Abstract
We analyzed the clinicobiological features and treatment outcome of a series of acute promyelocytic leukemias (APLs) occurring as a second tumor (APL-st's, n = 51) and compared these with a large group of de novo APL cases (n = 641), both observed by the Italian cooperative group GIMEMA. In the APL-st group, 37 patients had received radiotherapy and/or chemotherapy for their primary malignancy (PM), while 14 had been treated by surgery alone. Compared with de novo APL patients, APL-st patients were characterized by a predominance of females (P < .003), higher median age (P < .05), and worse performance status (P < .005). The median time elapsed between PM and APL-st was 36 months, with a longer latency for patients treated with surgery alone. No significant differences were found with regard to karyotypic lesions or type of promyelocytic leukemia/retinoic acid receptor α (PML/RARα) fusion in the 2 cohorts. A high prevalence of PMs of the reproductive system was observed among the female APL-st population (24 [71%] of 34 patients in this group had suffered from breast, uterine, or ovarian cancer). Thirty-one APL-st and 641 de novo APL patients received homogeneous APL therapy according to the all-trans retinoic acid (ATRA) and idarubicin regimen (the AIDA regimen). The complete remission (CR), 4-year event-free survival (EFS), and 4-year overall survival (OS) rates were 97% and 93%, 65% and 68%, and 85% and 78% in the APL-st and de novo APL groups, respectively. In spite of important clinical differences (older age and poorer performance status), the APL-st group responded as well as the de novo APL group to upfront ATRA plus chemotherapy, probably reflecting genetic similarity.
Introduction
In recent years, the incidence of acute leukemia occurring as a second tumor (AL-st) has increased as a consequence of the increasing number of long-term survivors of cancer. A specific etiopathogenetic role for AL-st development has been suggested for certain chemotherapeutic agents used for the treatment of the primary malignancy (PM). These agents are associated with the acquisition of molecular alterations leading to the development of AL-st with characteristic clinical and biological features.1-3 For example, consistent karyotypic changes are reported in AL-st patients receiving alkylating or topoisomerase II inhibitors for their PM.4-11 In addition, a significant fraction of AL-st's develop in patients who had previously received radiotherapy with or without chemotherapy. Together, these AL-st's are also referred to as therapy-related ALs. Finally, AL-st's may develop in individuals treated by surgery alone.12 In the latter instance, a cause-and-effect relationship with the PM is uncertain.
Compared with other acute myelocytic leukemia (AML) subtypes, APL shows specific genetic and clinical features, including a unique t(15;17) aberration leading to the formation of a PML/RARα hybrid13-16 and a striking response to differentiating agents such as all-trans retinoic acid (ATRA). The introduction of this agent in front-line association with chemotherapy has considerably improved the prognosis of the disease, and approximately 70% of patients receiving this treatment have been reported as long-term survivors.17-25
With regard to APL occurring as a second tumor (APL-st), some observations on single case reports or small series have suggested that these cases show the same molecular pathogenesis as de novo cases and a similarly good response to ATRA-containing therapy.26-29Such a favorable outcome is in marked contrast to other AL-st forms, whose prognosis is usually dismal and much poorer than that of their de novo counterparts.30 31 However, to the best of our knowledge no series of homogeneously treated APL-st's have been reported in the past few years, and therefore little is known about the prognostic outcome of APL-st patients receiving modern regimens including simultaneous ATRA and chemotherapy.
To better elucidate the clinicobiological features and outcome of APL-st's compared with newly diagnosed cases, we conducted a study on a large series of APL-st and de novo APL patients observed by the Gruppo Italiano per le Malattie Ematologiche dell'Adulto (GIMEMA).
Patients and methods
Patients
A total of 51 patients with APL-st were observed by GIMEMA during the period 1984-1998. Of these patients, 43 were recruited in multicenter national trials and 8 patients not eligible for intensive therapy were registered in the GIMEMA archive. This archive collects epidemiologic data on all new patients with acute leukemia observed in GIMEMA centers, regardless of their eligibility for clinical trials. Detailed information on demographics (race, age, sex), time and date of onset of primary cancer, related treatment (surgery, chemotherapy, radiotherapy), outcome of PM, and latency between PM and APL-st was obtained through the use of tailored questionnaires.
The diagnosis of APL-st was based on morphology and/or cytogenetics in patients diagnosed before 1993 and on reverse transcriptase–polymerase chain reaction (RT-PCR) positivity for the PML/RARα fusion in patients diagnosed thereafter. Karyotypic and/or molecular diagnostic confirmation with evidence of (15;17) and/or PML/RARα was available in 47 cases, while the diagnosis was based on morphology and immunophenotype in 4 cases.
Clinical and biologic features of patients with APL-st were compared with those of 641 patients with de novo APL enrolled in the ATRA and idarubicin (AIDA) clinical trial.17 All patients in this study had cytogenetic and/or molecular diagnostic confirmation as a mandatory prerequisite for study enrollment.
Treatment
Thirty-one patients with APL-st and 641 with de novo APL were homogeneously treated according to the AIDA protocol, consisting of a single-arm induction with simultaneous ATRA and idarubicin, followed by 3 consolidation courses and 4 randomization arms for maintenance (chemotherapy vs ATRA vs chemotherapy plus ATRA vs observation) as previously reported.17 Twelve patients with APL-st were treated with other chemotherapy regimens, including idarubicin and cytosine arabinoside (6 patients); daunorubicin alone (3 patients); idarubicin, cytosine arabinoside, and ATRA (2 patients); and daunorubicin and ATRA (1 patient). Finally, 8 APL-st patients in advanced age or with poor performance status were treated with ATRA alone.
Statistical analysis
Categorical and continuous variables were analyzed with the χ2 test and Student t test.
Event-free survival (EFS) was determined from diagnosis to the date of the first event (no complete remission [CR], relapse, death) or last follow-up. Overall survival (OS) was defined as the time from diagnosis to death or the date of last follow-up. Probabilities of survival were estimated by the Kaplan-Meier method.32
Results
APL secondary to a PM represented 4.8% of the APL GIMEMA population. This prevalence was obtained considering only the homogeneous population of patients enrolled in the AIDA protocol between October 1993 and June 1998 (31 of 641). The main clinical and biologic features of the total population of 51 APL-st patients are summarized in Table 1 and compared with the characteristics of patients with de novo APL. A significantly higher proportion of females, a higher median age, and a worse performance status were observed among patients with APL-st. No significant differences were found between the 2 cohorts in morphologic subsets, white cell and platelet counts, or type of PML/RARα fusion.
Clinical and laboratory features at presentation of APL secondary to a previous tumor (APL-st) and de novo APL
| . | APL-st . | de novo APL . | P . |
|---|---|---|---|
| Male patients, no. (%) | 17 of 51 (33.3) | 349 of 641 (55) | < .003 |
| Median age, y (range) | 57 (27-76) | 38.9 (1.4-74) | < .05 |
| PS (WHO) III-IV, no. (%) | 12/51 (24) | 24/641 (3.7) | < .005 |
| FAB M3v (hypogranular), no. (%) | 7/51 (14) | 71/565 (13) | NS |
| White blood cells × 109/L, median (range) | 1.4 (0.2-174) | 2.6 (0.3-140) | NS |
| Platelet count × 109/dL, median (range) | 26.5 (2.0-174) | 23.0 (1.0-117) | NS |
| Hemorrhage, no. (%) | 24/51 (47) | 405/641 (65) | < .02 |
| BCR1 | 21 (62) | 273 (56) | NS |
| BCR2 | 3 (9) | 30 (6) | NS |
| BCR3 | 10 (29) | 185 (38) | NS |
| . | APL-st . | de novo APL . | P . |
|---|---|---|---|
| Male patients, no. (%) | 17 of 51 (33.3) | 349 of 641 (55) | < .003 |
| Median age, y (range) | 57 (27-76) | 38.9 (1.4-74) | < .05 |
| PS (WHO) III-IV, no. (%) | 12/51 (24) | 24/641 (3.7) | < .005 |
| FAB M3v (hypogranular), no. (%) | 7/51 (14) | 71/565 (13) | NS |
| White blood cells × 109/L, median (range) | 1.4 (0.2-174) | 2.6 (0.3-140) | NS |
| Platelet count × 109/dL, median (range) | 26.5 (2.0-174) | 23.0 (1.0-117) | NS |
| Hemorrhage, no. (%) | 24/51 (47) | 405/641 (65) | < .02 |
| BCR1 | 21 (62) | 273 (56) | NS |
| BCR2 | 3 (9) | 30 (6) | NS |
| BCR3 | 10 (29) | 185 (38) | NS |
PS indicates performance status as defined by World Health Organization (WHO; information on PS was available for 533 of 641 patients with de novo APL); BCR1, 2, 3, proportional distribution of different types of PML/RARα fusion evaluated in 34 APL-st and 488 de novo APL patients; M3v, variant (hypogranular) cases.
The distinct types of PM in the group of APL-st patients are shown in Table 2. In the majority of cases the PM was breast cancer (15 patients); other frequent PMs were non-Hodgkin lymphoma (9 patients) and cancer of the uterus (7 patients). The majority of females with APL-st (71%) had had a cancer of the reproductive system (breast, uterus, ovary) as a PM, while the cumulative incidence of these tumors in the Italian female cancer patient population is 47.8%.33
Types of previous cancer in patients with APL-st's
| Type of cancer . | No. of patients . |
|---|---|
| Breast | 15 |
| Non-Hodgkin lymphoma | 9 |
| Uterus | 7 |
| Hodgkin disease | 3 |
| Bowel | 3 |
| Urinary bladder | 3 |
| Ovary | 2 |
| Thyroid, multiple myeloma, prostate, larynx, kidney, melanoma, central nervous system glioma, desmoid tumor, chondroma | 1 each |
| Type of cancer . | No. of patients . |
|---|---|
| Breast | 15 |
| Non-Hodgkin lymphoma | 9 |
| Uterus | 7 |
| Hodgkin disease | 3 |
| Bowel | 3 |
| Urinary bladder | 3 |
| Ovary | 2 |
| Thyroid, multiple myeloma, prostate, larynx, kidney, melanoma, central nervous system glioma, desmoid tumor, chondroma | 1 each |
Treatment of the PM consisted of surgery alone in 14 patients (27%), chemotherapy alone in 10 (20%), radiotherapy in 17 (33%), and chemotherapy combined with radiotherapy in 10 (20%). In the 20 patients treated with chemotherapy, with or without radiotherapy, the following agents were used either singly or in combination: alkylating agents in 13 patients, anthracyclines or anthracenedione in 11, antimetabolytes in 6, and epipodophyllotoxins in 5. The median time interval between PM and APL-st was 36 months (range, 8-366 months). As shown in Table 3, the latency varied according to the type of therapy received for the PM. In fact, the time interval between PM and APL-st diagnosis was longer for patients who received neither chemotherapy nor radiotherapy than for patients in other groups, although the difference did not reach statistical significance (P = .06 by the Kruskal-Wallis test).
Latency between primary tumor and APL-st development in relation to type of treatment of primary tumor
| Treatment . | No. of patients . | Latency, mo, median (range) . |
|---|---|---|
| Surgery only | 14 | 60 (8-358) |
| Chemotherapy | 10 | 29 (12-180) |
| Radiotherapy | 17 | 29 (10-366) |
| Chemotherapy + radiotherapy | 10 | 40 (21-324) |
| Overall | 51 | 36 (8-366) |
| Treatment . | No. of patients . | Latency, mo, median (range) . |
|---|---|---|
| Surgery only | 14 | 60 (8-358) |
| Chemotherapy | 10 | 29 (12-180) |
| Radiotherapy | 17 | 29 (10-366) |
| Chemotherapy + radiotherapy | 10 | 40 (21-324) |
| Overall | 51 | 36 (8-366) |
All patients received some type of treatment for APL-st (Table4). In the whole series, 43 (84%) of 51 patients attained hematologic CR. Of the 8 remaining patients, 1, treated with ATRA alone, died of progressive disease, whereas 7 patients (14%) died during induction of hemorrhage (4 patients), sepsis (2 patients), or myocardial infarction (1 patient).
APL as a second tumor: treatment results according to type of therapy
| Treatment . | No. of patients . | Complete remission, no. (%) . | Induction death, no. (%) . | Resistant disease, no. (%) . |
|---|---|---|---|---|
| AIDA | 31 | 30 (97) | 1 (3) | — |
| ATRA | 8 | 5 (62) | 2 (25) | 1 (12) |
| Other4-150 | 12 | 8 (67) | 4 (33) | — |
| Total | 51 | 43 (84) | 7 (14) | 1 (2) |
| Treatment . | No. of patients . | Complete remission, no. (%) . | Induction death, no. (%) . | Resistant disease, no. (%) . |
|---|---|---|---|---|
| AIDA | 31 | 30 (97) | 1 (3) | — |
| ATRA | 8 | 5 (62) | 2 (25) | 1 (12) |
| Other4-150 | 12 | 8 (67) | 4 (33) | — |
| Total | 51 | 43 (84) | 7 (14) | 1 (2) |
Other treatments include anthracycline or anthracenedione with or without cytosine-arabinoside with or without ATRA.
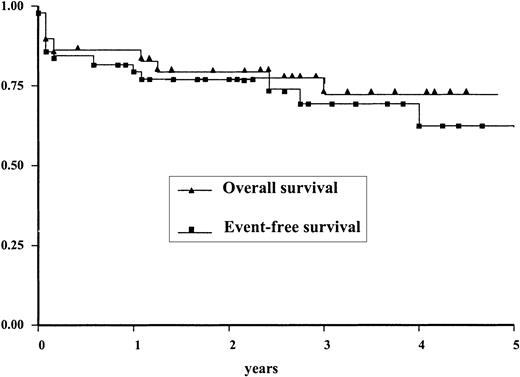
The results of treatment in therapy-related APL-st (ie, APL-st in patients who received chemotherapy and/or radiotherapy for their PM) were not different from those obtained in patients who had been treated with surgery alone: CR was obtained in 33 of 37 patients in the first group and in 10 of 14 patients in the second group (Fisher exact test = 0.1). No statistically significant differences were found between EFS and OS rates in the 2 cohorts (not shown). The EFS and OS curves of the entire group of patients with APL-st are shown in Figure1. The 4-year EFS and OS rates are, respectively, 61% and 72%.
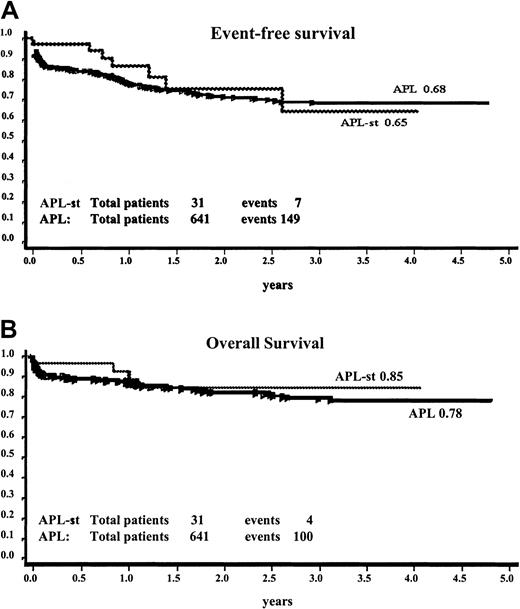
The comparison of treatment outcome in the 31 patients with APL-st and 641 patients with de novo APL homogeneously treated according to the AIDA protocol is shown in Figure 2. The 4-year EFS was 65% ± 13% and 68% ± 2.3% and the OS was 85% ± 7% and 78% ± 2.1% for the APL-st and de novo APL groups, respectively.
Comparison of EFS and OS of 31 patients with APL-st's and 641 patients with de novo APL among a population of homogeneously treated patients (AIDA protocol).
Panel A, EFS; panel B, OS.
Comparison of EFS and OS of 31 patients with APL-st's and 641 patients with de novo APL among a population of homogeneously treated patients (AIDA protocol).
Panel A, EFS; panel B, OS.
Discussion
The proportion of APL-st in the whole APL population reported here (4.8%) is similar to that described in a French study,26whereas other authors have found a higher incidence of APL-st (12%) in a lower total number of APL patients.27 The prevalence of APL-st within all APLs detected in our study is comparable to that described by GIMEMA for AML-st within all AML subtypes (6.0%), and significantly higher with respect to secondary acute lymphoid leukemia (2.3%).34
Compared with de novo APL patients, patients with APL-st had a higher median age and a remarkable predominance of females. The observation of older age also relates to other AL-st's35 and could simply reflect the longer life span required to develop 2 tumors, as well as prolonged risk exposure. With respect to the prevalence of females (67% of the APL-st patients were female), this finding was also reported in the 2 largest series published previously.26,27 Interestingly, such female predominance is observed neither in de novo APLs (see “Results,” Avvisati et al,36 and Pulsoni et al37) nor in AML-st's, thus representing an intriguing peculiarity of APL-st. In this respect, it is worth noting the high incidence of tumors of the female reproductive system among the PMs in our study. In fact, PMs of the cervix, uterus, ovary, or breast were recorded in 71% of females in our study and in 85% of females in the French series.26Although we are unable at present to provide a biologic explanation for this association, this higher prevalence of PMs of the female reproductive system may account for the observed higher incidence of APL-st's in females.
With respect to biologic characteristics such as genetic abnormalities and type of PML/RARα junction, APL-st patients appear remarkably similar to de novo APL patients, in sharp contrast to AML-st patients, whose karyotypic aberrations are usually different from those observed in de novo AML patients.
As to the pathogenetic role of previous treatments, the importance of chemotherapy including topoisomerase II inhibitors has been reported by several authors.6-12 These drugs had been employed for the treatment of PMs in 11 (22%) of our patients. A high proportion of patients in our study (53%) had previously been treated with radiotherapy (alone or combined with chemotherapy) for their PMs. A similar proportion of such patients has been reported in other series of secondary APL: 13 of 16 in the French study26 and 8 of 14 in the MD Anderson study.27 In a review of 51 patients with therapy-related APL, radiotherapy had been employed in 25 cases, and in 10 of them it was not associated with chemotherapy.28 A role of previous radiotherapy as a risk factor for APL-st development cannot be definitely demonstrated by these data, which could simply reflect the frequent employment of radiotherapy in cancer treatment.
A significant proportion of APL-st's cannot be labeled “therapy related.” In fact, 26% of patients in our study, as well as a similar proportion in the MD Anderson series,27 did not receive any chemotherapy or radiotherapy for the treatment of their PMs. Similarly, in the GIMEMA experience 48% of ALL-st's38 and 32% of AML-st's were not therapy related.13 In this proportion of patients the occurrence of 2 tumors may be the result of simple chance association, although some evidence supports the hypothesis of genetic predisposing factors to multiple tumors.39 40
AML-st's are usually characterized by very poor prognosis. Conversely, the response to treatment of APL-st's, therapy-related or not, is comparable to the response to treatment of de novo APL. In spite of being characterized by significantly more advanced age and worse performance status, the APL-st group in the present study responded equally well to therapy as did patients in the de novo APL group. In particular, the outcomes of the former group after treatment with the AIDA protocol appeared quite favorable, with a high proportion of potentially cured patients, as in the de novo group. Among the factors that may account for this favorable response, we highlight the following: (1) similar molecular pathogenesis and genetic lesions seem to be features of both APL-st and de novo APL, with production of a hybrid PML/RARα protein which represents the specific target of ATRA; and (2) the chemosensitivity of APL-st seems not to be compromised by previous chemotherapy and radiotherapy and may be related to low expression of multidrug resistance–related proteins in APL.41 42 Finally, our results further highlight the absolute peculiarity of APL among AMLs and underline the necessity of treating APL-st's similarly to the de novo forms.
Prepublished online as Blood First Edition Paper, DOI 10.1182/blood-2001-12-0312.
Supported by Associazione Italiana contro le Leucemie, Ministero dell'Università e della Ricerca Scientifica e Tecnologica, and Ministero della Salute.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Alessandro Pulsoni, Hematology, La Sapienza University, Via Benevento 6, 00161 Rome, Italy; e-mail:pulsoni@bce.uniroma1.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal