Crystal-storing histiocytosis (CSH) is a rare event in disorders associated with monoclonal gammopathy. The intracellular crystal formation is almost always accompanied by the expression of κ light chains. However, the exact mechanism for the storage has not been clarified until now. We report a case of generalized CSH in a 73-year-old man who presented with IgA κ paraproteinemia and paraproteinuria. The initially observed CSH in the bone marrow biopsy was associated with the clinical and pathomorphologic features of a monoclonal gammopathy of undetermined significance. The progression of disease could not be affected by steroid therapy and the patient died of septic shock 7 months after detection of CSH. At the time of autopsy there was evidence for multiple myeloma and generalized CSH. Two-dimensional gel electrophoresis of liver tissue combined with immunoblotting revealed the massive storage of heavy chains of α type and light chains of κ type, each in a monoclonal pattern. Analysis of the stored κ light chain by nanoelectrospray-ionization mass spectrometry indicated that it belongs to the variable κI variability subgroup. We identified some unusual amino acid substitutions including Leu59, usually important for hydrophobic interactions within a protein, at a position where it has never been previously described in plasma cell disorders. In conclusion, we present the first case of CSH with molecular identification of the stored κ subgroup and detection of unusual amino acid substitutions. Our results suggest that conformational alterations induced by amino acid exchanges represent a crucial pathogenic factor in CSH.
Introduction
Crystal-storing histiocytosis (CSH) is an uncommon phenomenon in disorders associated with the expression of monoclonal immunoglobulins (multiple myeloma [MM], monoclonal gammopathy of undetermined significance [MGUS], lymphoplasmacytic lymphoma [LPL], extramedullary plasmacytoma).1-34 It is presumed to be an intralysosomal accumulation of secreted paraproteins or immunoglobulins, which aggregate in crystals. Given their rarity, the appearance of crystal-laden macrophages in the bone marrow often presents diagnostic difficulties because those cells may mimic Gaucher cells or so-called pseudo-Gaucher cells in chronic myelogenous leukemia.7,19,21,27 CSH in extramedullary sites has been mistaken for adult rhabdomyoma,4,10fibrosclerosis,2 Weber-Christian disease,5 or other types of histiocytosis.35
Although CSH shows a variety of appearances, sometimes obscuring the underlying disorder, immunoglobulins of light-chain type κ have been almost exclusively involved without a consistent association with a particular heavy chain.1 This suggests that CSH results from the storage of crystals produced by plasma cell tumors that either overproduce κ light chain or express a structurally aberrant molecule. However, the exact mechanism has not been clarified yet.
Proteome studies provide the possibility of analyzing the protein pattern of several thousand proteins simultaneously to identify biologically important proteins, to characterize their modifications, and to describe their functions. High-resolution 2-dimensional electrophoresis and mass spectrometry are prerequisites for such proteome studies.
We present a case of generalized CSH associated with IgA κ gammopathy, discuss possible pathogenic mechanisms on the basis of our proteome studies of the stored proteins, and review the reported cases in the literature.
Patient, materials, and methods
Case report
A 73-year-old man was admitted to the hospital because of ascites and a 5-month history of an 8-kg weight loss and fatigue. Seven months before his admission, the erythrocyte sedimentation rate had risen to 120 mm/h. Hematochemical analysis showed hypochromic anemia with a hematocrit of 32.4%. Serum immunoelectrophoresis showed an IgA κ paraproteinemia. Nephelometric analysis resulted in a serum concentration of 2580 mg/dL IgA and of 1991 mg/dL κ light chain. Urine immunoelectrophoresis revealed an IgA paraproteinuria and an increased excretion of albumin. Quantitative urine analysis resulted in a concentration of 46 mg/dL κ light chain. Radiographic examinations of the skull, entire spine, pelvis, and left and right humerus and femur indicated no lytic bone lesions or diffuse osteopenia. Bone marrow aspirate showed the accumulation of enlarged macrophages with cytoplasmic storage phenomena that led initially to the suspicion of Gaucher disease. The subsequently performed bone marrow biopsy did not confirm this diagnosis but was consistent with CSH. The rarely interspersed plasma cells were inconspicuous and not sufficient for the diagnosis of MM. CSH was also seen in biopsies of the liver, peritoneum, and skin made in the next 3 months. Because of the rapid clinical course with portal congestion, increasing ascites, and arising cutaneous tumors, steroid therapy was given for 5 weeks but did not affect the progression of disease. Terminally, fever and diarrhea developed and the patient died of septic shock 7 months after diagnosis of CSH.
Histopathology
Bone marrow biopsies were fixed in Schaffer solution, routinely processed, and embedded in methyl methacrylate as described elsewhere.36 Sections were stained with Giemsa, periodic acid-Schiff (PAS), Gomori silver, Ladewig trichrome, Congo red, and Perls reaction for iron.
Biopsy tissue of liver, peritoneum, and skin as well as specimens obtained at the time of autopsy were fixed in 4% neutral-buffered formalin, routinely processed, and embedded in paraffin. Sections were stained with hematoxylin-eosin (H-E), Giemsa, PAS, Congo red, Elastica-van Gieson trichrome, and Perls reaction for iron.
Immunohistochemistry
For immunohistochemical analysis acrylate and paraffin sections were used. Immunolabeling was done according to the alkaline phosphatase-antialkaline phosphatase (APAAP) and avidin-biotin peroxidase complex (ABC) techniques, following methods previously described.36 The primary antibodies, pretreatment procedures, and antibody dilutions used in this study are listed in Table 1.
Immunohistochemical analysis on plastic and paraffin-embedded specimen
| Antigen . | Source . | Plastic sections . | Paraffin sections . | ||
|---|---|---|---|---|---|
| Pretreatment . | Dilution . | Pretreatment . | Dilution . | ||
| Myeloperoxidase | DAKO† | Protease | 1:5 | Protease | 1:10 |
| CD68 (KP1) | DAKO† | Protease | 1:60 | Protease | 1:100 |
| CD45 (LC) | DAKO† | None | 1:20 | None | 1:100 |
| CD3 | DAKO† | Microwave | 1:50 | Protease | 1:300 |
| CD20 (L26) | Immunotech‡ | Microwave | 1:2 | Microwave | 1:20 |
| CD79a (MB1) | Laboserv1-153 | Microwave | 1:10 | Microwave | 1:20 |
| Plasma cells | DAKO† | Microwave | 1:10 | Microwave | 1:30 |
| κ light chain* | DAKO† | Protease | 1:10 000 | Protease | 1:15 000 |
| λ light chain* | DAKO† | Protease | 1:10 000 | Protease | 1:15 000 |
| IgA* | DAKO† | Protease | 1:7 000 | Protease | 1:6 000 |
| IgD* | DAKO† | — | — | Microwave | 1:100 |
| IgG* | DAKO† | Protease | 1:2 000 | Protease | 1:7 000 |
| IgM* | DAKO† | — | — | Protease | 1:3 000 |
| Antigen . | Source . | Plastic sections . | Paraffin sections . | ||
|---|---|---|---|---|---|
| Pretreatment . | Dilution . | Pretreatment . | Dilution . | ||
| Myeloperoxidase | DAKO† | Protease | 1:5 | Protease | 1:10 |
| CD68 (KP1) | DAKO† | Protease | 1:60 | Protease | 1:100 |
| CD45 (LC) | DAKO† | None | 1:20 | None | 1:100 |
| CD3 | DAKO† | Microwave | 1:50 | Protease | 1:300 |
| CD20 (L26) | Immunotech‡ | Microwave | 1:2 | Microwave | 1:20 |
| CD79a (MB1) | Laboserv1-153 | Microwave | 1:10 | Microwave | 1:20 |
| Plasma cells | DAKO† | Microwave | 1:10 | Microwave | 1:30 |
| κ light chain* | DAKO† | Protease | 1:10 000 | Protease | 1:15 000 |
| λ light chain* | DAKO† | Protease | 1:10 000 | Protease | 1:15 000 |
| IgA* | DAKO† | Protease | 1:7 000 | Protease | 1:6 000 |
| IgD* | DAKO† | — | — | Microwave | 1:100 |
| IgG* | DAKO† | Protease | 1:2 000 | Protease | 1:7 000 |
| IgM* | DAKO† | — | — | Protease | 1:3 000 |
Polyspecific primary antibodies.
DAKO Diagnostika, Hamburg, Germany.
Coulter-Immunotech Diagnostics, Hamburg, Germany.
Laboserve, Gießen, Germany.
Electron microscopy
For ultrastructural analysis, fragments of the primarily formalin-fixed and paraffin-embedded biopsy samples were cut off the paraffin blocks and re-embedded in epoxy resin. Ultrathin sections were placed on uncoated grids, contrasted with uranyl acetate and lead citrate, and observed on a Philips 420 electron microscope (Philips, Einthoven, The Netherlands).
Protein analysis
To further characterize the stored proteins we performed 2-dimensional polyacrylamide gel electrophoresis (2-DE) supplemented by immunoblotting and mass spectrometry (MS) on tissue samples from the patient. Stored urine or serum samples from the patient and fresh or frozen tissue from the kidneys were not available for these protein analyses.
2-DE.
Soluble and membrane-bound proteins were extracted from the patient's liver tissue samples as from normal liver tissue (4 samples of age-related male donors) and were separated by high-resolution 2-DE according to Klose37-39 using the Iso-Dalt system. In brief, isoelectric focusing (IEF) was performed with 4% carrier ampholytes, the second dimension was run on gels (16 × 16 × 0.15 cm) with a 10% to 16% polyacrylamide gradient as described earlier.40-42 Then 400 μg protein (Lowry protein assay kit, Sigma-Aldrich, Deisenhofen, Germany) was separated for Coomassie brilliant blue-stained gels (CBB R-250, Merck, Darmstadt, Germany); 100μg protein was used for silver staining.43
Immunoblotting.
The technique used for immunoblotting has been described earlier in detail.44 After 2-DE separation of proteins (400 μg protein/sample), selected areas in the 2-DE gels were excised for immunoblotting. The heavy chain region of IgA, IgD, IgM (∼90-40 kDa, pI ∼5.0-7.0, gel area 7.5 × 6.5 cm) and IgG (∼75-35 kDa, pI ∼6.6-8.0, gel area 8.5 × 5cm) as well as light chain region of κ and λ (∼40-20 kDA, pI ∼5.0-8.0, gel area 10 × 3.5 cm) were blotted onto hydrophobic polyvinylidene difluoride (PVDF) membranes (0.2 μm, Bio-Rad, Hercules, CA). For the immunodetection of the immunoglobulins the polyclonal antibodies also used for immunohistochemistry (all from Dako, Glostrup, Denmark; listed in Table 1) directed against human IgA (dilution 1:3000), IgD (1:80), IgM (1:2000), IgG (1:4000) κ and λ light chains (1:5000 each) were applied. Alkaline phosphatase-conjugated goat antirabbit IgG (dilution 1:2000, Bio-Rad) was used as a link and visualization was performed by Naphtol AS MX Phosphate/Fast Red TR Salt (Sigma-Aldrich) followed by counterstaining of the blots with CBB R-250.44
Protein identification.
Peptide mass fingerprinting (PMF) by matrix-assisted laser desorption-ionization mass spectrometry (MALDI-MS) and nanoelectrospray ionization mass spectrometry (nano-ESI-MS/MS) were applied to identify distinct proteins. Conspicuous protein spots (∼kDa/∼pI) as compared to controls were cut out from the CBB-stained 2-DE gels of the liver tissue samples and analyzed; spot 25 (25/5.6) resulted from the soluble protein fraction, spots 23 (23/5.0), 22 (22/5.0), 20 (20/5.0) as well as 13-1, 13-2, and 13-3 (13/4.5, 4.7, and 5.1, respectively) from the membrane bound fraction. Tryptic in-gel digestion and PMF by MALDI-MS were performed according to Lamer and Jungblut.45 The mass spectra were obtained by a Voyager Elite MALDI-TOF mass spectrometer (Perseptive Biosystems, Framingham, MA). Proteins were identified by using the search program MS-Fit (http://falcon.ludwig.ucl.ac.uk/ucsfhtml3.2/msfit.com). The sequence database of the National Center for Biotechnology Information (NCBI) was reduced to the “human rodent” proteins. A mass tolerance of 0.1 Da and 2 incomplete cleavages were allowed.
Sequence support was obtained by nano-ESI-MS/MS46performed with a Q-Tof mass spectrometer (Micromass, Manchester, England). An additional in-gel digestion with endoproteinase Glu-C (Roche Diagnostics, Mannheim, Germany) was performed to obtain other peptides and potentially increase the sequence coverage. The peptides were fragmented in a collision cell. The resulting MS/MS spectra allowed us to determine parts of the peptide sequence or the whole sequence. The sequence tag method47 was used to search the proteins in the NCBI protein database. Similarly, a search was additionally performed at the SWISS-PROT database at the European Bioinformatics Institute (EMBL) with the program FASTA 348and at the BLAST server of the NCBI.
Results
Histopathology
Bone marrow biopsies performed 7 and 5 months before the patient died revealed an increasing accumulation of histiocytes stuffed with needlelike crystalline material in a proportion of 30% and 50% of the total cell volume, respectively (Figure1A). Additionally erythrophagocytosis was observed in these cells. Plasma cells without significant nuclear atypia were rarely admixed in the groups of crystal-laden histiocytes and hematopoietic cells (Figure 1B). Cytoplasmic inclusions were observed only occasionally in plasma cells, which were slightly increased in the second biopsy but comprised even less than one tenth of the cellularity of the bone marrow. The hematopoiesis was at least slightly repressed except for a minimal elevation of eosinophilic granulopoiesis.
CSH in the bone marrow and the liver.
(A,B) Initial bone marrow biopsy. (A) Bone marrow shows a diffuse infiltrate of histiocytes stuffed with crystalloid inclusions (Giemsa, × 1600). (B) Plasma cells (marked by arrows) are only rarely admixed (Giemsa, × 4000). (C-F) Immunohistochemistry reveals positive reactivity of the histiocytes not only for IgA (C) and κ (D), but also for IgG (E) and λ (F; × 1000 each). (G-I) Bone marrow at the time of autopsy. (G) Plasma cells are multiplied compared to the initial biopsies and stain immunohistochemically positively for a monoclonal plasma cell marker (× 1600). (H) Immunohistochemistry for κ light chains reveals positive reactivity in histiocytes and in plasma cells (marked by arrows). Plasma cells stain less intensely possibly due to the conformation of the produced κ light chain (× 4000). (I) They also stain positively for IgA heavy chain (× 4000). (J,K) Liver biopsy 5 months before death. (J) Swollen Kupffer cells obstruct sinusoidal spaces (H-E; × 1200). (K) The Kupffer cells are highlighted immunohistochemically by their expression of CD68 (× 2500).
CSH in the bone marrow and the liver.
(A,B) Initial bone marrow biopsy. (A) Bone marrow shows a diffuse infiltrate of histiocytes stuffed with crystalloid inclusions (Giemsa, × 1600). (B) Plasma cells (marked by arrows) are only rarely admixed (Giemsa, × 4000). (C-F) Immunohistochemistry reveals positive reactivity of the histiocytes not only for IgA (C) and κ (D), but also for IgG (E) and λ (F; × 1000 each). (G-I) Bone marrow at the time of autopsy. (G) Plasma cells are multiplied compared to the initial biopsies and stain immunohistochemically positively for a monoclonal plasma cell marker (× 1600). (H) Immunohistochemistry for κ light chains reveals positive reactivity in histiocytes and in plasma cells (marked by arrows). Plasma cells stain less intensely possibly due to the conformation of the produced κ light chain (× 4000). (I) They also stain positively for IgA heavy chain (× 4000). (J,K) Liver biopsy 5 months before death. (J) Swollen Kupffer cells obstruct sinusoidal spaces (H-E; × 1200). (K) The Kupffer cells are highlighted immunohistochemically by their expression of CD68 (× 2500).
In the liver biopsy performed 5 months before the patient died, swollen Kupffer cells containing crystalline inclusions that obstructed the sinusoidal spaces were evident predominantly in central areas (Figure 1J-K). Some laden histiocytes were also observed in the portal areas. Crystal-storing histiocytes were detected as well in the connective tissue of the peritoneum, cutis, and subcutis.
The crystalline inclusions stained pinkish red with H-E, reddish brown with Elastica-van Gieson, and dark blue with Giemsa in the acrylate sections but only weakly in paraffin sections. They reacted faintly positive with Perls reaction for iron. PAS and Congo red failed to stain.
Immunohistochemistry
The crystal-storing histiocytes in all localizations examined reacted positively with the anti-CD68 antibody KP1. Antibodies against CD20 (L26), CD79a (MB1), CD3, and myeloperoxidase gave negative reactions in these cells. Immunohistochemically, the majority of the crystal-storing histiocytes were positive for IgA and IgG heavy chains as well as κ and λ light chains (Figure 1C-F). However, IgM and IgD were not detected in the histiocytes. The exact assignment of immunoglobulin expression in the only rarely admixed plasma cells was not possible.
Electron microscopy
Electron microscopy showed crystalline inclusions in the macrophages of the bone marrow, liver, and skin. The electron-dense crystalline inclusions varied in dimension and structure. Rectangular and rhomboid shapes predominated. Similar inclusions were also detected occasionally in plasma cells of the bone marrow and in some hepatocytes (Figure 2A-B). No periodic organization of the substructure was observed.
Ultrastructure of different cell types containing crystalline inclusions.
(A) Kupffer cell containing rectangular and rhomboid crystals (× 10 000). (B) Similar crystalline intracytoplasmic inclusions in a hepatocyte. Note the mitochondria (M) and characteristic peroxisomes (P; × 21 000). (C) Geometrically shaped crystals in a plasma cell at the time of autopsy (× 5800).
Ultrastructure of different cell types containing crystalline inclusions.
(A) Kupffer cell containing rectangular and rhomboid crystals (× 10 000). (B) Similar crystalline intracytoplasmic inclusions in a hepatocyte. Note the mitochondria (M) and characteristic peroxisomes (P; × 21 000). (C) Geometrically shaped crystals in a plasma cell at the time of autopsy (× 5800).
Autopsy findings
Autopsy of the cachectic patient (164 cm, 56 kg) revealed a tremendous number of large histiocytes storing excess amounts of irregularly outlined crystals diffusely spread in the bone marrow. Most of the histiocytes were immunohistochemically positive for IgA, IgM, and IgG heavy chains as well as κ and λ light chains. Among the crystal-storing histiocytes, plasma cells were seen interspersed. In contrast to the bone marrow biopsies done 7 and 5 months before, the number had increased up to a portion of 10% to 15%. Consistent with MM the cells were at least partly arranged in groups up to 20 cells. Although small and monomorphic plasma cells predominated, some cells were enlarged and showed vesicular inclusions in their cytoplasm. Electron microscopy revealed crystalline inclusions in these cells identical to those observed in the histiocytes (Figure 2C). Immunohistochemistry confirmed the plasma cell nature of these cells (Figure 1G) and the expression of IgA heavy chains as well as κ light chains (Figure 1H-I). Compared to the histiocytes, plasma cells stained only faintly positive. A conspicuous increase of plasma cells was also found in the spleen, which showed a lymphatic depletion of the white pulpa. The spleen was enlarged (300 g) and the liver was approximately normal size (1450 g). CSH involved the reticuloendothelial system of bone marrow, liver, spleen, and lymph nodes as well as macrophages of the peritoneum, pericardium, pleura, skin, retroperitoneum, and several parenchymatous organs (lungs, myocardium, kidneys, adrenals, testes, mucosa of the gastrointestinal tract). CSH in the retroperitoneum was accompanied by fibrosis with sparsely intermingled lymphocytes. Lymph nodes showed lymphatic depletion in addition to the accumulation of crystal-storing histiocytes. Segmental sclerosis of occasional glomeruli was present in the kidneys in association with slight focal interstitial fibrosis and tubular atrophy most likely due to a moderate atherosclerosis. Hyaline protein casts were seen in different portions of the tubules without crystallike inclusions in tubular epithelial cells or the characteristic pathologic features of myeloma kidneys.
Light microscopy revealed bronchopneumonia in the upper lobe of the right lung with septicopyemic spread in the myocardium and the right adrenal gland with detection of fungal hyphae corresponding to aspergillus in the myocardium. There were serous effusions in each pleural (200 mL each) and peritoneal (5000 mL) cavity.
Additionally, a cavernous hemangioma was present in the left lobe of the liver (5 cm in diameter) and a pulmonary hamartoma was seen in the middle lobe of the right lung (2 cm in diameter).
Protein analysis
Use of 2-DE in combination with immunoblotting revealed the storage of great quantities of heavy chains of α type and light chains of κ type, each in a monoclonal pattern in accordance with the criteria of Tissot and Spertini.49 The remaining immunoglobulins were detected only in normal amounts showing a polyclonal pattern each.
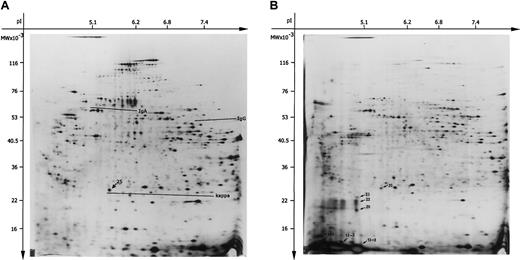
The 2-DE patterns of the soluble and membrane-bound protein fraction of the patient's liver tissue are shown in Figure3. IgA heavy chain was visible in a monoclonal pattern in the 2-DE gel of the soluble fraction (Figure 3A). In contrast to polyclonal heavy chains, monoclonal heavy chains are detectable as sets of well-resolved spots characterized by charge microheterogeneity.49 This result was confirmed by immunoblotting where a set of well-defined red spots corresponded to charge microheterogeneity of monoclonal IgA (Figure4A). Great amounts of κ light chain in a monoclonal pattern were also detected in the 2-DE gels corresponding to a dominant and well-defined protein spot located at 25 kDa/pI 5.6 (Figure 3A). A “fuzzy” red background was demonstrated by immunoblotting with less distinguishable zones interpreted as additionally stored polyclonal κ light chains according to Tissot and Spertini49 (Figure 4C). Compared to these observations, the remaining immunoglobulins were detected only in normal amounts showing a polyclonal pattern characterized by “fuzzy” red zones without distinct individualized spots in the immunoblots, as shown in Figure 4B for IgG heavy chains in the patient and control liver.
The 2-DE of the soluble and membrane-bound protein fractions from the patient's liver sample.
Proteins (100 μg) were separated in the first dimension by IEF using carrier ampholytes pH 3 to 10. Separation in the second dimension was performed using an acrylamide gradient (10%-16%) followed by silver staining. The relative molecular mass (Mr) axis was calibrated by standard proteins (Protein Mr Marker Kit, Pierce). (A) The 2-DE pattern of soluble protein fraction. The areas of IgA and IgG heavy chains as well as κ light chain are underlined, spot 25 (25 kDa/pI 5.6) is marked by arrow. (B) The 2-DE pattern of the membrane-bound protein fraction. The spots chosen for mass spectrometry were marked by arrows (spots: 25, 23, 22, 20, 13-1, 13-2, and 13-3). Unit for the x-axis is pI; y-axis, MW (molecular weight; kDa).
The 2-DE of the soluble and membrane-bound protein fractions from the patient's liver sample.
Proteins (100 μg) were separated in the first dimension by IEF using carrier ampholytes pH 3 to 10. Separation in the second dimension was performed using an acrylamide gradient (10%-16%) followed by silver staining. The relative molecular mass (Mr) axis was calibrated by standard proteins (Protein Mr Marker Kit, Pierce). (A) The 2-DE pattern of soluble protein fraction. The areas of IgA and IgG heavy chains as well as κ light chain are underlined, spot 25 (25 kDa/pI 5.6) is marked by arrow. (B) The 2-DE pattern of the membrane-bound protein fraction. The spots chosen for mass spectrometry were marked by arrows (spots: 25, 23, 22, 20, 13-1, 13-2, and 13-3). Unit for the x-axis is pI; y-axis, MW (molecular weight; kDa).
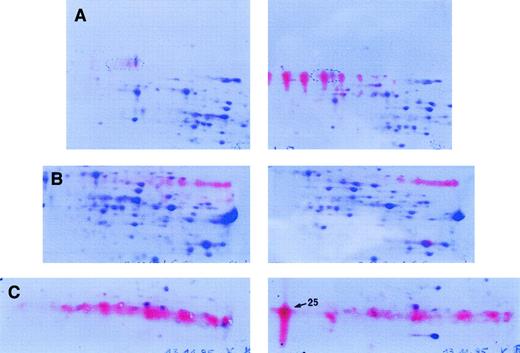
Immunoblot analysis.
Detection of IgA (A), IgG (B), and κ light chain (C) in soluble proteins of liver tissue probes. Immunodetection was performed on the soluble protein fraction of our patient (right) in comparison to control liver samples (left). After immunodetection, counterstaining of the spots was performed with CBB. (A) Monoclonal IgA heavy chain is detected by a set of well-resolved red spots characterized by charge microheterogeneity. (B) Polyclonal IgG heavy chains are observed as a “fuzzy” red zone, without distinct, individualized spots. (C) Monoclonal κ light chain corresponds to one dominant distinct red spot (spot 25) besides a “fuzzy” polyclonal background.
Immunoblot analysis.
Detection of IgA (A), IgG (B), and κ light chain (C) in soluble proteins of liver tissue probes. Immunodetection was performed on the soluble protein fraction of our patient (right) in comparison to control liver samples (left). After immunodetection, counterstaining of the spots was performed with CBB. (A) Monoclonal IgA heavy chain is detected by a set of well-resolved red spots characterized by charge microheterogeneity. (B) Polyclonal IgG heavy chains are observed as a “fuzzy” red zone, without distinct, individualized spots. (C) Monoclonal κ light chain corresponds to one dominant distinct red spot (spot 25) besides a “fuzzy” polyclonal background.
The membrane-bound protein fraction of the liver showed additional protein spots in the 2-DE gels. These proteins were distributed over a region from 23 kDa to about 13 kDa/pI 4.5 to 5.1 as shown in Figure 3B. Immunoblotting with antibodies directed against κ light chain showed only one positively reacting spot at 25 kDa/pI 5.6 that was already detected in the soluble fraction (data not shown). To identify some of these detected spots, PMF by MALDI-MS was performed on the following spots (∼kDa/∼pI) as indicated in Figure 3B: spot 25 (25/5.6), 23 (23/5.0), 22 (22/5.0), 20 (20/5.0) as well as 13-1, 13-2, and 13-3 (13/4.5, 4.7, and 5.1). Mass peaks of spot 25 were matched against the NCBI database. Immunoglobulin κ light chain was found to match with 4 mass peaks resulting in a sequence coverage of 22% (data not shown).
Nano-ESI-MS/MS was performed on spot 25 for sequence support to confirm the PMF data and to identify the nonmatching peaks. The results of this microsequencing that covered about 67% of the complete κ light-chain protein are listed in Table 2 and Figure5. Comparison of the light chain with κ germ line gene sequences showed the highest homology with variableκI germ line gene O8/O18. In the computer-assisted similarity search the sequences showed a 78% up to 100% identity with published sequences of Ig κ light chain belonging to the VκI variability subgroup. The detected sequences comprised residues 1 to 18 of the human Ig κ light chain V-I amyloid protein BAN (acc P04430).50 Residues 46 to 103 shared an identity of 78% with the κ V-I regions REI (acc P01607)51 and WAT (acc P80362).52 Residues 108 to 142 and 170 to 183 as well as 191 to 207 shared a 100% identity with the κ chain constant region of the Bence Jones protein ROY (acc P01834).53 We observed 8 unusual residues at different positions: Leu4, Val54, Leu59, Phe70, Tyr71, Ser72, Arg76, and Gln92 (Table 3). Unusual residues were defined as occurring in 1% and less of compared sequences at the corresponding positions in association with plasma cell disorders in the EMBL and NCBI databases. The exchanges were located in the framework region (FR) 1 (position 4), complementarity-determining region (CDR) 2 (position 54), FR 3 (positions 59, 70-72, and 76), and CDR 3 (position 92). Leu59 that replaced Pro has never been previously reported at this position in association with plasma cell disorders to the best of our knowledge. The observed unusual exchanges at positions 4 (Met→Leu), 70 (Asp→Phe), 71 (Phe→Tyr), 72 (Thr→Ser), and 76 (Ser→Arg) have been described mainly in association with primary amyloidosis (Table 3).
Results of mass spectrometric analysis of 2-DE spot 25 identified as κ light chain
| Mass (Da) . | Theoretical. mass (Da) . | Sequences . | From* . |
|---|---|---|---|
| 1860.98† | 1860.930 | DIQLTQSPSSLSASVGDR | 001 -018 |
| 1749.98† | 1749.938 | LLIYDASNVETGVLSR | 046 -061 |
| 1579.76† | 1579.766 | FSGSGSGTFYSLTIR | 062 -076 |
| 3141.70 | 3141.451 | SLQPEDIATYYCac°QQYQDFPLTFGGGTK | 077 -103 |
| 2102.05 | 2102.129 | RTVAAPSVFIFPPSDEQLK | 108 -126 |
| 1946.04 | 1946.027 | TVAAPSVFIFPPSDEQLK | 109 -126 |
| 1811.92 | 1811.911 | SGTASVVCac°LLNNFYPR | 127 -142 |
| 1503.80 | 1503.758 | DSTYSLSSTLTLSK | 170 -183 |
| 1903.96 | 1903.958 | LYACac°EVTHQGLSSPVTK | 191 -207 |
| Mass (Da) . | Theoretical. mass (Da) . | Sequences . | From* . |
|---|---|---|---|
| 1860.98† | 1860.930 | DIQLTQSPSSLSASVGDR | 001 -018 |
| 1749.98† | 1749.938 | LLIYDASNVETGVLSR | 046 -061 |
| 1579.76† | 1579.766 | FSGSGSGTFYSLTIR | 062 -076 |
| 3141.70 | 3141.451 | SLQPEDIATYYCac°QQYQDFPLTFGGGTK | 077 -103 |
| 2102.05 | 2102.129 | RTVAAPSVFIFPPSDEQLK | 108 -126 |
| 1946.04 | 1946.027 | TVAAPSVFIFPPSDEQLK | 109 -126 |
| 1811.92 | 1811.911 | SGTASVVCac°LLNNFYPR | 127 -142 |
| 1503.80 | 1503.758 | DSTYSLSSTLTLSK | 170 -183 |
| 1903.96 | 1903.958 | LYACac°EVTHQGLSSPVTK | 191 -207 |
Cac° indicates cysteine modified with acrylamide.
Residues are numbered according to the standard Kabat numbering system.77
Mass peaks identified in all spots under investigation.
Amino acid sequence alignment.
Comparison of the variable domain of the κ light chain of the present CSH patient with the most likely germ line counterpart LCO8/O18. Dashes indicate identity with the germ line sequence. Stars indicate lacking sequence data. Amino acids are numbered according to the Kabat numbering system.77
Amino acid sequence alignment.
Comparison of the variable domain of the κ light chain of the present CSH patient with the most likely germ line counterpart LCO8/O18. Dashes indicate identity with the germ line sequence. Stars indicate lacking sequence data. Amino acids are numbered according to the Kabat numbering system.77
Occurrence of CSH-associated unusual residues in other monoclonal human κ light chains associated with plasma cell disorders
| Position . | Residue . | Reported protein . | Protein deposition . |
|---|---|---|---|
| 4 | Leu | BAN (acc P04430)50 | AL amyloidosis |
| MUM78 | AL amyloidosis | ||
| ES30579 | AL amyloidosis | ||
| Acc AAD4414273 | AL amyloidosis | ||
| 54 | Val | AK680 | Unknown |
| 59 | Leu | Not reported | |
| 70 | Phe | Acc AAG4248073 | AL amyloidosis |
| 71 | Tyr | Bence Jones protein REI (acc P01607)51 | Unknown |
| Bence Jones protein BRE (acc I39154)78 | AL amyloidosis | ||
| ARN (acc S24320)72 | AL amyloidosis | ||
| 72 | Ser | Bence Jones protein DEL (acc 4388953)70 | Fanconi syndrome |
| ARN (acc S24320)72 | AL amyloidosis | ||
| 76 | Arg | MUM78 | AL amyloidosis |
| AK180 | Unknown | ||
| GK280 | Unknown | ||
| 92 | Gln | Bence Jones protein REI (acc P01607)51 | Unknown |
| IgM (POT) Fv fragment (acc 443041)81 | Unknown |
| Position . | Residue . | Reported protein . | Protein deposition . |
|---|---|---|---|
| 4 | Leu | BAN (acc P04430)50 | AL amyloidosis |
| MUM78 | AL amyloidosis | ||
| ES30579 | AL amyloidosis | ||
| Acc AAD4414273 | AL amyloidosis | ||
| 54 | Val | AK680 | Unknown |
| 59 | Leu | Not reported | |
| 70 | Phe | Acc AAG4248073 | AL amyloidosis |
| 71 | Tyr | Bence Jones protein REI (acc P01607)51 | Unknown |
| Bence Jones protein BRE (acc I39154)78 | AL amyloidosis | ||
| ARN (acc S24320)72 | AL amyloidosis | ||
| 72 | Ser | Bence Jones protein DEL (acc 4388953)70 | Fanconi syndrome |
| ARN (acc S24320)72 | AL amyloidosis | ||
| 76 | Arg | MUM78 | AL amyloidosis |
| AK180 | Unknown | ||
| GK280 | Unknown | ||
| 92 | Gln | Bence Jones protein REI (acc P01607)51 | Unknown |
| IgM (POT) Fv fragment (acc 443041)81 | Unknown |
AL indicates light chain amyloid; acc, NCBI database.
The peaks 1579.76 Da, 1749.98 Da, and 1860.98 Da (Table 2) were prominent in all spots under investigation, thus suggesting that spots 23, 22, 20, as well as 13-1, 13-2, and 13-3 represent κ light-chain fragments.
Provided that the membrane-bound protein fraction reflects especially the proteolytically resistant intralysosomal-stored immunoglobulins or their fragments, Figure 3B indicates that the amount of stored κ light chain corresponding to the marked spots 25, 23, 22, 20, as well as 13-1, 13-2, and 13-3 exceeded the amount of stored IgA.
Discussion
We present a case of generalized CSH associated with a monoclonal IgA κ gammopathy that preceded the complete development of MM and resulted in a fatal clinical course.
To date about 60 cases of CSH have been reported in the literature. Predominantly they were found in association with MM and LPL.1-35,54-56 Some (22 cases) have been detected primarily in extramedullary sites, associated with plasma cell granuloma, LPL, mucosa-associated lymphoid tissue (MALT) lymphoma, large-cell lymphoma, and extramedullary plasmocytoma.1,3-7,9,10,16,19,20,24,25,28,31,35,55However, the involvement of the bone marrow has been described in the majority of the cases. The association with crystalline storage in cells of the reticuloendothelial system at other sites has been reported in 21 cases so far (Table 4). Because of the rarity of the disease, the accumulation of crystal-storing histiocytes in the bone marrow often presents diagnostic difficulties. Sometimes the number of histiocytes in the bone marrow is so increased in proportion to the clonal plasma cells that the diagnosis of a storage disorder like Gaucher disease is initially considered, as in a bone marrow smear in our case. Because the crystal-storing macrophages may adopt the appearance of Gaucher cells, they have been referred to as pseudo-Gaucher cells.13,27 However, at least the ultrastructure of the crystalline inclusions distinguishes these cells from glucocerebroside-storing real Gaucher cells as well as from the so-called pseudo-Gaucher cells that are sometimes observed in chronic myeloid leukemia57 and thalassemia.58Therefore Schaefer7 proposed the term “pseudo-pseudo-Gaucher cells” (PPGCs) as a specific designation for this particular type of macrophage. The real Gaucher cells exhibit tubular structures in the electron microscope with a unique substructure of 10 or 12 fibrils that spiral in a right-handed screw sense along the length of the tubule.59 Pseudo-Gaucher cells in chronic myeloid leukemia discriminate from these by a microfibrillar ultrastructure.57 In contrast, macrophages in CSH present with many often membrane-bound electron-dense rhomboid, hexagonal, or more needlelike crystal profiles in the cytoplasm, as in our patient.7 At higher magnification these crystals often show a periodical hexagonal crystal lattice with a center-to-center distance of about 75 Å.7 In some cases the clonal myeloma or lymphoma cells also contain similar protein crystals.7This finding contributes to the view that the stored proteins represent crystallized immunoglobulins.
Generalized CSH: summary of clinical and pathologic features of reported cases
| Case/report . | Age, y/sex . | Presenting symptoms . | Diagnosis . | Paraprotein . | Sites of deposition . | Clinical course . |
|---|---|---|---|---|---|---|
| Jones 19991 | 50/M | Bone pain | MM | S | BM, LN | 10 mo |
| 66/M | Splenomegaly, cold agglutinin | LPL | S: biclonal U: biclonal | BM, spleen | 8 mo | |
| 70/M | Splenomegaly | LPL | ND | BM, LN | Transformed to LCL at 24 mo | |
| 70/M | Abdominal pain, splenomegaly | LPL | S: biclonal | BM, spleen, LN | 4 mo | |
| Garcia 19982 | 44/M | Transient dyspnea, bilateral proptosis | LPL | S: IgG κ | BM, liver, spleen, LN, and diverse additional sites4-150 | 18 mo |
| Schaefer 19967 | 75/M | Fatal renal failure, anemia, hyperbilirubinemia | LPL | S: biclonal IgM κ > IgG λ | BM, liver4-150 | DOD |
| 55/M | Not reported | MM | S: IgG κ | BM, liver | Not reported | |
| Yamamoto 199111 | 71/M | Blurred vision | MM | S: IgG κ U: κ | BM, liver, spleen, LN, and diverse additional sites4-150 | 4 y |
| Takahashi 198713 | 60/M | Fractures of the lumbar vertebrae; bone pain | MM, A4-150 | S: IgA κ U: BJP κ | BM, liver, spleen, LN, and diverse additional sites4-150 | 45 mo |
| Itagaki 198117 | 69/F | NA | MM | S: IgG κ | BM, systemic | NA |
| Mullen 198118 | 52/M | Low back pain, weight loss | MM | S: IgG κ U: κ | BM, liver, LN, and diverse additional sites4-150 | 6.5 y |
| Terashima 197822 | 58/M | Adult Fanconi syndrome | L-chain storage histiocytosis (MGUS) | S: κ U: κ BJP | BM, liver, spleen, LN, and diverse additional sites4-150 | 3 y |
| Kjeldsberg 197723 | 60/M | Anorexia, fatigue, and pallor | MM, A4-150 | S: κ | BM, liver, spleen4-150 | 11 mo |
| Finkel 197326 | 57/F | Adult Fanconi syndrome | KLP, A4-150 | U: κ BJP | BM, liver, abdominal LN, stomach4-150 | 10 y |
| Ito 197027 | 53/F | NA | MM | U: κ BJP | BM, liver, spleen, LN, adrenals, kidneys4-150 | 5 y |
| Dedmon 196329 | 51/M | Adult Fanconi syndrome | MM | Not reported | BM, LN | 10 mo |
| Engele 195730 | 38/M | Adult Fanconi syndrome | MM | U: BJP | BM, spleen | 2.5 y |
| Brass 194832 | 73/F | Not reported | MM | Not reported | BM, liver, spleen4-150 | Not reported |
| Agress 194056 | 55/F | NA | RE and PH | NA | BM, liver, spleen, LN, adrenals, kidneys4-150 | 1 mo |
| Ritchie 193633 | 36/F | NA | RE | U: BJP | BM, spleen | 1 mo |
| Glaus 191734 | 67/M | Cutaneous elastolysis | MM, A4-150 | U: BJP | BM, LN, skin, testes4-150 | 2 y |
| Present case | 73/M | Weight loss, fatigue | MM | S&U: IgA κ | BM, liver, spleen, LN, and diverse additional sites4-150 | 7 mo |
| Case/report . | Age, y/sex . | Presenting symptoms . | Diagnosis . | Paraprotein . | Sites of deposition . | Clinical course . |
|---|---|---|---|---|---|---|
| Jones 19991 | 50/M | Bone pain | MM | S | BM, LN | 10 mo |
| 66/M | Splenomegaly, cold agglutinin | LPL | S: biclonal U: biclonal | BM, spleen | 8 mo | |
| 70/M | Splenomegaly | LPL | ND | BM, LN | Transformed to LCL at 24 mo | |
| 70/M | Abdominal pain, splenomegaly | LPL | S: biclonal | BM, spleen, LN | 4 mo | |
| Garcia 19982 | 44/M | Transient dyspnea, bilateral proptosis | LPL | S: IgG κ | BM, liver, spleen, LN, and diverse additional sites4-150 | 18 mo |
| Schaefer 19967 | 75/M | Fatal renal failure, anemia, hyperbilirubinemia | LPL | S: biclonal IgM κ > IgG λ | BM, liver4-150 | DOD |
| 55/M | Not reported | MM | S: IgG κ | BM, liver | Not reported | |
| Yamamoto 199111 | 71/M | Blurred vision | MM | S: IgG κ U: κ | BM, liver, spleen, LN, and diverse additional sites4-150 | 4 y |
| Takahashi 198713 | 60/M | Fractures of the lumbar vertebrae; bone pain | MM, A4-150 | S: IgA κ U: BJP κ | BM, liver, spleen, LN, and diverse additional sites4-150 | 45 mo |
| Itagaki 198117 | 69/F | NA | MM | S: IgG κ | BM, systemic | NA |
| Mullen 198118 | 52/M | Low back pain, weight loss | MM | S: IgG κ U: κ | BM, liver, LN, and diverse additional sites4-150 | 6.5 y |
| Terashima 197822 | 58/M | Adult Fanconi syndrome | L-chain storage histiocytosis (MGUS) | S: κ U: κ BJP | BM, liver, spleen, LN, and diverse additional sites4-150 | 3 y |
| Kjeldsberg 197723 | 60/M | Anorexia, fatigue, and pallor | MM, A4-150 | S: κ | BM, liver, spleen4-150 | 11 mo |
| Finkel 197326 | 57/F | Adult Fanconi syndrome | KLP, A4-150 | U: κ BJP | BM, liver, abdominal LN, stomach4-150 | 10 y |
| Ito 197027 | 53/F | NA | MM | U: κ BJP | BM, liver, spleen, LN, adrenals, kidneys4-150 | 5 y |
| Dedmon 196329 | 51/M | Adult Fanconi syndrome | MM | Not reported | BM, LN | 10 mo |
| Engele 195730 | 38/M | Adult Fanconi syndrome | MM | U: BJP | BM, spleen | 2.5 y |
| Brass 194832 | 73/F | Not reported | MM | Not reported | BM, liver, spleen4-150 | Not reported |
| Agress 194056 | 55/F | NA | RE and PH | NA | BM, liver, spleen, LN, adrenals, kidneys4-150 | 1 mo |
| Ritchie 193633 | 36/F | NA | RE | U: BJP | BM, spleen | 1 mo |
| Glaus 191734 | 67/M | Cutaneous elastolysis | MM, A4-150 | U: BJP | BM, LN, skin, testes4-150 | 2 y |
| Present case | 73/M | Weight loss, fatigue | MM | S&U: IgA κ | BM, liver, spleen, LN, and diverse additional sites4-150 | 7 mo |
S indicates serum M spike; BM, bone marrow; LN, lymph nodes; U, urine M spike; ND, not determined; LCL, large-cell lymphoma; DOD, died of disease; A, amyloidosis; BJP, Bence Jones proteinuria; NA, not available data; KLP, κ light-chain proteinuria; RE, reticuloendotheliosis; PH, purpura hemorrhagica.
Autopsy findings.
In our case electron microscopy revealed crystal storage in the macrophages at different sites, just as described in the literature but without periodic organization of the crystalline substructure. Whether this is due to the composition of the stored proteins or the amino acid sequence of the immunoglobulins is unclear. Crystalline inclusions were also detected in hepatocytes as reported by Kjeldsberg et al23 in a case of MM associated with generalized CSH. Crystalline plasma cell inclusions were already occasionally seen in the initial bone marrow biopsy and at the time of autopsy when plasma cells were increased in number and fulfilled the diagnostic criteria of MM.
The phenomenon that the number of PPGCs may exceed by far the number of plasma cells has also been described by others.1,22 Therefore, the cell proliferation might not fulfill the criteria of MM at the time of initial presentation. The association of MGUS with CSH has been described twice in the literature.1,22 However regarding the rapid clinical course of the disorder in our patient and in others,22 it seems debatable if the diagnosis of MGUS in association with CSH reflects the prognosis sufficiently.
Immunohistochemistry of the stored material revealed conflicting results. In the cases of CSH reported in the literature, the stored protein in PPGCs remained either unstained5,7-10,12,16,18or exhibited only a weak reaction mainly restricted to the surfaces of the crystals.1,13,35 This failure to stain has been attributed to the coverage of the epitopes in the crystal structure and the degradation by limited lysosomal proteolysis. The storage of immunoglobulins other than the paraprotein has been reported only 3 times.1,6,19 The pathogenesis for this phenomenon as well as for the crystal storage in the histiocytes has not been clarified yet. Interestingly, every published case of CSH with MM and nearly all cases of LPL with known paraprotein type have been reported to produce monoclonal immunoglobulins of light-chain type κ.1 The expression of λ light chains has been described only in 5 cases; 4 of them were associated with LPL1,5,7 and 1 with the extramedullary detection of PPGCs at the site of MALT lymphoma.55 Information about the expressed type of immunoglobulin is available in 12 of 22 cases of generalized CSH reported so far, including our own (Table 4). All of them expressed κ light chains. The production of λ light chains was exclusively observed in one patient who suffered from LPL and showed biclonal gammopathy. On the other hand, the published data do not show any association between the type of heavy chain and CSH. IgA was observed in 2, IgG in 6, and IgM in 1 of the above-mentioned 12 patients with generalized CSH. In 4 of them no expression of heavy chains was detected. Thus crystal formation in CSH seems to be related to the type of light chain but not to a special type of heavy chain. The κ light chains have also been involved in light-chain deposition disease and adult Fanconi syndrome with only rare exceptions.60-65Therefore, κ light chains seem to be of poorer solubility under acid intralysosomal conditions. However, given the rarity of this disorder, additional factors have to be discussed that might contribute to this phenomenon.
A possible mechanism of crystal formation may be overproduction. This is supported by the observation that focal CSH occurs also in combination with polyclonal immunoglobulin expression such as plasma cell granuloma or posttransplantation plasmacytosis.1 But some of the patients reported in the literature have minimal or no serum or urine paraprotein levels, respectively, and the number of clonal plasma cells in the bone marrow might be low as in our case.1,4,10,12,19,22,54 55 Thus, more likely than overproduction is that the stored paraproteins have sequence abnormalities at specific sites that promote crystallization or adversely affect intralysosomal degradation or both.
To date there are only limited data on sequence analyses from κ light chains in patients with adult Fanconi syndrome. These studies have consistently shown the implication of the VκI variability subgroup as well as amino acid substitutions in the variable region similar to those in amyloidogenic molecules.66 All analyzed κ V domains from patients with intracellular crystals were found to be encoded by the LCO2/O12 germ line gene and had an unusual nonpolar residue exposed to the solvent in the CDR-L1 loop.67 A study on 4 patients with adult Fanconi syndrome showed that the variable domains of the isolated κ chains of the patients were resistant to proteolytic cleavage in vitro, suggesting that crystal accumulation in the lysosomes may result from resistance to degradation.68 In murine models it has been demonstrated that multiple peculiarities of the variable region are simultaneously needed to allow κ light-chain crystallization, which likely results from a unique light-chain tridimensional conformation imposed by concomitant somatic mutations of a specific germinally encoded framework.69 The exact structure of such immunoglobulin aggregates has been determined only in single cases.70 Thereby, the demonstration of structural defects in myeloma immunoglobulins seems to revive the old idea that paraproteins distinguish themselves not only by quantity and clonality but also in a qualitative manner by aberrant structure as suggested by Apitz71 when introducing the term of paraprotein.
However, there have not been any publications on the exact nature of the stored immunoglobulins in CSH. To the best of our knowledge this is the first report using 2-DE and protein identification of tissue samples of a patient with CSH. The stored proteins were characterized especially with regard to the observed unusual immunostaining pattern. The use of 2-DE in combination with immunoblotting of the extracted proteins demonstrated an accumulation of monoclonal immunoglobulins of type IgA κ accompanied by a normal amount of polyclonal immunoglobulins of other types.
Protein identification by MS of conspicuous protein spots confirmed the storage of tremendous amounts of κ light chain in the soluble and membrane-bound protein fractions of the patient's liver samples. Partial sequence analysis by nano-ESI-MS/MS yielded a sequence coverage of 67% with amino acid sequences corresponding to the VκI variability subgroup with unusual amino acid residues in position 4, 54, 59, 70-72, 76, and 92, located in CDR 2 and CDR 3 as well as FR 1 and FR 3. Leu that replaced Met in position 4 and Pro in position 59 is known to be important for hydrophobic interactions within the protein structure. The latter exchange has never been reported previously in the corresponding position in association with plasma cell disorders. The remaining unusual residues have been described predominantly in primary amyloidosis (Table3).50,70,72,73 Jarret and Lansbury74suggested that amyloid fibrils and protein crystal formation require the same nucleation-dependent starting polymerization step. Unusual amino acids at position 30 or in strand 43-50 and 51-61 may increase stability of monomer or dimer interactions, as supposed for amyloidogenic light chains,75 and could thus initiate the formation of crystal-forming units.74 The present sequence analysis may thus provide a link between those theories on nucleation and the pathogenesis of CSH. However, it seems highly unlikely that the nidus is the same for both.
The microsequencing with MS has not yielded the complete sequence of the stored κ light chain. It is a well-known phenomenon that the sequence coverage obtained by ESI-MS/MS depends on the peptides obtained after digestion. Some peptides are not efficiently ionized during MS. Because the amount of stored proteins available for protein analysis was limited, Edman degradation was not possible for sequence analysis. Consequently, we cannot exclude additional underlying sequence aberrations in the missing segments that might be sufficient to significantly change light-chain properties.
DNA and cDNA sequencing were not included in our investigations because neoplastic plasma cells were only rarely admixed in the bone marrow biopsies, not sufficient for the extraction of DNA or mRNA in adequate purity and quantity for sequence analysis. Thus, the role of the relative contribution of germ line versus somatic mutations in the pathogenesis of this disease remains to be elucidated.
The additional deposition of polyclonal immunoglobulins in our case might have been caused by the accumulation of the proteolytically resistant paraprotein that impaired the intralysosomal degradation of the remaining proteins especially for stereologic reasons. It seems unlikely that the storage of crystalline immunoglobulins is caused by an enzyme defect itself because the enzymes involved in the degradation of immunoglobulins are rather nonspecific and are not restricted to the cleavage of them.
Finally, some questions concerning the pathogenesis of CSH remain open: (1) While roughly one half of the cases with CSH occur localized at the site of lymphoplasmacellular proliferation, the other half develops a generalized accumulation of crystalline proteins in the reticuloendothelial system. The generalized distribution is often associated with a rapid, fatal clinical course, as in our patient. Therefore, it seems to be critical to find adequate therapeutic strategies especially for these patients. The application of plasmapheresis has been reported only in one case in the literature so far, used to control symptoms of hyperviscosity.1Nevertheless many myeloma patients with CSH have reported survivals of 5 to 15 years after diagnosis, which is longer than the median survival for MM alone.76 (2) With regard to the discussion of possible mechanisms that might cause CSH, the hypothesis of the expression of abnormal paraproteins by neoplastic plasma cells that are at least partly resistant to enzymatic degradation seems to be the most probable right now. But it is unclear whether resistance comes from crystal formation, high intrinsic stability, or fortuitous loss of proteolytic sites. Consequently, there is a need to obtain complete sequence data so that recombinant forms can be produced for laboratory studies. Sufficient serum, Bence Jones protein, or tissue samples from CSH patients should be obtained to support such studies. (3) The observation of localized CSH in individual cases of extramedullary polyclonal nonneoplastic plasma cell proliferations in the literature1 is not explained by the above-mentioned pathogenic suggestion.35 One might speculate that the accumulation of crystalline proteins is here, in fact, the result of overproduction. But this has not been proven yet. (4) Although crystallizable κ light chains are present in nearly all cases of renal failure due to adult Fanconi syndrome,65 adult Fanconi syndrome is an uncommon complication of patients with CSH. Forms of extracellular protein deposition that occur relatively frequently in MM, such as generalized amyloidosis or extracellular crystal formation in conjunctiva or cornea, have been observed only seldom in CSH. It has to be clarified if the kind or localization of the sequence abnormality in the expressed paraprotein is crucial for the kind of protein deposition.
In summary, we report a case that represents a very rare association of MGUS with generalized CSH. The case presented here and the review of the previous literature strongly suggest that the generalized form of CSH is highly associated with a poor prognosis, whereas localized forms of CSH have a more favorable clinical course. Although immunohistochemistry showed a polyclonal storage pattern of immunoglobulins, 2-DE revealed the prevalence of a monoclonal IgA κ paraprotein that was also detected in the serum. The striking fact that CSH is predominantly associated with the expression of κ light chain suggests that the crystal formation is determined by the type of light-chain molecule that might be abnormal. This hypothesis is supported by data presented here with molecular identification of the stored κ subgroup and detection of unusual amino acid substitutions in the variable region of the stored κ light chain for the first time in CSH. The fundamental conclusion of our results is that alterations induced by amino acid exchanges in the expressed κ light chain seem to be crucial in the pathogenesis of CSH.
Because collection of protein samples for sequence analysis has not been a standard procedure in CSH patients so far, we recommend the storage and analysis of serum, urine, and tissue samples from such patients to accumulate sequence data systematically.
We are grateful to Dr S. Liebmann, S. Madsen-Unverfärth, H. Muthmann, S. Schäfer, and A. Sendelhofert for skillful technical assistance. We thank Priv-Doz Dr R. Gruber for his excellent support and keen observation in the interpretation of the immunobiologic serum and urine data.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Annette Lebeau, Pathologisches Institut der Ludwig-Maximilians-Universität, Thalkirchner Str. 36, 80337 München, Germany; e-mail:a.lebeau@lrz.uni-muenchen.de.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal