Abstract
Aggregation of high-affinity IgE receptor FcεRI induces sequential activation of nonreceptor-type protein-tyrosine kinases and subsequent tyrosine phosphorylation of cellular proteins, leading to degranulation in mast cells. A hematopoietic cell–specific adaptor protein, 3BP2, that was originally identified as an Abl SH3-binding protein was rapidly tyrosine phosphorylated by the aggregation of FcεRI on rat basophilic leukemia RBL-2H3 cells. Tyrosine phosphorylation of 3BP2 did not depend on calcium influx from external sources. To examine the role of 3BP2 in mast cells, we overexpressed the SH2 domain of 3BP2 in the RBL-2H3 cells. Overexpression of 3BP2-SH2 domain resulted in a suppression of antigen-induced degranulation as assessed by β-hexosaminidase release. Even though overall tyrosine phosphorylation of cellular protein was not altered, antigen-mediated tyrosine phosphorylation of phospholipase C-γ (PLC-γ) and calcium mobilization were significantly suppressed in the cells overexpressing the 3BP2-SH2 domain. Furthermore, antigen stimulation induced the association of 3BP2-SH2 domain with LAT and other signaling molecule complexes in the RBL-2H3 cells. FcεRI-mediated phosphorylation of JNK and ERK was not affected by the overexpression of 3BP2-SH2 domain. These data indicate that 3BP2 functions to positively regulate the FcεRI-mediated tyrosine phosphorylation of PLC-γ and thereby the signals leading to degranulation.
Introduction
Aggregation of the high-affinity IgE receptor (FcεRI) on mast cells mediates the allergic response by the release of inflammatory mediators by degranulation and production of cytokines and arachidonic acid metabolites.1 FcεRI signals by activating nonreceptor-family protein-tyrosine kinases (PTKs) Lyn, Syk, and Btk. On aggregation of FcεRIα subunits, the Src family PTK Lyn phosphorylates the associated FcεRIβ and γ subunits on tyrosine residues in the immunoreceptor tyrosine-based activating motif. This leads to the recruitment of Syk to the plasma membrane, which is essential for FcεRI-mediated degranulation by phosphorylating downstream molecules.2 Similarly, Btk is membrane-targeted by phosphatidylinositol-3,4,5-triphosphate to its pleckstrin-homology (PH) domain. Two types of kinase Syk and Btk contribute tyrosine phosphorylation and activation of phospholipase C-γ (PLC-γ) and calcium mobilization.1 3
Several adaptors and scaffolds lacking enzymatic and transcriptional domains have been identified in the immune receptor–mediated signaling pathway. Proximal adaptor proteins, which are the substrates of PTKs, have important roles in early FcεRI signaling. They have multiple motifs and domains that allow their binding to the other molecules. Activated PTKs phosphorylate the substrate adaptor proteins to assemble the cytoplasmic signaling molecules in the juxtamembrane region for effective interaction, presumably in glycolipid enriched microdomains (GEMs). Mice deficient in the adaptor protein linker for activation of T cells (LAT) SLP-76 (SH2-containing leukocyte phosphoprotein of 76 kDa) or Gab2 (Grb2-associated binder-2) were resistant to IgE-mediated passive systemic anaphylaxis.4-6 Analysis of bone marrow–derived mast cells (BMMCs) from mice revealed their essential roles in FcεRI-mediated degranulation and/or cytokine production.
3BP2 was originally isolated as an Abl SH3-binding protein of unknown function.7 In addition to the proline-rich region that mediates SH3 binding, it has PH and Src homology 2 (SH2) domains. 3BP2 was also identified as one of the Syk kinase-interacting proteins by a yeast 2-hybrid screen.8 C-terminal SH2 domain of 3BP2 is required to interact with Syk in yeast. Expression of 3BP2 has been reported in T, B, natural killer (NK), and monocytic cell lines. Transient overexpression of 3BP2 in T cells induces transcriptional activation of the interleukin 2 (IL-2) gene. In NK cells, overexpression of 3BP2 by vaccinia virus enhances cytotoxicity.9 Prior to these findings, a specific motif recognized by 3BP2-SH2 domain was examined by in vitro degenerated peptide library screening.10 The optimal sequence for the 3BP2-SH2 domain is Tyr-Glu-Asn (YEN) motif.
The present experiments demonstrated the expression of 3BP2 in mast cells and the functional importance of 3BP2-SH2 domain in FcεRI-mediated signal transduction. Overexpression of 3BP2-SH2 domains in the rat basophilic leukemia RBL-2H3 cells resulted in a dramatic suppression of the FcεRI-mediated tyrosine phosphorylation of PLC-γ and degranulation, suggesting that 3BP2-SH2 inhibited the function of endogenous 3BP2, which positively regulates those signaling. FcεRI-mediated activation of mitogen-activated protein kinases (MAPKs) was not affected by the overexpression of 3BP2-SH2 domain. These findings raised the possibility that the adaptor molecule 3BP2 might be a new target for discovery of a drug to treat allergic diseases.
Materials and methods
Materials and antibodies
Protein A-agarose, mouse monoclonal anti–dinitrophenyl IgE (anti–DNP IgE, clone SPE-7), and thapsigargin were purchased from Sigma (St Louis, MO). Anti–phosphotyrosine monoclonal antibody (mAb) 4G10, anti–PLC-γ1 mAb, and anti-LAT antibody were obtained from Upstate Biotechnology (Lake Placid, NY). Anti–hemagglutinin epitope (HA) (Y-11), anti-3BP2 (N-18 and C-19) and anti–PLC-γ2 antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-FcεRIβ mAb was kindly provided by Dr Reuben P. Siraganian (National Institutes of Health, Bethesda, MD). Antigen 2,4-dinitrophenylated bovine serum albumin (DNP-BSA, 30 mol of DNP/1 mol of BSA) was from LSL (Tokyo, Japan).11 The HA-tagged expression constructs pMT3-HA-3BP2 and pMT3-HA-3BP2-SH2 (454-559) cDNA were kindly provided by Dr Amnon Altman (La Jolla Institute, La Jolla, CA).8 Point mutation of Arg486 of pMT3-HA-3BP2 cDNA to Lys (R486K) was prepared by the Site-Directed Mutagenesis kit from Stratagene (La Jolla, CA).
Cell culture and transfections
Rat basophilic leukemia RBL-2H3 cells were maintained as monolayer cultures in Dulbecco modified Eagle medium (DMEM) with 2mM L-glutamine, 100 U/mL of penicillin, and 10% heat-inactivated fetal calf serum. For stable transfection, 20 μg of linearized expression constructs and 2 μg of pSV2-neo vector were cotransfected into 5×106 RBL-2H3 cells by electroporation (950 microfarads [μF], 310 V) as described previously.12 13 Stably transfected cell lines were selected with 0.4 mg/mL of active G418 (Life Technologies, Gaithersburg, MD). Cell lines were screened by level of protein expression by immunoblotting of total cell lysates with anti-HA antibody and anti-FcεRIβ mAb as an internal control. Two positive cloned lines transfected with different kinds of cDNA were selected for further analysis, in which the level of 3BP2 expression was the highest among the clones. For control cells, linearized empty pMT3 vector and pSV2-neo vector were cotransfected by electroporation.
Cell activation, immunoprecipitation, and immunoblotting
The cell monolayers cultured overnight with anti-DNP IgE (1:5000) were washed once with Tyrode-Hepes buffer (10 mM Hepes, pH 7.4, 127 mM NaCl, 4 mM KCl, 0.5 mM KH2PO4, 1 mM CaCl2, 0.6 mM MgCl2, 10 mM LiCl2, 5.6 mM glucose, and 0.1% BSA) and then stimulated with 10 ng/mL of antigen DNP-BSA in the same buffer for the indicated times. For the immunoprecipitation studies, cells were washed with ice-cold phosphate-buffered saline (PBS) twice and solubilized in lysis buffer (1% Triton X-100, 50 mM Tris, pH 7.4, 150 mM NaCl, 10 mM EDTA (ethylenediaminetetraacetic acid), 100 mM NaF, 1 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride [PMSF], and 2 μg/mL aprotinin). In some experiments, cells were solubilized in the denatured buffer (lysis buffer containing 1% sodium deoxycholate and 0.1% sodium dodecyl sulfate [SDS]). Cell lysates were precleared by centrifugation and then incubated with the indicated primary antibodies prebound to protein A–agarose. After rotation for 1 hour at 4°C, the beads were washed 4 times with lysis buffer. Immunoprecipitated proteins were eluted by heat treatment at 100°C for 5 minutes with 2 × sampling buffer. For preparation of total cell lysates, monolayers were rinsed with PBS and lysed by direct addition of 2 × sampling buffer. Immunoprecipitates and total cell lysates were separated by SDS–polyacrylamide gel electrophoresis (SDS-PAGE) and electrotransferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA). The blots were probed with the indicated antibodies. In all blots, proteins were visualized by enhanced chemiluminescence (PerkinElmer Life Sciences, Boston, MA).12 13
Analysis of β-hexosaminidase release
Degranulation of the transfected cells was determined by measurement of β-hexosaminidase release. Cells (105) were seeded in a 24-well plate and cultured overnight with or without anti-DNP IgE. The cell monolayers were washed once with Tyrode-Hepes buffer and then stimulated with different concentrations of the antigen DNP-BSA, or thapsigargin calcium ionophore A23187 in the same buffer. After incubation for 1 hour at 37°C, the medium was recovered for analysis of β-hexosaminidase release. Total cell lysates were obtained by addition of 1% NP-40 in the same medium. The medium and total cell lysates were incubated with 1.3 mg/mL ofP-nitrophenyl-N-acetyl-β-d-glucopyranoside (Nacalai, Osaka, Japan) in 0.1 M sodium citrate buffer (pH 4.5) for 40 minutes at 37°C. The reaction was terminated by the addition of 0.2 M glycine buffer (pH 10.7). The release of the product 4-P-nitrophenol was monitored by absorbance at 405 nm. The released β-hexosaminidase activities were expressed as a percentage of the total.
Measurement of [Ca++]i
Intracellular free calcium concentration ([Ca++]i) was measured by means of fluorescent indicator fura-2 (Dijindo, Kumamoto, Japan). The cell monolayers of the different cell lines sensitized with anti-DNP IgE were detached from the tissue culture dish with 5 mM EDTA, washed twice with Tyrode-Hepes buffer, then loaded with 3 μM of fura-2 for 30 minutes at 37°C. After incubation, cells were washed twice and resuspended in Tyrode-Hepes buffer at 106cells/mL. Cells were stimulated by either 10 ng/mL of antigen DNP-BSA or 1 μM thapsigargin. Fura-2 fluorescence was monitored with a fluorescence spectrophotometer (Hitachi F-4500, Tokyo, Japan) by the excitation wavelength at 340 and 380 nm and the emission wavelength at 510 nm.
Results
Aggregation of FcεRI-induced tyrosine phosphorylation of 3BP2 in RBL-2H3 cells
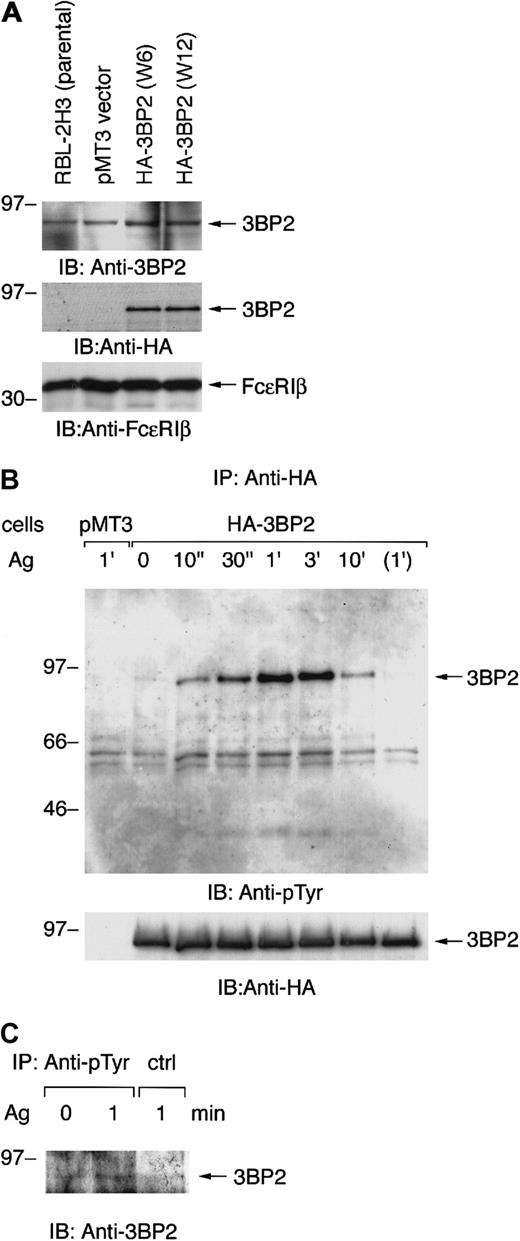
Our preliminary experiments showed that cotransfection of nonreceptor-type PTKs with 3BP2 resulted in tyrosine phosphorylation of 3BP2. Among these PTKs, Syk predominantly phosphorylated 3BP2 in COS cells (data not shown). Syk is known to play an essential role in early signaling from FcεRI in mast cells. Therefore, we first tested the expression of 3BP2 in the mast cell line RBL-2H3. Expression of 3BP2 in RBL-2H3 cells was identified by immunoblotting of total cell lysate with anti-3BP2 antibody (Figure1A, top panel). Since 2 kinds of anti-3BP2 antibodies (N-18 and C-19) were not working for immunoprecipitation, we generated the stable cell lines expressing epitope-tagged full-length 3BP2. The cDNA of HA-tagged 3BP2 was transfected into RBL-2H3 cells by electroporation. Stable clones were screened by immunoblotting with anti-HA and anti-FcεRIβ antibodies. Two positive cloned lines (W6 and W12) were selected for further analysis in which the levels of 3BP2 expression were highest among all these clones (Figure 1A). Densitometric analysis indicated that transfection of 3BP2 resulted in a 2-fold increase over endogenous protein, which does not appear to be dramatic (data not shown).
Aggregation of FcεRI induces tyrosine phosphorylation of 3BP2 in RBL-2H3 cells.
(A) Generation of cell lines expressing HA-tagged 3BP2 wild type. The RBL-2H3 cells were stably transfected with either empty pMT3 vector or pMT3-HA-3BP2, together with pSV2-neo, by electroporation (950 μF, 310 V). Clones resistant to G418 were selected and screened by level of protein expression. Two positive cloned lines with the highest expression were chosen for further analysis. Total cell lysates of the parental RBL-2H3 and transfected cells were analyzed by immunoblotting (IB) with anti-3BP2, anti-HA, and anti-FcεRIβ antibodies used as an internal control. (B) Analysis of tyrosine phosphorylation of 3BP2. Cell lines expressing HA-3BP2 and vector-transfected control cells (pMT3) were cultured overnight with anti-DNP IgE and then stimulated with 10 ng/mL of antigen (Ag) DNP-BSA for the indicated times or incubated without antigen for 1 minute. Tyrosine phosphorylation of 3BP2 was analyzed by immunoprecipitation (IP). Cells were lysed in 1% Triton lysis buffer and cell lysates were immunoprecipitated with anti-HA antibody. Immunoprecipitates were separated by 10% SDS-PAGE and analyzed by immunoblotting with anti-pTyr mAb. The membrane was stripped and reprobed with anti-HA antibody. Similar results were obtained when the other lines were examined. (C) Tyrosine phosphorylation of endogenous 3BP2. RBL-2H3 cells sensitized with anti-DNP IgE were either unstimulated or stimulated with antigen for 1 minute. Cells were lysed in the denature buffer and cell lysates were immunoprecipitated with anti-pTyr mAb or control IgG. Immunoprecipitates were analyzed by immunoblotting with anti-3BP2 antibody. Molecular size markers are indicated at the left in kilodaltons.
Aggregation of FcεRI induces tyrosine phosphorylation of 3BP2 in RBL-2H3 cells.
(A) Generation of cell lines expressing HA-tagged 3BP2 wild type. The RBL-2H3 cells were stably transfected with either empty pMT3 vector or pMT3-HA-3BP2, together with pSV2-neo, by electroporation (950 μF, 310 V). Clones resistant to G418 were selected and screened by level of protein expression. Two positive cloned lines with the highest expression were chosen for further analysis. Total cell lysates of the parental RBL-2H3 and transfected cells were analyzed by immunoblotting (IB) with anti-3BP2, anti-HA, and anti-FcεRIβ antibodies used as an internal control. (B) Analysis of tyrosine phosphorylation of 3BP2. Cell lines expressing HA-3BP2 and vector-transfected control cells (pMT3) were cultured overnight with anti-DNP IgE and then stimulated with 10 ng/mL of antigen (Ag) DNP-BSA for the indicated times or incubated without antigen for 1 minute. Tyrosine phosphorylation of 3BP2 was analyzed by immunoprecipitation (IP). Cells were lysed in 1% Triton lysis buffer and cell lysates were immunoprecipitated with anti-HA antibody. Immunoprecipitates were separated by 10% SDS-PAGE and analyzed by immunoblotting with anti-pTyr mAb. The membrane was stripped and reprobed with anti-HA antibody. Similar results were obtained when the other lines were examined. (C) Tyrosine phosphorylation of endogenous 3BP2. RBL-2H3 cells sensitized with anti-DNP IgE were either unstimulated or stimulated with antigen for 1 minute. Cells were lysed in the denature buffer and cell lysates were immunoprecipitated with anti-pTyr mAb or control IgG. Immunoprecipitates were analyzed by immunoblotting with anti-3BP2 antibody. Molecular size markers are indicated at the left in kilodaltons.
Aggregation of FcεRI induced rapid activation of nonreceptor-type PTKs in RBL-2H3 cells. To examine whether 3BP2 is involved in the FcεRI-mediated signaling pathway, we examined tyrosine phosphorylation of 3BP2 by using these cells. Cells transfected with either HA-3BP2 or vector alone were primed with anti-DNP IgE and then stimulated with antigen DNP-BSA. Cell lysates were immunoprecipitated with anti-HA antibody and the immunoprecipitates were analyzed by immunoblotting with anti-pTyr and anti-HA antibodies (Figure 1B). Aggregation of FcεRI induced a rapid tyrosine phosphorylation of 3BP2 at peak time 1 minute. Subsequently, 3BP2 was dephosphorylated to the basal level by 10 minutes after stimulation. Furthermore, endogenous 3BP2 was precipitated with anti-pTyr mAb from antigen-stimulated cell lysate demonstrating that endogenous 3BP2 is tyrosine phosphorylated by antigen stimulation in RBL-2H3 cells (Figure 1C). Therefore, these results indicate that 3BP2 is regulated by PTKs and protein-tyrosine phosphatases (PTPases) that are downstream of FcεRI in RBL-2H3 cells.
FcεRI-mediated tyrosine phosphorylation of 3BP2 does not require calcium influx
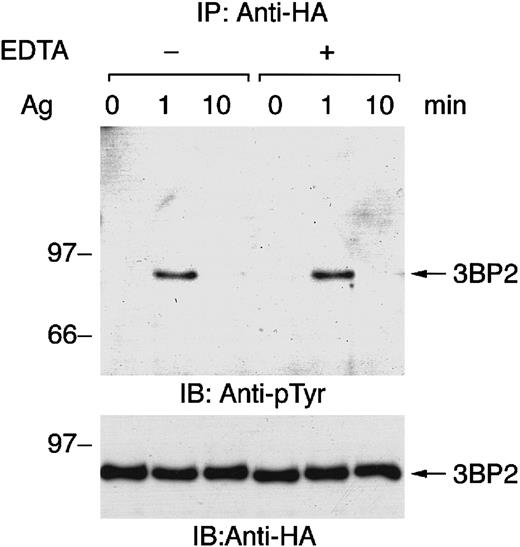
Genetic studies have revealed that Syk is essential for FcεRI-mediated tyrosine phosphorylation of PLC-γ, calcium mobilization, and degranulation in mast cells.14 15Elevation of intracellular free calcium causes the activation of some PTKs, such as Pyk2, but not others. Therefore, we tested whether FcεRI-mediated tyrosine phosphorylation of 3BP2 is independent of calcium influx by depleting free calcium with EDTA pretreatment followed by receptor stimulation (Figure2). As shown, FcεRI-mediated tyrosine phosphorylation and dephosphorylation of 3BP2 were similar in cells activated in complete medium and cells activated in calcium-free medium. Thus, antigen stimulation–induced tyrosine phosphorylation and dephosphorylation of 3BP2 are independent of the presence of extracellular free calcium.
FcεRI-mediated tyrosine phosphorylation of 3BP2 does not depend on calcium influx from external sources.
Cell lines expressing HA-3BP2 sensitized with anti-DNP IgE were stimulated with 10 ng/mL of antigen DNP-BSA for the indicated times without or with the presence of 0.5 mM EDTA in medium. Cell lysates were immunoprecipitated with anti-HA antibody, and then immunoprecipitates were separated by 10% SDS-PAGE and analyzed by immunoblotting with anti-pTyr and anti-HA antibodies. Similar results were obtained when the other lines were examined.
FcεRI-mediated tyrosine phosphorylation of 3BP2 does not depend on calcium influx from external sources.
Cell lines expressing HA-3BP2 sensitized with anti-DNP IgE were stimulated with 10 ng/mL of antigen DNP-BSA for the indicated times without or with the presence of 0.5 mM EDTA in medium. Cell lysates were immunoprecipitated with anti-HA antibody, and then immunoprecipitates were separated by 10% SDS-PAGE and analyzed by immunoblotting with anti-pTyr and anti-HA antibodies. Similar results were obtained when the other lines were examined.
Overexpression of 3BP2-SH2 domain suppresses FcεRI-mediated β-hexosaminidase release
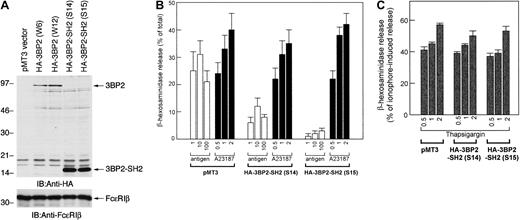
To examine the function of 3BP2 in FcεRI-mediated signaling in mast cells, we transfected the truncated mutant form of 3BP2 that had a dominant negative effect. The cDNA of HA-3BP2-SH2 domain was transfected into RBL-2H3 cells to generate stable clones. After the selection and screening of the clones expressing mutant protein, 2 positive clones (S14 and S15) that eventually overexpressed 3BP2-SH2 domain were selected for further analysis (Figure3A).
Overexpression of 3BP2-SH2 domain suppresses FcεRI-mediated degranulation in RBL-2H3 cells.
(A) Generation of cell lines expressing the HA-tagged SH2 domain of 3BP2. The RBL-2H3 cells were stably transfected with pMT3-HA-3BP2-SH2 (454-559) by electroporation. G418-resistant clones were screened by immunoblotting and 2 positive cloned lines with highest expression were selected for further analysis. Total cell lysates of the transfected cell lines were analyzed with anti-HA and anti-FcεRIβ antibodies used as an internal control. (B) Analysis of FcεRI-mediated β-hexosaminidase release. Control cells transfected with pMT3 vector alone and cells overexpressing 3BP2-SH2 domain were cultured overnight with anti-DNP IgE and then stimulated with the indicated concentrations of the antigen DNP-BSA (ng/mL) or with the calcium ionophore A23187 (μM). The antigen- or A23187-induced releases are normalized by expression as a percentage of the total β-hexosaminidase activity. (C) Analysis of thapsigargin-induced β-hexosaminidase release. Cells were stimulated with the indicated concentrations of thapsigargin (μM); releases are normalized by expression as a percentage of β-hexosaminidase activity induced by 1 μM A23187. The results are the mean values ± SE from 3 independent experiments.
Overexpression of 3BP2-SH2 domain suppresses FcεRI-mediated degranulation in RBL-2H3 cells.
(A) Generation of cell lines expressing the HA-tagged SH2 domain of 3BP2. The RBL-2H3 cells were stably transfected with pMT3-HA-3BP2-SH2 (454-559) by electroporation. G418-resistant clones were screened by immunoblotting and 2 positive cloned lines with highest expression were selected for further analysis. Total cell lysates of the transfected cell lines were analyzed with anti-HA and anti-FcεRIβ antibodies used as an internal control. (B) Analysis of FcεRI-mediated β-hexosaminidase release. Control cells transfected with pMT3 vector alone and cells overexpressing 3BP2-SH2 domain were cultured overnight with anti-DNP IgE and then stimulated with the indicated concentrations of the antigen DNP-BSA (ng/mL) or with the calcium ionophore A23187 (μM). The antigen- or A23187-induced releases are normalized by expression as a percentage of the total β-hexosaminidase activity. (C) Analysis of thapsigargin-induced β-hexosaminidase release. Cells were stimulated with the indicated concentrations of thapsigargin (μM); releases are normalized by expression as a percentage of β-hexosaminidase activity induced by 1 μM A23187. The results are the mean values ± SE from 3 independent experiments.
Using these stable clones, we analyzed FcεRI-mediated degranulation by measuring β-hexosaminidase release (Figure 3B). The control cells transfected with empty vector and 3BP2-SH2 domain were stimulated either with FcεRI aggregation, the calcium ionophore A23187, or thapsigargin. Antigen-induced β-hexosaminidase release in control cells was similar to that in parental RBL-2H3 cells (data not shown). Antigen-induced β-hexosaminidase release in the cells overexpressing 3BP2-SH2 domain was dramatically suppressed compared with that in control cells. On the other hand, β-hexosaminidase release by stimulation with A23187 or thapsigargin was similar in 3 cell lines. Thus, these results demonstrate that overexpression of 3BP2-SH2 domain results in a suppression of FcεRI-mediated degranulation.
FcεRI-induced tyrosine phosphorylation of cellular proteins is not affected by the overexpression of 3BP2-SH2 domain
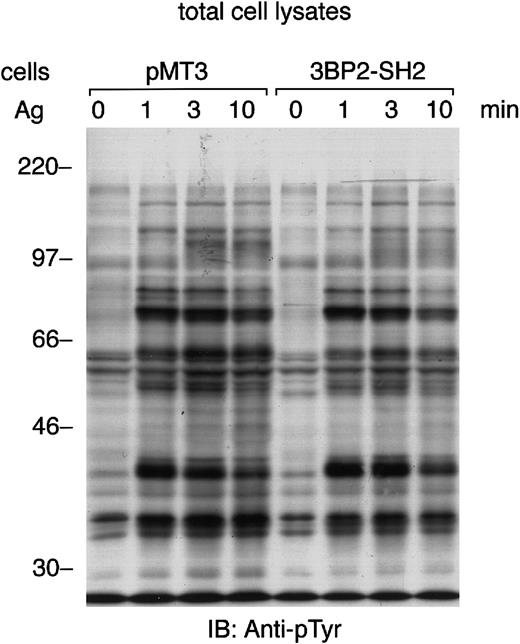
3BP2 was originally identified as an Syk-kinase interacting protein in yeast.8 Aggregation of FcεRI in mast cells lacking the expression of Syk results in minimal tyrosine phosphorylation of cellular proteins.14 Therefore, we tested the FcεRI-mediated tyrosine phosphorylation of cellular proteins in the cells overexpressing 3BP2-SH2 domain (Figure4). The pattern of protein-tyrosine phosphorylation induced by the aggregation of FcεRI was almost the same as in the control cells. Immunoprecipitation studies indicated that tyrosine phosphorylation of FcεRI, Syk, and LAT was not affected by the overexpression of 3BP2-SH2 domain in RBL-2H3 cells (data not shown). Therefore, this result suggests that overexpression of 3BP2-SH2 domain does not affect the FcεRI-mediated tyrosine phosphorylation of most of the cellular proteins, including LAT adaptor protein. The overexpression of 3BP2-SH2 domain has little effect on many of the tyrosine kinase substrates seen in cell lysates.
FcεRI-induced tyrosine phosphorylation of cellular proteins is not affected by the overexpression of 3BP2-SH2 domain.
Control cells (pMT3) and cells overexpressing SH2 domain of 3BP2 were primed with anti-DNP IgE and stimulated with 10 ng/mL antigen DNP-BSA for the indicated times. Total cell lysates (105 cell equivalents per lane) were separated by 10% SDS-PAGE and analyzed by immunoblotting with anti-pTyr mAb. Similar results were obtained when the other lines were examined.
FcεRI-induced tyrosine phosphorylation of cellular proteins is not affected by the overexpression of 3BP2-SH2 domain.
Control cells (pMT3) and cells overexpressing SH2 domain of 3BP2 were primed with anti-DNP IgE and stimulated with 10 ng/mL antigen DNP-BSA for the indicated times. Total cell lysates (105 cell equivalents per lane) were separated by 10% SDS-PAGE and analyzed by immunoblotting with anti-pTyr mAb. Similar results were obtained when the other lines were examined.
Positive role of 3BP2 in FcεRI-mediated tyrosine phosphorylation of PLC-γ
The elevation of intracellular calcium is critical for FcεRI-mediated degranulation. PLC-γ catalyzes the hydrolysis of phospholipids with the formation of diacylglycerol and inositol 1,4,5-triphosphate that is required for calcium mobilization. Transient overexpression of 3BP2 in T cells enhanced transcriptional activities of IL-2 promoter and its nuclear factor of activated T cell (NFAT) and activator protein 1 (AP-1) elements. This indicated that 3BP2 regulates calcium mobilization in T cells.8Since FcεRI-mediated tyrosine phosphorylation of LAT was not affected by the overexpression of 3BP2-SH2 domain (Figure 4), we tested antigen-induced tyrosine phosphorylation of PLC-γ. FcεRI-induced tyrosine phosphorylation of PLC-γ1 and PLC-γ2 was suppressed in the cells overexpressing 3BP2-SH2 domain (Figure5A-B). This suggested that the overexpression of 3BP2-SH2 domain inhibits the function of endogenous 3BP2, which transmit the activating signal from FcεRI to PLC-γ.
Overexpression of 3BP2-SH2 domain suppresses FcεRI-mediated tyrosine phosphorylation of PLC-γ and calcium mobilization.
(A and B) Cells transfected with vector alone (pMT3) and cells overexpressing 3BP2-SH2 domain were stimulated with antigen for the indicated times; then cell lysates were immunoprecipitated with either anti–PLC-γ2 (A) or anti–PLC-γ1 (B) antibody. The immunoprecipitated proteins were separated by 10% SDS-PAGE and analyzed by immunoblotting with anti-pTyr mAb. The membrane was then stripped and reprobed with anti–PLC-γ2 or anti–PLC-γ1 antibody. Similar results were obtained when the other lines were examined. (C) Intracellular Ca++ concentration ([Ca++]i) was measured by means of fluorescent indicator fura-2. Sensitized cells were loaded with fura-2 and then activated with either 10 ng/mL antigen (top) or 1 μM thapsigargin (bottom). The traces show the 340:380 fluorescence ratio. Similar results were obtained when the other lines were examined.
Overexpression of 3BP2-SH2 domain suppresses FcεRI-mediated tyrosine phosphorylation of PLC-γ and calcium mobilization.
(A and B) Cells transfected with vector alone (pMT3) and cells overexpressing 3BP2-SH2 domain were stimulated with antigen for the indicated times; then cell lysates were immunoprecipitated with either anti–PLC-γ2 (A) or anti–PLC-γ1 (B) antibody. The immunoprecipitated proteins were separated by 10% SDS-PAGE and analyzed by immunoblotting with anti-pTyr mAb. The membrane was then stripped and reprobed with anti–PLC-γ2 or anti–PLC-γ1 antibody. Similar results were obtained when the other lines were examined. (C) Intracellular Ca++ concentration ([Ca++]i) was measured by means of fluorescent indicator fura-2. Sensitized cells were loaded with fura-2 and then activated with either 10 ng/mL antigen (top) or 1 μM thapsigargin (bottom). The traces show the 340:380 fluorescence ratio. Similar results were obtained when the other lines were examined.
Moreover, FcεRI-mediated elevation of [Ca++]i was suppressed in the cells overexpressing 3BP2-SH2 domain (Figure 5C). The responses of these cell lines to thapsigargin were equivalent. Therefore, these results indicate that endogenous 3BP2 functions prior to calcium mobilization to positively regulate FcεRI-mediated tyrosine phosphorylation of PLC-γ in the FcεRI-mediated signaling pathway.
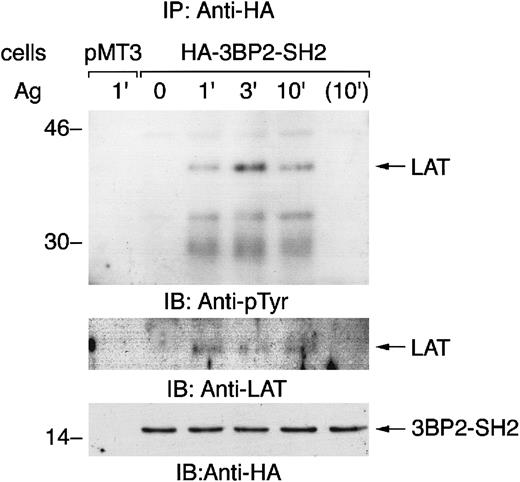
Aggregation of FcεRI induces the association of 3BP2-SH2 domain with LAT
The 3BP2-SH2 domain is predicted to bind to the YEN motif when it is tyrosine phosphorylated.10 An adaptor protein LAT has 2 copies of the YEN motif (Tyr120 and Tyr226). To examine the mechanism of the cellular effect of overexpression of 3BP2-SH2 domain, we characterized the proteins that associated with 3BP2-SH2 domain in FcεRI-mediated signaling (Figure6). Immunoprecipitation studies demonstrated that 3BP2-SH2 domain associated with LAT when cells were stimulated with antigen. 3BP2-SH2 domain also transiently associated with FcεRIβ, indicating that 3BP2 may be complexed with other signaling molecules by binding with FcεRI and LAT in the GEMs (data not shown).16,17 In yeast, 3BP2-SH2 domain binds to autophosphorylation sites in the linker or kinase domain of Syk family PTKs.8 However, we could not detect the association of Syk with 3BP2-SH2 domain by immunoprecipitation studies or pull-down experiments using glutathione S-transferase (GST)–3BP2-SH2 domain fusion protein (data not shown). Therefore, this result suggested that 3BP2-SH2 domain associates with LAT in the FcεRI-mediated signaling pathway.
Aggregation of FcεRI induces the association of 3BP2-SH2 domain with LAT.
Cells transfected with vector alone (pMT3) and cells overexpressing 3BP2-SH2 domain were stimulated with antigen for the indicated times and cell lysates were then immunoprecipitated with anti-HA antibody. The immunoprecipitated proteins were separated by 10% and 14% SDS-PAGE and analyzed by immunoblotting with anti-pTyr, anti-LAT, and anti-HA antibodies.
Aggregation of FcεRI induces the association of 3BP2-SH2 domain with LAT.
Cells transfected with vector alone (pMT3) and cells overexpressing 3BP2-SH2 domain were stimulated with antigen for the indicated times and cell lysates were then immunoprecipitated with anti-HA antibody. The immunoprecipitated proteins were separated by 10% and 14% SDS-PAGE and analyzed by immunoblotting with anti-pTyr, anti-LAT, and anti-HA antibodies.
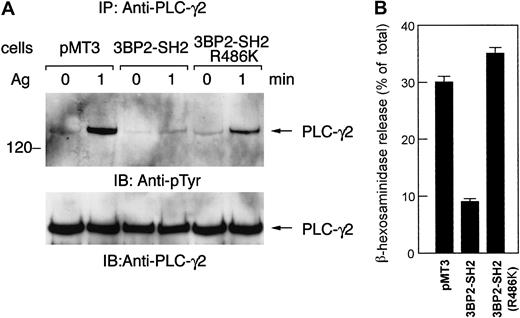
Expression of the mutant form of the 3BP2-SH2 domain (R486K) does not affect FcεRI-mediated signaling
A number of molecules involved in the FcεRI signaling pathway contain SH2 domain. FcεRI aggregation causes the activation of PTKs and phosphorylated tyrosines become the docking sites for SH2 domain-containing proteins, which then propagate the downstream signals. Introduction of Syk-SH2 domain into streptolysin O–permeabilized RBL-2H3 cells results in an inhibition of degranulation, leukotriene production, and tyrosine phosphorylation of cellular proteins.18 On the other hand, PLC-γ1-SH2 domain inhibits degranulation at lower affinity. Furthermore, Src-SH2 domain does not inhibit degranulation, indicating that there is specificity in the inhibition by the SH2 domain in FcεRI-mediated signaling. We have demonstrated that antigen-mediated tyrosine phosphorylation of PLC-γ and release of β-hexosaminidase were suppressed in cells expressing the 3BP2-SH2 domain (Figures 3 and 5). However, this suppression was not observed in cells expressing the mutant form of the 3BP2-SH2 domain (R486K) (Figure7). Expression of the mutant form of 3BP2-SH2 domain does not suppress FcεRI-mediated tyrosine phosphorylation of PLC-γ and degranulation. Therefore, these observations support the idea that overexpression of the 3BP2-SH2 domain specifically suppresses the FcεRI-mediated degranulation pathway in RBL-2H3 cells.
The mutant form of 3BP2-SH2 domain does not suppress FcεRI-mediated degranulation.
Control cells and cells expressing 3BP2-SH2 domain or the mutant form of 3BP2-SH2 domain (R486K) were stimulated with 10 ng/mL antigen. (A) Cell lysates were immunoprecipitated with anti–PLC-γ2 antibody. The immunoprecipitated proteins were separated by 10% SDS-PAGE and analyzed by immunoblotting with anti-pTyr mAb and anti–PLC-γ2. Similar results were obtained when the other lines were examined. (B) Analysis of β-hexosaminidase release. The antigen-induced releases are normalized by expression as a percentage of the total β-hexosaminidase activity. The results are the mean values ± SE from 3 independent experiments.
The mutant form of 3BP2-SH2 domain does not suppress FcεRI-mediated degranulation.
Control cells and cells expressing 3BP2-SH2 domain or the mutant form of 3BP2-SH2 domain (R486K) were stimulated with 10 ng/mL antigen. (A) Cell lysates were immunoprecipitated with anti–PLC-γ2 antibody. The immunoprecipitated proteins were separated by 10% SDS-PAGE and analyzed by immunoblotting with anti-pTyr mAb and anti–PLC-γ2. Similar results were obtained when the other lines were examined. (B) Analysis of β-hexosaminidase release. The antigen-induced releases are normalized by expression as a percentage of the total β-hexosaminidase activity. The results are the mean values ± SE from 3 independent experiments.
Discussion
These results demonstrate that 3BP2 is the substrate of PTKs that are activated by antigen stimulation. Moreover, although overall tyrosine phosphorylation of cellular proteins was not changed, overexpression of 3BP2-SH2 domain selectively suppressed FcεRI-mediated tyrosine phosphorylation of PLC-γ, calcium mobilization, and degranulation in RBL-2H3 cells (Figure 3B, Figure 5). FcεRI-mediated tyrosine phosphorylation of overall cellular proteins, FcεRIβ and γ subunits, Syk, and LAT was not affected (Figure 4and data not shown). Although 3BP2 was originally identified as an Syk kinase–interacting protein in the yeast system, 3BP2-SH2 was associated with tyrosine-phosphorylated LAT in RBL-2H3 cells (Figure6). These observations suggest that endogenous 3BP2 positively regulates the signals between FcεRI-mediated tyrosine phosphorylation of LAT, PLC-γ, and calcium mobilization to induce degranulation in mast cells via its SH2 domain.
An in vitro binding study demonstrated that tyrosine kinase substrate that has YEN motif is optimal for 3BP2-SH2 domain. LAT has multiple tyrosine phosphorylation sites, and Tyr120 and Tyr226 are in this motif. Mutational analysis of LAT in T cells suggested that Tyr226 is one of the responsible sites that bind to Grb2.19 Furthermore, T cell receptor–mediated association of LAT with PLC-γ is dependent on LAT Tyr132, and mutation of this site affects calcium influx and NFAT activation. Therefore, 3BP2-SH2 domain could bind to a distinct phosphorylation site that interacts with PLC-γ-SH2 domain on LAT. This suggests that suppression of FcεRI-mediated tyrosine phosphorylation PLC-γ by the overexpression of 3BP2-SH2 domain was not simply due to the competitive effect between the SH2 domains of 3BP2 and PLC-γ (Figure 5).
Our preliminary experiments have shown that 3BP2 is tyrosine phosphorylated by Lyn, Syk, or Btk in COS cells. Among them Syk predominantly phosphorylated 3BP2 in COS cells (data not shown). Syk is mainly expressed in hematopoietic lineage cells and is essential for immune receptor–mediated cell activation during lymphocyte development and acquired immunity.20,21 In addition, recent findings provided evidence that Syk is expressed in nonhematopoietic cells.22,23 A phage display study revealed the requirement of Asp-Tyr-Glu (DYE) sequence for Syk substrate.24 3BP2 has 2 tyrosine residues in this sequence, Tyr174 and Tyr446. Presumably, these tyrosine residues are phosphorylated by Syk and interact with the downstream molecules to transmit the activation signals from FcεRI. In addition, it is possible that Syk phosphorylates the other tyrosine residues in 3BP2. However, it remains unclear which PTPase dephosphorylates 3BP2 after mast cell activation.
Aggregation of FcεRI induces translocation of activated Syk to the GEMs in RBL-2H3 cells. After cell activation, 3BP2 associates with LAT and FcεRI, suggesting that 3BP2 may be complexed with other signaling molecules in the GEMs (Figure 6).25,26 Recently, electron microscopic analysis revealed the membrane topography of FcεRI signaling in RBL-2H3 cells.16 17 Redistribution of cross-linked FcεRI in the membrane excludes Lyn and then accumulates Syk. Secondary LAT rafts are larger after FcεRI cross-linking, and it allows the interaction of signaling molecules to propagate downstream events. Although it is not clear which PTK phosphorylates 3BP2 in mast cells, tyrosine-phosphorylated 3BP2 may redistribute to LAT rafts to regulate tyrosine phosphorylation of PLC-γ. Subcellular fractionation studies also provided evidence that 3BP2 translocated to the plasma membrane after FcεRI aggregation in RBL-2H3 cells (data not shown).
Our observations have shown that overexpression of 3BP2-SH2 domain only affects the FcεRI-mediated degranulation process leading to the release of inflammatory mediators, such as histamine. In contrast, antigen-induced activation of c-Jun N-terminal kinase (JNK) and extracellular signal-related kinase (ERK) were unaffected by overexpression of 3BP2-SH2 domain (data not shown). Therefore, rather than upstream PTK, adaptor molecules may selectively function for specific pathways. A selective inhibitor of intracellular signaling molecules might have therapeutic potential. It has been reported that the compounds inhibiting release of antigen-induced allergic mediators from mast cells suppressed FcεRI-mediated Syk activation. Hence, we propose that 3BP2 is a candidate molecule for developing the novel antiallergic drugs because of its selective function in mast-cell signaling cascades.
We thank Dr Reuben P. Siraganian for providing the reagents and helpful discussion; Dr Amnon Altman for providing 3BP2 constructs; and Dr Chisei Ra (Nihon University) for instruction in β-hexosaminidase release analysis.
Prepublished online as Blood First Edition Paper, May 17, 2002; DOI 10.1182/blood-2001-12-0340.
Supported in part by the Uehara Memorial Foundation (K.S.); the AstraZeneca Asthma Research Award (K.S.); and a grant-in-aid for scientific research from the Japan Society for the Promotion of Science and the Ministry of Education, Culture, Sports, Science and Technology, Japan.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Kiyonao Sada, Division of Proteomics, Department of Genome Sciences, Kobe University Graduate School of Medicine, 7-5-1, Kusunoki-cho, Chuo-ku, Kobe 650-0017, Japan; e-mail:ksada@med.kobe-u.ac.jp.

![Fig. 5. Overexpression of 3BP2-SH2 domain suppresses FcεRI-mediated tyrosine phosphorylation of PLC-γ and calcium mobilization. / (A and B) Cells transfected with vector alone (pMT3) and cells overexpressing 3BP2-SH2 domain were stimulated with antigen for the indicated times; then cell lysates were immunoprecipitated with either anti–PLC-γ2 (A) or anti–PLC-γ1 (B) antibody. The immunoprecipitated proteins were separated by 10% SDS-PAGE and analyzed by immunoblotting with anti-pTyr mAb. The membrane was then stripped and reprobed with anti–PLC-γ2 or anti–PLC-γ1 antibody. Similar results were obtained when the other lines were examined. (C) Intracellular Ca++ concentration ([Ca++]i) was measured by means of fluorescent indicator fura-2. Sensitized cells were loaded with fura-2 and then activated with either 10 ng/mL antigen (top) or 1 μM thapsigargin (bottom). The traces show the 340:380 fluorescence ratio. Similar results were obtained when the other lines were examined.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/6/10.1182_blood-2001-12-0340/4/m_h81823126005.jpeg?Expires=1767720854&Signature=HnTGQnYMthNiIcuTKJzXFBO7-o7cIXPbrTy8HDI-EQYypiXhd6U4zN1lHb72vFpvMjd50Iy2HA38MzAZLLO9kskVCXT5DkKDnF9wF-DUPBjlcvsI~qvV1HOk28dA1TkNq~b3j5HiPkAlN0T3Ly2ee0I6ip6INEbPdzSLdqYjC8sPPW6CAVJSLNMTz0OvX91UR5eNSwbzxxn75ZDFKINHPgLxJbSgNzaO1GZgklE4MeCq6BxH6NUj5E4uvV0jzNuEs-2h8uJodSHN5Zl5GG~ASC9EklTAqpCueFeKHJArPgUuSBkkXLOCXFHpcm0P4zvdmwKRNdzYKg-THXdVw4N8XQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal