The translocation t(8;21), AML1/ETO, represents a frequent aberration in de novo acute myelogenous leukemia (AML) and is detectable in up to 40% of AML FAB M2.1 In constitutively transgenic mice, AML1/ETO abrogates fetal hematopoiesis, but in inducible transgenic mice AML1/ETO is not leukemogenic per se.2-4 AML1/ETO is detectable in stem cells of patients in complete continuous remission (CCR) and only an increasing transcript number indicates a forthcoming clinical relapse.5 6 We investigated whether AML1/ETO transcripts are also present in bone marrow (BM) aspirates of 18 adults (22 to 76 years old) without neoplasia, 4 adults (25 to 76 years old) with hematopoietic neoplasia (non-Hodgkin lymphoma [NHL], myelodysplasia syndrome [MDS]), and 156 cord blood (CB) samples from healthy newborns. The samples were investigated by 3 independent laboratories in Goettingen, Vienna, and Hannover.
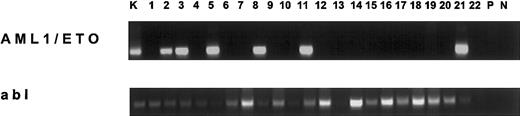
Of 22 adult bone marrow samples, 6 (27%) were AML1/ETO-positive (Figure 1), of which 2 derived from patients with NHL. Of all samples, 2 exhibited neoplastic cells (NHL, MDS), of which one was AML1/ETO-positive.
Transcription of AML1/ETO in adult bone marrow from individuals without AML.
In the upper panel the 185-bp amplification product of the nested reverse trancriptase (RT)–PCR is shown in the positive control cell lineage Kasumi-1 (K) and 6 bone marrow samples of individuals without AML followed by the primary and nested negative controls (P, N, respectively). In the lower panel, the corresponding internal positive controls using an abl RT-PCR are shown. We used 2% to 3% of the total cDNA of each sample as PCR template. Goettingen primary PCR, AML1-A 5′-ACC TCAGGTTTGTCGGTCG-3′ (bp 1976 to 1974), ETO-B 5′-GAACTGGTTCTTGGAGCTCCT-3′ (bp 2211 to 2231). Goettingen nested PCR, AML1-C 5′-AAA AGCTTCACTCTGACCATCA-3′ (bp 2008 to 2029), ETO-D 5′-GGCATTGTTGGAGGAGTCAG-3′ (bp 2173 to 2192). Vienna primary PCR, AML1-1 5′-AGCCATGAAGAACCAGG-3′ (bp 1941 to 1960), ETO-1 5′-AGGCTGTAGGAGAATGG-3′ (bp 2265 to 2281). Vienna nested PCR, AML1-2 5′-TACCACAGAGCCATCAAA-3′ (bp 2062 to 2079), ETO-2 5′-GTTGTCGGTGTAAATGAA-3′ (bp 2229 to 2246). Hannover real-time PCR was performed as described.7 All nucleotide sequences refer to Miyoshi et al.8 An abl RT-PCR served as internal positive control and specificity of amplification was confirmed by cycle sequencing as described.4 The sensitivity of the AML1/ETO RT-PCRs was 10−6 (cell-in-cell dilution of Kasumi-1 in HL60). RNA preparation, reverse transcription, and PCR were performed in separate laboratories and in repetitive negative controls contaminations were not observed.
Transcription of AML1/ETO in adult bone marrow from individuals without AML.
In the upper panel the 185-bp amplification product of the nested reverse trancriptase (RT)–PCR is shown in the positive control cell lineage Kasumi-1 (K) and 6 bone marrow samples of individuals without AML followed by the primary and nested negative controls (P, N, respectively). In the lower panel, the corresponding internal positive controls using an abl RT-PCR are shown. We used 2% to 3% of the total cDNA of each sample as PCR template. Goettingen primary PCR, AML1-A 5′-ACC TCAGGTTTGTCGGTCG-3′ (bp 1976 to 1974), ETO-B 5′-GAACTGGTTCTTGGAGCTCCT-3′ (bp 2211 to 2231). Goettingen nested PCR, AML1-C 5′-AAA AGCTTCACTCTGACCATCA-3′ (bp 2008 to 2029), ETO-D 5′-GGCATTGTTGGAGGAGTCAG-3′ (bp 2173 to 2192). Vienna primary PCR, AML1-1 5′-AGCCATGAAGAACCAGG-3′ (bp 1941 to 1960), ETO-1 5′-AGGCTGTAGGAGAATGG-3′ (bp 2265 to 2281). Vienna nested PCR, AML1-2 5′-TACCACAGAGCCATCAAA-3′ (bp 2062 to 2079), ETO-2 5′-GTTGTCGGTGTAAATGAA-3′ (bp 2229 to 2246). Hannover real-time PCR was performed as described.7 All nucleotide sequences refer to Miyoshi et al.8 An abl RT-PCR served as internal positive control and specificity of amplification was confirmed by cycle sequencing as described.4 The sensitivity of the AML1/ETO RT-PCRs was 10−6 (cell-in-cell dilution of Kasumi-1 in HL60). RNA preparation, reverse transcription, and PCR were performed in separate laboratories and in repetitive negative controls contaminations were not observed.
Of 156 CB samples, 63 (40%) were AML1/ETO-positive. We subjected 6 positive CB samples to real-time polymerase chain reaction (PCR) to determine the AML1/ETO copy number. Typical normalized ratios (AML1/ETO/housekeeping gene copies) of patients in CCR are 5 × 10−5 to 1 × 10−3, whereas patients with newly diagnosed AML range between 0.1 and 2.14.7 The ratios of the 6 CB samples ranged between 1.9 × 10−5and 7.8 × 10−3, of which 3 out of 6 were comparable to patients with CCR. Assuming similar transcriptional levels, the number of AML1/ETO-positive cells in half of all positive healthy newborns may resemble that of patients with AML in CCR.
We postulate that positive cells are either generated by permanent mutagenesis or are derived from aberrant hematopoietic stem cells. Since the gene fusion AML1/ETO is prone to be induced by radiation in vitro,9 an ongoing generation in all age groups by external mutagens may explain our observations. In this model it seems unlikely that AML1/ETO-positive cells have a survival advantage; otherwise, a much higher incidence in the elderly should be expected, though our observations may be influenced by the better cDNA quality of the cord blood samples.
On the other hand, the t(8;21) may be generated in early hematopoiesis. Positive cells will then permanently derive from a positive stem cell pool but only few positive individuals may attract secondary genetic alterations and progress to AML. The latter mechanism is supported by the recent report of Wiemels et al on the detection of AML1/ETO in neonatal blood spots of children who developed a corresponding AML with more than 10 years latency.10
J.B. is supported by a grant from the Deutsche JoséCarreras Leukämie-Stiftung. We are grateful to Professor B. Wormann (Klinikum Braunschweig, Germany) for stimulating discussion and scientific support and to Midia Jlussi for technical assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal