Autoimmune hemolytic anemia results from an IgM antibody (Ab)–red cell interaction and is referred to as cold hemagglutinin disease (CHD). CHD is most commonly associated with infectious or lymphoproliferative diseases but may also be coupled with malignancies or autoimmune or immunodeficiency syndromes. Cold agglutinin–mediated hemolysis occurs at low temperatures, may be severe, and is notoriously difficult to treat. CHD patients with infections often have a short clinical course, whereas those with lymphoma require therapy. Therapeutic maneuvers successful in patients with warm Ab–associated autoimmune hemolytic anemia such as corticosteroids, intravenous immunoglobulin G (IgG), and splenectomy are usually ineffective in CHD.1,2 Novel treatment approaches of CHD are based on a better understanding of the immunologic abnormalities associated with this disorder, as well as the availability of sophisticated biotechnology products.1-5 Rituximab is a genetically engineered chimeric monoclonal antibody that targets the CD20 antigen on B cells currently used for the treatment of non-Hodgkin lymphoma. In vitro studies have demonstrated that the antibody binds human C1q and induces complement-dependent cytotoxicity, antibody-dependent, cell-mediated cytotoxicity, and apoptosis. In clinical trials of patients with malignant lymphoma, rituximab depleted circulating B cells with the first doses, leading to ongoing remissions and remaining effective for several months.3-5 In these diseases, as in autoimmune thrombocytopenia (ITP), rituximab presumably acts by elimination of CD20+ clonotypic precursor B cells and/or CD20+ plasma cells, applying to immunoglobulin-mediated diseases of B lymphocytes.6 Here we report on 2 patients with severe CHD, who were both successfully treated with the chimeric anti-CD20 monoclonal Ab rituximab. The patients' disease course and response to rituximab are displayed in Figure1. Both patients were seronegative for HIV-1 and HIV-2, and other infections were ruled out by appropriate diagnostic procedures.
Changes in biologic variables before and after rituximab therapy.
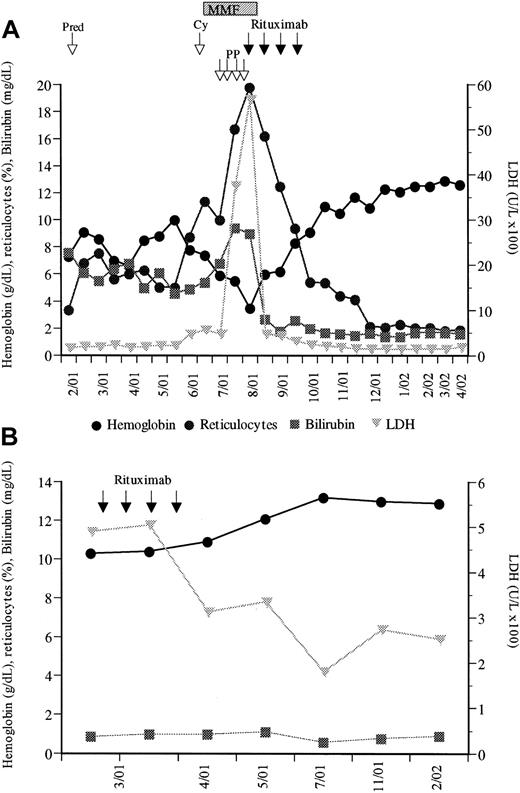
(A) First patient; (B) second patient. Pred indicates prednisone; PP, plasmapheresis; Cy, cyclophosphamide; and MMF, mycophenolate mofetil.
Changes in biologic variables before and after rituximab therapy.
(A) First patient; (B) second patient. Pred indicates prednisone; PP, plasmapheresis; Cy, cyclophosphamide; and MMF, mycophenolate mofetil.
The first patient, a 50-year-old female, was admitted to our hospital in February 2001 because of severe anemia (hemoglobin level, 7.3g/dL), jaundice, weakness, and dyspnea. Respiratory sounds were normal, and the spleen and liver were not enlarged. Laboratory data showed marked reticulocytosis (7.54%) and bilirubinemia (7.8 mg/dL), positive direct Coombs test, a high cold agglutinin titer of 1:256 (normal, 1:32), and low haptoglobin level (21 mg/dL). A bone marrow (BM) biopsy revealed a markedly hyperplastic erythropoiesis and agglutination of erythroid precursors. Immune phenotyping showed only polyclonal B cells and no cytogenetic abnormalities. Initially, prednisone (2 mg/kg/d) was administered and slowly tapered (Figure 1A). But 5 months later the direct Coombs test became positive again, with a concomitant increase in cold agglutinins (1:1024), impressive decrease of hemoglobin level to 5.9 g/dL, elevated reticulocyte percentage (10.1%), elevated lactate dehydrogenase (LDH) level (458 U/L), elevated bilirubin level (6.8 mg/dL), and haptoglobin level below 5 mg/dL. Cyclophosphamide (1000 mg/m2) was given but did not improve the clinical course. Therefore, mycophenolate mofetil (MMF; 1 g/d, then 2 g/d) was started, which has been described as beneficial in autoimmune hematolytic anemia.2Nevertheless, the above parameters did not improve; on the contrary, severe illness of the patient persisted with daily worsening of the clinical condition. Emergency plasmapheresis was carried out, resulting in only brief clearance of cold agglutinins. Since the effect of plasmapheresis did not lead to a marked improvement in the clinical course, rituximab was given (375 mg/m2, intravenously, weekly for 4 courses). Already with the first infusion, this led to a marked improvement of the patient's clinical condition and to a continuously rising hemoglobin level and marked decrease of reticulocyte percentage and bilirubin and LDH levels (Figure 1A). MMF was discontinued with the first rituximab infusion, and only the latter continued for 3 additional weeks. Tolerance to treatment was excellent, with no side effects. At last follow-up, 9 months after rituximab therapy, the hemoglobin level is 12.6 g/dL, bilirubin level, 1.6 mg/dL, and LDH level, 188 U/L, indicating continuing remission of CHD.
The second patient, a 60-year-old woman, presented to our hospital in March 2001. In 1990, laboratory studies had shown a positive direct Coombs test, and an idiopathic CHD was diagnosed. Initially, only mild signs of hemolysis with normal hemoglobin level did not require specific therapy. During the year 2000, however, pre-existing Raynaud phenomenon worsened and the hemoglobin level decreased to 10.0 g/dL, reticulocyte percentage was 5.7%, haptoglobin level was below 20 mg/dL, and LDH level was 583 U/L. Physical examination showed neither adenopathy nor liver or spleen enlargement. The direct Coombs test was positive, attributable to complement (C3d, 4+). Serum protein electropheresis revealed a monoclonal protein (IgM kappa). The BM aspirate and biopsy showed erythroid hyperplasia and lymphoplasmocytic infiltrates (10%), with abnormal B cells by immunophenotyping. Since this patient refused cyclophosphamide chemotherapy, she was treated with rituximab (4 × 375 mg/m2). No infusion-related side effects were observed. Five weeks and 11 months after the last rituximab infusion, the hemoglobin level had increased to, respectively, 12.1 g/dL and 12.9 g/dL, reticulocyte percentage had decreased to, respectively, 2.5% and 2.0%, and LDH level had decreased to, respectively, 335 U/L and 253 U/L (Figure 1B).
Our report demonstrates that both CHD patients were successfully treated with rituximab. No side effects occurred, and no additional immunosuppressive or chemotherapeutic agents had to be administered to maintain the response. The effect of the elimination of B cells by this anti-CD20 therapy is novel and has rarely been described. One small trial and only 2 case reports have reported on the use of rituximab in patients with autoimmune hemolytic anemia.7-9 The largest report comprises 6 children, showing an ongoing complete remission (CR) with rituximab, but with warm reactive Ab (IgG)–associated autoimmune hemolytic anemia, which is vastly better to treat than CHD.7 The 2 case reports describe a CR to rituximab each in a single patient with refractory CHD, including the diagnosis of an indolent clonal lymphoproliferative disease similar to our second patient.8,9 Nevertheless, these patients received a combination of rituximab plus cyclophosphamide and corticosteroids,8 or rituximab plus α-interferon,9 whereas in our patients previous nonresponsive medication was stopped with the beginning of rituximab in the first patient, and, even more striking and not reported to date, a complete remission was rapidly achieved with rituximab alone in the second patient. Moreover, in these previous reports8,9 the follow-up was shorter, the disease course milder,8,9 and recurrence of CHD arose 7 months later, with the patient dying from a stroke 14 months after the treatment initiation.9 In our 2 patients treatment response is ongoing 9 and 11 months after the start of rituximab, with no CHD recurrence either in the first patient, with no underlying primary lymphoma, or in the second patient, with no further treatment necessity for the lymphoplasmocytic lymphoma.
Of interest is, finally, that pure red cell aplasia (PRCA), as a rare complication in chronic lymphocytic leukemia (CLL), has recently been demonstrated in 2 B-cell CLL patients to also dramatically respond to rituximab,9 and its action in Waldenström macroglobulinemia, immune thrombocytopenic purpura (ITP), and cryoglobulinemia has clearly been verified.10-12 The mechanism of action of rituximab seems to be that of an immune modulation, presumably in Waldenström macroglobulinemia due to the elimination of either CD20+clonotypic precursor B cells or CD20+ plasma cells. The same rationale may well apply to other immunoglobulin-mediated diseases of B lymphocytes, such as cryoglobulinemia, ITP, and CHD. In summary, our report strongly suggests that rituximab can successfully control refractory CHD. With the increasing interest in Ab-based therapies, rituximab appears to be a promising alternative to the conventional medication in CHD. In view of its mild toxicity and the lack of effective alternative treatments, it should be strongly considered in severely affected patients who do not respond to standard therapy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal