A tetracycline-controlled expression system was adapted to the human promyelocytic HL-60 cell line by placement of the transactivator (tTA-off) sequence under the control of the human EF-1α promoter region. Constitutively active and dominant-inhibitory forms of Cdc42 (Cdc42V12 and Cdc42N17, respectively) were conditionally expressed in this system. The expression of Cdc42V12 had no marked effect on chemoattractant-mediated superoxide production, corroborating previous results indicating that the guanosine 5′-triphosphate (GTP)–bound form of Cdc42 is ineffective in directly activating nicotinamide adenine dinucleotide phosphate (NADPH) oxidase in a cell-free system. However, the N17 mutant potently inhibited chemoattractant-induced superoxide production. The expression of Cdc42N17 interfered with the GTP-loading of Rac and Ras and with the activation of the MAP-kinase pathway. A drastic reduction of chemoattractant-induced inositol-1,4,5-trisphosphate formation and calcium mobilization was observed, corroborating previous in vitro study results identifying PLCβ2 as a Rac/Cdc42 effector. Cdc42N17 was also found to inhibit the translocation of Ras-GRF2, a guanine nucleotide exchange factor for Ras and Rac but not for Cdc42. Thus, the dominant-inhibitory mutant Cdc42N17 was found to interfere at multiple levels in the signaling pathways. The pleiotropic inhibitory effects of Cdc42N17 illustrate the potential pitfalls of using dominant-inhibitory proteins to study the function of Ras-family GTPases. In this regard, a number of conclusions drawn from the use of dominant-inhibitory mutants in myeloid cells might have to be reconsidered.
Introduction
Transendothelial migration of leukocytes and their accumulation at sites of inflammation are of particular importance for host defense against invading pathogens. Chemotactic factors, including the N-formyl peptides, C5a, and interleukin-8 (IL-8), which bind to specific heterotrimeric G-protein–coupled transmembrane receptors, regulate cell migration and activate microbicidal and cytotoxic functions through the release of proteolytic enzymes from specific granules and the generation of superoxide anions by the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase. Activation of this enzyme proceeds through a multistep assembly of several components including the 2 transmembrane subunits of cytochrome b558 (p22phox and gp91phox) and 4 cytosolic proteins (p47phox, p67phox, p40phox, and the small GTPase Rac).1,2 The triggering and control of bactericidal functions require a coordinated activation of several signaling pathways, namely the activation of tyrosine kinases, phospholipases (PLCβ2, PLD, and PLA2), phosphatidylinositol 3-kinase (PI3K), and mitogen-activated protein kinase (MAPK) cascades.3
Low–molecular-weight GTPases are key regulators of a wide spectrum of cellular functions.4-7 The binding of guanine nucleotide regulates all members of the Ras-superfamily. Activation of GTPases through guanosine 5′-diphosphate (GDP)–GTP exchange is promoted by guanine nucleotide exchange factors (GEFs). In leukocytes, GTPases of the Rho family (Rho, Rac, and Cdc42) participate in the remodeling of actin cytoskeleton and in the regulation of cell adhesion. In addition to its contribution as part of the oxidase enzymatic complex, Rac also appears to be connected with upstream signaling mechanisms regulating NADPH oxidase activation. p21-activated kinase (PAK), a direct target of Rac, has been shown to phosphorylate p47phox in vitro in a Rac-GTP–dependent manner8 and to regulate the stress-activated kinase p38 SAPK,9 which has been involved in the signal transduction pathway leading to superoxide production.10 11 Like Rho, Rac and Cdc42 appear to interact with distinct regulators and downstream target proteins that may allow them to contribute to unique cellular functions. However, the understanding of their mechanism of signaling in neutrophils remains limited, mainly because the expression of exogenous proteins in these cells is particularly difficult to perform.
The human promyelocytic HL-60 cell line provides a model system to study the regulatory mechanisms involved in triggering and control of neutrophil bactericidal functions. Appropriate expression vectors and transfection methods have been developed that allow the successful expression of exogenous proteins in this cell line.12,13Yet, the yield of transfection is low, excluding transient transfection. Because of clonal variations, the effects produced by the stable expression of dominant-interfering proteins are often difficult to interpret. Moreover, overexpression of exogenous proteins may severely upset metabolic pathways, making stable cell lines difficult to establish. The tetracycline-regulated system for tightly controlled expression of exogenous gene products described by Gossen and Bujard14 appears to be ideally suited to circumvent these general problems.
In the present paper, we describe the adaptation of such a tetracycline-controlled expression system to HL-60 cells. Using luciferase as a reporter gene, we show that the system allows a strong expression that can be tightly controlled in a tetracycline-responsive manner. This conditional expression system was used to decipher the chemoattractant-activated signaling pathways that lead to NADPH oxidase activation. A dominant-inhibitory form (Cdc42N17) and a constitutively active form (Cdc42V12) of Cdc42 were conditionally expressed. The expression of Cdc42V12 had no major increasing effect on chemoattractant-mediated NADPH activity and calcium mobilization, whereas Cdc42N17 was found to be an effective inhibitor that appeared to down-modulate specific parallel signaling pathways far upstream of NADPH oxidase. It interfered with PLCβ2 activation, the formation of Rac-GTP, and the activation of the Ras/MAPK pathway. Moreover, Cdc42N17 also appeared to exert a regulatory effect on the translocation of Ras-GRF2, a GEF for Ras and Rac but not for Cdc42.
Materials and methods
Plasmid constructions
The plasmid pUHD15-1 containing the tetracycline-inhibited transactivator (tTA-off), the plasmid pUHD10-3 encoding a minimal promoter fused to tetracycline operator sequences, and the luciferase reporter plasmid pUHC 13-314,15 were provided by Dr H. Bujard. The plasmid PEF-pgk-Neo,12 provided by Dr M. Dinauer, was modified by inserting a DNA fragment containing the cloning sites BamHI and SmaI between theEcoRI and NotI sites.
A new transactivator plasmid, pEF-tTA, was constructed by inserting the fragment BamHI-EcoRI of pUHD15-1 between theBamHI and NotI sites of the modified pEF-pgk-neo plasmid. The luciferase reporter plasmid pUHC13-3 was modified by inserting a SalI-NruI fragment of the plasmid pCEP4 (Invitrogen, Groningen, The Netherlands) containing the hygromycin resistance gene into the HpaI site.
To construct the response plasmid pTet-Bsd-IRES-EGFP, theXhoI-XbaI fragment of the pUHD10-3 plasmid was inserted into the vector pcDNA3.1/hygro(-) (Invitrogen) after cleavage by NruI and XbaI. The hygromycin resistance gene was excised with NaeI and replaced by anEcoRV-SmaI fragment of the plasmid pEF/Bsd (Invitrogen) encoding the blasticidin resistance gene. ABamHI site located at the end of the Tet operator was eliminated using the Quick change site-directed mutagenesis kit (Stratagene, La Jolla, CA). A BamHI-NotI fragment of the plasmid pIRES2–enhanced green fluorescence protein (EGFP) (Clontech, Palo Alto, CA) containing an internal ribosomal entry site (IRES) sequence and the coding sequence for EGFP were inserted between the BamHI and PmeI sites in the multicloning site of the mutated plasmid.
The myc-tagged Cdc42N17 and Cdc42V12 cDNAs provided by Dr P. Chavrier (Paris, France) were subcloned in the multicloning site of the response plasmid pTet-Bsd-IRES-EGFP, between the NotI andBamHI sites.
Generation of stable cell lines expressing the transactivator tTA
Cells were cultured in RPMI 1640-glutaMAX 1 medium (Life Technologies, Grand Island, NY) supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin, and 10% Tet system approved heat-inactivated fetal bovine serum (Clontech). Cells were transfected by electroporation with the plasmid pEF-tTA, and cellular clones were isolated as described.16 To assay for the functional expression of the transactivator tTA, each G418-resistant clone was transfected by electroporation with the modified luciferase reporter plasmid. Clones resistant to hygromycin B (200 μg/mL) were assayed for luciferase expression using a luciferase assay system (Promega, Madison, WI). Luciferase activity was measured in a Lumat LB9507 luminometer.
Expression of tTA-regulated gene products
A selected tTA-expressing clone was stably transfected with themyc-Cdc42N17- or myc-Cdc42V12-IRES-EGFP expression plasmids. Selection was performed in the presence of blasticidin (10 μg/mL). Resistant clones were analyzed for EGFP expression by using a FACScalibur flow cytometer (Becton Dickinson) and the Cell Quest analysis program.
For detection of myc-tagged proteins, cells lysates were subjected to immunoprecipitation with anti–c-myc antibodies (9E10; Roche Diagnostics, Meylan, France) coupled to protein-G Sepharose beads. After sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), immunoblotting was performed with specific antibodies to Cdc42 (sc-87; Santa Cruz Biotechnology, Santa Cruz, CA) and the enhanced chemiluminescence detection system (ECL) (Amersham, les Ulis, France).
Superoxide production assays
HL-60 cell differentiation was induced withN,6O-2′-dibutyryl adenosine 3′,5′ cyclic monophosphate (Bt2cAMP) or, when specified, with dimethyl sulfoxide (DMSO).16 Superoxide production was assayed by following cytochrome c reduction upon the application ofN-formyl Met-Leu-Phe-Lys-OH (fMLFK) or phorbol 12-myristate 13-acetate (PMA) (1 μM and 1 μg/mL, respectively).16
A cell-free NADPH activation assay was performed with sonicated membranes, and cytosolic fractions were prepared from bovine neutrophils17 and from HL-60 cells16differentiated with Bt2cAMP in the presence (50 ng/mL) or the absence of doxycycline. Superoxide generation was measured in 96-well microplates.18
Recombinant myc-Cdc42N17 was expressed in Escherichia coli as a glutathione S-transferase (GST) fusion protein. After purification by affinity on glutathione Sepharose beads, themyc-Cdc42N17 protein was enzymatically cleaved from GST.19
[35S]GTPγS binding assay
[35S]GTPγS binding to Rac was measured by adding 1 μM [35S]GTPγS to the cell-free system preincubation mixture. GTPγS-loaded Rac was pulled down with a bacterially synthesized GST-fusion of the PAK1 Rac-binding domain.20
In vivo phosphorylation of p47phoxand p40phox
HL-60 cells were grown and differentiated in the presence (50 ng/mL) or absence of doxycycline and were subjected to metabolic labeling with [33P] orthophosphate16Immunoprecipitations of p47phox and p40phox were performed as described.16,21 After SDS-PAGE, 33P-labeled proteins were detected by autoradiography. The presence of p47phox and p67phox was assayed in lysates of unlabeled cells using specific antibodies22 and ECL.
IP3 formation, cytosolic calcium mobilization, and kinase assays
Inositol–1,4,5-trisphosphate (IP3) level was determined using an [3H] IP3 radioreceptor assay kit (NEN Life Science Products, Boston, MA). Cytosolic calcium measurements were performed as described.23
Akt kinase activity was measured in cell lysates using a nonradioactive Akt kinase assay kit (Cell Signaling Technology, Beverly, MA). Extracellular signal-related kinase 1/2 (Erk1/2) phosphorylation was detected by immunoblotting with a phosphospecific antibody, p-ERK (E4) (sc-7383; Santa Cruz Biotechnology). Total Erk2 in lysates was estimated by immunoblotting with anti-Erk2 antibodies (sc-154; Santa Cruz Biotechnology) and ECL.
Rac2-GTP and Ras-GTP pull-down
GTP-bound Rac2 was pulled down with a bacterially synthesized GST fusion of the PAK1 Rac-binding domain.20 Similarly, the amount of Ras-GTP was measured by using a GST fusion of the Ras-binding domain of Raf1.24 Cell lysis and pull-down assays were performed as described.20 Rac2 and Ras were detected by ECL after immunoblotting with specific antibodies to human Rac2 (sc-96; Santa Cruz Biotechnology) and to human H-, K- and N-Ras (Ab3; Oncogene Research Products, Cambridge, MA).
Ras-GRF cellular distribution
Cells, differentiated in the presence (50 ng/mL) or absence of doxycycline, were stimulated for various periods of time with fMLFK (1 μM) at 37°C. After subcellular fractionation,16solubilized membrane fractions were submitted to SDS-PAGE and immunoblotting was performed with a polyclonal antibody to Ras-GRF (sc-963; Santa Cruz Biotechnology).
Results
Adaptation of a tetracycline-controlled expression system to the human promyelocytic HL-60 cell line
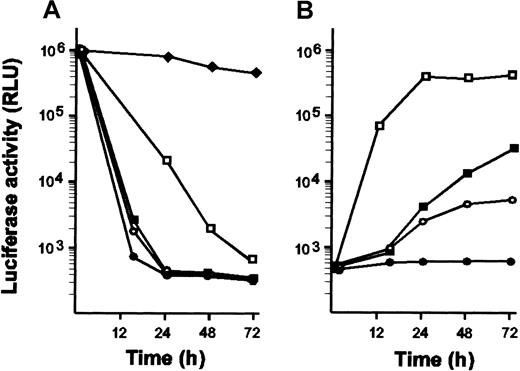
The tTA-off sequence14 was placed under the control of the promoter of the human EF-1α chromosomal gene. This promoter has previously been shown to efficiently stimulate transcription in myeloid cells.12 Luciferase was used as a reporter gene to assay for the functional expression of the transactivator and for the tetracycline control of target gene expression. Two clones were found to express a functional transactivator. As reported for other mammalian cells, the tTA-off transactivator tightly controlled protein expression in a tetracycline-responsive manner in HL-60 cells. Luciferase activity dropped by 3 orders of magnitude for tetracycline concentrations ranging from 20 to 100 ng/mL (Figure 1A). Luciferase activity could be reinduced after tetracycline removal (Figure 1B). However, several passages were required for full reinduction when doses of tetracycline greater than 50 ng/mL had been applied.
Kinetics of tetracycline action and dose response analysis of tetracycline-regulated luciferase expression.
(A) HL-60 cells expressing luciferase under the control of tTA were grown in the absence of tetracycline. At time 0, cells were separated in several batches and grown in the presence of various doses of tetracycline: 1 ng/mL (♦), 10 ng/mL (■), 20 ng/mL (▪), 50 ng/mL (○), and 100 ng/mL (●). At indicated times, aliquots containing the same amounts of cells were withdrawn and assayed for luciferase activity. No change in growth behavior or cell morphology occurred for any concentration of antibiotic. (B) Cells maintained in the presence of doses of tetracycline that repress luciferase expression were washed twice with phosphate-buffered saline and recultured in the absence of tetracycline. At indicated times, aliquots containing the same amount of cells were withdrawn and assayed for luciferase activity. A substantial increase in luciferase activity could be detected only after several passages when cells had been treated with 100 ng/mL tetracycline (●). Results are given in relative luciferase activity (RLU). Average values of 2 independent experiments are given.
Kinetics of tetracycline action and dose response analysis of tetracycline-regulated luciferase expression.
(A) HL-60 cells expressing luciferase under the control of tTA were grown in the absence of tetracycline. At time 0, cells were separated in several batches and grown in the presence of various doses of tetracycline: 1 ng/mL (♦), 10 ng/mL (■), 20 ng/mL (▪), 50 ng/mL (○), and 100 ng/mL (●). At indicated times, aliquots containing the same amounts of cells were withdrawn and assayed for luciferase activity. No change in growth behavior or cell morphology occurred for any concentration of antibiotic. (B) Cells maintained in the presence of doses of tetracycline that repress luciferase expression were washed twice with phosphate-buffered saline and recultured in the absence of tetracycline. At indicated times, aliquots containing the same amount of cells were withdrawn and assayed for luciferase activity. A substantial increase in luciferase activity could be detected only after several passages when cells had been treated with 100 ng/mL tetracycline (●). Results are given in relative luciferase activity (RLU). Average values of 2 independent experiments are given.
This system offers the advantage of comparing the effect of an exogenous protein by simply turning on and off its expression, thereby avoiding comparison with independent clones that may not have the same genetic background. Furthermore, the expression of potentially toxic proteins can be maintained at a moderate level by the presence of appropriate doses of tetracycline.
In the following study, the tetracycline analogue doxycycline, which is more efficient than tetracycline, was used to repress the system. To alleviate the laborious analysis of positive clones and to identify clones with the highest level of expression, a response plasmid was used that allows the bicistronic expression of both the protein of interest and the EGFP. It has been previously shown that the expression of EGFP in neutrophils does not affect NADPH oxidase activity or chemotaxis.25
Conditional expression of dominant-inhibitory and constitutively active forms of the GTPase Cdc42
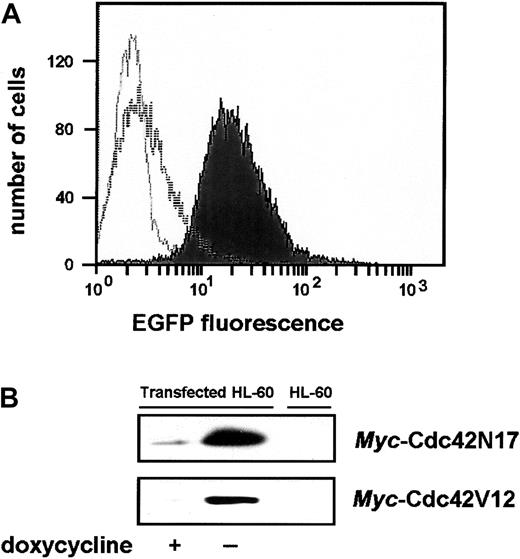
tTA-expressing HL-60 cells, referred to as parental cells, were used to investigate the effects produced in intact cells by the expression of a dominant-inhibitory (N17) or a constitutively active (V12) mutant of the Rho-GTPase Cdc42, a candidate molecule for the regulation of myeloid cell microbicidal and cytotoxic functions. Preliminary experiments indicated that the expression of the Cdc42 mutants did not affect cell growth. Cells could thus be cultured in the absence of doxycycline during the selection step. FACS was used to analyze resistant clones for fluorescence marker expression. As illustrated in Figure 2A for a Cdc42N17-transfected clone, the fluorescence of expressing cells was much above the background fluorescence of parental cells. When EGFP expression was turned off by the addition of doxycycline, the whole cell population was uniformly shifted to low fluorescence levels. An overall 10-fold induction in the absence of doxycycline could be estimated, with more than 90% of the induced cells expressing levels of EGFP that were never reached in the population of uninduced cells.
Analysis of the doxycycline-induced bicistronic protein expression.
(A) Fluorescence-activated cell sorting (FACS) analysis of EGFP expression. Overlays of histogram plots, scoring the number of cells for a given fluorescence intensity, are presented. The unfilled light gray curve corresponds to the background fluorescence profile of tTA-expressing parental cells. The unfilled and filled dark gray curves correspond to the fluorescence profiles of cells grown in the presence or absence of doxycycline, respectively. (B) Expression of themyc-tagged Cdc42N17 or Cdc42V12 proteins. Cell lysates were subjected to immunoprecipitation with anti–c-mycantibodies. Dominant-inhibitory proteins were detected by immunoblot with anti-Cdc42 antibodies. Untransfected HL-60 cells were used as control.
Analysis of the doxycycline-induced bicistronic protein expression.
(A) Fluorescence-activated cell sorting (FACS) analysis of EGFP expression. Overlays of histogram plots, scoring the number of cells for a given fluorescence intensity, are presented. The unfilled light gray curve corresponds to the background fluorescence profile of tTA-expressing parental cells. The unfilled and filled dark gray curves correspond to the fluorescence profiles of cells grown in the presence or absence of doxycycline, respectively. (B) Expression of themyc-tagged Cdc42N17 or Cdc42V12 proteins. Cell lysates were subjected to immunoprecipitation with anti–c-mycantibodies. Dominant-inhibitory proteins were detected by immunoblot with anti-Cdc42 antibodies. Untransfected HL-60 cells were used as control.
Cdc42 mutants were expressed as myc-tagged proteins. Immunoprecipitation with anti-myc antibodies and Western blot analysis using an antibody to human Cdc42 were used to directly monitor myc-Cdc42N17 or myc-Cdc42V12 expression. As shown in Figure 2B for selected clones, cells grown in the absence of doxycycline expressed a high level of the exogenous proteins. Their expression was almost undetectable in cells grown in the presence of doxycycline, reflecting a relatively tight control of the system. Densitometric quantification of the immunoblots indicated at least a 20-fold increase in exogenous protein expression in induced cells.
Phenotype induced by the expression of the Cdc42N17 dominant-inhibitory mutant
Promyelocytic human leukemia HL-60 cells differentiate to a granulocytic cell type phenotype and acquire maturation markers when treated by Bt2cAMP or DMSO. Following maturation into granulocytes, HL-60 cells express NADPH oxidase components and produce superoxide upon chemoattractant application. Although p22phox, p40phox, and Rac2 are detected in promyelocytes, gp91phox and the cytosolic proteins p67phox and p47phox appear only after myelocyte stages and can be considered differentiation markers.26,27 Likewise, the N-formyl peptide and C5a receptors are expressed in HL-60 cells only after differentiation to neutrophil-like cells.28 Western blot analysis showed that the differentiation of HL-60 cells was unaffected by the expression of Cdc42N17 or by the presence of doxycycline. Although the maturation markers p67phox and p47phox were hardly detectable in undifferentiated cells, they were expressed at the same levels in cells grown in the presence or absence of doxycycline (Figure3A). Uninduced and induced differentiated cells displayed roughly the same number of N-formyl peptide or C5a receptors at the cell surface in radioactive ligand–binding assays (data not shown).
Expression of Cdc42N17 did not affect cell differentiation.
(A) Western blot analysis of 2 cytosolic components, p47phox and p67phox, of the NADPH oxidase complex in undifferentiated cells (nd) and in Bt2cAMP-differentiated cells (d). Data are representative of 3 separate experiments. (B, C) Chemoattractant-induced phosphorylation of p47phox and p40phox. Cells grown and differentiated in the presence or absence of doxycycline (50 ng/mL) were subjected to metabolic labeling with [33P] orthophosphate and stimulated with 1 μM fMLFK. At indicated times, aliquots were withdrawn and lysed. p47phox and p40phox were immunoprecipitated, and33P-labeled proteins were detected by SDS-PAGE and autoradiography. The figure is representative of 2 separate experiments.
Expression of Cdc42N17 did not affect cell differentiation.
(A) Western blot analysis of 2 cytosolic components, p47phox and p67phox, of the NADPH oxidase complex in undifferentiated cells (nd) and in Bt2cAMP-differentiated cells (d). Data are representative of 3 separate experiments. (B, C) Chemoattractant-induced phosphorylation of p47phox and p40phox. Cells grown and differentiated in the presence or absence of doxycycline (50 ng/mL) were subjected to metabolic labeling with [33P] orthophosphate and stimulated with 1 μM fMLFK. At indicated times, aliquots were withdrawn and lysed. p47phox and p40phox were immunoprecipitated, and33P-labeled proteins were detected by SDS-PAGE and autoradiography. The figure is representative of 2 separate experiments.
Phosphorylation of the cytosolic factor p47phoxis thought to be a key step in agonist-mediated NADPH oxidase activation.2 It has been shown that recombinant p47phox prephosphorylated by protein kinase C (PKC) can specifically activate NADPH oxidase in a cell-free system.29 Additionally, p40phoxtakes up phosphate when NADPH oxidase is activated,22 but the exact role of p40phox in enzyme activation remains controversial. We thus examined whether p47phox and p40phox were differentially phosphorylated in cells expressing Cdc42N17. Immunoprecipitations of p47phox or p40phox from lysates of cells metabolically labeled with [33P] orthophosphoric acid indicated that the chemotactic peptide fMLFK induced a comparable increase in the phosphorylation of p47phox (Figure 3B) and p40phox (Figure 3C), regardless of whether cells expressed Cdc42N17.
When assayed for NADPH oxidase activity, differentiated parental tTA-expressing cells responded equally well to chemoattractants whether they had been grown in the presence or absence of doxycycline (data not shown), confirming that doxycycline had no effect on cell differentiation or cellular function. The response of parental cells to fMLFK was better observed after differentiation with 1 mM Bt2cAMP, whereas the response to PMA was more potent when cells were differentiated with DMSO. Transfected cells grown in the presence of 50 ng/mL doxycycline produced an amount of superoxide that was similar to that produced by the parental cells, whether they were differentiated with Bt2cAMP and stimulated with fMLFK (Figure 4A, left panel) or differentiated with DMSO and stimulated with PMA (Figure 4B, left panel). The expression of Cdc42V12 did not markedly potentiate chemoattractant-mediated NADPH oxidase activity (116% ± 21% of the response observed in uninduced cells; n = 6). In contrast, when Cdc42N17-transfected cells were grown and differentiated in the absence of doxycycline, cells did not respond at all to either agonist. The same phenotype was observed when cells were stimulated with the C5a anaphylatoxin or with the synthetic peptide WKYMVM, an agonist for theN-formyl peptide receptor homologue FPRL130(data not shown). Dose responses for doxycycline showed that the oxidative response correlated with the dose-dependent expression of Cdc42N17 (Figure 4A-B, right panels). The inhibition of fMLFK- and PMA-stimulated superoxide production occurred roughly at the same levels of Cdc42N17 expression, indicating that there was no clear differential sensitivity of the 2 pathways.
Effect of the expression of Cdc42N17 on superoxide production.
Kinetics of superoxide production are shown on the left side of panels A and B. Transfected HL-60 cells were grown and differentiated into neutrophil-like cells with Bt2cAMP (A) or DMSO (B) in the absence or presence of doxycycline (50 ng/mL). Superoxide formation was initiated by the addition of fMLFK or PMA (arrow), and cytochrome c reduction was continuously recorded at 550 nm. Data are representative of more than 10 independent experiments. On the right side of panels A and B, superoxide production was measured in differentiated cells grown in intermediate doses of doxycycline. Results are expressed as percentage of the response obtained in uninduced cells. Values represent the means ± SD of 3 independent experiments. An immunoblot representative of the dose-dependent expression ofmyc-Cdc42N17 is presented. (C) Oxidase activity assay in a cell-free system. Membrane and cytosolic fractions from induced (black bars) and uninduced (gray bars) Bt2cAMP-differentiated HL-60 cells (dHL-60) were assayed for their ability to stimulate superoxide production in an amphiphile-activated heterologous cell-free system. Results are expressed as the percentage of the response obtained with cytosol of doxycycline-treated cells and bovine membrane or with membrane of doxycycline-treated cells and bovine cytosol. Values represent the means ± SD of 5 independent experiments performed in duplicate. (D) [35S]GTPγS loading of Rac. [35S]GTPγS was added to the cell-free system in the preincubation step. GTPγS-loaded Rac was pulled down with a GST fusion of the PAK1 Rac-binding domain. The figure is representative of 3 independent experiments.
Effect of the expression of Cdc42N17 on superoxide production.
Kinetics of superoxide production are shown on the left side of panels A and B. Transfected HL-60 cells were grown and differentiated into neutrophil-like cells with Bt2cAMP (A) or DMSO (B) in the absence or presence of doxycycline (50 ng/mL). Superoxide formation was initiated by the addition of fMLFK or PMA (arrow), and cytochrome c reduction was continuously recorded at 550 nm. Data are representative of more than 10 independent experiments. On the right side of panels A and B, superoxide production was measured in differentiated cells grown in intermediate doses of doxycycline. Results are expressed as percentage of the response obtained in uninduced cells. Values represent the means ± SD of 3 independent experiments. An immunoblot representative of the dose-dependent expression ofmyc-Cdc42N17 is presented. (C) Oxidase activity assay in a cell-free system. Membrane and cytosolic fractions from induced (black bars) and uninduced (gray bars) Bt2cAMP-differentiated HL-60 cells (dHL-60) were assayed for their ability to stimulate superoxide production in an amphiphile-activated heterologous cell-free system. Results are expressed as the percentage of the response obtained with cytosol of doxycycline-treated cells and bovine membrane or with membrane of doxycycline-treated cells and bovine cytosol. Values represent the means ± SD of 5 independent experiments performed in duplicate. (D) [35S]GTPγS loading of Rac. [35S]GTPγS was added to the cell-free system in the preincubation step. GTPγS-loaded Rac was pulled down with a GST fusion of the PAK1 Rac-binding domain. The figure is representative of 3 independent experiments.
To assess the functionality of the NADPH oxidase complex, superoxide production was assayed by measuring cytochrome c reduction in a cell-free reconstitution system after the addition of GTPγS and NADPH.18 Oxidase activation was performed on a mixture of membrane and cytosol preparations from either bovine neutrophils or Bt2cAMP-differentiated HL-60 cells conditionally expressing Cdc42N17. Reconstitution of NADPH oxidase activity was obtained with membrane and cytosolic fractions of differentiated cells expressing Cdc42N17, corroborating the lack of effect of Cdc42N17 on HL-60 cell differentiation (Figure 4C). Membrane preparations from HL-60 cells grown in the presence or absence of doxycycline were indistinguishable in their ability to complement bovine cytosol for NADPH oxidase activation. However, a modest reduction (approximately 30%) of NADPH oxidase activity was observed when bovine membrane preparations were complemented with cytosol preparations from cells expressing Cdc42N17. Addition of purified recombinant myc-Cdc42N17, at concentrations ranging from 5 to 50 μg/mL, did not inhibit the cell-free NADPH oxidase system (data not shown). This indicates that Cdc42N17 does not act as a direct competitor of Rac at the level of the oxidase complex. Moreover, [35S]GTPγS binding to Rac in the cell-free system was not affected by the expression of Cdc42N17, indicating that Cdc42N17 did not interfere with Rac GTP loading (Figure4D).
Thus, although the GTP-bound form of Cdc42 has been shown to be ineffective at directly activating NADPH oxidase in a superoxide-generating cell-free system31 and Cdc42V12 expression was not found to increase NADPH oxidase activity in intact cells, Cdc42N17 expression unexpectedly interfered with the production of superoxide.
Cdc42N17 expression does not affect PI3K/Akt signaling pathway
Studies performed with knock-out mice lacking the γ isoform of phosphatidylinositol-3 kinase (PI3Kγ) have shown that PI3Kγ is the sole isoform involved in chemoattractant-induced superoxide production in myeloid cells.32-34 This isoform can be directly activated by G protein βγ subunits in vitro.35 Taken together, these results link G protein–coupled receptor stimulation to the formation of phosphatidylinositol-3,4,5-trisphosphate and the activation of the pathways regulated by phosphoinositides bearing phosphate in the D3 position. In addition, PI3Kγ deficiency results in a lack of chemoattractant-mediated activation of Akt.34
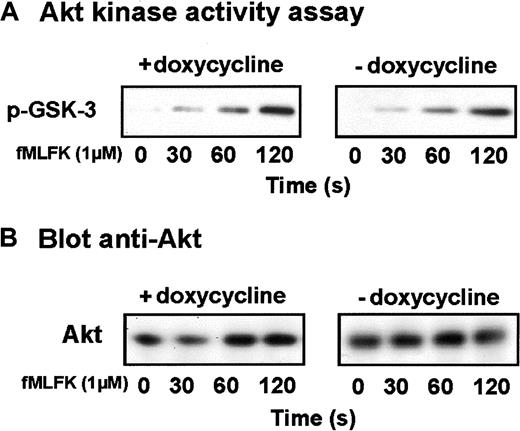
To examine the impact of Cdc42N17 expression on the PI3Kγ pathway, Akt was selectively immunoprecipitated from lysates of induced and uninduced cells, and the ability of Akt to phosphorylate its substrate GSK-3 was assayed. As illustrated in Figure5, the expression of Cdc42N17 had no significant effect on Akt activation in response to chemoattractant receptor stimulation. Wortmannin and LY294002 inhibited the activation of Akt (not shown), indicating that in human myeloid cells, as in mouse cells, Akt is a downstream effector of PI3K. Thus, the inhibition of superoxide generation in cells expressing Cdc42N17 does not result from an inhibition of the PI3K signaling pathway.
Cdc42N17 has no effect on PI3Kγ activation.
(A) HL-60 cells expressing Cdc42N17 were differentiated with Bt2cAMP in the presence or absence of doxycycline and stimulated for various periods of time with fMLFK. The PI3Kγ-downstream effector Akt was selectively immunoprecipitated from cell lysates. In a kinase assay, phosphorylation of the Akt substrate GSK-3 was measured by Western immunoblotting using a phospho-GSK3 antibody. Results are representative of 2 independent experiments. (B) Western blot analysis with anti-Akt antibody was performed to assess the amount of immunoprecipitated Akt kinase.
Cdc42N17 has no effect on PI3Kγ activation.
(A) HL-60 cells expressing Cdc42N17 were differentiated with Bt2cAMP in the presence or absence of doxycycline and stimulated for various periods of time with fMLFK. The PI3Kγ-downstream effector Akt was selectively immunoprecipitated from cell lysates. In a kinase assay, phosphorylation of the Akt substrate GSK-3 was measured by Western immunoblotting using a phospho-GSK3 antibody. Results are representative of 2 independent experiments. (B) Western blot analysis with anti-Akt antibody was performed to assess the amount of immunoprecipitated Akt kinase.
Cdc42N17 interferes with agonist-induced intracellular calcium mobilization, Ras/MAPK signaling, and Rac2 activation
The exposure of neutrophils to chemoattractants generates multiple intracellular second messengers and signaling molecules through the activation of phospholipases and protein kinases.3,36These include isoforms of phosphatidylinositol-specific phospholipase C (PLCβ2/β3), the aforementioned PI3Kγ, protein kinase C, mitogen-activated protein kinases p44/42 (Erk1/2),11,37and p38 SAPK.10,38,39 The strict requirement of PLC in superoxide generation has recently been established with mice lacking the 2 PLCβ2/β3 isoforms.33,34 Moreover, recent studies have shown that both Cdc42 and Rac can stimulate the activity of PLCβ2 in vitro40 and of PLCγ1 in RBL-2H3 mast cells.41 42 These 2 studies provide evidence that small G proteins may interact at an early step in receptor-mediated intracellular signaling.
To evaluate a possible effect of Cdc42N17 on the signaling pathway mediated by PLCβ2, we examined the kinetics of fMLFK-mediated IP3 formation and intracellular calcium mobilization in induced and uninduced cells after differentiation with Bt2cAMP. As illustrated in Figure 6A, the expression of Cdc42N17 markedly reduced the initial increase in IP3 levels upon fMLFK stimulation (black bars). Similarly, a drastic reduction of the fMLFK-induced intracellular calcium mobilization occurred in cells expressing Cdc42N17 in the absence (Figure 6B) or the presence (not shown) of extracellular calcium. However, only a modest increase of the fMLFK-induced intracellular calcium mobilization was observed in cells expressing the constitutively active Cdc42V12 mutant (Figure 6C). The inability of Cdc42N17-expressing cells to produce superoxide on fMLFK addition may primarily result from a weaker ability to mobilize intracellular calcium because previous studies have emphasized the essential role of calcium mobilization in NADPH oxidase activation.43 44 This observation prompted us to examine whether an ionophore-induced calcium influx could restore a substantial fMLFK-mediated superoxide response in cells expressing Cdc42N17. As expected, 2-minute pretreatment with ionomycine restored superoxide production in these cells (80% ± 7%; n = 5; of the amount produced by uninduced cells) (Figure 6D). In uninduced cells, ionomycine was unable to induce superoxide production or increase the effects of chemoattractants (not shown).
Effect of the expression of Cdc42N17 on the activation of PLCβ2.
(A) fMLFK-mediated IP3 formation. IP3 levels in differentiated Cdc42N17-expressing cells (black bars) and uninduced cells (gray bars) were determined at time intervals after stimulation with fMLFK (1 μM) using an inositol-1,4,5-trisphosphate [3H] radioreceptor assay kit. Error bars represent the standard deviation of 3 independent experiments performed in duplicate. (B, C) Kinetics of fMLFK-mediated intracellular calcium mobilization. Cells were loaded with Fura-2, and fMLFK-mediated calcium increase was assayed in the absence of extracellular calcium. Mean responses ± SE (n = 7) in differentiated Cdc42N17- (B) or Cdc42V12- (C) expressing cells grown in the absence (triangles) or in the presence (circles) of doxycycline are presented. (D) Effect of ionomycine on superoxide production in Cdc42N17-expressing HL-60 cells. Cells grown and differentiated in the absence of doxycycline were treated or not for 2 minutes with 1 μM ionomycine at 37°C before stimulation with fMLFK. Data are representative of 2 independent experiments.
Effect of the expression of Cdc42N17 on the activation of PLCβ2.
(A) fMLFK-mediated IP3 formation. IP3 levels in differentiated Cdc42N17-expressing cells (black bars) and uninduced cells (gray bars) were determined at time intervals after stimulation with fMLFK (1 μM) using an inositol-1,4,5-trisphosphate [3H] radioreceptor assay kit. Error bars represent the standard deviation of 3 independent experiments performed in duplicate. (B, C) Kinetics of fMLFK-mediated intracellular calcium mobilization. Cells were loaded with Fura-2, and fMLFK-mediated calcium increase was assayed in the absence of extracellular calcium. Mean responses ± SE (n = 7) in differentiated Cdc42N17- (B) or Cdc42V12- (C) expressing cells grown in the absence (triangles) or in the presence (circles) of doxycycline are presented. (D) Effect of ionomycine on superoxide production in Cdc42N17-expressing HL-60 cells. Cells grown and differentiated in the absence of doxycycline were treated or not for 2 minutes with 1 μM ionomycine at 37°C before stimulation with fMLFK. Data are representative of 2 independent experiments.
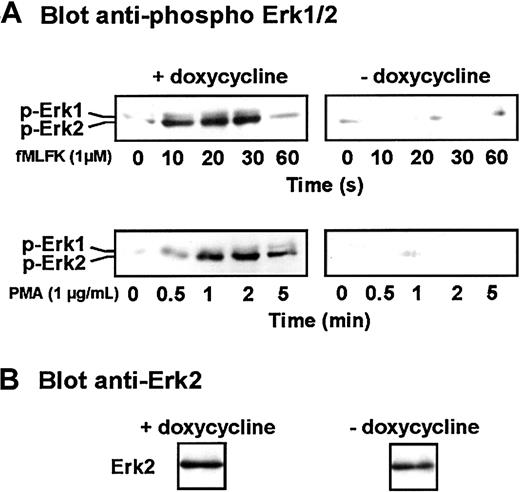
We next investigated whether the expression of Cdc42N17 had an effect further downstream in the chemoattractant-mediated signaling pathway. Erk1/2 activation was examined after immunoblotting of lysates from stimulated cells with antibodies that specifically recognize the phosphorylated forms of both kinases. As shown in Figure7, fMLFK triggered rapid and transient (10 to 30 seconds) Erk1/2 phosphorylation in cells grown and differentiated in the presence of doxycycline. Upon stimulation with PMA, transient Erk1/2 phosphorylation was also observed but with a different time course (1 to 5 minutes). With both agonists, Erk2 was the predominant phosphorylated species. However, neither fMLFK nor PMA was able to induce Erk1/2 activation in cells expressing Cdc42N17. This suggests that the dominant-inhibitory form of Cdc42 interferes upstream of the MAP kinases.
Effect of the expression of Cdc42N17 on MAP kinase activation.
(A) Differentiated induced or uninduced cells were stimulated for various periods of time with fMLFK or PMA. Erk1/2 phosphorylation was detected after SDS-PAGE by Western immunoblotting with a phosphospecific antibody. (B) A fraction of an unstimulated cell lysate was probed with an anti-Erk2 antibody. Results are representative of 3 independent experiments.
Effect of the expression of Cdc42N17 on MAP kinase activation.
(A) Differentiated induced or uninduced cells were stimulated for various periods of time with fMLFK or PMA. Erk1/2 phosphorylation was detected after SDS-PAGE by Western immunoblotting with a phosphospecific antibody. (B) A fraction of an unstimulated cell lysate was probed with an anti-Erk2 antibody. Results are representative of 3 independent experiments.
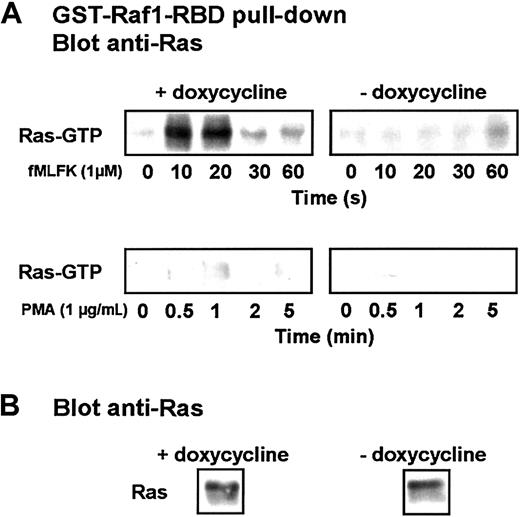
An activation pathway has emerged in which Gβγ recruits PI3Kγ to the plasma membrane, enhancing the activity of Src-like tyrosine kinases. A complex—including adapter proteins, a guanine exchange factor, and the small GTPase Ras—is formed that leads to the sequential activation of the downstream kinases Raf/MEKK-1, MEK, and MAPKs.45 We therefore assayed the activation of Ras by using the Ras-binding domain of Raf1 in fusion with GST to specifically isolate the GTP-bound form of Ras from lysates of stimulated cells.24 The presence of GTP-bound Ras was detected after immunoblotting with anti–pan-Ras antibodies (Figure8). Although the chemotactic factor fMLFK triggered rapid and transient activation of Ras in cells grown and differentiated in the presence of doxycycline, it stimulated only weak GTP loading of Ras after 1 minute of stimulation in cells expressing Cdc42N17. When PMA was applied, only a slight activation of Ras was observed in uninduced cells, and no GTP-bound Ras was detected in cells expressing Cdc42N17.
The expression of Cdc42N17 inhibits Ras activation.
(A) Differentiated induced or uninduced cells were stimulated for indicated time with 1 μM fMLFK or 1 μg/mL PMA. Cells from withdrawn aliquots were lysed, and Ras-GTP pull-down was performed using a GST-fusion of the Raf1 Ras-binding domain. The isolated Ras was detected after SDS-PAGE by Western blot analysis using an anti-Ras antibody. (B) A fraction of an unstimulated cell lysate was probed with an anti-Ras antibody. The figure is representative of 3 independent experiments.
The expression of Cdc42N17 inhibits Ras activation.
(A) Differentiated induced or uninduced cells were stimulated for indicated time with 1 μM fMLFK or 1 μg/mL PMA. Cells from withdrawn aliquots were lysed, and Ras-GTP pull-down was performed using a GST-fusion of the Raf1 Ras-binding domain. The isolated Ras was detected after SDS-PAGE by Western blot analysis using an anti-Ras antibody. (B) A fraction of an unstimulated cell lysate was probed with an anti-Ras antibody. The figure is representative of 3 independent experiments.
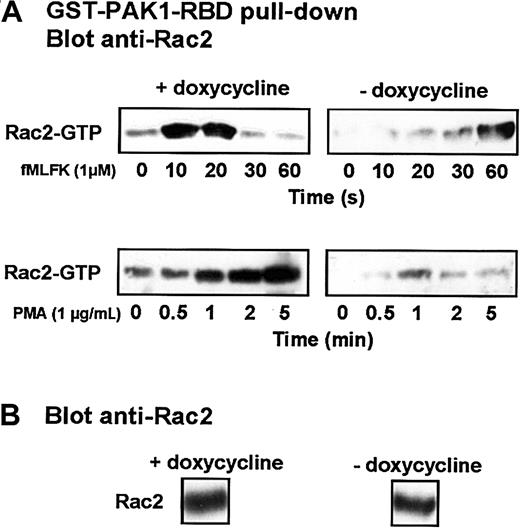
Similarly, we used the Rac-binding domain of PAK1, which associates exclusively with the GTP-bound state of Rac, to isolate the active form of Rac2 from stimulated cells.20 Rac2 is the major isoform present in differentiated HL-60 cells.27 As shown in Figure 9, in cells grown and differentiated in the presence of doxycycline, fMLFK induced a rapid and transient conversion of Rac2 to the GTP-bound active state, which culminated at 10 to 20 seconds. In the absence of doxycycline, the basal level of Rac2 in its GTP-bound form (no fMLFK) was consistently lower, and fMLFK-mediated Rac2 activation was markedly delayed. The application of PMA to uninduced cells also stimulated GTP loading of Rac2, but the kinetics of activation was much slower and more sustained. The expression of Cdc42N17 led to a drastic inhibition of PMA-mediated Rac2 activation.
Expression of Cdc42N17 interferes with Rac2 activation.
(A) Differentiated induced or uninduced cells were stimulated with 1 μM fMLFK or 1 μg/mL PMA. Aliquots were withdrawn at indicated times. Cells were lysed, and Rac-GTP pull-down was performed using a GST-fusion of the PAK1 Rac-binding domain. The isolated Rac2 was detected after SDS-PAGE by Western blot analysis using an anti-Rac2 antibody. (B) A fraction of an unstimulated cell lysate was probed with an anti-Rac2 antibody. Results are representative of 4 different experiments.
Expression of Cdc42N17 interferes with Rac2 activation.
(A) Differentiated induced or uninduced cells were stimulated with 1 μM fMLFK or 1 μg/mL PMA. Aliquots were withdrawn at indicated times. Cells were lysed, and Rac-GTP pull-down was performed using a GST-fusion of the PAK1 Rac-binding domain. The isolated Rac2 was detected after SDS-PAGE by Western blot analysis using an anti-Rac2 antibody. (B) A fraction of an unstimulated cell lysate was probed with an anti-Rac2 antibody. Results are representative of 4 different experiments.
Expression of Cdc42N17 prevents Ras-GRF recruitment to the membrane fraction
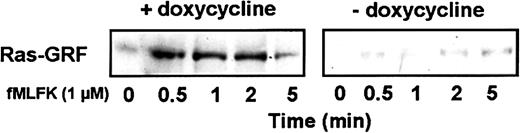
A consensus has been reached that small GTPase dominant-inhibitory mutants work in cells by competing with their normal counterparts for binding to their specific GEFs. Because many GEFs for the Rho family GTPases (Rho, Rac, and Cdc42) activate more than one Rho family member, Cdc42N17 is expected to block the activation of Rac by directly interfering with and sequestering common GEFs. Impaired GTP-loading on Ras may also result from the sequestration of a GEF common to Cdc42/Rac and Ras. However, in a recent study, Cdc42N17 has been shown to inhibit translocation to the membrane of Ras-GRF1, a brain-restricted GEF for Ras but not for Cdc42.46,47 The activity of Ras-GRF1 is inhibited by the GDP-bound form of Cdc42 but is unaffected by its GTP-bound form,47 suggesting an indirect regulation of this GEF. Similarly, in differentiated HL-60 cells, the activation of Ras-GRF2, a more ubiquitous isoform that has been shown to be a GEF for Ras and Rac but not for Cdc42,48 could be indirectly regulated by Cdc42. When activated, Ras-GRFs translocate to the membrane.
The subcellular localization of Ras-GRF2 was therefore examined in differentiated HL-60 cells when the expression of Cdc42N17 was either induced or repressed. Induced and uninduced cells expressed the same amount of Ras-GRF2 (not shown). On stimulation with fMLFK, Ras-GRF2 rapidly and transiently translocated to the membrane of uninduced cells (Figure 10). In cells expressing Cdc42N17, translocation did not occur.
Expression of Cdc42N17 inhibits the agonist-induced translocation of Ras-GRF to the membrane fraction.
Cells were differentiated with Bt2cAMP in the presence or absence of doxycycline. Aliquots were treated with 1 μM fMLFK for the indicated times and lysed. After subcellular fractionation, membrane fractions were submitted to SDS-PAGE. The amount of Ras-GRF at the membrane was determined by Western blotting with an anti–Ras-GRF antibody. The figure is representative of 3 different experiments.
Expression of Cdc42N17 inhibits the agonist-induced translocation of Ras-GRF to the membrane fraction.
Cells were differentiated with Bt2cAMP in the presence or absence of doxycycline. Aliquots were treated with 1 μM fMLFK for the indicated times and lysed. After subcellular fractionation, membrane fractions were submitted to SDS-PAGE. The amount of Ras-GRF at the membrane was determined by Western blotting with an anti–Ras-GRF antibody. The figure is representative of 3 different experiments.
Discussion
In the present study, we show that the conditional expression of Cdc42N17 in neutrophil-like differentiated HL-60 cells has a dramatic inhibitory effect on chemoattractant-induced superoxide production. This inhibitory effect is not attributable to a loss of function of any of the components that form the active NADPH oxidase complex.
On cell activation, p47phox is phosphorylated and translocates to the plasma membrane. It functions as an adapter protein that promotes the binding of p67phox, Rac, or both to the flavocytochrome b558. PKC is at least one of the enzymes able to convert p47phox to a functional molecule.29,49,50 The respective contribution of each PKC isoform has not been completely elucidated. Although an antisense approach has shown that PKCβ modulates the respiratory burst in differentiated HL-60 cells,51 another study has shown that PKCζ phosphorylates specific residues in p47phox and participates in the signaling cascade between the N-formyl peptide receptor and NADPH oxidase activation.52 Because N-formyl peptides induce rapid phosphorylation of p47phox in differentiated HL-60 cells expressing Cdc42N17, the inability of these cells to stimulate superoxide production is unlikely to result from a dysfunction of p47phox. Interestingly, the phosphorylation of p47phox occurs in these cells, though the PLCβ2 pathway (diacylglycerol [DAG] and calcium-second messenger production) and possibly calcium-mediated PKC activation are perturbed. Therefore, additional signaling pathways may contribute to the regulation of the phosphorylation of p47phox and its subsequent redistribution. For instance, phosphatidic acid, generated by phospholipase D, has been linked to the activation of NADPH oxidase53 through the activation of a novel calcium-independent kinase that has been shown to phosphorylate p47phox in vitro.54This is consistent with the observation that p47phox and Rac2 are still translocated to the plasma membrane in neutrophils from PI3Kγ or PLCβ2/β3 knock-out mice.32 33
Rac binds to p67phox, to the flavocytochrome b558, and to the membrane through distinct regions.55-60 The recent crystal structure of a fragment encompassing amino acids 1 to 203 of p67phox in complex with Rac-GTP60 reveals significant differences in the way p67phox interacts with Rac, in comparison with other structures of Rho family effector complexes. Although Cdc42 does not normally activate NADPH oxidase,31it becomes active by replacing amino acids 27 and 30 within the effector loop by the corresponding residues of Rac.61 The crystal structure clearly shows that Gly-30 in Rac is indeed part of the protein–protein interface. The replacement in Rac of Ala-27 by the lysine present in Cdc42 leads to a steric clash of the lysine side chain with the p67phox structure, thereby explaining the specific interaction of Rac and p67phox. In addition, p67phox fragment 1-192 inhibits Rac-mediated membrane ruffling but does not inhibit the Cdc42-mediated development of filopodia and RhoA-mediated stress fiber formation.56The Cdc42 binding to p67phox observed initially62 has been proved to be an artifact caused by the presence in the assay of a Cdc42-binding PAK-like protein.56 Purified recombinant Cdc42N17 did not inhibit the cell-free NADPH oxidase system, indicating that the inhibition of NADPH oxidase activity when Cdc42N17 is expressed did not result from a direct competition between Cdc42N17 and Rac for the formation of an active complex.
Tight temporal control of Rac activation is apparently required for its contribution to NADPH oxidase activation. In cells expressing Cdc42N17, there is no N-formyl peptide-mediated superoxide production in spite of the GTP-loading on Rac2 at later time points (Figure 9). The expression of Cdc42N17 seems to have adverse effects on superoxide generation through interference at multiple levels in the signaling pathways downstream of chemoattractant receptors. The second messenger-generating enzyme PLCβ2, a PLC isoform restricted to myeloid cells, generates IP3 that in turn binds to the IP3 receptor calcium channel, thereby promoting the release of intraluminal calcium into the cytoplasm.63 PLCβ2 has been characterized as a target for Cdc42 and Rac in an in vitro study.40 A purified complex between either Cdc42 or Rac and the Rho guanine nucleotide dissociation inhibitor LyGDI mediates GTP-dependent activation of PLCβ2. The activation of PLCβ2 by Cdc42/Rac appears to be specific because the GTPases cannot enhance the activity of PLCδ1 or PLCβ1.64 More recently, in RBL-2H3 mast cells, the GTP-bound form of both Cdc42 and Rac has been shown to participate in IgE receptor (Fcε–RI) signaling to the exocytosis of secretory granules by interacting with the PLCγ1 isoform.41 42 In myeloid cells, PLCβ2 could thus represent a focal point from which the 2 GTPases may influence calcium changes critical for the neutrophil bactericidal functions. Our results are consistent with this view. In differentiated HL-60 cells, the expression of Cdc42N17 results in a drastic reduction ofN-formyl peptide–induced IP3 formation and subsequently in a reduced mobilization of intracellular calcium. The adverse effect of Cdc42N17 on PLCβ2 activation and calcium release is expected to have an impact on downstream signaling pathways leading to the activation of the diverse components involved in NADPH oxidase activation.
GTPase N17 mutants produce their inhibitory effects through several mechanisms. They either interfere with the endogenous counterpart for the activation of a downstream effector or sequester an upstream positive regulator, such as a GEF. In the latter case, endogenous GTPase is maintained in a GDP-bound inactive conformation. In fact, the Rho family GTPases are switched to their active conformation by a large number of GEFs that are not always selective for a particular GTPase.4,65,66 Thus, a given dominant-negative GTPase can sequester more than one GEF and produce pleiotropic effects.67
The Cdc42N17 mutant may also produce an inhibitory effect by interfering with GEF activation through a reduced DAG formation and calcium release. For instance, FGD1, a GEF specific for Cdc42, and Vav, which activates multiple Rho family proteins, share a potential DAG-binding domain.66 Because they may thus be regulated by phorbol esters, the inhibition of the PMA-induced NADPH oxidase and the Rac activation we observed are likely to be related to the interference of Cdc42N17 at the GEF level. Phosphorylation and membrane localization of Tiam1, a GEF for Rac1 and Cdc42, are induced by the activation of calcium–calmodulin kinase II and PKC either by ionomycine or by stimulation of lysophosphatidic acid, bombesin, or bradykinin receptors.68,69 Likewise, calcium signaling regulates Ras-GRF1, a brain-restricted GEF for Ras, linking G protein-coupled receptors to the activation of the Ras/MAPK cascade.70 Ras-GRF1 activation is mediated by calmodulin that binds to a calmodulin-binding motif. The widely distributed homologue Ras-GRF2, which activates both Ras and Rac but not Cdc42, seems to be regulated by similar mechanisms.48 Thus, if one assumes that Ras-GRF2 is involved in chemoattractant-mediated signaling to Ras or Rac, a reduced calcium increase may participate in the impairment of Rac and Ras GTP-loading and MAPK cascade activation in Cdc42N17-expressing cells. This view is consistent with the observation that an ionomycin-induced calcium influx restores superoxide production in these cells.
In addition, recent studies47 have shown that Cdc42 controls the translocation of Ras-GRF1 to the plasma membrane, but a direct interaction between Ras-GRF1 and Cdc42 has not been observed. In differentiated HL-60 cells expressing Cdc42N17, the inhibition of Ras GTP-loading and Ras-GRF2 translocation to the plasma membrane suggests that there is a signaling cascade consisting of Cdc42, Ras-GRF2, and Ras. By specifically sequestering FGD1, the Cdc42N17 mutant protein would maintain endogenous Cdc42 in the GDP-bound inactive conformation, thereby reducing the activity of PLCβ2 and the activation of the Cdc42-regulated ACK tyrosine kinases. The Ras guanine nucleotide exchange activity of RasGRF1 is enhanced on tyrosine phosphorylation by the Cdc42-regulated kinase ACK1.71
Therefore, this study provides evidence for an unexpected inhibitory role of Cdc42N17 in NADPH oxidase activation through interference at multiple levels in the signaling pathways. Most important, as already pointed out by Feig,67 the pleiotropic inhibitory effects of Cdc42N17 illustrate the potential pitfalls of using dominant-inhibitory proteins to study the function of Ras family GTPases. In this regard, a number of conclusions drawn from the use of dominant-inhibitory mutants in myeloid cells might have to be reconsidered.
We thank Laurence Macari for technical assistance and Marie-Claire Dagher and Ruth Griffin-Shea for helpful discussion and critical reading of the manuscript.
Prepublished online as Blood First Edition Paper, May 13, 2002; DOI 10.1182/blood-2001-12-0193.
Supported by grants from the Commissariat à l'Energie Atomique, the Centre National de la Recherche Scientifique, and the University Joseph Fourier (Unité Mixte de Recherche 5092 Commisariat àl'Energie Atomique/Centre National de la Recherche Scientifique/University Joseph Fourier).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Marie-Josèphe Rabiet, DRDC/BBSI-UMR 5092 CEA-CNRS, 17 rue des Martyrs, F-38054 Grenoble Cedex 9, France; e-mail:mjrabiet@cea.fr.

![Fig. 3. Expression of Cdc42N17 did not affect cell differentiation. / (A) Western blot analysis of 2 cytosolic components, p47phox and p67phox, of the NADPH oxidase complex in undifferentiated cells (nd) and in Bt2cAMP-differentiated cells (d). Data are representative of 3 separate experiments. (B, C) Chemoattractant-induced phosphorylation of p47phox and p40phox. Cells grown and differentiated in the presence or absence of doxycycline (50 ng/mL) were subjected to metabolic labeling with [33P] orthophosphate and stimulated with 1 μM fMLFK. At indicated times, aliquots were withdrawn and lysed. p47phox and p40phox were immunoprecipitated, and33P-labeled proteins were detected by SDS-PAGE and autoradiography. The figure is representative of 2 separate experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/5/10.1182_blood-2001-12-0193/3/m_h81723025003.jpeg?Expires=1769415483&Signature=ew~KPBhRGTFBKflUvMuFNtJrlY37PxMWVHI~~6MyE2RUzxqlI9Mq62gqK0lvsHhnFRkh9Jfoa7BiYM9G2SBBR9Fv6yTr0eG~vIUJNBfKSV7IwrupCVQpTbwWBKIsiJjdVhr1cFSr-Cac~MXw8Wl4I~Z0R~gXVUkWaNRoTrNDVCl1LfF~6xzZdSX8RZL5hK27bjqgvZThexJtYRyXGagqDpWGElQLY9L939DyN9gvsxQNePLbhd7jw7KcWHivpwnK-i7~kRoWhxk-MXgFG-Sf2a8rB~SRrHvxzaOyIn0WKKg6RG8aqIkk7uWoE88VCbpx0CT1LRA2ngmn5fnsNXU8VA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Effect of the expression of Cdc42N17 on superoxide production. / Kinetics of superoxide production are shown on the left side of panels A and B. Transfected HL-60 cells were grown and differentiated into neutrophil-like cells with Bt2cAMP (A) or DMSO (B) in the absence or presence of doxycycline (50 ng/mL). Superoxide formation was initiated by the addition of fMLFK or PMA (arrow), and cytochrome c reduction was continuously recorded at 550 nm. Data are representative of more than 10 independent experiments. On the right side of panels A and B, superoxide production was measured in differentiated cells grown in intermediate doses of doxycycline. Results are expressed as percentage of the response obtained in uninduced cells. Values represent the means ± SD of 3 independent experiments. An immunoblot representative of the dose-dependent expression ofmyc-Cdc42N17 is presented. (C) Oxidase activity assay in a cell-free system. Membrane and cytosolic fractions from induced (black bars) and uninduced (gray bars) Bt2cAMP-differentiated HL-60 cells (dHL-60) were assayed for their ability to stimulate superoxide production in an amphiphile-activated heterologous cell-free system. Results are expressed as the percentage of the response obtained with cytosol of doxycycline-treated cells and bovine membrane or with membrane of doxycycline-treated cells and bovine cytosol. Values represent the means ± SD of 5 independent experiments performed in duplicate. (D) [35S]GTPγS loading of Rac. [35S]GTPγS was added to the cell-free system in the preincubation step. GTPγS-loaded Rac was pulled down with a GST fusion of the PAK1 Rac-binding domain. The figure is representative of 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/5/10.1182_blood-2001-12-0193/3/m_h81723025004.jpeg?Expires=1769415483&Signature=2JYgurolTysEdkZdwZSh9IhoPY0mINoivY26PilnBOhyVdVtVvmxhkM85L5HiqIhgUceUcMFToCNeNXFd02D65Vjs5dEfGbYplNDht3dDDoZkykAPwuBvxHJaACM0EH0fNsXGqxO6Guabc8ytD6FY5pustOJ7iLBkoZY4QjcIXIEP5xh1VAD4mkBWv~uVQ-HRsZfJhunnv-K60Ppz2aUy8m3DT0hrvPGhs3c5zBETcOCV1VntXvxt~DSbVyGeRkPv0BR56eq6bJCzoYasDLbyG2rxhnbXw9ftP-IcMkquifZ1DQu0jv0PX7fqm6A~KKOqCaJ7aZBHgSQDxiQ0Ax3Ew__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Effect of the expression of Cdc42N17 on the activation of PLCβ2. / (A) fMLFK-mediated IP3 formation. IP3 levels in differentiated Cdc42N17-expressing cells (black bars) and uninduced cells (gray bars) were determined at time intervals after stimulation with fMLFK (1 μM) using an inositol-1,4,5-trisphosphate [3H] radioreceptor assay kit. Error bars represent the standard deviation of 3 independent experiments performed in duplicate. (B, C) Kinetics of fMLFK-mediated intracellular calcium mobilization. Cells were loaded with Fura-2, and fMLFK-mediated calcium increase was assayed in the absence of extracellular calcium. Mean responses ± SE (n = 7) in differentiated Cdc42N17- (B) or Cdc42V12- (C) expressing cells grown in the absence (triangles) or in the presence (circles) of doxycycline are presented. (D) Effect of ionomycine on superoxide production in Cdc42N17-expressing HL-60 cells. Cells grown and differentiated in the absence of doxycycline were treated or not for 2 minutes with 1 μM ionomycine at 37°C before stimulation with fMLFK. Data are representative of 2 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/5/10.1182_blood-2001-12-0193/3/m_h81723025006.jpeg?Expires=1769415483&Signature=ACcBS3dx9zIYtmQpTb5rwhhpT-KNGADvlZjuUExjPWHvxZ3z98rsl2VsocTyVMqCZjFIfp-CRaUJvyQvs-ULv5jnm7JtbssJV4mprR5uAdTD2b2SAkjDX9ooNkRwlnkphe1Glt20JUw892iBFHtYXjmqz58jfc1fB8tVunWjBtE7Mdb0k5NLTV-zHdFO-ZCjTK7gN2ucmf1nfu0nCEcIFoWbM~IqsjhfuON7L6rplncufDZUjYnOwVMr73MtBwYSHsUNV8fRswP1xyHMWY0p8uAd8w1KTMliKnbdD1M4Jjvqu8b0U7yFiyK0b5OxRxTgezK4mJUzN0mTKrHKaIGXDA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal