Childhood idiopathic thrombocytopenic purpura (ITP) resolves usually after the first episode, although it may recur, and in 10% to 20% of patients develops into a chronic disorder. Evidence of the immunoregulatory role of Th1/Th2 responses in autoimmune diseases prompted us to perform a prospective study of Th1/Th2 gene expression profiles and transforming growth factor β (TGF-β) plasma levels in 18 children (median age, 6.4 years) with acute ITP, before and after intravenous immunoglobulin G (IVIg) infusion, and during a follow-up period (0.5-5 years). Initially, 12 of 18 patients had either low Th0/Th1 plus interleukin 10 (IL-10) or no in vivo cytokine gene expression (0). At 24 hours after IVIg infusion this pattern became 0 or Th2 (9 of 12) or remained low Th0/Th1 (3 of 12). During follow-up these patients did not relapse and maintained 0 or Th2 pattern without IL-10. Of the remaining 6 patients, 4 presented with a Th1 or Th0/Th1 pattern plus IL-10 that persisted after IVIg treatment (although interferon γ [IFN-γ] expression diminished) and stabilized to Th1 plus IL-10 at follow-up, which was marked by infrequent episodes of ITP. Two patients presenting with a strict Th1 pattern characterized by high expression of IFN-γ, which remained unchanged after IVIg and at follow-up, can be characterized as chronic ITP. TGF-β plasma levels were low in patients with active disease and increased in remission. Overall, acute ITP presents with Th1, Th0/Th1, or 0 in vivo cytokine gene expression. Stable remission is associated with a 0 or Th2 pattern. A 0 or Th2 pattern after IVIg gave the best prognosis, whereas sustained high expression of IFN-γ and refractoriness to IVIg were the main indicators of poor prognosis.
Introduction
Idiopathic thrombocytopenic purpura (ITP) is an acquired autoimmune disorder characterized by the production of antibodies against antigens on the membranes of platelets, resulting in enhanced Fc-mediated destruction of the platelets by macrophages in the reticuloendothelial system. ITP is mainly classified into 2 forms, chronic ITP, typically an adult disease persisting for years, and acute ITP, a self-limited childhood disorder usually occurring within weeks after a viral infection. ITP is more common in children than in adults and approximately 40% of all patients are younger than 10 years. In children, both sexes are equally affected; in adults women predominate 3:1.1-6
Although autoreactive B lymphocytes secreting antiplatelet antibodies are considered as the primary immunologic defect in ITP, several T-lymphocyte abnormalities have also been described. Cell-mediated cytotoxicity against platelets has been demonstrated using lymphocytes and T-cell clones from patients with ITP. In addition, abnormalities have also been described in the analysis of T-cell subsets, mainly a decreased CD4 population and reversed CD4/CD8 ratios, higher numbers of CD45RA, and lower numbers of CD45RO T cells, all reminiscent of the association of HIV infection and thrombocytopenia as well as other autoimmune phenomena, such as systemic lupus erythematosus, the initial presentation of which may be ITP.7-11
Administration of intravenous immunoglobulin G (IVIg) is the standard therapy used in recent years for ITP and is effective in a significant proportion of patients.2,12 The proposed mechanisms of action of IVIg include the saturation of phagocytic Fc receptors or the neutralization of antiplatelet autoantibodies by anti-idiotypic antibodies in the preparations.2 It has been reported that antibodies against interleukin 1 (IL-1) and IL-6 were detected in IVIg preparations.13 Because IL-1α, associated with the cytoplasmic membrane of antigen-presenting cells, appears to be particularly important in the triggering of T lymphocytes,14 its neutralization by anti–IL-1α antibodies contained in IVIg preparations may result in immunosuppression in vivo. In addition, IgG1 and IgG2 antibodies to IL-1α may trigger cytotoxic processes directed against both IL-1α–producing and IL-1α–responding cells, which may result in a rapid decrease in the number of circulating T and B cells.14
Another possible effect of IVIg administration may be the masking of superantigen-binding sites on T cells, thus modulating the superantigen-induced cytokine production in T cells.15 It has also been shown in vitro that IVIg down-regulates the synthesis of certain cytokines as well as cell-surface IL-2 receptor expression.16 It has also been reported that transforming growth factor β (TGF-β) contained in varied quantities in IVIg preparations contributes to the therapeutic effect of IVIg in autoimmune diseases17; this finding is disputed by others.18
In recent years, numerous studies19-23 have shown that patients suffering from autoimmune diseases have polarized Th1 or Th2 responses. Childhood ITP does not seem to fit the definition of an established autoimmune disease and although spontaneous remission occurs in the majority of children, it still remains impossible to predict at the time of diagnosis which child will develop an acute self-resolving disorder and which a chronic disorder.2,3 5To this end, we studied the expression of a panel of Th1 and Th2 cytokine genes in a group of children who presented with ITP and assessed the effect of IVIg administration on the cytokine gene expression of the above patients. In addition, we performed follow-up studies in the same children 0.5 to 5 years later to investigate whether the primary findings can be of prognostic value. Although the data obtained are based on a small number of cases studied, it appears that the Th cytokine profile at presentation and after IVIg infusion can predict the clinical course of childhood ITP.
Patients, materials, and methods
Patients
Eighteen patients with acute ITP and 14 healthy children as controls were studied. All subjects presented to the Pediatric Department of the Patras University Hospital (PUH). Samples of heparinized blood (0.5-5 mL) were drawn from patients prior to any treatment and 24 hours after completion of IVIg administration, and from controls only once during a routine visit to PUH for minor problems. Samples were also collected at follow-up visits at least once for each patient. Informed consent was obtained from each participating patient (when old enough) and the patient's parent or guardian. Human experimentation guidelines were submitted to and approved by the internal review board and scientific advisory committee of PUH. PUH abides by the Helsinki declaration on ethical principles for medical research involving human subjects.
IVIg
The IVIg preparation used was Sandoglobulin (Novartis, Basel, Switzerland). When reconstituted for therapeutic use it contained 50 mg/mL IgG, 25 to 35 mg/mL sucrose, 6 to 10 mg/mL glucose, and 40 to 100 mM NaCl. For in vitro studies, 1 mL of 15 different reconstituted IVIg preparations was kept stored at −20°C.
Cell cultures
Heparinized venous blood was collected from the ITP patients at presentation, 24 hours after IVIg treatment, and at follow-up visits as well as from healthy pediatric controls. Peripheral blood mononuclear cells (PBMCs) were prepared by centrifugation over a Ficoll-Paque gradient (Pharmacia, Uppsala, Sweden). Plasma was collected and stored at −80°C. The cells (106 PBMC/group) were processed immediately or cultured for 8 hours in RPMI 1640 culture medium (Gibco BRL, Gaithersburg, MD) containing 10% fetal calf serum (FCS; Gibco BRL) and other supplements as previously described,23 in the presence of 5 ng/mL phorbol myristate acetate (PMA) and 1 μM ionomycine (Sigma, St Louis, MO). The cells were counted using a Sysmex NE-8000 counter (Kyoto, Japan) and their viability was estimated by the trypan blue exclusion method as described previously.24
TGF-β1 ELISA
Determination of TGF-β1 levels in the plasma and also in different IVIg preparations was performed by an enzyme-linked immunoassay (ELISA) as instructed by the manufacturer (Quantikine; R & D Systems, Minneapolis, MN).
Reverse transcriptase–polymerase chain reaction (RT-PCR)
Total cellular RNA isolated by the guanidinium thiocyanate-phenol-chloroform extraction procedure, as described by Chomczynski and Sacchi,25 was reverse transcribed (RT) after heat denaturation and annealing, with Random Hexamer (Promega, Madison, WI), in the presence of 200 U Superscript RT (Gibco BRL) and 0.5 mM of each deoxynucleotide (Promega), in 50 μL, for 1 hour at 37°C. Then 1 μL of the RT mixture was submitted to polymerase chain reaction (PCR), in a volume of 50 μL, in the presence of 150 μM of each deoxynucleotide, 2.5 U Taqpolymerase (Gibco BRL), and 0.25 μM of the upstream and downstream primers (Institute of Molecular Biology, Crete, Greece). Each reaction was carried out with RNA extracted from 6.5 × 103 viable PBMCs. The PCR products were run on 2% agarose gels and stained with ethidium bromide for UV light visualization and photography. For quantitative evaluation, the bands were scanned and the data analyzed using ImageTool V1.28 software (University of Texas Health Science Center, San Antonio, TX). The RT-PCR signal generated by β2-microglobulin (β2m) mRNA was chosen to estimate the amounts of cDNA obtained from different cell samples. Each RT-PCR included controls for RNA extraction (lysis buffer alone treated as a normal sample), RT (RT reagents without RNA), and PCR (PCR reagents without cDNA). The PCR primer pairs26-32 used in this study are shown in Table 1.
Primers and conditions for the RT-PCR experiments performed in this study
| Gene . | Sequence of primers (5′ → 3′) . | T (°C) . | Product . |
|---|---|---|---|
| IL-2 | GCAACTCCTGTCTTGCATTG | 59 | 173 |
| AATGTGAGCATCCTGGTGAG | |||
| IFN-γ | AGCTCTGCATCGTTTTGGGTTC | 64 | 492 |
| CAAATATTGCAGGCAGGACAACC | |||
| IL-3 | GCTCCCATGACCCAGACAACGTCC | 61 | 400 |
| CTAAAAGATCGCGAGGCTCAAAGTCGT | |||
| IL-4 | CTGTGCTCCGGCAGTTCTAC | 58 | 176 |
| ACGTACTCTGGTTGGCTTCC | |||
| IL-6 | GCCAGAGCTGTGCAGATGAG | 58 | 187 |
| AGGAACTCCTTAAAGCTGCG | |||
| IL-10 | ACCCAGTCTGAGAACAGCTGC | 61 | 260 |
| GTTCACATGCGCCTTGATGTCT | |||
| IL-13 | GACCACGGTCATTGCTCTCACT | 61 | 234 |
| TCTGGGTCTTCTCGATGGCACT | |||
| β2m | CCCCCACTGAAAAAGATGAG | 56 | 150 |
| TCACTCAATCCAAATGCGGC |
| Gene . | Sequence of primers (5′ → 3′) . | T (°C) . | Product . |
|---|---|---|---|
| IL-2 | GCAACTCCTGTCTTGCATTG | 59 | 173 |
| AATGTGAGCATCCTGGTGAG | |||
| IFN-γ | AGCTCTGCATCGTTTTGGGTTC | 64 | 492 |
| CAAATATTGCAGGCAGGACAACC | |||
| IL-3 | GCTCCCATGACCCAGACAACGTCC | 61 | 400 |
| CTAAAAGATCGCGAGGCTCAAAGTCGT | |||
| IL-4 | CTGTGCTCCGGCAGTTCTAC | 58 | 176 |
| ACGTACTCTGGTTGGCTTCC | |||
| IL-6 | GCCAGAGCTGTGCAGATGAG | 58 | 187 |
| AGGAACTCCTTAAAGCTGCG | |||
| IL-10 | ACCCAGTCTGAGAACAGCTGC | 61 | 260 |
| GTTCACATGCGCCTTGATGTCT | |||
| IL-13 | GACCACGGTCATTGCTCTCACT | 61 | 234 |
| TCTGGGTCTTCTCGATGGCACT | |||
| β2m | CCCCCACTGAAAAAGATGAG | 56 | 150 |
| TCACTCAATCCAAATGCGGC |
T (°C) indicates annealing temperature; product, PCR product size (bp).
EMSAs
Electrophoretic mobility shift assays (EMSAs) were performed to assay for the presence and function of the lymphotropic transcription factors (TFs) nuclear factor-κB (NF-κB),33 activator protein-1 (AP-1)34, and nuclear factor of activated T cells (NFAT)35 as described previously36 with modifications. Briefly, the cellular membranes were broken by sonication on ice and a 3 to 4 × pellet volume of a buffer containing 5 mM HEPES (N-2-hydroxyethylpiperazine-n′-2-ethanesulfonic acid), pH 7.9, 26% glycerol, 1.5 mM MgCl2, 0.2 mM EDTA (ethylenediaminetetraacetic acid), 0.5 mM DTT (dichlorodiphenyltrichloroethane), 0.5 μM PMSF (phenylmethylsulfonyl fluoride), and 1 μg/mL leupeptin (all from Sigma) was added and then salt (KCl) adjusted to 300 mM to elute the proteins from chromatin. After an incubation of 30 minutes on ice, the cell lysates were centrifuged at 27 000g, for 60 minutes, at 4°C to sediment chromatin. Protein concentration was determined using the Bradford assay (Bio-Rad, Hercules, CA). As oligonucleotide probes we used the sequences AGTTGAGGGATTTCACTT for NF-κB, GTGACTCAGCGCG for AP-1, and AAGAAAGGAGGAAAAACTGTTT for NFAT.
Results
Patients
Table 2 shows the age, sex, and platelet counts of patients and controls. Table3 shows clinical and laboratory parameters of patients (P1-P18) divided into 2 groups based on the outcome of the disease. Group 1 includes 12 patients (P1-P12) followed for 1 to 4 years who had only one episode of thrombocytopenia and are considered cured; patients P8 and P9 received no treatment at all, but were tested for Th cytokine expression before leaving the hospital. Group 2 includes 6 children (P13-P18); patients P13 to P16 have infrequent episodes of thrombocytopenia triggered by viral infections and require no further treatment except occasionally a brief IVIg infusion. P17 and P18 have required maintenance with corticosteroids. At presentation, patients P1, P2, P5, P13, P15, P17, and P18 required steroids after only a moderate response to IVIg.
Data of study subjects
| . | Children with ITP . | Healthy children . |
|---|---|---|
| No. | 18 | 14 |
| Male/female | 12/6 | 11/3 |
| Age | 6.4 (0.7-14) | 10.1 (5-14) |
| Platelet count | 18 (3-41) | 230 (175-335) |
| . | Children with ITP . | Healthy children . |
|---|---|---|
| No. | 18 | 14 |
| Male/female | 12/6 | 11/3 |
| Age | 6.4 (0.7-14) | 10.1 (5-14) |
| Platelet count | 18 (3-41) | 230 (175-335) |
Age is in years (mean with range in parentheses); platelets × 109/L (median with range in parentheses); data from patients at presentation.
Clinical and laboratory parameters of ITP patients
| Patient . | Sex . | At presentation . | Follow-up . | |||||
|---|---|---|---|---|---|---|---|---|
| Age . | PLT . | IVIg (d) . | PLT (24 h after IVIg) . | Time after . | PLT . | Current condition . | ||
| Group 1 | ||||||||
| P1 | M | 4 | 3 | 2 | 22 | 1 | 250 | Cured |
| P2 | F | 4 | 26 | 2 | 75 | 3 | 271 | Cured |
| P3 | M | 8 | 16 | 5 | 380 | 3 | 341 | Cured |
| P4 | M | 12 | 8 | 5 | 288 | 1 | 257 | Cured |
| P5 | M | 0.7 | 12 | 4 | 24 | 1 | 249 | Cured |
| P6 | F | 10 | 26 | 5 | 218 | 1 | 235 | Cured |
| P7 | F | 4.5 | 21 | 2 | 249 | 1 | 321 | Cured |
| P8 | M | 8 | 10 | 0 | NA | 1 | 235 | Cured |
| P9 | M | 1 | 30 | 0 | NA | 3.5 | 315 | Cured |
| P10 | M | 2 | 5 | 5 | 311 | 3 | 330 | Cured |
| P11 | F | 5.5 | 10 | 2 | 268 | 3 | 341 | Cured |
| P12 | F | 4 | 20 | 5 | 183 | 4 | 201 | Cured |
| Group 2 | ||||||||
| P13 | M | 14 | 28 | 2 | 29 | 2 | 218 | Relapsing ITP |
| P14 | M | 1 | 13 | 4 | 117 | 1 | 216 | Relapsing ITP |
| P15 | M | 8 | 25 | 2 | 66 | 0.5 | 341 | Relapsing ITP |
| 0.6 | 80 | |||||||
| 0.7 | 130 | |||||||
| P16 | F | 10 | 5 | 2 | 106 | 2 | 209 | Relapsing ITP |
| 5 | 216 | |||||||
| P17 | M | 8 | 20 | 1 | 40 | 0.8 | 70 | Chronic ITP |
| P18 | M | 10 | 41 | 1 | 30 | 0.9 | 30 | Chronic ITP |
| 1 | 72 | |||||||
| 1.1 | 35 | |||||||
| Patient . | Sex . | At presentation . | Follow-up . | |||||
|---|---|---|---|---|---|---|---|---|
| Age . | PLT . | IVIg (d) . | PLT (24 h after IVIg) . | Time after . | PLT . | Current condition . | ||
| Group 1 | ||||||||
| P1 | M | 4 | 3 | 2 | 22 | 1 | 250 | Cured |
| P2 | F | 4 | 26 | 2 | 75 | 3 | 271 | Cured |
| P3 | M | 8 | 16 | 5 | 380 | 3 | 341 | Cured |
| P4 | M | 12 | 8 | 5 | 288 | 1 | 257 | Cured |
| P5 | M | 0.7 | 12 | 4 | 24 | 1 | 249 | Cured |
| P6 | F | 10 | 26 | 5 | 218 | 1 | 235 | Cured |
| P7 | F | 4.5 | 21 | 2 | 249 | 1 | 321 | Cured |
| P8 | M | 8 | 10 | 0 | NA | 1 | 235 | Cured |
| P9 | M | 1 | 30 | 0 | NA | 3.5 | 315 | Cured |
| P10 | M | 2 | 5 | 5 | 311 | 3 | 330 | Cured |
| P11 | F | 5.5 | 10 | 2 | 268 | 3 | 341 | Cured |
| P12 | F | 4 | 20 | 5 | 183 | 4 | 201 | Cured |
| Group 2 | ||||||||
| P13 | M | 14 | 28 | 2 | 29 | 2 | 218 | Relapsing ITP |
| P14 | M | 1 | 13 | 4 | 117 | 1 | 216 | Relapsing ITP |
| P15 | M | 8 | 25 | 2 | 66 | 0.5 | 341 | Relapsing ITP |
| 0.6 | 80 | |||||||
| 0.7 | 130 | |||||||
| P16 | F | 10 | 5 | 2 | 106 | 2 | 209 | Relapsing ITP |
| 5 | 216 | |||||||
| P17 | M | 8 | 20 | 1 | 40 | 0.8 | 70 | Chronic ITP |
| P18 | M | 10 | 41 | 1 | 30 | 0.9 | 30 | Chronic ITP |
| 1 | 72 | |||||||
| 1.1 | 35 | |||||||
Group 1 (P1-P12), patients with acute ITP followed by long-term remission, P8 and P9 received no treatment; group 2 (P13-P18), patients P13 to P16 with acute ITP followed by infrequent relapses triggered by viral infections; P17 and P18 had acute ITP followed by frequent episodes occurring within periods of less than 1 month, regardless of infection, requiring continuous monitoring. Only the times when blood samples were given for the present study are shown in the follow-up phase.
PLT indicates number of platelets (× 109/L); IVIg, 2 g/kg body weight (daily dose); NA, not applicable; time after, time in years after the first episode when blood sample was taken for the follow-up study.
Ex vivo Th1/Th2 cytokine gene expression in the ITP patients versus healthy pediatric controls
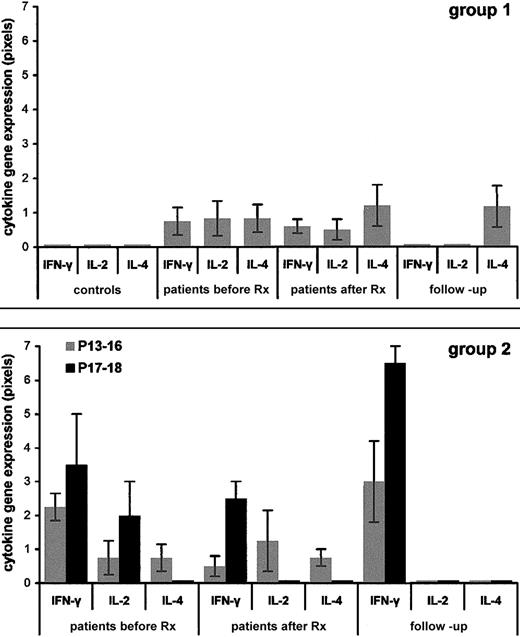
Figures 1 and2 depict the data obtained from the pediatric controls and the patients. The results are from RT-PCR performed on PBMCs isolated from blood and processed immediately (ex vivo) and are given in pixels (mean values ± SEM).
Ex-vivo Th1/Th2 cytokine gene expression profiles in patients with ITP versus healthy pediatric controls.
PBMCs were isolated from the patients and controls and RNA was extracted immediately (ex vivo). RT-PCR was performed and the results were quantified and given as pixels/102 ± SEM (as described in “Patients, materials, and methods”). Panel A shows 14 healthy pediatric controls (left) and group 1 ITP patients P1 to P12 with first episode followed by stable remission (gray bars); Panel B shows group 2 ITP patients P13 to P16 with first episode followed by infrequent relapses triggered by viral infection (gray bars) and ITP patients P17 and P18 with first episode followed by frequent episodes occurring within periods of less than 1 month, regardless of infection, requiring continuous monitoring (black bars). Before Rx indicates at presentation; after Rx, 24 hours after IVIg therapy; follow-up, recall patients.
Ex-vivo Th1/Th2 cytokine gene expression profiles in patients with ITP versus healthy pediatric controls.
PBMCs were isolated from the patients and controls and RNA was extracted immediately (ex vivo). RT-PCR was performed and the results were quantified and given as pixels/102 ± SEM (as described in “Patients, materials, and methods”). Panel A shows 14 healthy pediatric controls (left) and group 1 ITP patients P1 to P12 with first episode followed by stable remission (gray bars); Panel B shows group 2 ITP patients P13 to P16 with first episode followed by infrequent relapses triggered by viral infection (gray bars) and ITP patients P17 and P18 with first episode followed by frequent episodes occurring within periods of less than 1 month, regardless of infection, requiring continuous monitoring (black bars). Before Rx indicates at presentation; after Rx, 24 hours after IVIg therapy; follow-up, recall patients.
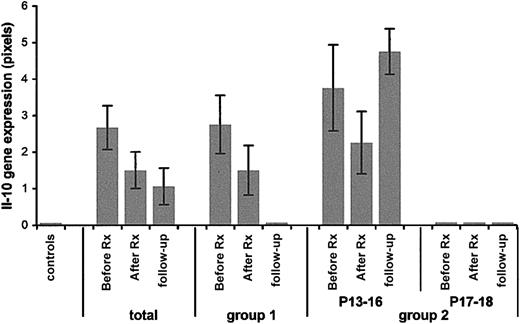
Expression of the IL-10 gene in ITP patients compared to pediatric controls.
Pediatric controls, far left; total: results from all 18 patients. Group 1 indicates patients P1 to P12; group 2, patients P13 to P18; P13 to P16 patients with relapsing ITP; P17 and P18 patients with chronic ITP. Before Rx indicates at presentation; after Rx, 24 hours after IVIg therapy; follow-up, recall patients.
Expression of the IL-10 gene in ITP patients compared to pediatric controls.
Pediatric controls, far left; total: results from all 18 patients. Group 1 indicates patients P1 to P12; group 2, patients P13 to P18; P13 to P16 patients with relapsing ITP; P17 and P18 patients with chronic ITP. Before Rx indicates at presentation; after Rx, 24 hours after IVIg therapy; follow-up, recall patients.
For the sake of brevity, we state at this point that (1) PBMCs isolated from all the pediatric controls expressed none of the cytokine genes tested ex vivo, whereas all expressed these cytokines when cultured with mitogens. (2) IL-3, IL-6, and IL-13 expression was within control range in all patients (data not shown). (3) All cytokine genes tested in this study were able to be induced by mitogens in all the patients (data not shown). (4) For the Th pattern analysis of the patients we used the data obtained for the cytokines IL-2, interferon γ (IFN-γ), and IL-4 (Figure 1); IL-10 data are shown separately (Figure 2) and were not included in this analysis because non-T cells such as monocytes can produce high amounts of this cytokine also.23
Patients P1 to P12 (Figure 1, group 1) are those who had one acute episode only. They presented with a low Th0/Th1 pattern (7 of 12) or no ex vivo cytokine expression (5 of 12). After IVIg infusion the majority of these patients expressed no cytokine in vivo or IL-4 only (Th2) exactly like patients P8 and P9 of this group who required no therapy. Patients P1, P2, and P5, who required steroids after a moderate response to IVIg infusion, are the ones who presented with a Th0/Th1 pattern with the highest values of IL-2/IFN-γ of this group; this pattern was maintained after IVIg infusion though the intensities of IL-2 and IFN-γ gene expression were lower. At follow-up, 1 to 4 years later, all 12 patients expressed none of the cytokines tested in vivo or they expressed IL-4 only (Th2).
Patients P13 to P16 (Figure 1, group 2, gray histograms) are the ones with an occasional episode of thrombocytopenia triggered by viral infections, who require no maintenance treatment. They presented with Th1 or Th0/Th1 pattern that was maintained after IVIg infusion but with lower expression of IFN-γ. At follow-up, 0.5 to 5 years later, they all have a Th1 (IFN-γ) in vivo pattern.
Patients P17 and P18 (Figure 1, group 2, black histograms) are those with chronic disease requiring maintenance steroid therapy. They have a constant Th1-high–IFN-γ pattern in all phases of the disease studied. At follow-up, 0.8 to 1.1 years later, they maintain the highest IFN-γ levels of all patients with ITP.
Ex vivo IL-10 gene expression
On the whole, IL-10 gene expression (Figure 2) correlated negatively with disease activity (Figure 2, total). In group 1, it was highly expressed in the acute phase; its expression decreased after IVIg infusion and reached zero levels at follow-up.
In group 2, IL-10 expression was highly expressed at all times in the relapsing category with a transient decrease after IVIg infusion, whereas it was not expressed at any point of the study in the children with the chronic active disease.
TGF-β1 levels in sera and IVIg preparations
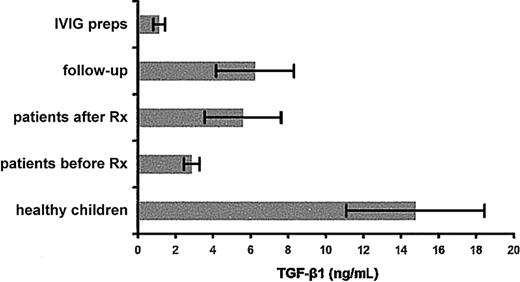
An ELISA was used to measure TGF-β1 levels in the plasma of the children with ITP before and 24 hours after IVIg treatment and at follow-up at the times indicated in Table 3 in pediatric controls and 15 randomly selected preparations of IVIg. The results are depicted in Figure 3 and show that TGF-β1 plasma levels increased immediately after treatment in the majority of the patients and the IVIg preparations tested contained negligible levels of TGF-β1(mean = 1.14 ng/mL).
TGF-β1 levels in plasma of patients and controls and in IVIg preparations.
TGF-β1 concentrations (mean values ± SEM) measured by ELISA in the plasma of the pediatric controls and of all the patients at presentation (before Rx), after IVIg treatment (after Rx), and at follow-up, and in different IVIg preparations.
TGF-β1 levels in plasma of patients and controls and in IVIg preparations.
TGF-β1 concentrations (mean values ± SEM) measured by ELISA in the plasma of the pediatric controls and of all the patients at presentation (before Rx), after IVIg treatment (after Rx), and at follow-up, and in different IVIg preparations.
Absence of a transcriptional silencer in patients with ITP
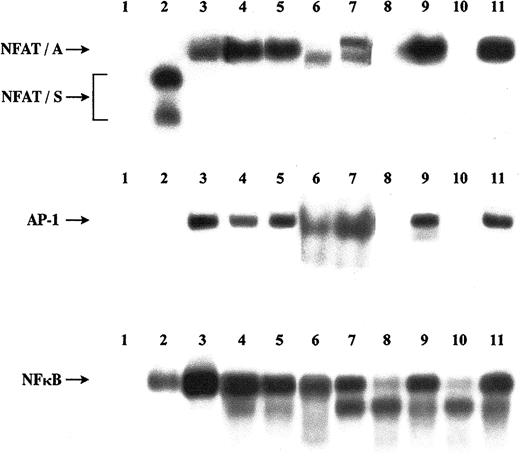
The TFs AP-1, NF-κB, and NFAT regulate cytokine transcription.34,35,37 Of the 3 TFs, AP-1 and NF-κB are exclusively positive regulators, whereas NFAT TFs play a dual role. An NFAT-silencer (NFAT-S) down-regulates transcription and an NFAT-transcription activator (NFAT-A) up-regulates transcription. The silencer is only detectable in resting naive CD4 T cells and is lost in recently activated cells (effectors) and resting memory cells. The activator is detectable in activated and memory cells.24,38 It was thus suggested that the presence of a silencer contributes to the more stringent activation requirements of naive CD4 T cells, thus safeguarding against autoimmunity.24 To test this hypothesis, we performed EMSA experiments with AP-1, NF-κB, and NFAT probes and nuclear extracts from ex vivo or mitogenically stimulated peripheral blood lymphocytes (PBLs) from a healthy child and 2 children with ITP from group 1, in the acute phase and in remission. Phenotypic analysis performed in remission showed that these patients, like the healthy child, had over 50% resting naive CD4 T cells (data not shown). The results are shown in Figure 4. At presentation, the patients, in contrast to the healthy control, had AP-1, NF-κB, and NFAT-A activity in their ex vivo T cells and, similarly to the healthy control, in their mitogenically stimulated cells. In remission, none of these TFs were present in the nucleus of the patients' ex vivo cells but were present in their stimulated cells, that is, the same pattern with that in the healthy control, with the exception that the NFAT-S was missing.
Activity of the lymphotropic TFs.
Activities of AP-1, NF-κB, and NFAT in nuclear extracts isolated from PBLs of 2 ITP patients in acute phase and in remission and a healthy pediatric control are shown. EMSAs were performed with labeled probes, as described in “Patients, materials, and methods,” and protein extracts from PBLs of the patients and the healthy control before or after mitogenic stimulation of the cells with PMA and ionomycine. Lane 1, free probes; lane 2, healthy control, ex vivo cells; lane 3, healthy control, stimulated cells; lane 4, first patient in acute phase, ex vivo cells; lane 5, first patient in acute phase, stimulated cells; lane 6, second patient in acute phase, ex vivo cells; lane 7: second patient in acute phase, stimulated cells; lane 8, first patient in remission, ex vivo cells; lane 9, first patient in remission, stimulated cells; lane 10, second patient in remission, ex vivo cells; lane 11, second patient in remission, stimulated cells. Arrows show active TF complexes. NFAT-A indicates NFAT-binding activator; NFAT-S, NFAT-binding silencer.
Activity of the lymphotropic TFs.
Activities of AP-1, NF-κB, and NFAT in nuclear extracts isolated from PBLs of 2 ITP patients in acute phase and in remission and a healthy pediatric control are shown. EMSAs were performed with labeled probes, as described in “Patients, materials, and methods,” and protein extracts from PBLs of the patients and the healthy control before or after mitogenic stimulation of the cells with PMA and ionomycine. Lane 1, free probes; lane 2, healthy control, ex vivo cells; lane 3, healthy control, stimulated cells; lane 4, first patient in acute phase, ex vivo cells; lane 5, first patient in acute phase, stimulated cells; lane 6, second patient in acute phase, ex vivo cells; lane 7: second patient in acute phase, stimulated cells; lane 8, first patient in remission, ex vivo cells; lane 9, first patient in remission, stimulated cells; lane 10, second patient in remission, ex vivo cells; lane 11, second patient in remission, stimulated cells. Arrows show active TF complexes. NFAT-A indicates NFAT-binding activator; NFAT-S, NFAT-binding silencer.
Discussion
Established autoimmune diseases have polarized Th1 and Th2 responses.19-23 Acute ITP is a childhood autoimmune disorder that either resolves after the first episode or develops into a relapsing form with rare episodes triggered by viral infections or, rarely, into a more serious chronic form that requires maintenance treatment.2,3,5 Studies performed with a selected population of chronic adult ITP patients with active disease observed neither a clear-cut Th1 nor a Th2 serum cytokine profile,39 whereas studies performed with a mixed population of patients with acute or chronic or chronic-complex ITP showed a Th0/Th1 pattern of T-cell activation.10
We analyzed the Th cytokine expression patterns in 18 children with acute ITP, before and after IVIg administration, and at follow-up. In parallel, we determined the expression pattern of IL-10 and plasma levels of TGF-β.
Compared to the healthy pediatric controls who expressed no Th cytokines in vivo, the majority of the children who presented with acute ITP were expressing IL-2, IFN-γ with or without IL-4 cytokine genes in vivo (72% of the sample), suggesting an early CD4 Th0 and Th1 cell activation, in accordance with an earlier study.10 A minority of the patients (28% of the sample) presented with no cytokine expression and these were 5 patients of group 1 who went into complete remission after IVIg infusion or with no treatment at all (2 patients).
Of the patients expressing Th1 or Th0/Th1, the first differentiating factor was their response to IVIg infusion. Those who expressed no cytokine or Th2 after IVIg treatment required no other therapy and went into long-term remission maintaining the 0 or Th2 pattern at follow-up. Those who maintained the Th1 or Th0/Th1 pattern after IVIg treatment were either refractory to IVIg or had a transient response to it. The differential effect of IVIg treatment in ITP patients probably reflects different degrees of imbalance of the immune system of patients,12 which could, in turn, reflect the relative frequency of autoreactive clones in the periphery. It has been shown that IVIg induces direct apoptosis of activated T and B lymphocytes and also monocytes/macrophages.40 We may thus hypothesize that the effectiveness of IVIg treatment correlates negatively with the severity of the disease.
The second differentiating factor was the relative intensity of IFN-γ gene expression: The patients with low IFN-γ expression at presentation (group 1) either responded completely to IVIg (0 or Th2 pattern after termination of treatment) or required steroids in addition (maintenance of low Th0/Th1 expression after termination of treatment), but then went into stable remission.
The patients with high expression of IFN-γ at presentation (group 2) had either a transient response to IVIg (low IFN-γ, Th1, or Th0/Th1) or were refractory to IVIg (high IFN-γ, Th1). An important differentiating factor between the milder relapsing form and the aggressive chronic form of ITP in group 2 is the presence or absence of the anti-inflammatory cytokine IL-10. IL-10 provides a negative feedback mechanism that counteracts the activation of Th1 cells and monocytes/macrophages,23 41 so the complete absence of IL-10–expressing cells in the peripheral blood of patients P17 and P18 probably contributes to the enhanced Th1 cytokine synthesis by the autoreactive T-cell clones, which, in turn, inhibits IL-4 synthesis in these patients.
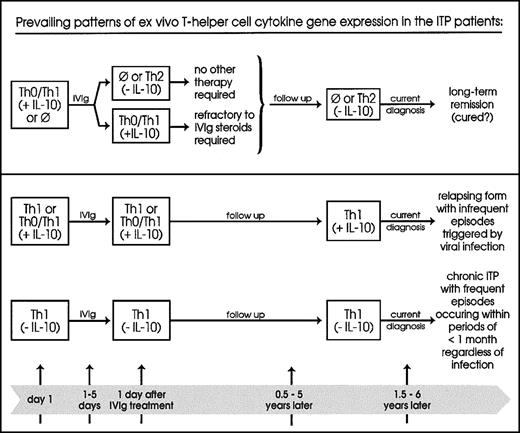
Based on the above, we propose the model depicted in Figure5. Multicenter studies of children presenting with ITP (RT-PCR/cytokine gene expression) and receiving none or IVIg-only treatment, and followed for at least 6 months, will test the validity of our pilot study.
Classification model.
This proposed model classifies patients with acute ITP according to their prevailing ex vivo Th1/Th2 cytokine gene expression patterns in relation to the phase of the disease and clinical profiles. ⊘ indicates no expression; upper panel, 12 patients (67%); lower panel, above, 4 patients (22%); below, 2 patients (11%).
Classification model.
This proposed model classifies patients with acute ITP according to their prevailing ex vivo Th1/Th2 cytokine gene expression patterns in relation to the phase of the disease and clinical profiles. ⊘ indicates no expression; upper panel, 12 patients (67%); lower panel, above, 4 patients (22%); below, 2 patients (11%).
The TGF-β levels in the IVIg preparations tested were negligible, thus appearing inadequate to influence cytokine expression in the patients. The very low plasma TGF-β concentrations at the time of disease onset increased after IVIg treatment. We may assume that the low TGF-β1 levels during active disease leave the autoantibody production against autologous platelets uncontrolled.42 In addition, because TGF-β1promotes thrombopoietin production,43 which, in turn, leads to increased platelet production,44 low TGF-β1 levels may also account directly for low platelet numbers during active disease. The relative increase of TGF-β1 levels in the patients after treatment and in remission may mediate a bystander immune suppression,39 43which may be adequate for the majority of the patients who recover or have rare episodes of thrombocytopenia but inadequate for the patients with chronic active disease.
A noteworthy aspect of this study is the EMSA finding (Figure 4) that the NFAT transcriptional silencer was absent from the nucleus of the ex vivo T cells of the 2 patients tested who were in remission. The positive TFs AP-1 and NF-κB were not active in these cells, indicating that the cells were not in vivo activated, whereas all positive factors including NFAT-A were activated in the in vitro–stimulated cells indicating that the cells were not anergic.45,46 Acquisition of the NFAT-S24,36 38 could play a role in converting proliferating precursors into resting cells during T-cell maturation. We hypothesize that an error at this stage of lymphocyte maturation causes a failure in the acquisition of the NFAT-S by some otherwise mature thymocytes and results in an increased number in the periphery of naive T cells, which are more prone to develop into autoreactive clones on antigenic stimulation.
We thank Prof Nicolaos Beratis, Prof George Maniatis, Patras, and Drs Cynthia Dunbar and George Chrousos, National Institutes of Health, Bethesda, MD, for reviewing the manuscript; Drs Evagelia Farri-Kostopoulou, Ioanna Foutzoula, and the staff of the Department of Pediatrics of PUH for their help in this study.
Supported by grants PENED/1274 from the Greek Ministry of Research and Technology, KARATHEODORIS/1952 from the University of Patras, and a grant from Novartis (Basel, Switzerland).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Note added in proof
Since this manuscript was submitted for publication a year has passed during which the patients included in this study were monitored, and their follow-up diagnosis remains the same.
Author notes
Athanasia Mouzaki, Laboratory Hematology and Transfusion Medicine, School of Medicine, University of Patras, Patras GR-261.10, Greece; e-mail: mouzaki@med.upatras.gr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal