Functional studies on native human dendritic cells (DCs) are hampered by technical difficulties in preparing fresh DCs. Recently, with the help of the monoclonal antibody M-DC8, we succeeded in isolating a major subpopulation of human blood DCs by a one-step immunomagnetic separation procedure. These cells strongly express FcγRIII (CD16) and FcγRII (CD32) and are quite efficient in the antigen-specific activation of naive T cells. Because some Fcγ receptor-bearing cell types are known as effector cells in antibody-dependent cellular cytotoxicity (ADCC), we investigated whether M-DC8+ DCs are capable of effectuating ADCC. In this report we show that freshly prepared M-DC8+ DCs efficiently mediate tumor-directed ADCC and that both types of Fcγ receptors as well as tumor necrosis factor α essentially contribute to the cytotoxic activity. The results provide evidence that, in addition to their pivotal role in primary T-cell activation, a subset of blood DCs displays efficient cytotoxicity in ADCC.
Introduction
Dendritic cells (DCs) are professional antigen-presenting cells1-3 that are widely used in the experimental immunotherapy of cancer.4 Although in these approaches DCs mediate their antitumor effect by stimulating tumor-specific T lymphocytes, recent observations suggest that DCs themselves have multiple effector cell functions in antitumor reactivity. Thus, DCs were shown to inhibit growth and to induce apoptosis of tumor cell lines in vitro.5,6 In addition, rat spleen DCs expressing natural killer (NK) cell receptor protein 1 were found to lyse tumor cells.7 Recently, we defined a novel major subset of human blood DCs by the use of the monoclonal antibody M-DC8, which recognizes a surface structure selectively expressed on these cells.8 M-DC8+ DCs, which account for 1% to 2% of peripheral blood mononuclear cells (PBMCs) strongly express major histocompatibility complex class II molecules and costimulatory molecules such as CD86. In addition, they efficiently activate neoantigen-specific CD4+ T cells and tumor peptide–specific CD8+ cytotoxic T cells.8 Two blood DC populations, DC1 and DC2, have previously been defined based on their phenotypes (CD11c+/CD123dim and CD11c−/CD123high, respectively).3M-DC8+ DCs, like DC1 cells, are CD11c+/CD123dim yet differ from DC1 and DC2 subsets by the strong expression of FcγRIII (CD16) in addition to the M-DC8 antigen. Prompted by the crucial role of FcγRIII (CD16) in antibody-dependent cellular cytotoxicity (ADCC) mediated by NK cells,9 we investigated whether M-DC8+ DCs are capable of effecting ADCC. So far, reports have demonstrated that human DCs are unable to efficiently mediate ADCC.10 11 Here, we describe that M-DC8+ DCs are able to effectuate tumor-directed ADCC, which is critically dependent on the engagement of FcγRIII (CD16) and FcγRII (CD32) as well as on the production of tumor necrosis factor α (TNF-α).
Study design
Cell lines and antibodies
Colorectal adenocarcinoma cell line COLO 205 and breast cancer cell line SK-BR-3 (American Type Culture Collection, Manassas, VA) were cultured according to the provider's instructions. The following murine monoclonal antibodies were used: 17-1A antibody (IgG2a, Glaxo Wellcome, Bad Oldesloe, Germany) recognizing the epithelial cellular adhesion molecule, isotype-matched control antibody (Becton Dickinson, Heidelberg, Germany), anti-FcγRIII (CD16) antibody (clone 3G8, IgG1, Becton Dickinson), anti-FcγRII (CD32) antibody (clone AT10, IgG1, Biozol, Eching, Germany), and anti–TNF-α antibody (IgG2a, Sigma-Aldrich, Taufkirchen, Germany). The purified isotype-matched control anti-CD3 antibody M-T301 (IgG1)12was generated in our laboratory. Furthermore, the humanized antibodies Herceptin, directed against the human epidermal growth factor receptor-2, and the isotype-matched control antibody, rituximab, directed against CD20 (IgG1, both from Hoffmann-La Roche, Grenzach-Wyhlen, Germany), were used.
Isolation of effector cells
Blood samples were obtained from healthy donors with informed consent. Isolation of M-DC8+ DCs was performed as described.8 Briefly, PBMCs were prepared by Ficoll-Hypaque (Biochrom, Berlin, Germany) density centrifugation and incubated for 15 minutes at 4°C with M-DC8 hybridoma supernatant containing 10 μg/mL antibody. After washing with phosphate-buffered saline (PBS) 1 × 108 cells were resuspended in 500 μL PBS and labeled with 10 μL rat anti–mouse IgM coupled to paramagnetic microbeads (Miltenyi Biotec, Bergisch-Gladbach, Germany) for another 15 minutes at 4°C. After washing, cells were sorted on separation columns (Miltenyi Biotec). PBS containing 1% human serum (CC pro, Neustadt, Germany) was used as running and elution buffer.
CD14+ monocytes and CD56+ NK cells were isolated from freshly prepared PBMCs using immunomagnetic separation (Miltenyi Biotec) according to the manufacturer's instructions.
Chromium release assay
Cytotoxic activity of M-DC8+ DCs was determined against COLO 205 and SK-BR-3 cells in a 51Cr release assay. Briefly, tumor cells (1 × 106) were labeled with 100 μCi (3.7 MBq) 51Cr (sodium chromate, NEN, Zaventem, Belgium) for 1 hour at 37°C and then washed 4 times with PBS. Labeled target cells were plated as triplicates in round bottomed 96-well plates at 5 × 103/well and incubated with various numbers of effector cells for 18 hours in the presence of 10 μg/mL 17-1A antibody, Herceptin, or isotype-matched control antibodies. Released 51Cr was determined in a β-counter (Wallac, Freiburg, Germany). Maximal and minimal release were measured by treating labeled cells with 1% Nonidet P-40 or medium alone, respectively. The specific cytotoxicity was calculated according to the formula: percent specific lysis = 100 × [(cpm experimental release − cpm spontaneous release)/(cpm maximal release − cpm spontaneous release)].
To inhibit Fcγ receptor function during ADCC, M-DC8+ DCs were preincubated with various concentrations of anti-FcγRIII (CD16), anti-FcγRII (CD32), or an isotype-matched control antibody for 1 hour at 4°C, and then added to tumor cells.
To block TNF-α function in M-DC8+ DC-mediated ADCC,51Cr release assay was repeated in the presence of a neutralizing anti–TNF-α antibody at a concentration of 10 μg/mL.
For statistical evaluation, the Student t test was used.P < .05 was considered significant.
Results and discussion
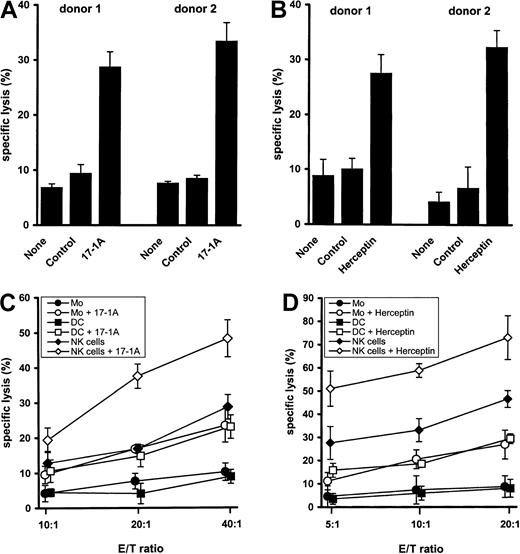
To determine the capacity of M-DC8+ DCs to mediate ADCC, DCs were isolated at high purity (> 95%) by immunomagnetic separation from freshly drawn blood of healthy donors and were cocultured with COLO 205 cells expressing the epithelial cellular adhesion molecule or SK-BR-3 cells expressing the human epidermal growth factor receptor-2. As targeting antibodies, either the murine 17-1A antibody or the humanized antibody Herceptin was chosen, both of which have already been used in adjuvant immunotherapy of cancer.13,14 In the presence of 17-1A antibody M-DC8+ DCs efficiently lysed COLO 205 cells (Figure1A), whereas only marginal tumor cell lysis was found in the absence of antibody or in the presence of an isotype-matched control antibody. In addition, DC8+ DCs efficiently lysed SK-BR-3 cells in the presence of Herceptin antibody (Figure 1B). In further experiments, M-DC8+ DCs were compared with monocytes and NK cells that are regarded as important mediators of ADCC.9 15 All effector cell populations were isolated at high purity (> 95%) as determined by fluorescence-activated cell sorter analysis (data not shown). The cytotoxicity of M-DC8+ DCs was similar to that observed with autologous monocytes, whereas NK cells emerged as the most potent mediators of ADCC in this experimental setting (Figure 1C,D).
M-DC8+ DC-mediated antibody-dependent cytotoxicity toward COLO 205 and SK-BR-3 tumor cells.
(A) Freshly isolated M-DC8+ DCs were cocultured in 96-well plates with 51Cr-labeled COLO 205 cells (5 × 103 cells/well) in the presence of 17-1A antibody (10 μg/mL), an isotype-matched control antibody (10 μg/mL), or in the absence of antibody, at an effector-target (E/T) ratio of 40:1. After 18 hours of incubation, chromium release was measured. The results of 2 different donors are presented as mean ± SE of triplicate wells. (B) M-DC8+ DCs were cocultured in 96-well plates with 51Cr-labeled SK-BR-3 cells (5 × 103 cells/well) in the presence of Herceptin antibody (10 μg/mL), an isotype-matched control antibody (10 μg/mL), or in the absence of antibody, at an E/T ratio of 20:1. After 18 hours of incubation, chromium release was measured. The results of 2 different donors are presented as mean ± SE of triplicate wells. (C) 51Cr-labeled COLO 205 cells (5 × 103cells/well) were incubated either with M-DC8+ DCs, monocytes, or NK cells in the presence or absence of 10 μg/mL 17-1A antibody at different E/T ratios. After 18 hours of incubation, chromium release was determined. Symbols represent means ± SE of results obtained from 4 different donors. For each donor the mean of triplicate determination was taken. (D) 51Cr-labeled SK-BR-3 cells (5 × 103 cells/well) were incubated either with M-DC8+ DCs, monocytes, or NK cells in the presence or absence of 10 μg/mL Herceptin antibody at different E/T ratios. After 18 hours of incubation, chromium release was determined. Symbols represent means ± SE of results obtained from 4 different donors. For each donor the mean of triplicate determination was taken.
M-DC8+ DC-mediated antibody-dependent cytotoxicity toward COLO 205 and SK-BR-3 tumor cells.
(A) Freshly isolated M-DC8+ DCs were cocultured in 96-well plates with 51Cr-labeled COLO 205 cells (5 × 103 cells/well) in the presence of 17-1A antibody (10 μg/mL), an isotype-matched control antibody (10 μg/mL), or in the absence of antibody, at an effector-target (E/T) ratio of 40:1. After 18 hours of incubation, chromium release was measured. The results of 2 different donors are presented as mean ± SE of triplicate wells. (B) M-DC8+ DCs were cocultured in 96-well plates with 51Cr-labeled SK-BR-3 cells (5 × 103 cells/well) in the presence of Herceptin antibody (10 μg/mL), an isotype-matched control antibody (10 μg/mL), or in the absence of antibody, at an E/T ratio of 20:1. After 18 hours of incubation, chromium release was measured. The results of 2 different donors are presented as mean ± SE of triplicate wells. (C) 51Cr-labeled COLO 205 cells (5 × 103cells/well) were incubated either with M-DC8+ DCs, monocytes, or NK cells in the presence or absence of 10 μg/mL 17-1A antibody at different E/T ratios. After 18 hours of incubation, chromium release was determined. Symbols represent means ± SE of results obtained from 4 different donors. For each donor the mean of triplicate determination was taken. (D) 51Cr-labeled SK-BR-3 cells (5 × 103 cells/well) were incubated either with M-DC8+ DCs, monocytes, or NK cells in the presence or absence of 10 μg/mL Herceptin antibody at different E/T ratios. After 18 hours of incubation, chromium release was determined. Symbols represent means ± SE of results obtained from 4 different donors. For each donor the mean of triplicate determination was taken.
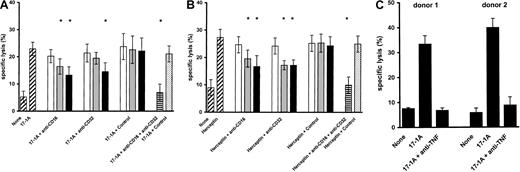
The crucial role of Fcγ receptors in ADCC is well documented.16 Monocytes have been shown to mediate ADCC through FcγRI (CD64) and FcγRII (CD32).17 This ADCC activity can be enhanced by pretreatment of monocytes with interferon γ.18 In contrast, ADCC mediated by NK cells is dependent mainly on activation via FcγRIII (CD16).9 Because freshly isolated M-DC8+ DCs are characterized by the strong expression of FcγRIII (CD16) and FcγRII (CD32),8 the differential role of these Fcγ receptors in M-DC8+DC-mediated ADCC was evaluated. Anti-FcγRIII (CD16) antibody and anti-FcγRII (CD32) antibody inhibited ADCC mediated by murine (Figure2A) as well as humanized antibodies (Figure 2B) to a similar degree. Together with the almost complete reduction of ADCC obtained by the combination of both inhibiting antibodies, these data suggest that the antibody-mediated cytotoxic effect is critically dependent on the simultaneous engagement of both Fcγ receptors.
Evaluation of mechanisms underlying ADCC mediated by M-DC8+ DCs.
(A) Freshly isolated M-DC8+ DCs were preincubated for 1 hour at 4°C with 50 μg/mL (■), 100 μg/mL (░), or 200 μg/mL (▪) of either an anti-FcγRIII (CD16), or an anti-FcγRII (CD32), or an isotype-matched control antibody. In addition, M-DC8+DCs were preincubated with a combination of an anti-FcγRIII (CD16) and an anti-FcγRII (CD32) antibody, each at 200 μg/mL ( ). In this experiment an isotype-matched antibody at 400 μg/mL was used as control (
). In this experiment an isotype-matched antibody at 400 μg/mL was used as control ( ). DCs were cocultured either with 51Cr-labeled COLO 205 cells in the presence of 17-1A (10 μg/mL) at an E/T ratio of 40:1 (A) or with51Cr-labeled SK-BR-3 cells in the presence of Herceptin (10 μg/mL) at an E/T ratio of 10:1 (B). After 18 hours of incubation, chromium release was measured. Columns represent means ± SE of results obtained from 4 different donors. For each donor the mean of triplicate determination was taken. Asterisks indicate a statistically significant difference (P < .05) between the inhibition of ADCC in the presence of anti-FcγRIII (CD16) or anti-FcγRII (CD32) antibody or a combination of both anti-FcγR antibodies and the isotype-matched control antibody. (C) 51Cr-labeled COLO 205 cells were incubated with M-DC8+ DCs at an E/T ratio of 40:1 in the presence of 10 μg/mL 17-1A antibody with or without a neutralizing anti–TNF-α antibody at a concentration of 10 μg/mL. After 18 hours of incubation, chromium release was determined. The results of 2 different donors are presented as mean ± SE of triplicate wells.
). DCs were cocultured either with 51Cr-labeled COLO 205 cells in the presence of 17-1A (10 μg/mL) at an E/T ratio of 40:1 (A) or with51Cr-labeled SK-BR-3 cells in the presence of Herceptin (10 μg/mL) at an E/T ratio of 10:1 (B). After 18 hours of incubation, chromium release was measured. Columns represent means ± SE of results obtained from 4 different donors. For each donor the mean of triplicate determination was taken. Asterisks indicate a statistically significant difference (P < .05) between the inhibition of ADCC in the presence of anti-FcγRIII (CD16) or anti-FcγRII (CD32) antibody or a combination of both anti-FcγR antibodies and the isotype-matched control antibody. (C) 51Cr-labeled COLO 205 cells were incubated with M-DC8+ DCs at an E/T ratio of 40:1 in the presence of 10 μg/mL 17-1A antibody with or without a neutralizing anti–TNF-α antibody at a concentration of 10 μg/mL. After 18 hours of incubation, chromium release was determined. The results of 2 different donors are presented as mean ± SE of triplicate wells.
Evaluation of mechanisms underlying ADCC mediated by M-DC8+ DCs.
(A) Freshly isolated M-DC8+ DCs were preincubated for 1 hour at 4°C with 50 μg/mL (■), 100 μg/mL (░), or 200 μg/mL (▪) of either an anti-FcγRIII (CD16), or an anti-FcγRII (CD32), or an isotype-matched control antibody. In addition, M-DC8+DCs were preincubated with a combination of an anti-FcγRIII (CD16) and an anti-FcγRII (CD32) antibody, each at 200 μg/mL ( ). In this experiment an isotype-matched antibody at 400 μg/mL was used as control (
). In this experiment an isotype-matched antibody at 400 μg/mL was used as control ( ). DCs were cocultured either with 51Cr-labeled COLO 205 cells in the presence of 17-1A (10 μg/mL) at an E/T ratio of 40:1 (A) or with51Cr-labeled SK-BR-3 cells in the presence of Herceptin (10 μg/mL) at an E/T ratio of 10:1 (B). After 18 hours of incubation, chromium release was measured. Columns represent means ± SE of results obtained from 4 different donors. For each donor the mean of triplicate determination was taken. Asterisks indicate a statistically significant difference (P < .05) between the inhibition of ADCC in the presence of anti-FcγRIII (CD16) or anti-FcγRII (CD32) antibody or a combination of both anti-FcγR antibodies and the isotype-matched control antibody. (C) 51Cr-labeled COLO 205 cells were incubated with M-DC8+ DCs at an E/T ratio of 40:1 in the presence of 10 μg/mL 17-1A antibody with or without a neutralizing anti–TNF-α antibody at a concentration of 10 μg/mL. After 18 hours of incubation, chromium release was determined. The results of 2 different donors are presented as mean ± SE of triplicate wells.
). DCs were cocultured either with 51Cr-labeled COLO 205 cells in the presence of 17-1A (10 μg/mL) at an E/T ratio of 40:1 (A) or with51Cr-labeled SK-BR-3 cells in the presence of Herceptin (10 μg/mL) at an E/T ratio of 10:1 (B). After 18 hours of incubation, chromium release was measured. Columns represent means ± SE of results obtained from 4 different donors. For each donor the mean of triplicate determination was taken. Asterisks indicate a statistically significant difference (P < .05) between the inhibition of ADCC in the presence of anti-FcγRIII (CD16) or anti-FcγRII (CD32) antibody or a combination of both anti-FcγR antibodies and the isotype-matched control antibody. (C) 51Cr-labeled COLO 205 cells were incubated with M-DC8+ DCs at an E/T ratio of 40:1 in the presence of 10 μg/mL 17-1A antibody with or without a neutralizing anti–TNF-α antibody at a concentration of 10 μg/mL. After 18 hours of incubation, chromium release was determined. The results of 2 different donors are presented as mean ± SE of triplicate wells.
Tumor necrosis factor α is regarded as an important effector molecule of tumor cell killing.19,20 TNF-α production by activated DCs is well documented.21 We observed that during ADCC, M-DC8+ DCs secreted TNF-α at a concentration surmounting that observed with autologous monocytes (data not shown). To evaluate the contribution of TNF-α to the cytotoxic effect of DCs, ADCC was determined in the presence of a neutralizing anti–TNF-α antibody. As demonstrated in Figure 2C, tumor-directed ADCC was almost completely abrogated by the anti–TNF-α antibody suggesting an important role of TNF-α in ADCC mediated by M-DC8+ DCs.
In summary, our data provide for the first time evidence that a major subpopulation of circulating human blood DCs efficiently mediates tumor-directed ADCC through a mechanism that is dependent on the engagement of FcγRIII (CD16) and FcγRII (CD32) as well as on the production of TNF-α. Because immature CD1a+ DCs have been shown to infiltrate tumor tissues22 this novel DC effector function may play an important role in natural and therapeutic antitumor reactions.
The technical assistance of Karin Günther, Bärbel Löbel, and Verona Schwarze is greatly appreciated.
Supported by the Wilhelm Sander-Stiftung and by the Medical Faculty, Technical University of Dresden.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Ernst Peter Rieber, Institute of Immunology, Medical Faculty, Technical University of Dresden, Fetscherstr 74, 01307 Dresden, Germany; e-mail: rieber@rcs.urz.tu-dresden.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal