
Distribution patterns of LacCer and transducer molecules in fractions separated by sucrose density gradient centrifugation
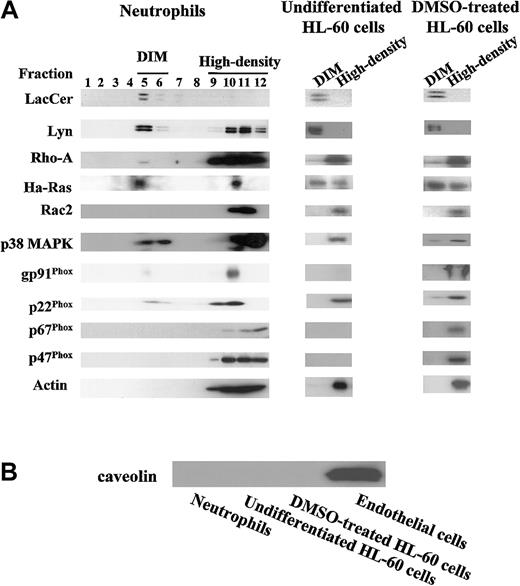
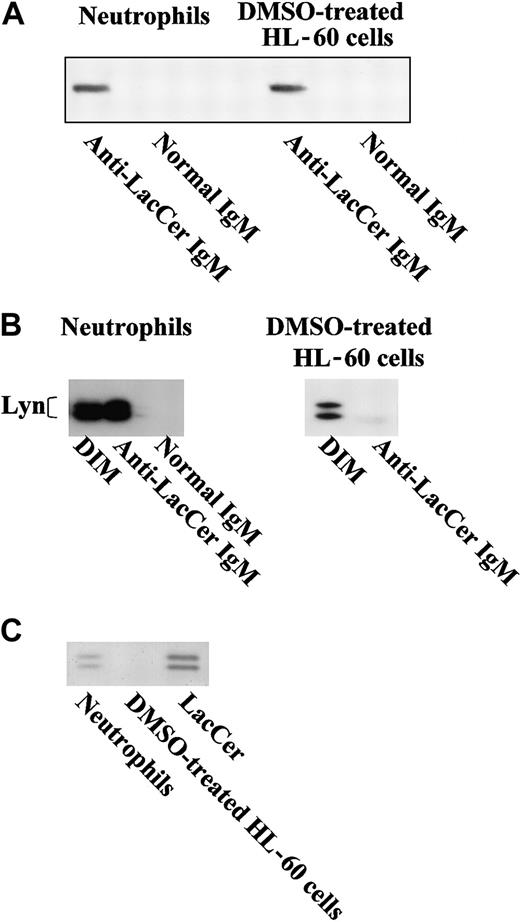
It has been shown that GSLs cluster to form microdomains in the cell surface membrane and are associated with various transducer molecules.26 The microdomains are insoluble in a detergent Triton X-100 at 4°C and float with associated proteins to a low density during gradient centrifugation.27 Figure3 shows the distribution patterns of LacCer, transducer molecules, and other cellular proteins in each fraction obtained from neutrophil and HL-60 cell lysates by sucrose density gradient centrifugation. More than 95% of LacCer in the cell lysates was recovered in DIM fraction, especially fractions 5 and 6 (DIM), which only contained 2.5% of total cellular protein (Figure3A). Moreover, about 35% of Lyn, 60% of Ha-Ras, 5% of p38 MAPK, and less than 1% of RhoA were recovered in DIM fraction. In contrast, actin was localized in the high-density fraction (fractions 9-12) that contained more than 95% of cellular proteins. Other transducers and receptors such as focal adhesion kinase and β1 and β2 integrins were not detected in DIM fraction but detected in the high-density fractions (data not shown). Furthermore, p22phox and gp91phox, membrane components of NADPH oxidase,28 were recovered 12% and 6%, respectively, in DIM fraction, whereas p47phox and p67phox, cytosolic components of NADPH oxidase, and small G-protein Rac2 were recovered in high-density fractions.
Distribution patterns of LacCer, transducer molecules, and NADPH oxidase components in fractions separated by sucrose density gradient centrifugation.
(A) Neutrophils, undifferentiated, and DMSO-treated HL-60 cells were lysed in the Triton X-100–contained lysis buffer, homogenized, and subjected to sucrose density gradient centrifugation. A series of 1 mL fractions were taken starting from the top and subjected to HPTLC (LacCer) or SDS-PAGE/immunoblotting (transducer molecules, NADPH oxidase components, and actin), respectively. DIM and high-density in undifferentiated and DMSO-treated HL-60 cells represent the mixture of fractions 5 and 6 and the mixture of fractions 9 to 12, respectively. (B) Neutrophils, undifferentiated and DMSO-treated HL-60 cells, and human umbilical vein endotheial cells (Endothelial cells) were lysed in the lysis buffer B, and the postnuclear supernatants of these cells were subjected to SDS-PAGE/immunoblotting with anticaveolin IgG.
Distribution patterns of LacCer, transducer molecules, and NADPH oxidase components in fractions separated by sucrose density gradient centrifugation.
(A) Neutrophils, undifferentiated, and DMSO-treated HL-60 cells were lysed in the Triton X-100–contained lysis buffer, homogenized, and subjected to sucrose density gradient centrifugation. A series of 1 mL fractions were taken starting from the top and subjected to HPTLC (LacCer) or SDS-PAGE/immunoblotting (transducer molecules, NADPH oxidase components, and actin), respectively. DIM and high-density in undifferentiated and DMSO-treated HL-60 cells represent the mixture of fractions 5 and 6 and the mixture of fractions 9 to 12, respectively. (B) Neutrophils, undifferentiated and DMSO-treated HL-60 cells, and human umbilical vein endotheial cells (Endothelial cells) were lysed in the lysis buffer B, and the postnuclear supernatants of these cells were subjected to SDS-PAGE/immunoblotting with anticaveolin IgG.
In case of HL-60 cells, regardless of DMSO treatment, LacCer and Lyn molecules were exclusively recovered in DIM fraction (Figure 3A). The distribution patterns of small G-protein RhoA, a Rho family GTPase, small G-protein Ha-Ras, a Ras family GTPase, p38 MAPK, and Rac2 in undifferentiated and DMSO-treated HL-60 cells were almost the same as those in neutrophils. As reported previously,19 expression of gp91phox, p22phox, p47phox, and p67phox were increased in DMSO-treated HL-60 cells, and these components were recovered in the high-density fractions.
The integral membrane protein caveolin is known as a principal structural component of caveolae.29 In our experimental conditions, we could detect caveolin neither in neutrophils nor HL-60 cells, although caveolin was expressed in human umbilical vein endothelial cells, which was consistent with the data of other groups30 31 (Figure 3B).
Lipid composition of DIM
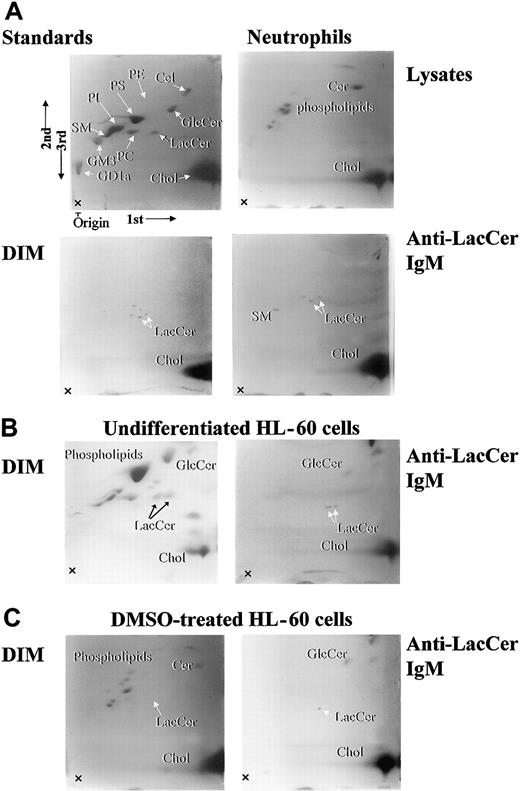
The lipid components of cell lysates and DIM fractions were separated by 2-dimensional HPTLC (Figure4, lysate). In neutrophil lysates, major lipid components were phospholipids and cholesterol, and LacCer was hardly detected (Figure 4, Lysate). Importantly, neutrophil DIM fraction was embraced with LacCer, cholesterol, and some lipids around LacCer, which likely represent LacCer with different length of fatty acid chains (Figure 4). Immunoprecipitation of neutrophil DIM with anti-LacCer mAb Huly-m13 revealed that LacCer, sphingomyelin, and cholesterol were recovered in the immunoprecipitants. In case of undifferentiated and DMSO-treated HL-60 cells, ceramide, phospholipids, and cholesterol were major components of DIM from the cells. Immunoprecipited DIM with anti-LacCer mAb Huly-m13 contained LacCer and glucosylceramide and cholesterol but not sphingomyelin in undifferentiated and DMSO-treated HL-60 cells. These data clearly indicate that lipid compositions in the LacCer-enriched DIM are different between neutrophils and undifferentiated or DMSO-treated HL-60 cells.
Two-dimensional HPTLC analysis of lipid components in DIM fractions of neutrophils, undifferentiated, and DMSO-treated HL-60 cells.
Lipid compositions of neutrophils, undifferentiated, and DMSO-treated HL-60 cells were analyzed by 2-dimensional HPTLC, and 10-cm thin-layer chromatography (TLC) plates were sprayed with primulin. 1st, 2nd, and 3rd arrows indicate the directions of the first, second, and third chromatographic runs, respectively. Lysate indicates whole cell lysates of neutrophils; DIM, fraction 5; anti-LacCer IgM, immunoprecipitated fraction of DIM with anti-LacCer Huly-m13; standards, standard lipids; PE, phosphatidylethanolamine; PS, phosphatidylserine; PC, phosphatidylcholine; GlcCer, glucosylceramide; Cer, ceramide; SM, sphingomyelin; Chol, cholesterol; GM3, NeuAc3Ga14Glc1Cer; GD1a, IV3NeuNAcII3NeuNAcGgOse4Cer.
Two-dimensional HPTLC analysis of lipid components in DIM fractions of neutrophils, undifferentiated, and DMSO-treated HL-60 cells.
Lipid compositions of neutrophils, undifferentiated, and DMSO-treated HL-60 cells were analyzed by 2-dimensional HPTLC, and 10-cm thin-layer chromatography (TLC) plates were sprayed with primulin. 1st, 2nd, and 3rd arrows indicate the directions of the first, second, and third chromatographic runs, respectively. Lysate indicates whole cell lysates of neutrophils; DIM, fraction 5; anti-LacCer IgM, immunoprecipitated fraction of DIM with anti-LacCer Huly-m13; standards, standard lipids; PE, phosphatidylethanolamine; PS, phosphatidylserine; PC, phosphatidylcholine; GlcCer, glucosylceramide; Cer, ceramide; SM, sphingomyelin; Chol, cholesterol; GM3, NeuAc3Ga14Glc1Cer; GD1a, IV3NeuNAcII3NeuNAcGgOse4Cer.
Association of LacCer with Lyn
When DIM fractions were immunoprecipitated with anti-LacCer mAb Huly-m13, comparable amounts of LacCer were recovered from neutrophils and DMSO-treated HL-60 cells in the immunoprecipitants (Figure5A). However, Lyn was recovered from neutrophil DIM but not HL-60 cell DIM by immunoprecipitation with anti-LacCer mAb Huly-m13 (Figure 5B), although LacCer and Lyn molecules were localized in DIM fractions of both neutrophils and DMSO-treated HL-60 cells (Figure 3A). Furthermore, association of LacCer with Lyn was confirmed by immunoprecipitation using anti-Lyn mAb. Bands corresponding to LacCer were recovered from neutrophil DIM but not HL-60 cells by immunoprecipitation with anti-Lyn mAb (Figure 5C). These data suggest that LacCer is associated with Lyn in neutrophil DIM but not DIM of DMSO-treated HL-60 cells. In addition, we confirmed that p38 MAPK, RhoA, and Ha-Ras were not immunoprecipitated with anti-LacCer mAb Huly-m13 (data not shown), although LacCer and these transducer molecules were recovered in DIM fraction of neutrophils and HL-60 cells (Figure 3A).
Association of Lyn with LacCer in neutrophils but not DMSO-treated HL-60 cells.
(A) DIM fractions of neutrophils and DMSO-treated HL-60 cells were immunoprecipitated with mouse anti-LacCer antibody Huly-m13 (anti-LacCer IgM) or normal mouse IgM (normal IgM). After extraction with chloroform/methanol (2/1) solution and purification using Bond Elut-C18 packed columns, aliquots of the immunoprecipitants were also analyzed by immunoslot blotting using anti-LacCer mAb Huly-m13. (B) Immunoprecipitants with Huly m-13 shown in panel A were also analyzed by SDS-PAGE/immunoblotting using rabbit anti-Lyn IgG. (C) DIM of neutrophils or DMSO-treated HL-60 cells was immunoprecipitated with mouse anti-Lyn monoclonal IgG and subjected to HPTLC, followed by staining of LacCer with primulin. LacCer indicates LacCer standard.
Association of Lyn with LacCer in neutrophils but not DMSO-treated HL-60 cells.
(A) DIM fractions of neutrophils and DMSO-treated HL-60 cells were immunoprecipitated with mouse anti-LacCer antibody Huly-m13 (anti-LacCer IgM) or normal mouse IgM (normal IgM). After extraction with chloroform/methanol (2/1) solution and purification using Bond Elut-C18 packed columns, aliquots of the immunoprecipitants were also analyzed by immunoslot blotting using anti-LacCer mAb Huly-m13. (B) Immunoprecipitants with Huly m-13 shown in panel A were also analyzed by SDS-PAGE/immunoblotting using rabbit anti-Lyn IgG. (C) DIM of neutrophils or DMSO-treated HL-60 cells was immunoprecipitated with mouse anti-Lyn monoclonal IgG and subjected to HPTLC, followed by staining of LacCer with primulin. LacCer indicates LacCer standard.
Involvement of Lyn and other kinases in the LacCer-induced superoxide generation
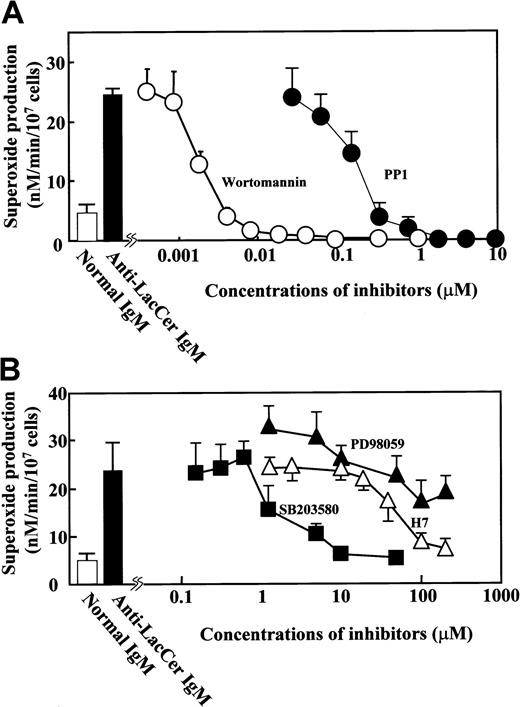
Recently, Src family tyrosine kinases, PI-3K, and MAPKs are reported to be involved in superoxide generation by neutrophils.32-34 As shown in Figure6, PP1, a Src family kinase–specific inhibitor, and wortmannin, a PI-3K inhibitor, dose dependently inhibited anti-LacCer mAb T5A7–induced superoxide generation by neutrophils with IC50 (inhibitory concentration of 50%) values of 0.04 and 0.3 μM, respectively. SB203580, which specifically inhibits p38 MAPK activity, abolished the anti-LacCer mAb-induced neutrophil superoxide generation in a dose-dependent manner (IC50 = 2 μM). H7, a serine/threonine kinase inhibitor such as protein kinase C, weakly suppressed the T5A7-induced superoxide generation by neutrophils (IC50 = 80 μM). In contrast, PD98059, which inhibits MAPK-1 and extracellular signal–regulated kinase 1/2 through blockade of c-Raf, hardly affected the T5A7-induced superoxide generation by neutrophils. Taken together, these results suggest that anti-LacCer mAb-induced superoxide generation is mediated by Src family tyrosine kinase (probably Lyn), PI-3K, p38 MAPK, and protein kinase C, and extracellular signal–regulated kinase 1/2 may not be involved in the LacCer-mediated neutrophil superoxide generation.
Effect of PP1, wortmannin, SB203580, H7, and PD98059 on T5A7-induced neutrophil superoxide generation.
Neutrophils were preincubated for 30 minutes in the presence of 0.0005 to 200 μM PP1(●), wortmannin(○), SB203580(▪), H7(▵), or PD98059(▴) at 4°C and then incubated in anti-LacCer IgM–coated 96-well plates at 37°C for 30 minutes. Neutrophils were also incubated in anti-LacCer IgM–coated wells (solid bar) or in normal IgM–coated wells (open bar) in the absence of inhibitors. Data show the mean ± SD of 4 independent experiments.
Effect of PP1, wortmannin, SB203580, H7, and PD98059 on T5A7-induced neutrophil superoxide generation.
Neutrophils were preincubated for 30 minutes in the presence of 0.0005 to 200 μM PP1(●), wortmannin(○), SB203580(▪), H7(▵), or PD98059(▴) at 4°C and then incubated in anti-LacCer IgM–coated 96-well plates at 37°C for 30 minutes. Neutrophils were also incubated in anti-LacCer IgM–coated wells (solid bar) or in normal IgM–coated wells (open bar) in the absence of inhibitors. Data show the mean ± SD of 4 independent experiments.
To confirm the activation of Lyn via the stimulation with anti-LacCer antibodies, DIM from neutrophils was incubated with [γ-32P]-ATP in an anti-LacCer mAb T5A7–coated dish and then immunoprecipitated with anti-Lyn mAb using Triton X-100–contained lysis buffer in which DIM was not solubilized. As shown in Figure7A, Lyn and other proteins were phosphorylated by T5A7, whereas Lyn was hardly phosphorylated when DIM was incubated in a normal IgM–coated dish. Interestingly, PP1 (100 nM) almost completely suppressed the T5A7-induced phosphorylation of the proteins. Furthermore, immunoprecipitation was performed in RIPA buffer in which proteins of DIM were completely separated. Under these conditions, the treatment with T5A7 also increased phosphorylation of Lyn, which was abolished by PP1 (Figure 7B). Moreover, DIM was incubated with [γ-32P]-ATP in a T5A7-coated dish and immunoprecipitated with anti-pp38 MAPK mAb in RIPA buffer. p38 MAPK was phosphorylated, and the phosphorylation was also completely suppressed by PP1 (Figure 7C). These observations suggest that anti-LacCer antibody-mediated signaling activates Lyn and p38 MAPK in DIM.
T5A7-induced phosphorylation of Lyn and p38 MAPK in DIM.
DIM isolated from neutrophils was kept on ice in a polypropylene tube (resting) or placed in normal IgM (normal IgM) or anti-LacCer IgM T5A7–coated dishes (anti-LacCer IgM) at 4°C for 15 hours. Then the mixtures were incubated with [γ-32P]-ATP in the absence or presence of 100 nM PP1 for 5 minutes at 37°C, followed by trichloroacetic acid (TCA) precipitation. (A) The TCA precipitate was solubilized in lysis buffer B, immunoprecipitated with mouse anti-Lyn mAb, and subjected to SDS-PAGE, electroblotting transfer, and autoradiography (autoradiogram). To evaluate the recovery of Lyn, the blotted membrane was probed with rabbit anti-Lyn IgG (immunoblot). (B) The TCA precipitate was solubilized in RIPA buffer and immunoprecipitated with mouse anti-Lyn mAb. The immunoprecipitate was washed with RIPA buffer and subjected to SDS-PAGE, electroblotting, and autoradiography. (C) The TCA precipitate was solubilized in RIPA buffer and immunoprecipitated with mouse anti-pp38 MAPK mAb and subjected to SDS-PAGE, electroblotting, and autoradiography. In all 3 panels, locations of molecular markers are indicated on the left.
T5A7-induced phosphorylation of Lyn and p38 MAPK in DIM.
DIM isolated from neutrophils was kept on ice in a polypropylene tube (resting) or placed in normal IgM (normal IgM) or anti-LacCer IgM T5A7–coated dishes (anti-LacCer IgM) at 4°C for 15 hours. Then the mixtures were incubated with [γ-32P]-ATP in the absence or presence of 100 nM PP1 for 5 minutes at 37°C, followed by trichloroacetic acid (TCA) precipitation. (A) The TCA precipitate was solubilized in lysis buffer B, immunoprecipitated with mouse anti-Lyn mAb, and subjected to SDS-PAGE, electroblotting transfer, and autoradiography (autoradiogram). To evaluate the recovery of Lyn, the blotted membrane was probed with rabbit anti-Lyn IgG (immunoblot). (B) The TCA precipitate was solubilized in RIPA buffer and immunoprecipitated with mouse anti-Lyn mAb. The immunoprecipitate was washed with RIPA buffer and subjected to SDS-PAGE, electroblotting, and autoradiography. (C) The TCA precipitate was solubilized in RIPA buffer and immunoprecipitated with mouse anti-pp38 MAPK mAb and subjected to SDS-PAGE, electroblotting, and autoradiography. In all 3 panels, locations of molecular markers are indicated on the left.
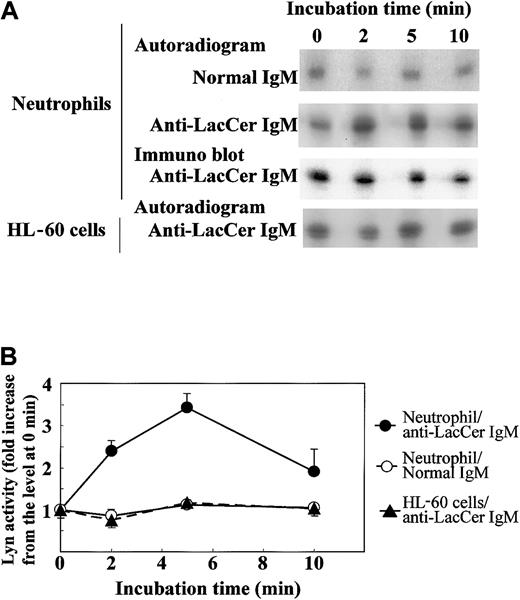
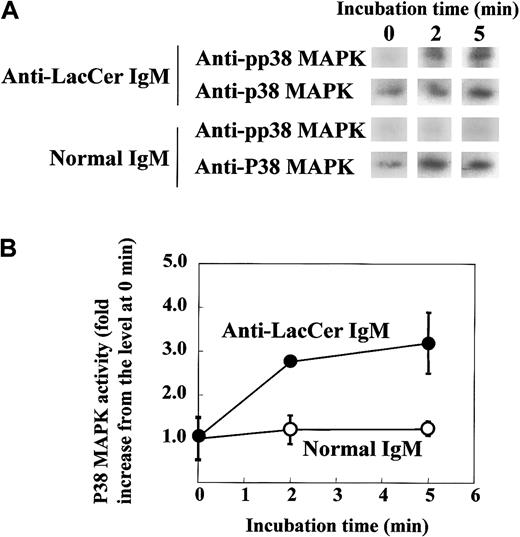
To further clarify the involvement of Lyn in the LacCer-mediated signal transduction, we evaluated the activation of Lyn by treatment of neutrophils with anti-LacCer mAb T5A7. After incubation of neutrophils with T5A7 for 2 to 10 minutes, autophosphorylation of Lyn was measured by immunoprecipitation with anti-Lyn mAb. As shown in Figure8, T5A7 treatment increased the Lyn activity in neutrophils, and the maximum stimulation (3.4-fold) was observed 5 minutes after stimulation. In contrast, T5A7 could not activate Lyn in DMSO-treated HL-60 cells. To determine whether anti-LacCer antibody-mediated signaling activates p38 MAPK, phosphorylation of p38 MAPK was also evaluated using neutrophils incubated with T5A7. As shown in Figure9, phosphorylation of p38 MAPK was increased after T5A7 treatment of neutrophils, suggesting that LacCer-mediated signaling activates p38 MAPK in neutrophils.
T5A7-induced Lyn activation in neutrophils.
(A) Neutrophils and DMSO-treated HL-60 cells were incubated in normal IgM–coated (normal IgM) or anti-LacCer IgM T5A7–coated (anti-LacCer IgM) 10 cm dishes at 37°C for indicated periods. Kinase activity was measured by in vitro autophosphorylation as described in “Materials and methods.” Data show the representative time course of 3 independent experiments. To evaluate the recovery of Lyn, the blotted membrane using anti-LacCer IgM T5A7–induced neutrophils was probed with rabbit anti-Lyn IgG (immunoblot). (B) Phosphorylation of Lyn was calculated by scanning the signals of phosphorylated and recovered Lyn by Scion image. The Lyn activity is expressed as fold increase compared with that of cells kept on ice (0 minute). Each point shows the mean ± SD of 3 independent experiments.
T5A7-induced Lyn activation in neutrophils.
(A) Neutrophils and DMSO-treated HL-60 cells were incubated in normal IgM–coated (normal IgM) or anti-LacCer IgM T5A7–coated (anti-LacCer IgM) 10 cm dishes at 37°C for indicated periods. Kinase activity was measured by in vitro autophosphorylation as described in “Materials and methods.” Data show the representative time course of 3 independent experiments. To evaluate the recovery of Lyn, the blotted membrane using anti-LacCer IgM T5A7–induced neutrophils was probed with rabbit anti-Lyn IgG (immunoblot). (B) Phosphorylation of Lyn was calculated by scanning the signals of phosphorylated and recovered Lyn by Scion image. The Lyn activity is expressed as fold increase compared with that of cells kept on ice (0 minute). Each point shows the mean ± SD of 3 independent experiments.
T5A7-induced p38 MAPK activation in neutrophils.
(A) Neutrophils were incubated in anti-LacCer IgM T5A7–coated (anti-LacCer IgM) or normal IgM–coated (normal IgM) 10 cm dishes at 37°C for indicated periods. After incubation, lysates were prepared and immunoprecipitated by rabbit anti–p38 MAPK IgG, followed by SDS-PAGE and electroblotting. The membranes were probed with mouse anti–pp38 MAPK IgM (pp38 MAPK). Further, membranes were reprobed with rabbit anti–p38 MAPK IgG (p38 MAPK). (B) Phosphorylation of p38 MAPK was calculated by scanning the signals of phosphorylated and recovered p38 MAPK by Scion image. The activity of p38 MAPK is expressed as fold increase compared with that of cells kept on ice (0 minute). Each point shows the mean ± SD of independent 3 experiments.
T5A7-induced p38 MAPK activation in neutrophils.
(A) Neutrophils were incubated in anti-LacCer IgM T5A7–coated (anti-LacCer IgM) or normal IgM–coated (normal IgM) 10 cm dishes at 37°C for indicated periods. After incubation, lysates were prepared and immunoprecipitated by rabbit anti–p38 MAPK IgG, followed by SDS-PAGE and electroblotting. The membranes were probed with mouse anti–pp38 MAPK IgM (pp38 MAPK). Further, membranes were reprobed with rabbit anti–p38 MAPK IgG (p38 MAPK). (B) Phosphorylation of p38 MAPK was calculated by scanning the signals of phosphorylated and recovered p38 MAPK by Scion image. The activity of p38 MAPK is expressed as fold increase compared with that of cells kept on ice (0 minute). Each point shows the mean ± SD of independent 3 experiments.
Subcellular localization of Lyn and LacCer in neutrophils
GSLs are present in plasma membrane of various types of cells.26 However, in neutrophils, LacCer is reported to be mainly localized in granules.35 In this study, we revealed that Lyn was associated with LacCer in DIM (Figure 5) and activated by the treatment of neutrophils with anti-LacCer mAb (Figure 8), suggesting that LacCer and Lyn are colocalized in DIM fraction of the plasma membrane. To confirm this possibility, we evaluated subcellular localization of LacCer and Lyn in neutrophils by performing Percoll density gradient centrifugation. As shown in Table1, lactate dehydrogenase (a cytosol marker) and lactoferrin (a specific granule marker) were almost completely recovered in the cytosol and specific granule fractions, respectively. About 70% of myeloperoxidase (an azurophil granule marker) was recovered in the azurophil granule fraction. Alkaline phosphatase (a marker for plasma membrane and secretory vesicle) was recovered 56% in the plasma membrane fraction. Gelatinase (a marker for gelatinase and specific granules) was recovered 47% and 42% in the gelatinase and specific granule fractions, respectively, as previously observed.24 LacCer was found approximately 90% in the gelatinase, specific, and azurophil granule fractions, whereas only 10% of LacCer was recovered in the plasma membrane fraction. In contrast, Lyn molecules were almost completely recovered in the plasma membrane fraction. Moreover, we confirmed that Lyn was coimmunoprecipitated with LacCer by anti-LacCer mAb Huly-m13 in DIM fraction isolated from neutrophil plasma membrane (data not shown), indicating that Lyn is associated with LacCer in DIM of neutrophil plasma membrane.
Distribution of LacCer, Lyn, and marker enzymes in subcellular fractions of neutrophils
| Fraction . | MPO . | Lactoferrin . | Gelatinase . | ALP . | LDH . | LacCer . | Lyn . |
|---|---|---|---|---|---|---|---|
| Cytosol | 0.7 ± 0.2 | 0.0 | 2.7 ± 2.3 | 1.0 ± 0.3 | 95.4 ± 3.5 | 0.9 ± 0.2 | 0.0 |
| Plasma membrane | 1.5 ± 0.3 | 0.0 | 5.5 ± 1.3 | 55.8 ± 3.2 | 0.0 | 10.0 ± 0.9 | 99.5 ± 2.1 |
| Gelatinase glanules | 5.2 ± 1.2 | 7.9 ± 2.2 | 47.3 ± 1.2 | 15.4 ± 1.7 | 0.0 | 15.5 ± 2.1 | 0.0 |
| Specific granules | 11.6 ± 2.4 | 89.9 ± 5.1 | 42.3 ± 2.3 | 7.9 ± 0.8 | 0.0 | 36.5 ± 2.7 | 0.0 |
| Azurophil granules | 68.2 ± 4.2 | 0.2 ± 0.1 | 4.3 ± 2.4 | 10.3 ± 1.4 | 0.0 | 37.1 ± 3.2 | 0.0 |
| Recovery | 87.2 ± 5.1 | 98.0 ± 3.2 | 103.5 ± 4.1 | 90.3 ± 3.1 | 95.4 ± 3.5 | 99.0 ± 4.1 | 99.5 ± 2.1 |
| Fraction . | MPO . | Lactoferrin . | Gelatinase . | ALP . | LDH . | LacCer . | Lyn . |
|---|---|---|---|---|---|---|---|
| Cytosol | 0.7 ± 0.2 | 0.0 | 2.7 ± 2.3 | 1.0 ± 0.3 | 95.4 ± 3.5 | 0.9 ± 0.2 | 0.0 |
| Plasma membrane | 1.5 ± 0.3 | 0.0 | 5.5 ± 1.3 | 55.8 ± 3.2 | 0.0 | 10.0 ± 0.9 | 99.5 ± 2.1 |
| Gelatinase glanules | 5.2 ± 1.2 | 7.9 ± 2.2 | 47.3 ± 1.2 | 15.4 ± 1.7 | 0.0 | 15.5 ± 2.1 | 0.0 |
| Specific granules | 11.6 ± 2.4 | 89.9 ± 5.1 | 42.3 ± 2.3 | 7.9 ± 0.8 | 0.0 | 36.5 ± 2.7 | 0.0 |
| Azurophil granules | 68.2 ± 4.2 | 0.2 ± 0.1 | 4.3 ± 2.4 | 10.3 ± 1.4 | 0.0 | 37.1 ± 3.2 | 0.0 |
| Recovery | 87.2 ± 5.1 | 98.0 ± 3.2 | 103.5 ± 4.1 | 90.3 ± 3.1 | 95.4 ± 3.5 | 99.0 ± 4.1 | 99.5 ± 2.1 |
Neutrophils were disrupted by nitrogen cavitation, and the postnuclear supernatant was applied on top of a 3-layer Percoll gradient and centrifuged. The resultant 4 visible bands (corresponding to the azurophil granule, the specific granule, the gelatinase granule, and the plasma membrane fractions) and the cytosol fraction were collected. The contents of LacCer, Lyn, and marker enzymes in each fraction were expressed as percentage of total lysates. Data represent the means ± SD of 3 experiments.
MPO indicates myeloperoxidase; ALP, alkaline phosphatase; LDH, lactate dehydrogenase.
Effects of β-cyclodextrin and nystatin on LacCer-mediated Lyn activation in DIM from neutrophil plasma membrane
Several investigators have demonstrated that cholesterol plays an important role in the biologic functions of microdomains or caveolae.36 37 Figure 4 indicates that cholesterol is present in the LacCer-enriched DIM. To elucidate the role of cholesterol in LacCer-dependent Lyn activation, we examined the effects of methyl-β-cyclodextrin (a cholesterol-scavenging reagent) and nystatin (a cholesterol-binding reagent). When DIM from the neutrophil plasma membrane was incubated with anti-LacCer mAb T5A7, phosphorylation of Lyn increased (Figure10A) and was abolished by 100 nM PP1 (lane 3). Interestingly, phosphorylation of Lyn was approximately 2-fold and 5-fold enhanced by the treatment with methyl-β-cyclodextrin and nystatin, respectively. These results suggest that LacCer-dependent Lyn activation in the plasma membrane is regulated by cholesterol.
Effects of cyclodextrin and nystatin on anti-LacCer antibody-induced Lyn activation.
DIM prepared from neutrophil plasma membrane was diluted and incubated without or with 10 mM methyl-β-cyclodextrin (β-cyclodextrin) or 60 μg/ mL nystatin (nystatin) for 30 minutes at 25°C. After incubation, the reaction mixtures were ultracentrifuged and the pellets were resuspended in 5 mL of the kinase buffer, followed by incubation in normal IgM– or anti-LacCer IgM T5A7–coated dishes for 15 hours at 4°C. Then an aliquot of 50 μCi [γ32P]-ATP was added to each dish, and the mixture was further incubated at 37°C for 5 minutes. The reaction was stopped by placing on ice. In some experiments, anti-LacCer–induced Lyn activation was assessed in the presence of 100 nM PP1 (PP1). (A) Phosphorylation of Lyn was analyzed by immunoprecipitation with mouse anti-Lyn IgM, followed by SDS-PAGE and blotting onto PVDF membrane and autoradiography (autoradiogram). To evaluate the recovery of Lyn, the blotted membrane was probed with anti-Lyn rabbit IgG (Immunoblot). (B) Lyn activity is expressed as fold increase compared with that of DIM incubated in normal IgM–coated dishes (normal IgM). Each bar shows the mean ± SD of independent 3 experiments.
Effects of cyclodextrin and nystatin on anti-LacCer antibody-induced Lyn activation.
DIM prepared from neutrophil plasma membrane was diluted and incubated without or with 10 mM methyl-β-cyclodextrin (β-cyclodextrin) or 60 μg/ mL nystatin (nystatin) for 30 minutes at 25°C. After incubation, the reaction mixtures were ultracentrifuged and the pellets were resuspended in 5 mL of the kinase buffer, followed by incubation in normal IgM– or anti-LacCer IgM T5A7–coated dishes for 15 hours at 4°C. Then an aliquot of 50 μCi [γ32P]-ATP was added to each dish, and the mixture was further incubated at 37°C for 5 minutes. The reaction was stopped by placing on ice. In some experiments, anti-LacCer–induced Lyn activation was assessed in the presence of 100 nM PP1 (PP1). (A) Phosphorylation of Lyn was analyzed by immunoprecipitation with mouse anti-Lyn IgM, followed by SDS-PAGE and blotting onto PVDF membrane and autoradiography (autoradiogram). To evaluate the recovery of Lyn, the blotted membrane was probed with anti-Lyn rabbit IgG (Immunoblot). (B) Lyn activity is expressed as fold increase compared with that of DIM incubated in normal IgM–coated dishes (normal IgM). Each bar shows the mean ± SD of independent 3 experiments.
Reconstitution experiments
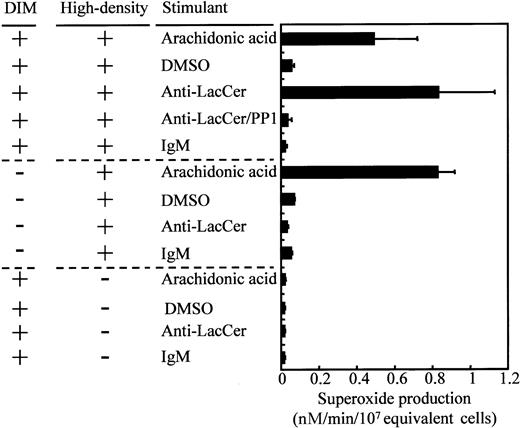
Sucrose density gradient centrifugation experiments indicated that DIM fraction contained LacCer, Lyn, p22phox, and gp91phox but not p47phox, p67phox, or Rac2 and that the high-density fraction contained Lyn, all of NADPH components, and a trace amount of LacCer (Figure 3). To evaluate the components involved in the anti-LacCer antibody-induced superoxide generation, we performed reconstitution experiments using DIM fraction and the high-density fraction. As shown in Figure11, anti-LacCer IgM did not induce superoxide generation from DIM or the high-density fraction alone, whereas anti-LacCer IgM induced the superoxide generation from the mixed fractions of DIM and the high-density fractions. These observations suggest that LacCer and Lyn in DIM fraction and, also, membranous and cytosolic NADPH oxidase components supplemented by high-density fractions are necessary for the activation of NADPH oxidase by anti-LacCer antibodies. In contrast, arachidonic acid, a stimulating agent for NADPH oxidase in a cell-free system, could induce the superoxide generation from the high-density fraction, because almost all NADPH oxidase components were recovered in that fraction.
Reconstitution experiments of anti-LacCer antibodiy-induced superoxide generation.
Neutrophil DIM and the high-density fractions were isolated as described in Figure 3, and each fraction and mixture of the fractions (2 × 107 equivalent cells per milliliter) were incubated with 1 μg/mL anti-LacCer IgM T5A7 (anti-LacCer), 1 μg/mL normal mouse IgM, 0.1 μM arachidonic acid (arachidonic acid), or 0.5% DMSO (DMSO) for 30 minutes at 37°C in the presence or absence of superoxide dismutase. Superoxide production was calculated by measurement of the superoxide dismutase–inhibitable reduction of cytochrome c at 550 nm. Each bar shows the mean ± SD of independent 3 experiments. Anti-LacCer/PP1, the mixtures of DIM and high-density fractions incubated with 1 μg/mL T5A7 in the presence of 1 μM PP1.
Reconstitution experiments of anti-LacCer antibodiy-induced superoxide generation.
Neutrophil DIM and the high-density fractions were isolated as described in Figure 3, and each fraction and mixture of the fractions (2 × 107 equivalent cells per milliliter) were incubated with 1 μg/mL anti-LacCer IgM T5A7 (anti-LacCer), 1 μg/mL normal mouse IgM, 0.1 μM arachidonic acid (arachidonic acid), or 0.5% DMSO (DMSO) for 30 minutes at 37°C in the presence or absence of superoxide dismutase. Superoxide production was calculated by measurement of the superoxide dismutase–inhibitable reduction of cytochrome c at 550 nm. Each bar shows the mean ± SD of independent 3 experiments. Anti-LacCer/PP1, the mixtures of DIM and high-density fractions incubated with 1 μg/mL T5A7 in the presence of 1 μM PP1.
Discussion
The present study clarifies the role of LacCer through its interaction with Lyn in microdomains of neutrophil plasma membrane. Human neutrophils produced superoxide anion in response to anti-LacCer antibodies (Figure 1). In contrast, DMSO-treated HL-60 cells failed to produce superoxide generation by stimulation with anti-LacCer antibodies. Interestingly, LacCer was essentially recovered in Triton X-100–insoluble DIM fractions of neutrophils as well as DMSO-treated HL-60 cells (Figure 3). In addition, neutrophil DIM contained Lyn and small G proteins, which are assumed to be anchored to the inner face of the plasma membrane via C-terminal acylation. Lyn was immunoprecipitated by anti-LacCer antibody from neutrophil DIM fraction (Figure 5B). Moreover, all of Lyn molecules were essentially localized in plasma membrane (Table 1), and LacCer was immunoprecipitated by anti-Lyn antibody in neutrophils (Figure 5C). Thus, it is likely that LacCer forms GSDs coupled with Lyn on neutrophil plasma membrane. Importantly, Lyn was not immunoprecipitated by anti-LacCer antibody from DIM fraction of DMSO-treated HL-60 cells, although Lyn molecules were recovered in the DIM fraction of HL-60 cells (Figures 3 and 4), suggesting that Lyn is not closely associated with LacCer in HL-60 cells. Consistent with the above observations, stimulation with anti-LacCer antibody induced the phosphorylation of Lyn in neutrophils but not in DMSO-treated HL-60 cells (Figures 7 and 8). Therefore, the association of Lyn with LacCer seems to be a key for LacCer-mediated activation of Lyn, leading to superoxide generation by neutrophils. p38 MAPK, as well as Lyn, was also recovered in the DIM fraction of DMSO-treated HL-60 cells (Figure 3), raising the possibility that p38 MAPK may be involved in the LacCer-mediated Lyn activation in HL-60 cells. However, Lyn was not associated with LacCer in DMSO-treated HL-60 cells. Therefore, p38 MAPK is unlikely involved in LacCer-mediated superoxide generation in this cell type, although p38 MAPK was recovered in DIM fraction of these cells.
LacCer, like other GSLs, may not be located in the cytoplasmic leaflet of membrane bilayer. The connection of LacCer, located in the external leaflet of the bilayer, with Lyn, located in a cytoplasmic site of membrane, is unclear at this time. The same question applies to various GSLs and other Src family kinases, G proteins, and signal transducers. Several protein kinases including Lyn have been found in DIM and associated with the cytoplasmic leaflet through double acylation.38 Because tetraspan proteolipid proteins such as transmembrane proteins have been found in DIM,39connection of GSLs to signal transducers may be mediated by such proteins. However, a recent study with reconstituted membrane containing GM3 ganglioside, cSrc, sphingomyelin, and cholesterol indicated that stimulation of GM3 induces activation of cSrc without any protein.40
Another possibility is that cholesterol plays a functional role in connecting LacCer in exoplasmic leaflet to cytoplasmic proteins, because cholesterol exists in both exoplasmic and cytoplasmic leaflets, and forms dimers between the leaflets under certain conditions.3 Glycosylphosphatidylinositol-anchored proteins are localized on the leukocyte surface with cholesterol within microdomains, and cholesterol-binding reagents are reported to inhibit the glycosylphosphatidylinositol-anchored protein-mediated activation of Src family kinases in DIM.41 Interestingly, the present study showed that the depletion (by methyl-β-cyclodextrin) or the sequestration (by nystatin) of cholesterol did not inhibit but enhanced the LacCer-mediated Lyn activation. Because cholesterol functions as a spacer in the leaflets by filling voids created by interdigitating fatty acid chains,27 cholesterol depletion from LacCer-enriched GSDs may cause narrowing of the spaces in exoplasmic and cytoplasmic leaflets, resulting in the packing of LacCer and Lyn and enhancement of LacCer-Lyn interaction. Moreover, we examined the effect of methyl-β-cyclodextrin on anti-LacCer IgM–induced superoxide generation from neutrophils. Methyl-β-cyclodextrin inhibited the superoxide generation by not only anti-LacCer antibody T5A7 but also 4β-phorbol 12-myristate 13-acetate, a protein kinase C activator (data not shown). Reconstitution experiments have demonstrated that mixtures of cholesterol, phospholipids, and sphingomyelin restore the active conformation of purified cytochrome b558,42 a membrane component of NADPH oxidase.28 Thus, cholesterol may be indispensable for the conformation and/or function of cytochrome b558 in neutrophils, while it suppressibly regulates the association of LacCer with Lyn in GSDs.
Furthermore, it is possible that interdigitation of long-chain LacCer into the opposing membrane leaflet could lead to the preferential binding of LacCer to acyl chains of Lyn.3LacCer molecules of neutrophils have been reported to possess C24 acyl chains,43 which could interdigitate with the cytoplasmic leaflet of the bilayer.44 In this study, cross-linking of LacCer by anti-LacCer antibody induced LacCer-mediated signaling. Thus, the clustering of LacCer at exoplasmic leaflet may activate the cytoplasmic Lyn molecules via the interaction between acyl chains of these molecules.
HPTLC analysis showed that, in addition to LacCer, sphingomyelin and cholesterol were major components in the anti-LacCer antibody-immunoprecipitated neutrophil DIM, whereas glucosylceramide and cholesterol with LacCer were recovered in the anti-LacCer antibody-immunoprecipitated DIM of DMSO-treated HL-60 cells (Figure 4). GSLs and sphingomyelin are relatively rich in long and saturated fatty acyl chains with high melting temperature, which allows tight packing.3 Thus, in neutrophils, LacCer-enriched microdomain, mainly composed of LacCer, sphingomyelin, and cholesterol, may exist in the liquid ordered phase, which is characterized by tight acyl-chain packing and rapid lateral mobility in the bilayer.45 The lack in the association of LacCer with Lyn in DMSO-treated HL-60 cells may be due to the differences in lipid compositions and/or structures of ceramide moieties of LacCer and sphingomyelin between neutrophil and the HL-60 cell DIM. p38 MAPK was recovered in the DIM fractions of not only neutrophils but also DMSO-treated HL-60 cells (Figure 3). The phosphorylation of DIM-associated p38 MAPK was completely suppressed by PP1 (Figure 7C).
Several signal transduction pathways have been reported to be involved in superoxide generation from neutrophils.28 The present study demonstrated that the Src family kinase inhibitor PP1 and PI-3K inhibitor wortmannin completely inhibited the anti-LacCer antibody-induced superoxide generation by neutrophils (Figure 6). A p38 MAPK inhibitor SB203580 and protein kinase C inhibitor H7 partially inhibited the anti-LacCer antibody-induced superoxide generation from neutrophils. It has been reported that, in neutrophils, activated Lyn associates with PI-3K for the activation of PI-3K.46,47PI-3K regulates the activation of p38 MAPK and protein kinase C, which results in the activation of NADPH oxidase.48 49Therefore, it seems that PI-3K is involved in the downstream events of anti-LacCer antibody-induced Lyn activation. Furthermore, based on the effect of p38 MAPK and protein kinase C inhibitors on the superoxide generation shown in Figure 6, p38 MAPK rather than protein kinase C is mainly involved in the LacCer-mediated superoxide generation.
Reconstitution experiments using DIM and the high-density fractions indicated that LacCer-enriched microdomains (GSDs) are necessary for the anti-LacCer antibody–mediated superoxide generation from neutrophils. In separate experiments, we performed the immunoprecipitation of p22phox and gp91phoxfrom DIM fraction of neutrophil plasma membrane using anti-LacCer antibody Huly-m13. Those 2 components were not immunoprecipitated with the antibody (data not shown), suggesting that LacCer-mediated signaling molecules are not directly associated with cytochrome b558, a membrane component of NADPH oxidase composing gp91phox and p22phox, in neutrophil GSDs.
In neutrophils, about 90% of LacCer is localized in granules (Table 1) and likely forms microdomains (Figure 3). However, Lyn was not localized in the granules but exclusively present in the plasma membrane, suggesting that granular LacCer-enriched microdomain may be involved in Lyn-independent neutrophil functions.
Physiologic ligands and functions for LacCer are still unclear. However, microorganisms can interact with LacCer,50,51 and the soluble β-(1,6)-branched β-(1,3)-glucan, a cell wall component of microorganisms, specifically binds to LacCer expressed on neutrophils and enhances not only superoxide generation but also phagocytosis.52 Furthermore, LacCer mediates up-regulation of CD11b/CD18 on neutrophils.15 We demonstrated here that LacCer exists as a component of GSDs on neutrophil plasma membrane and is involved in the superoxide generation. Therefore, it could be postulated that neutrophils phagocytose and kill microorganisms by superoxide generation via LacCer-enriched GSD-mediated signaling.
We are grateful to Dr Kazuko Handa (Pacific Northwest Research Institute) for her helpful discussion and to Dr Sen-itiroh Hakomori (University of Washington) for his encouragement and invaluable comments throughout this study and for supply of mouse anti-LacCer IgM T5A7 and mouse anti-GM3 IgG3 DH2. We thank Dr Akimasa Someya (Juntendo University School of Medicine) for providing anti-p22phox and anti-gp91phox antisera and Dr Stephen Anderson (Pacific Northwest Research Institute) for scientific editing of the manuscript.
Supported in part by grants-in-aid 12680616 (to K.I.) for scientific research and 14021114 (to K.I.) for scientific research on priority areas from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Kazuhisa Iwabuchi, Dept of Biochemistry, Juntendo University School of Medicine, 2-1-1 Hongo, Bunkyo-ku, Tokyo 113-8421, Japan; e-mail: iwabuchi@med.juntendo.ac.jp.

![Fig. 7. T5A7-induced phosphorylation of Lyn and p38 MAPK in DIM. / DIM isolated from neutrophils was kept on ice in a polypropylene tube (resting) or placed in normal IgM (normal IgM) or anti-LacCer IgM T5A7–coated dishes (anti-LacCer IgM) at 4°C for 15 hours. Then the mixtures were incubated with [γ-32P]-ATP in the absence or presence of 100 nM PP1 for 5 minutes at 37°C, followed by trichloroacetic acid (TCA) precipitation. (A) The TCA precipitate was solubilized in lysis buffer B, immunoprecipitated with mouse anti-Lyn mAb, and subjected to SDS-PAGE, electroblotting transfer, and autoradiography (autoradiogram). To evaluate the recovery of Lyn, the blotted membrane was probed with rabbit anti-Lyn IgG (immunoblot). (B) The TCA precipitate was solubilized in RIPA buffer and immunoprecipitated with mouse anti-Lyn mAb. The immunoprecipitate was washed with RIPA buffer and subjected to SDS-PAGE, electroblotting, and autoradiography. (C) The TCA precipitate was solubilized in RIPA buffer and immunoprecipitated with mouse anti-pp38 MAPK mAb and subjected to SDS-PAGE, electroblotting, and autoradiography. In all 3 panels, locations of molecular markers are indicated on the left.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/4/10.1182_blood.v100.4.1454.h81602001454_1454_1464/6/m_h81622979007.jpeg?Expires=1769102042&Signature=3PHoCZJPqs7dtZxU32Ygq5iwE2YDiV5hP8lZm0tgjTh6dOsWNN2KF9Djcx6yYxwKYMuA6jphmPcBrQS~SSJFcvPDwpSSYx5zvBqLdf8ljme8zGiLsIKDwb4Yy7lr4EFVHz61sjy6Pa3NhfNR6Rp~0UvcYrR0y1EUgT9HmBD~7tOeLxwI5oxd3uTChUnQTHILDXFXI5fDip6jEfIzV6MAioAnvf5pGgCE9PbZLwd6ZJAATTn~xUOTGKtIUPR0GDnIxzFZ~-28uZqaG8WAyOJO0rzqCg1GblDFTDnQijyW-d87beFZR9sr4pRn~eGNZTVhTlLKKuHa8hQe3zuRcHeJZQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 10. Effects of cyclodextrin and nystatin on anti-LacCer antibody-induced Lyn activation. / DIM prepared from neutrophil plasma membrane was diluted and incubated without or with 10 mM methyl-β-cyclodextrin (β-cyclodextrin) or 60 μg/ mL nystatin (nystatin) for 30 minutes at 25°C. After incubation, the reaction mixtures were ultracentrifuged and the pellets were resuspended in 5 mL of the kinase buffer, followed by incubation in normal IgM– or anti-LacCer IgM T5A7–coated dishes for 15 hours at 4°C. Then an aliquot of 50 μCi [γ32P]-ATP was added to each dish, and the mixture was further incubated at 37°C for 5 minutes. The reaction was stopped by placing on ice. In some experiments, anti-LacCer–induced Lyn activation was assessed in the presence of 100 nM PP1 (PP1). (A) Phosphorylation of Lyn was analyzed by immunoprecipitation with mouse anti-Lyn IgM, followed by SDS-PAGE and blotting onto PVDF membrane and autoradiography (autoradiogram). To evaluate the recovery of Lyn, the blotted membrane was probed with anti-Lyn rabbit IgG (Immunoblot). (B) Lyn activity is expressed as fold increase compared with that of DIM incubated in normal IgM–coated dishes (normal IgM). Each bar shows the mean ± SD of independent 3 experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/4/10.1182_blood.v100.4.1454.h81602001454_1454_1464/6/m_h81622979010.jpeg?Expires=1769102042&Signature=T0XNeDY6v6hCE90VEHU0sIY6sm0stXFXdwc2z~tldyJJsDGnJgk~I2e7epfdHb-GWRygzEydfmHakUYkLbFYmpsjZRFHRxVUf7D-Uw5OXxRiFwHJXFM9Z5PwLBoX0TzWBUPuwZZUNrbO8v6l0BGO3j4C59JvpM9tXOkwr7bXg2Ec0lSU146vAXgg3Z9~J3rhFpwm9RxfbGVIftRDQAMSt~VmsqRzjFMmytTte51wL6W6LL9fpbSYcH6mKbBffuvHA1UFFfkTRfGPLycYB~8STfLT-y0knB~ruzO3yccV69cGejX9t5vbwR-BlTrEEczt7x3ZDyW64ZQ3PdQcyDbBAw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal