Abstract
The long-term immunologic effects of intermittent interleukin 2 (IL-2) therapy were evaluated in a cross-sectional study by comparing 3 groups: HIV-seronegative volunteers, HIV-infected (HIV+) patients receiving highly active antiretroviral therapy (HAART), and HIV+ patients receiving HAART and intermittent IL-2. Whole-blood immunophenotyping was performed to study expression of the IL-2 receptor chains on T lymphocytes and natural killer cells and to further characterize CD4+/CD25+ T cells. Increased CD25 expression, especially in CD4+ T cells but also in CD8+ T cells, without increases in expression of the β and γ chains of the IL-2 receptor was detected in the IL-2 group. Up to 79% of naive CD4+ T cells (median, 61%) from patients in the IL-2 group expressed CD25, and the number of naive CD4+/CD25+ T cells correlated positively with both the total and naive CD4+ T-cell counts. A discrete population of CD45 double intermediate RA+/RO+CD4+ cells was also preferentially expanded in the IL-2 group, and the number of these cells strongly correlated with the total CD4+ count. Despite increases in CD25 expression, T lymphocytes from patients treated with IL-2 did not have increased expression of early (CD69) or late (CD95) activation markers or evidence of recent proliferation (Ki67). Both CD4+/CD25+ and CD4+/CD25− cells from IL-2–treated HIV+ patients proliferated in response to mitogens, specific antigens, and T-cell-receptor–mediated stimuli. Thus, intermittent administration of IL-2 in HIV+ patients leads to preferential expansion of a unique subset of CD4+ T cells that may represent a critical population in T-cell homeostasis.
Introduction
Interleukin 2 (IL-2) or T-cell growth factor is currently approved for the treatment of metastatic renal cell carcinoma and melanoma. IL-2 has also been tested experimentally in HIV infection in phase I and II studies since the early years of the AIDS epidemic. In randomized controlled clinical trials, intermittent administration of IL-2 in HIV-infected (HIV+) patients has been shown to lead to substantial and sustained expansion of the CD4+T-cell pool.1,2 This expansion is enriched for naive CD4+ T cells and is not associated with increases in HIV-1 viral load.3,4 Subcutaneous administration of IL-2 was found to be equally effective and better tolerated than intravenous administration, thus facilitating outpatient management.5-7 In combination with highly active antiretroviral therapy (HAART), intermittent administration of IL-2 can lead to increases in CD4+ cell count even in cases in which the baseline count is as low as 50 cells/μL.8-12 Phase III studies with clinical end points (SILCCAT: Subcutaneous IL-2 in HIV Infected Subjects with Low CD4 Counts under Active Antiretroviral Therapy and ESPRIT: Evaluation of Subcutaneous Proleukin in a Randomized International Trial) are under way to assess the clinical benefit of the increases in CD4+ T cells induced by IL-2.
IL-2 binds to a cellular receptor that is composed of 3 chains: the α chain (CD25), β chain (CD122), and γc or common cytokine chain (CD132). IL-15 shares both the β and γcchains of the IL-2 receptor, whereas the γc chain is also shared by IL-4, IL-7, and IL-9 and is constitutively transcribed in lymphocytes.13-15 Two forms of a functional receptor exist: the βγ or intermediate-affinity receptor (Kd 10−9) and the αβγ or high-affinity receptor (Kd 10−11).16 The α chain (CD25) is necessary for the formation of the high-affinity receptor but has limited binding ability on its own (low-affinity receptor [Kd 10−8]); also, it has a short intracytoplasmic tail that lacks signaling function.17-19 Although expression of CD25 is classically induced by antigenic stimulation, IL-2 alone is sufficient to induce expression of CD25 and progression of T cells through the cell cycle.20
Despite some disagreement on the exact percentage of human peripheral blood T cells expressing CD25—likely reflecting differences in the monoclonal antibodies used, staining techniques, or populations under study—a consistent finding has been that CD4+ T cells express higher levels of CD25 and lower levels of CD122 than CD8+ T cells.19,21-23 Natural killer (NK) cells typically express very low levels of CD25 (< 5%), whereas most NK cells are CD122+.24
On the basis of data from prospective controlled studies, it has been reported that HIV+ patients treated with IL-2 have an expansion of CD4+ T cells bearing the α chain (CD25) of the IL-2 receptor.1 25 It was previously assumed that these CD25+ cells coexpress increased levels of the β and γc chains of the IL-2 receptor and are thus more sensitive to IL-2 signaling. Their presumed increased IL-2–binding potential was thought to contribute to a preferential use of endogenously produced IL-2, thus sustaining a higher basal CD4+ T-cell proliferation and enhancing responses to subsequent administration of IL-2. In the previous studies, however, the levels of expression of the β and γ chains of the IL-2 receptor were not tested; therefore, it remained unknown whether the expression of CD25 was an indication of cells expressing the high-affinity IL-2 receptor and thus being more responsive to IL-2 signaling. Additionally, the phenotypic characteristics of CD25-bearing CD4+ T cells in patients treated with IL-2 were not previously studied in detail with respect to their differentiation or activation status. Similarly, their functional and proliferative potentials have never been compared with those of CD4+/CD25− cells.
To better characterize the effect of IL-2 on expression of the IL-2 receptor by T cells and NK cells, we examined α- and β-chain expression in HIV-seronegative (HIV−) volunteers, HIV+ patients with high CD4+ counts given HAART, and HIV+ patients given both HAART and intermittent IL-2 therapy. The long-term effects of IL-2 treatment on CD25 expression in HIV+ patients were also studied. We tested the hypothesis that higher levels of expression of the α chain would be associated with higher baseline proliferation rates in T lymphocytes in the patients who received IL-2. In addition, because of the recent description of a subset of regulatory CD4+/CD25+ cells in animal models and humans,26 27 we sought to identify similarities or differences between these cells and the CD4+/CD25+ cells induced by intermittent administration of IL-2.
Patients and methods
Study participants
A cross-sectional study compared HIV− volunteers and HIV+ patients who had been receiving HAART for at least 6 months either alone (HAART group) or in combination with intermittent IL-2 therapy (IL-2+HAART group; Proleukin; Chiron, Emeryville, CA). Consecutively seen persons in each group who agreed to participate at the time of routine follow-up visits and on prespecified days of the week were included in the study. Patients receiving IL-2 were enrolled in institutional review board–approved trials at the National Institutes of Health evaluating the role of IL-2 as an experimental agent in the treatment of HIV+ patients. Informed consent was provided according to 45 Code of Federal Regulations (CFR) governing human subjects research. The maintenance regimen used in these trials is 1.5 to 7.5 million units (U) given subcutaneously twice a day for 5 days at intervals determined by the patients' CD4+ T-cell counts. On initiation of IL-2 therapy, cycles are typically administered every 8 weeks until the CD4+ T-cell count increases to more than 1000/μL or twice the baseline value. Subsequently, the cycling interval is individualized to maintain these values and can range from several months to years. Patients receiving HAART were chosen with the goal of achieving CD4 cell counts comparable to those in the IL-2 cohort. Viral burden was tested by using USbDNA, version 3 (Bayer Diagnostics, Tarrytown, NY; sensitivity, < 50 copies/mL).
Immunophenotyping
Blood was collected in a heparin-coated Vacutainer and processed within 4 hours after it was drawn (Becton Dickinson, Franklin Lakes, NJ). The whole-blood lysis technique for surface staining was used (BD Immunocytometry, San Jose, CA). The following monoclonal antibodies were used: CD25 phycoerythrin (PE) or fluorescein isothiocyanate, conjugated (FITC; clone 2A3); CD122 PE (clone TU27); CD4 peridinin chlorophyll protein (PerCP), FITC, or allophycocyanin (APC; clone SK3); CD8 FITC or PerCP (clone SK1); CD3 FITC, PerCP, or APC (clone SK7); CD16 FITC (clone NKP15); CD56 FITC (clone NCAM16.2); CD45RO APC (clone UCHL-1); CD62L PE (clone SK11); CD45RA FITC or PE (clone L48); CD27 FITC or PE (clone L128); CD95 APC (clone DX2); CD69 APC (clone L78), and IgG1 FITC, PE, or APC (clone X40; all from BD Immunocytometry). Samples were analyzed with a 4-color multiparameter flow cytometer (FACS Calibur; BD Immunocytometry). CD3+/CD4+ gating and CD3+/CD8+ gating were used for CD4+and CD8+ T cells, respectively, and CD3−/CD16+-56+ gating was used to identify NK cells (Figure 1). CD25+ and CD122+ populations were identified with the use of isotype controls. The statistical comparison of CD132 expression was restricted to the mean fluorescence intensity (MFI) measurement, which is more accurate when distinct populations are not easily differentiated by flow cytometry.
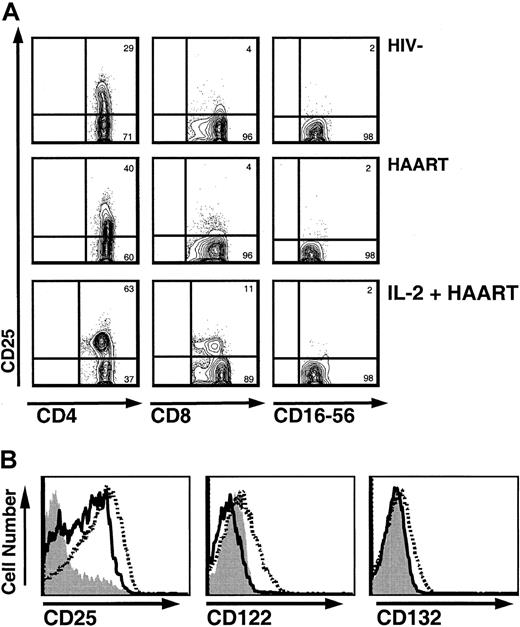
Intermittent IL-2 administration leads to persistent increased CD25 expression on T lymphocytes, with transient increases in the expression of CD122 and CD132 during cycles of IL-2.
(A) CD25 expression on T cells and NK cells in HIV−controls, HIV+ patients treated with HAART, and HIV+ patients treated with HAART and IL-2 (contour plots). Gating was done in CD3+/CD4+ and CD3+/CD8+ in the lymphocyte gate for CD4+ and CD8+ T cells, respectively, and in CD3−/CD16+-56+ for NK cells. In IL-2–treated patients, very distinct CD4+/CD25+ populations were observed. Of note, a slightly decreased fluorescence intensity for CD4 was observed in the CD25+ cells. (B) Increase in CD25 expression on CD4+ T cells accompanied by transient but not persistent increases in CD122 and CD132 expression. Histograms for CD4+ T cells of an IL-2–treated patient before initiation of IL-2 therapy (solid gray), at the end of an IL-2 cycle (broken black), and 12 months later (solid black).
Intermittent IL-2 administration leads to persistent increased CD25 expression on T lymphocytes, with transient increases in the expression of CD122 and CD132 during cycles of IL-2.
(A) CD25 expression on T cells and NK cells in HIV−controls, HIV+ patients treated with HAART, and HIV+ patients treated with HAART and IL-2 (contour plots). Gating was done in CD3+/CD4+ and CD3+/CD8+ in the lymphocyte gate for CD4+ and CD8+ T cells, respectively, and in CD3−/CD16+-56+ for NK cells. In IL-2–treated patients, very distinct CD4+/CD25+ populations were observed. Of note, a slightly decreased fluorescence intensity for CD4 was observed in the CD25+ cells. (B) Increase in CD25 expression on CD4+ T cells accompanied by transient but not persistent increases in CD122 and CD132 expression. Histograms for CD4+ T cells of an IL-2–treated patient before initiation of IL-2 therapy (solid gray), at the end of an IL-2 cycle (broken black), and 12 months later (solid black).
To study expression of CD25 on naive CD4+ cells, gating was done in the CD4+/CD45RO− population. CD45RO− gating was strictly defined so that all CD45RO− cells were also CD45RA bright, CD27+, and CD62L+ as tested in parallel tubes. Cryopreserved cells were stained to confirm induction of changes by IL-2 by studying specimens collected before initiation of IL-2 therapy, and in those cells, the naive population was defined as CD45RO−/CD27+. Cryopreserved cells were also stained to confirm the increased percentage of the dull RA+/RO+ CD4 subset in patients given IL-2. Approximately 1 to 1.5 × 105 total events and a minimum of 5000 events in the CD4+ gate were collected per sample. Intracellular staining for the nuclear antigen Ki67 was performed as previously described28 with Ki67 PE antibody (clone B56; BD Biosciences Pharmingen, San Diego, CA) in cryopreserved peripheral blood mononuclear cells (PBMCs) obtained at the same times the samples used for the immunophenotypic analyses were collected. Approximately 1.5 to 2 × 105 total events and a minimum of 10 000 events in the CD4+ or CD8+ gate were collected per sample. FlowJo software (Tree Star, San Carlos, CA) was used for all flow cytometric data analyses.
Proliferation assays of CD4+/CD25+ and CD4+/CD25− cells
Thirteen patients given IL-2+HAART were recruited for functional studies of CD25+ and CD25−CD4+ T cells. Separated cell subsets were used for all or some of the experimental conditions, depending on the yield of the cell separations (cells from one patient were used exclusively for the suppression experiment). The median CD4 count in this subgroup was 1022 cells/μL, the CD4:CD8 ratio was 1, and the median time since the last IL-2 cycle was 22 months (range, 3-48). Two of the 13 patients had a plasma viral load higher than 50 copies/mL. Fresh PBMCs were isolated by Ficoll-Hypaque lymphocyte separation. A fraction of PBMCs was irradiated for use as antigen-presenting cells and a second fraction was used as a control for the proliferation assays. CD4+cells were isolated by the multisort CD4+positive-selection procedure or the CD4+ T-cell negative-isolation procedure (Miltenyi Biotec, Auburn, CA) according to the manufacturer's recommended protocol. The resulting population had a median of 97% CD4+ cells (range, 91%-99%) and was further separated into CD25+ and CD25−fractions by CD25 microbeads (Miltenyi Biotec). The median purity of the separated populations was 93% for the CD4+/CD25− fraction (range, 85%-98%) and 90% for the CD4+/CD25+ fraction (range, 81%-98%). Separated fractions had similar percentages of naive cells (32% in the CD25+ fraction and 36% in the CD25− fraction), but compared with the CD25−fraction, the CD25+ fraction was highly enriched in dull intermediate RA+/RO+ cells and had a higher ratio of long-term or central memory cells (62L+, CD27+) to effector memory (62L−, CD27−) cells.
Cells (PBMCs, CD4+/CD25+, and CD4+/CD25−) were cultured in triplicate in 96-well, round-bottomed plates (Nalge Nunc International, Rochester, NY) at 1.5 × 105 cells/per well in complete medium in a final volume of 200 μL. Cells were stimulated with irradiated (30 Gy [3000 rad]) antigen-presenting cells (1 × 105cells in each well) and the following proliferation stimuli: phytohemagglutinin (PHA; Sigma-Aldrich, St Louis, MO) at a final concentration 3 μg/mL, anti-CD3 (OKT3; Ortho Biotech, Raritan, NJ) with and without anti-CD28 (BD Biosciences Pharmingen) at 1 μg/mL, cytomegalovirus (CMV; whole lysate, Bio-Whittaker, Walkersville, MD) at 2.5 μL/mL, tetanus toxoid (TT; Aventis Pasteur, Swiftwater, PA) at 3 μg/mL, pokeweed mitogen (PWM; Gibco BRL Life Technologies, Rockville, MD) at 1:200, or IL-2 at 100 IU/mL (Aldesleukin; Chiron).
In 6 experiments designed to test specifically for suppression, 5 × 104 CD4+/CD25− cells were stimulated with equal numbers of irradiated antigen-presenting cells and anti-CD3, anti-CD3 with anti-CD28, or PHA as described above, either alone or in the presence of incremental numbers of CD4+/CD25+ cells, according to the method described by Thornton and Shevach.29 Plates were incubated at 37°C with 5% carbon dioxide, and wells were pulsed with 1 μCi (.037 MBq) tritium-thymidine for 6 hours on the third day (PHA, anti-CD3 with or without anti-CD28, and IL-2) or fifth day (PWM, CMV, TT, and IL-2) of incubation. Harvesting was performed in an automatic plate harvester (Wallac, Gaithersburg, MD). Radioactivity was measured as tritium-thymidine incorporation in a β counter (Wallac). Wells containing cells with medium alone were used as negative controls. Net counts per minute (cpm) were calculated by subtracting the cpm of the wells with the medium (background controls) from the cpm of the wells with the specific stimuli. In all experiments, CD25+ and CD25−CD4 cells had background cpm of below 1500.
Statistical methods
All 3-group comparisons were done by using the Kruskal-Wallis test. When significant differences (P = .05) were detected, 2-group comparisons were done with the Wilcoxon 2-sample test. The Student t test was used to compare normally distributed variables: the percentage of RA+/RO+ dull intermediate CD4 cells, the percentage of CD25 expression on the RA+/RO+dull intermediate CD4 subset, and the percentage of Ki67+ T cells in the HAART and IL-2+HAART groups. The paired Studentt test was used to compare the mean CD25 expression on naive and RA+/RO+ dull CD4 cells and the geometric means of the proliferative responses of CD25+ and CD25−CD4 cells. The associations between variables were determined by using the Spearman rank correlation method. Adjustment ofP values for multiple testing was done with the Bonferroni method.
Results
Patient characteristics
The characteristics of participants at study entry are shown in Table 1. Patients in the IL-2 group had received a minimum of 3 cycles of IL-2 in the past and were tested several months after the last IL-2 cycle (range, 3-54 months), with 3 of them studied immediately before initiation of an IL-2 cycle. The 3 groups did not differ significantly with respect to their total CD4+ count, percentage of naive and memory CD4+cells, age, or viral load. Significant differences were observed for the following factors: CD4:CD8 ratio (HIV− versus HAART or IL-2+HAART group and IL-2+HAART versus HAART group,P = .01), percentage of CD4+ T cells (HIV− versus HAART group and IL-2+HAART versus HAART group, P = .005) and percentage and total CD8+T cells (HAART and IL-2+HAART versus HIV− group,P < .001).
Characteristics of study participants
| Participant group . | Age, y . | CD4 count (cells/μL) . | CD4 percentage . | CD8 count (cells/μL) . | CD8 percentage . | CD4:CD8 ratio . | HIV RNA level (copies/mL) . | Patients with viral load < 50 copies/mL . |
|---|---|---|---|---|---|---|---|---|
| HIV− controls (n = 16) | 39 (23-57) | 643 (410-1 053) | 44 (30-61) | 338 (150-753) | 22 (11-41) | 2.10 (0.85-4.27) | — | — |
| HIV+/HAART (n = 15) | 44 (27-59) | 609 (102-1 198) | 23 (12-48) | 1091 (254-3 749) | 42 (35-72) | 0.54 (0.19-1.37) | < 50 (< 50-46 775) | 10 of 15 (67%) |
| HIV+/IL-2+ HAART (n = 16)* | 44 (32-59) | 847 (488-1 253) | 39 (26-49) | 976 (504-1 754) | 43 (34-56) | 0.84 (0.55-1.35) | < 50 (< 50-30 024) | 9 of 16 (56%) |
| Participant group . | Age, y . | CD4 count (cells/μL) . | CD4 percentage . | CD8 count (cells/μL) . | CD8 percentage . | CD4:CD8 ratio . | HIV RNA level (copies/mL) . | Patients with viral load < 50 copies/mL . |
|---|---|---|---|---|---|---|---|---|
| HIV− controls (n = 16) | 39 (23-57) | 643 (410-1 053) | 44 (30-61) | 338 (150-753) | 22 (11-41) | 2.10 (0.85-4.27) | — | — |
| HIV+/HAART (n = 15) | 44 (27-59) | 609 (102-1 198) | 23 (12-48) | 1091 (254-3 749) | 42 (35-72) | 0.54 (0.19-1.37) | < 50 (< 50-46 775) | 10 of 15 (67%) |
| HIV+/IL-2+ HAART (n = 16)* | 44 (32-59) | 847 (488-1 253) | 39 (26-49) | 976 (504-1 754) | 43 (34-56) | 0.84 (0.55-1.35) | < 50 (< 50-30 024) | 9 of 16 (56%) |
Values are median (range).
Median time from last IL-2 cycle, 13 months (range 3-54); time from first IL-2 cycle, 3-8 years; and median total number of IL-2 cycles, 11 (range, 5-23).
Expression of the chains of the IL-2 receptor on CD4+ and CD8+ T cells and NK cells
The results of the immunophenotypic analysis of expression of the 3 chains of the IL-2 receptor on T cells and NK cells are shown in Table 2. We found, as others did previously,21 that the percentage of CD4+ T cells expressing CD25 was higher than the percentage of CD8+ T cells or NK cells expressing CD25 in all 3 groups (Figure 1A and Table 2). The percentage of CD4+ T lymphocytes expressing CD25 was higher in the IL-2 group than in the other 2 groups. The same was observed for MFI of the CD25 population. No differences were observed in CD25 expression or MFI between the HAART group and the HIV− volunteers. When β-chain (CD122) expression was evaluated, the percentage of CD4+ T cells expressing CD122 was found to be lower in the IL-2+HAART group than in the HIV− group. Finally, we observed significantly lower expression of CD132 (MFI) on CD4+ T cells from the IL-2+HAART group than on CD4+ T cells from the HAART group. Transient increases in expression of β and γ chains were detected during IL-2 cycles (Figure 1B).
Expression of CD25, CD122, and CD132 in the 3 groups of participants
| Cell type/chain . | Participant group . | P . | ||||
|---|---|---|---|---|---|---|
| HIV− (n = 16) . | HAART (n = 16) . | IL-2 + HAART (n = 16) . | HIV−vs HAART . | HIV− vs IL-2 + HAART . | HAART vs IL-2 + HAART . | |
| CD4+ T cells | ||||||
| CD25 (%) | 28.3 (17.3-45.8) | 23 (11.1-48.8) | 53.6 (23.0-69.8) | NS | < .001 | < .001 |
| CD25 (MFI) | 28.0 (23.0-36.8) | 28 (23.1-40.8) | 41.3 (27.9-68.5) | NS | < .001 | < .001 |
| CD122 (%) | 1.8 (1.4-7.0) | 1.8 (0.7-3.6) | 1.2 (0.3-4.3) | NS | .02 | NS |
| CD122 (MFI) | 2.6 (1.7-3.9) | 2.9 (2.1-4.2) | 2.7 (2.0-3.7) | NS | NS | NS |
| CD132 (MFI; n = 7) | 4.4 (3.3-7.9) | 7.4 (5.0-9.7) | 4.6 (3.2-5.6) | NS | NS | .01 |
| CD8+ T cells | ||||||
| CD25 (%) | 3 (1.2-7.3) | 1.2 (0.5-7.4) | 5.9 (1.6-45.9) | NS | .01 | .01 |
| CD25 (MFI) | 21.8 (18.5-25.7) | 21.8 (18.7-36.3) | 28.5 (21.0-42.5) | NS | .02 | .02 |
| CD122 (%) | 13.1 (5.2-33.6) | 16.4 (5.3-59.5) | 14.6 (2.2-34.5) | NS | NS | NS |
| CD122 (MFI) | 4.6 (2.9-8.4) | 6.0 (4.2-13.7) | 6.0 (3.8-11.9) | .03 | .03 | NS |
| CD132 (MFI; n = 7) | 4.9 (3.9-8.5) | 7.6 (5.5-13.9) | 6.6 (4.4-7.6) | NS | NS | NS |
| NK cells | ||||||
| CD25 (%) | 1.9 (0.7-4.3) | 2.4 (0.9-6.0) | 2.1 (0.4-6.2) | NS | NS | NS |
| CD122 (%) | 94.3 (27.7-97.8) | 93.4 (80.2-96.9) | 93.6 (49.0-97.6) | NS | NS | NS |
| Cell type/chain . | Participant group . | P . | ||||
|---|---|---|---|---|---|---|
| HIV− (n = 16) . | HAART (n = 16) . | IL-2 + HAART (n = 16) . | HIV−vs HAART . | HIV− vs IL-2 + HAART . | HAART vs IL-2 + HAART . | |
| CD4+ T cells | ||||||
| CD25 (%) | 28.3 (17.3-45.8) | 23 (11.1-48.8) | 53.6 (23.0-69.8) | NS | < .001 | < .001 |
| CD25 (MFI) | 28.0 (23.0-36.8) | 28 (23.1-40.8) | 41.3 (27.9-68.5) | NS | < .001 | < .001 |
| CD122 (%) | 1.8 (1.4-7.0) | 1.8 (0.7-3.6) | 1.2 (0.3-4.3) | NS | .02 | NS |
| CD122 (MFI) | 2.6 (1.7-3.9) | 2.9 (2.1-4.2) | 2.7 (2.0-3.7) | NS | NS | NS |
| CD132 (MFI; n = 7) | 4.4 (3.3-7.9) | 7.4 (5.0-9.7) | 4.6 (3.2-5.6) | NS | NS | .01 |
| CD8+ T cells | ||||||
| CD25 (%) | 3 (1.2-7.3) | 1.2 (0.5-7.4) | 5.9 (1.6-45.9) | NS | .01 | .01 |
| CD25 (MFI) | 21.8 (18.5-25.7) | 21.8 (18.7-36.3) | 28.5 (21.0-42.5) | NS | .02 | .02 |
| CD122 (%) | 13.1 (5.2-33.6) | 16.4 (5.3-59.5) | 14.6 (2.2-34.5) | NS | NS | NS |
| CD122 (MFI) | 4.6 (2.9-8.4) | 6.0 (4.2-13.7) | 6.0 (3.8-11.9) | .03 | .03 | NS |
| CD132 (MFI; n = 7) | 4.9 (3.9-8.5) | 7.6 (5.5-13.9) | 6.6 (4.4-7.6) | NS | NS | NS |
| NK cells | ||||||
| CD25 (%) | 1.9 (0.7-4.3) | 2.4 (0.9-6.0) | 2.1 (0.4-6.2) | NS | NS | NS |
| CD122 (%) | 94.3 (27.7-97.8) | 93.4 (80.2-96.9) | 93.6 (49.0-97.6) | NS | NS | NS |
Values are medians (range).
NS indicates nonsignificant (higher than or equal to .05); comparisons were higher than or equal to P = .1 in all instances.
CD25 expression and MFI were also elevated in CD8+ T cells from the IL-2 group compared with the other 2 groups. The MFI for CD122 on CD8+ T cells was higher in both the HAART and IL-2+HAART groups than in HIV− controls. No other significant differences were observed among the groups in expression of CD122 or CD132 on CD8+ T cells. In the staining experiments in which both anti-CD25 and anti-CD122 antibodies were used simultaneously, the percentages of double-positive (CD25+/CD122+) CD4+ and CD8+ T cells were very low (< 5%) in all 3 groups (data not shown). No differences among the 3 groups were detected in the expression of α or β chains of the IL-2 receptor on NK cells. Similar to previous observations, we found that the median percentage of NK cells expressing CD122 (> 90%) was higher than the median percentage of CD8+ T cells (13%-16%) or CD4+ T cells (< 2%) expressing CD122.
Enhanced expression of CD25 on CD4+ T cells in patients treated with IL-2 is persistent and occurs predominantly in the naive subset of CD4+ T cells
In HIV− volunteers, 39% of CD4+ T cells (range, 9%-61%) were naive and 10% of these cells expressed CD25. In HIV+ patients treated with HAART, 24% of CD4+T cells (range, 10%-57%) were naive and 13% of these expressed CD25 (P > .5 versus HIV− controls). In patients given IL-2, 30% of total CD4+ T cells (range, 3%-54%) were naive, and in contrast to findings in the other groups, 61% of naive CD4+ cells were CD25+(P < .001 versus the HIV− and HAART groups). The absolute counts of naive CD4+/CD25+ cells reflected the same differences (Figure2A). No significant differences were observed in the percentages of memory CD4+ T cells expressing CD25 (56% in HIV− volunteers, 52% in HAART-treated patients, and 64% in IL-2+HAART–treated patients;P > .11). As demonstrated by the shaded histograms in Figure 2B, the differences in CD25 expression on naive CD4+cells of patients receiving IL-2 and participants in the other 2 groups were striking.
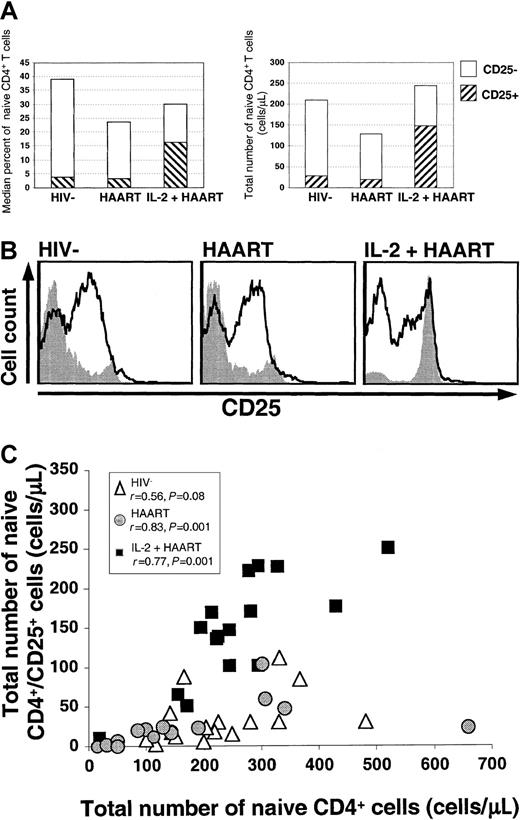
A large fraction of the naive (CD45RO−) CD4+ T cells in IL-2–treated patients expressed the α chain of the IL-2 receptor.
(A) Median percentages and absolute counts of naive CD4+ T cells are shown. The hatched areas represent the fraction of cells that were CD25+. (B) A CD25+ population of naive CD4+ cells was identified in all groups and was largest in the IL-2+HAART group. Levels of expression of CD25 on memory (solid black lines) and naive (gray shaded histograms) CD4+ T cells are shown for all 3 groups. The differences in CD25 expression in the memory subsets were less consistent than the differences in the naive subset and were not significant. (C) Correlation between the number of naive CD4+/CD25+ cells and the total number of naive CD4+ T cells. The absolute number of naive CD4+/CD25+ cells was associated with the total number of naive CD4+ T cells in HIV−volunteers (open triangles), HAART-treated patients (gray circles), and IL-2+HAART–treated patients (black squares). Spearman rank correlation coefficients and P values are shown.
A large fraction of the naive (CD45RO−) CD4+ T cells in IL-2–treated patients expressed the α chain of the IL-2 receptor.
(A) Median percentages and absolute counts of naive CD4+ T cells are shown. The hatched areas represent the fraction of cells that were CD25+. (B) A CD25+ population of naive CD4+ cells was identified in all groups and was largest in the IL-2+HAART group. Levels of expression of CD25 on memory (solid black lines) and naive (gray shaded histograms) CD4+ T cells are shown for all 3 groups. The differences in CD25 expression in the memory subsets were less consistent than the differences in the naive subset and were not significant. (C) Correlation between the number of naive CD4+/CD25+ cells and the total number of naive CD4+ T cells. The absolute number of naive CD4+/CD25+ cells was associated with the total number of naive CD4+ T cells in HIV−volunteers (open triangles), HAART-treated patients (gray circles), and IL-2+HAART–treated patients (black squares). Spearman rank correlation coefficients and P values are shown.
To determine the contribution of the expansion of the naive CD4+/CD25+ subset to the increases in the overall size of the CD4+ pool in the HAART and IL-2+HAART groups, we looked for correlations between the number of these cells and the numbers of total naive and total CD4+ T cells. In the HAART group, a strong correlation was found between the number of naive CD4+/CD25+ cells and the total CD4+ T-cell count (r = 0.75; P = .03) or the total number of naive CD4+ T cells (r = 0.83;P = .001; Figure 2C). Similarly, in the IL-2+HAART group, a significant correlation was observed between the number of naive CD4+/CD25+ T cells and the total CD4+ T-cell counts (r = 0.61; P = .03) or the absolute number of naive CD4+ T cells (r = 0.77;P = .001). In HIV− volunteers, a weaker correlation was found between the naive CD4+/CD25+ cell counts and the total naive CD4+ counts (r = 0.55; P = .08). In this group, no correlation was found between the number of naive CD4+/CD25+ cells and the total CD4+T-cell count.
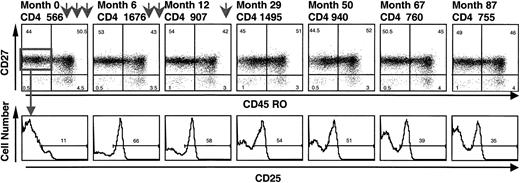
Cryopreserved cells obtained at various time points before and after initiation of IL-2 therapy were tested to provide further documentation of the emergence of naive CD4+/CD25+ cells after administration of IL-2 and their persistence after an IL-2 cycle. After initiation of IL-2, a distinct CD25+ population emerged in the CD45RO−/CD27+ (naive) CD4+ pool (Figure 3). In the patient whose results are shown in Figure 3, this population has persisted for 5 years since the last IL-2 cycle (administered at month 28).
A distinct population of naive CD4+/CD25+ cells emerged after initiation of IL-2 treatment and remained present for long periods after IL-2 cycles.
Frozen PBMCs were stained before and at different time points (months) after initiation of IL-2. Each arrow represents a 5-day IL-2 cycle (months 2, 4, 6, 8, 10, and 28). Naive CD4+ cells were defined as CD45RO−/CD27+, and the CD25 histograms were gated on these naive CD4+ cells.
A distinct population of naive CD4+/CD25+ cells emerged after initiation of IL-2 treatment and remained present for long periods after IL-2 cycles.
Frozen PBMCs were stained before and at different time points (months) after initiation of IL-2. Each arrow represents a 5-day IL-2 cycle (months 2, 4, 6, 8, 10, and 28). Naive CD4+ cells were defined as CD45RO−/CD27+, and the CD25 histograms were gated on these naive CD4+ cells.
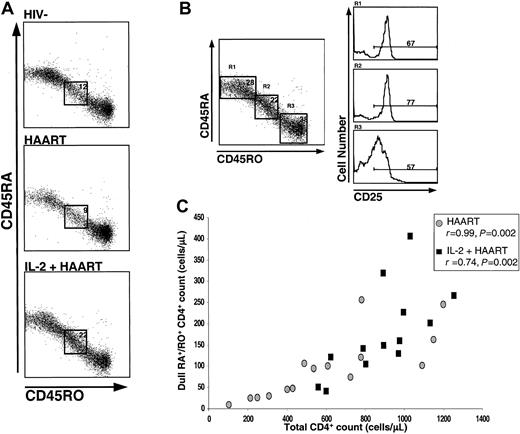
On staining for CD45RA/CD45RO, a CD4+ population that stained positive but had intermediate fluorescence intensity for both CD45RA and CD45RO isoforms was observed in all groups (Figure4). This population was distinct from RA+/RO− and RA−/RO+cells and has been previously referred to as a dull RA/RO double-positive population.30 This CD4+ subset was particularly prominent in IL-2–treated patients, in whom it was also observed to express high levels of CD25 (Figure 4A,B). It thus appeared to represent a phenotypically unique population that had preferentially expanded in patients given IL-2. In the HAART group, 12.3% of CD4+ cells (95% confidence interval [CI], 10.7%-13.9%) had this phenotype (dull RA+/RO+). In a previous HIV−cohort (I.S. unpublished data, May 2001), we found that this population represented 10.7% of the CD4 pool. In contrast, in the IL-2+HAART group, 20.2% (95% CI, 15.8%-24.6%) had this phenotype (P = .01 versus the HAART group).
CD4+ T cells that were dull double-positive for CD45RA+/CD45RO+ were increased in IL-2–treated patients and expressed high levels of CD25.
For the results shown in panel A, gating was done on CD3+/CD4+ cells. A representative example from each group is shown. In panel B, the histograms of CD25 expression on the different CD4+ T cell subsets are shown for an IL-2–treated patient (R1, CD45RA+/RO− cells; R2, dull double-positive CD45RA+/CD45RO+ cells, and R3, CD45RO+/RA−). In panel C, the number of dull RA+/RO+ CD4+ cells is shown to be associated with the total CD4+ T-cell count in both the HAART group (circles) and the IL-2+HAART group (squares). Spearman rank correlation coefficients and P values are shown.
CD4+ T cells that were dull double-positive for CD45RA+/CD45RO+ were increased in IL-2–treated patients and expressed high levels of CD25.
For the results shown in panel A, gating was done on CD3+/CD4+ cells. A representative example from each group is shown. In panel B, the histograms of CD25 expression on the different CD4+ T cell subsets are shown for an IL-2–treated patient (R1, CD45RA+/RO− cells; R2, dull double-positive CD45RA+/CD45RO+ cells, and R3, CD45RO+/RA−). In panel C, the number of dull RA+/RO+ CD4+ cells is shown to be associated with the total CD4+ T-cell count in both the HAART group (circles) and the IL-2+HAART group (squares). Spearman rank correlation coefficients and P values are shown.
The mean absolute numbers of dull RA+/RO+ cells were 81/μL (95% CI, 48-114/μL) in the HAART group and 174 (95% CI, 125-223/μL) in the IL-2+HAART group (P = .01). In both groups, the total number (but not the percentage) of these cells correlated strongly with the total CD4+ count (r = 0.99;P = .002 in the HAART group and r = 0.74;P = .002 in the IL-2 group; Figure 4C), suggesting that expansion of these cells plays a role in the CD4+ increases observed with HAART or IL-2+HAART. When expression of CD25 in the dull RA+/RO+ CD4 subsets in these 2 groups was compared, a significant difference was observed (mean values, 75% in the IL-2+HAART group versus 35% in the HAART group;P < .001). Finally, in patients treated with IL-2+HAART, expression of CD25 was higher in the dull RA+/RO+ CD4 cells than in either the memory or naive CD4 subsets (both paired differences were significant;P = .002). This was in contrast to findings in the HAART group, in which progressive increases in CD25 expression from the naive to the dull RA+/RO+ to the memory CD4+ T-cell subsets were observed.
CD25 up-regulation in patients treated with IL-2 is not accompanied by increased expression of other activation markers
Staining with early (CD69) and late (CD95) activation markers was performed to investigate the possibility that increased CD25 expression reflected an overall state of immune activation similar to that occurring after antigenic stimulation. The percentage of either CD4+ or CD8+ T cells expressing CD69 was similar in all 3 groups (data not shown). CD95 expression on CD4+ T cells was similar in the 2 HIV+ groups but was higher than that in the HIV− volunteers (56% in the HIV− group versus 83% in the HAART group and versus 80% in the IL-2+ HAART group; P = .02 for both comparisons). A similar observation was made in the CD8+T-cell pool (54% versus 87% and versus 83%;P = .01).
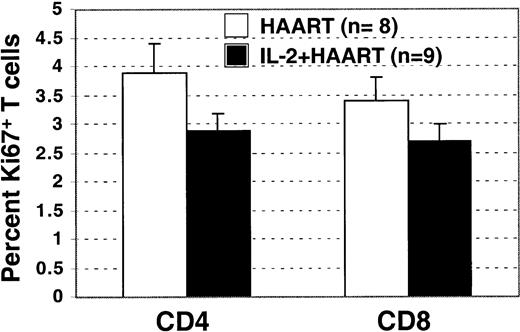
To investigate the possibility that continuous increased levels of CD4+ T-cell proliferation were sustaining the higher CD4+ counts in patients treated with IL-2, intracellular staining for the nuclear antigen Ki67 was performed in a subset of HIV+ patients who had plasma HIV RNA levels below 50 copies/mL and for whom cryopreserved cells from the study-entry time point were available (8 of 10 patients in the HAART group and 9 of 9 patients in the IL-2+HAART group). Ki67 is expressed during the late G1, S, G2, and M phases of the cell cycle and is considered a marker of recent proliferation. An increased percentage of Ki67-positive staining cells has been reported in HIV+patients before initiation of HAART.28 Surprisingly, we found that patients given IL-2 had a lower fraction of T cells (CD4+ or CD8+) that stained positive for Ki67 (Figure 5), a result suggesting that after IL-2 therapy, continuous higher rates of proliferation do not account for the sustained increases in CD4+ T-cell counts. Although the differences were not statistically significant (P = .14 for the CD4+ andP = .48 for the CD8+ T cells), the data suggest that decreased turnover and increased survival may be responsible for the increases in CD4+ T cells observed in patients who have received IL-2.
Persistent increased T-cell proliferation is not the mechanism that maintains high CD4+ counts in IL-2 recipients.
In patients with an HIV viral load below 50 copies/mL, the percentage of Ki67+ CD4+ and CD8+ T cells was not higher in the IL-2+HAART group than in HAART group.
Persistent increased T-cell proliferation is not the mechanism that maintains high CD4+ counts in IL-2 recipients.
In patients with an HIV viral load below 50 copies/mL, the percentage of Ki67+ CD4+ and CD8+ T cells was not higher in the IL-2+HAART group than in HAART group.
Both CD4+/CD25+ and CD4+/CD25− fractions in patients treated with IL-2 can proliferate in response to mitogenic or T-cell-receptor (TCR)–mediated signals
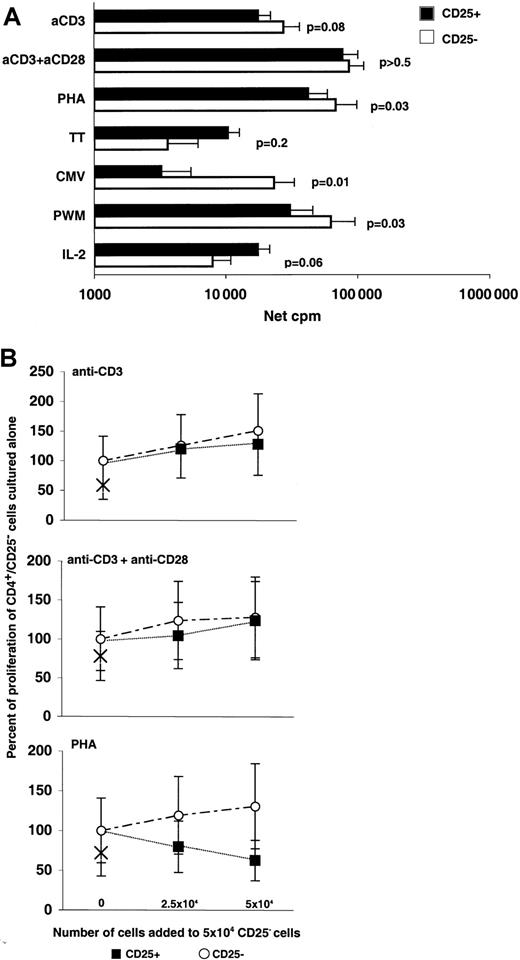
A population of anergic CD4+/CD25+ cells capable of suppressing proliferation of other cells has been reported to be important in regulation of autoimmunity in both animal models and humans.27 31 To examine the possibility that the CD4+/CD25+ population that expands in patients given IL-2 represents an expansion of CD4+/CD25+ immunoregulatory cells, we conducted experiments to study the proliferative capacity of these cells. As shown in Figure 6A, CD4+/CD25+ cells from patients given IL-2 were not anergic and showed various degrees of proliferative responses to all tested stimuli. CD4+/CD25+ cells had weaker responses than CD4+/CD25− cells to the mitogens PHA and PWM (P = .03) and to CMV antigen (P = .01). A trend toward higher responses to IL-2 was also observed (P = .06). Additionally, in all experiments, the background proliferation of CD4+/CD25−cells was higher than that of CD4+/CD25+ cells (P < .01). In experiments designed to detect suppression (Figure 6B), no evidence of suppression was found in the response to TCR stimulation with anti-CD3 or anti-CD3 with anti-CD28. Interestingly, a blunting of the response to PHA that did not lead to complete suppression was evident. This observation is under further investigation.
CD4+/CD25+ cells from IL-2–treated patients are not anergic.
(A) Separated CD4+/CD25+ (black bars) and CD4+/CD25− (white bars) subsets from cells of IL-2–treated patients were stimulated with anti-CD3 with or without anti-CD28 (n = 12), PHA (n = 12), TT (n = 7), CMV (n = 10), PWM (n = 11), and IL-2 (n = 8). Geometric means (net cpm), SE bars, andP values for paired comparisons (paired Student ttest) are shown. (B) CD4+/CD25+ cells from IL-2–treated patients do not suppress TCR-mediated responses of CD4+/CD25− cells. In 6 experiments, incremental numbers of CD4+/CD25+ cells (black solid squares) were added to a fixed number (5 × 104) of CD4+/CD25− cells to test for suppression. Addition of equal numbers of CD4+/CD25− cells (open circles) was used as a control. Results are expressed as the percentage of the proliferative response of the CD4+/CD25− cells cultured alone, and × signs represent the proliferative response of 5 × 104CD4+/CD25+ cells cultured alone. Mean values with SE bars are shown.
CD4+/CD25+ cells from IL-2–treated patients are not anergic.
(A) Separated CD4+/CD25+ (black bars) and CD4+/CD25− (white bars) subsets from cells of IL-2–treated patients were stimulated with anti-CD3 with or without anti-CD28 (n = 12), PHA (n = 12), TT (n = 7), CMV (n = 10), PWM (n = 11), and IL-2 (n = 8). Geometric means (net cpm), SE bars, andP values for paired comparisons (paired Student ttest) are shown. (B) CD4+/CD25+ cells from IL-2–treated patients do not suppress TCR-mediated responses of CD4+/CD25− cells. In 6 experiments, incremental numbers of CD4+/CD25+ cells (black solid squares) were added to a fixed number (5 × 104) of CD4+/CD25− cells to test for suppression. Addition of equal numbers of CD4+/CD25− cells (open circles) was used as a control. Results are expressed as the percentage of the proliferative response of the CD4+/CD25− cells cultured alone, and × signs represent the proliferative response of 5 × 104CD4+/CD25+ cells cultured alone. Mean values with SE bars are shown.
Discussion
This study clearly demonstrated the emergence of a unique population of CD4+/CD25+ T cells in HIV+ patients who had received intermittent IL-2 therapy. These cells are not anergic, do not proliferate continuously or express increased levels of the trimeric IL-2 receptor, and may represent a long-lived population of cells that are predominantly responsible for the increases in CD4+ T cells observed with IL-2 therapy. It is likely that these cells represent the product of IL-2–induced T-cell expansion in the absence of antigenic stimulation.
It was previously reported that naive human T cells can proliferate in vitro and retain their naive phenotype if stimulated in the absence of antigen with a combination of IL-2, IL-6, and tumor necrosis factor α (TNF-α).32,33 In one of these reports,33Unutmaz et al also observed the presence of a small fraction of CD25+ naive CD4+ cells in human peripheral blood and hypothesized that these cells represented the product of cytokine-driven peripheral expansion of the naive CD4+pool. A similar population of naive CD4+/CD25+cells has also been described in human cord blood.34During administration of IL-2, a transient 6- to 10-fold increase in the proliferation rates of both naive and memory CD4+ and CD8+ T cells has been observed.35,36Additionally, during the cycle, serum levels of proinflammatory cytokines (such as IL-6 and TNF-α) increase.37 It thus appears likely that the naive CD4+/CD25+ cells that expand preferentially with IL-2 administration are the product of cytokine-driven proliferation and an in vivo reflection of the previously described in vitro data. This hypothesis seems even more plausible given the strong correlation between the absolute numbers of these cells and the total CD4+ count and the total naive CD4+ counts in HIV+ patients. The weaker correlation in the HIV− volunteers could reflect the fact that far fewer of the cells in the CD4+ pool in these individuals are the products of recent cell division.38Naive T cells with similar phenotypes have been found in studies in animals during cytokine-driven homeostatic proliferation in lymphopenic hosts.39
In our study, we also found a population of dull double-positive RA+/RO+ CD4+ T cells that constituted approximately 10% of the total CD4+ pool in HIV− volunteers and patients in the HAART group and was preferentially expanded in patients given IL-2. This population of cells expressed very high levels of CD25 in IL-2 recipients and decreased proportionally with decreases in CD4+ counts. The source of these cells is unknown. We believe it is most likely that these cells represent naive cells assuming an intermediate phenotype as a result of cytokine-driven peripheral expansion similar to what has been observed in lymphopenic animals.40 It is also possible that they represent memory cells that are reverting to a naive phenotype as a result of cytokine-driven proliferation in the absence of their cognate antigen. In this regard, these cells may be analogous to the population of dull double-positive CD45RA+/RO+ CD4+ T cells described by Hamann et al.30 Those authors concluded that cells of this phenotype were able to produce cytokines such as IL-4 and interferon γ at levels much higher than those produced by naive or double RA+/RO+ bright cells and that they contained substantial numbers of TT-specific precursors. Alternatively, the expanded CD45RA+/RO+CD4+ cells in IL-2 recipients could represent naive cells that have recently encountered antigen. According to Picker et al, that transition, when induced by antigen, is a short-lived event and is associated with bright double-positive CD45RA+/RO+ CD4+cells,41 which are found predominantly in lymphoid tissue. The characteristics of the cells observed in the current study, which were not bright double-positive, were stable, and lacked evidence of recent proliferation, constitute evidence against this hypothesis.
The current study highlights the fact that increased expression of the α chain may not always reflect a heightened immune-activation state. As compared with the expression of CD25 on naive and dull CD45RA+/RO+ CD4+ T cells in IL-2 recipients, CD25 expression on memory CD4+ T cells was not as pronounced and appeared to be less stable over time. Patients studied several months after an IL-2 cycle had only similar or slightly increased levels of CD25 on memory CD4+ cells compared with persons not given IL-2. In agreement with this observation, other markers of activation, such as CD69 and CD95, were not expressed at higher levels in patients treated with IL-2. Additionally, despite persistent long-term increases in CD25 expression, increased expression of the β and γ chains on CD4+ cells of IL-2–treated patients occurred only transiently during the period of IL-2 administration. The short half-lives of the β and γ chains on the cellular surface and the limitations of the flow cytometric technique in identifying low-level expression preclude definitive statements about the degree of expression of the trimeric receptor on the CD4+/CD25+ cells in the patients who received IL-2. Binding studies will be necessary to clarify this issue.
Intracellular staining for the nuclear activation antigen Ki67 was used as an indirect measurement of recent proliferation and activation in a subset of study participants who had an HIV burden of less than 50 copies/mL. No evidence of increased Ki67 expression was detected in the IL-2 group, suggesting that persistent, increased T-cell proliferation in response to endogenously produced IL-2 cannot account for the sustained high CD4+ counts observed in that group. Given this finding, decreased turnover and prolonged survival of these cells is a more plausible explanation for this observation. Other data seem to support this hypothesis.42 Ongoing studies that allow longitudinal evaluation of cell survival in vivo with tracking of T lymphocytes labeled with bromodeoxyuridine or deuterium-glucose should shed additional light on these fundamental areas.43
A CD4+/CD25+ cell population has been identified in studies in animals and humans as a subset of cells with regulatory function that are anergic and immunosuppressive.27,44 These cells have been studied extensively in animal models and are considered to be of thymic origin. Once they encounter their cognate antigen, they become anergic (do not proliferate in response to TCR-mediated signals) and acquire an immunosuppressive function that is not antigen specific.45Their ability to suppress the cytotoxic T lymphocytes of CD8+ T cells has also been reported.46 These immunoregulatory CD4+/CD25+ cells have been observed in humans and identified as terminally differentiated memory cells that do not proliferate in response to anti-CD3, anti-CD3 with anti-CD28, or mitogens such as PHA and concanavalin A; are prone to apoptosis; and express CD25 with high fluorescence intensity.47,48 Despite some similarities (such as the high MFI for CD25 and the expression of homing molecules such as 62L), there are important phenotypic differences between these cells and the CD4+/CD25+ cells in our IL-2–treated group. In animal models, the immunoregulatory cells have been described as CD45RB low, indicating a memory phenotype. Similarly, studies in humans found that these cells were present exclusively in the CD45RO+fraction of CD4+ T cells.49 In the current study, the expanded CD4+/CD25+ cells in patients given IL-2 included a high proportion of naive or dull intermediate RA+/RO+ cells. In addition, no evidence of anergy or suppression of TCR responses was detected in the proliferative responses of the CD25+ and CD25−subsets of CD4+ cells from IL-2 recipients. Together, these data suggest that the CD25+ cells expanded in the presence of IL-2 are not the described CD4+/CD25+suppressor cells, although we cannot exclude the possibility that some of these clones expand during administration of IL-2.
In summary, in HIV+ patients receiving intermittent IL-2 therapy, a sustained increase in CD4+ T-cell counts was observed with a preferential expansion of CD4+ T cells with a naive phenotype. A high proportion of these cells expressed CD25 but not the other chains of the IL-2 receptor. Preliminary data suggest that prolonged survival of this population may represent the main mechanism of the sustained increases in CD4+ count observed after administration of IL-2. Additional studies focusing on the origin, functional characteristics, and properties of these cell subsets will further clarify their role in T-cell homeostasis. The results of ongoing phase III trials of intermittent IL-2 therapy in HIV infection that are assessing clinical end points will be critical in clarifying the clinical implications of our observations.
We thank all the participating patients and the staff of the National Institute of Allergy and Infectious Diseases/Critical Care Medicine Department Clinic for their commitment and enthusiasm, Dr Anthony Fauci for his continued encouragement and support, and Mary Rust for assistance in the preparation of the manuscript.
Funded in whole or in part with federal funds under National Cancer Institute contract NO1-CO-56000. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organization imply endorsement by the US Government.
The US Government has been granted a use patent for intermittent interleukin-2 therapy including Drs. H. Clifford Lane and Joseph A. Kovacs as inventors.
Partly presented in abstract form at the American Association of Immunologists meeting, Experimental Biology 2001, Orlando, Florida, March 31-April 4, 2001. Abstract B-816.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
H. Clifford Lane, Clinical and Molecular Retrovirology Section, Laboratory of Immunoregulation, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Building 10, Room 11S-231, 10 Center Drive, MSC 1876, Bethesda, MD 20892; e-mail: clane@niaid.nih.gov.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal