Nramp2 (DMT1) is a pH-dependent divalent cation transporter that acts as the transferrin-independent iron uptake system at the intestinal brush border and also transports iron released from transferrin across the membrane of acidified endosomes. In this study, RAW264.7 macrophages and 2 independently derived murine Sertoli cells lines, TM4 and 15P-1, were used to further study the subcellular localization of Nramp2/DMT1 in phagocytic cells, including possible recruitment to the phagosomal membrane. Nramp2/DMT1 was localized primarily to the EEA1-positive recycling endosome compartment, with some overlapping staining with Lamp1-positive late endosomes. After phagocytosis, immunofluorescence analysis and in vitro biochemical studies using purified latex bead-containing phagosomes indicated Nramp2/DMT1 recruitment to the membrane of Lamp1, cathepsin D, and rab7-positive phagosomes. Nramp2/DMT1 was also found associated with erythrocyte-containing phagosomes in RAW macrophages and with the periphery of sperm-containing phagosomes in Sertoli cells. These results suggest that, as for the macrophage-specific Nramp1 protein, Nramp2/DMT1 may transport divalent metals from the phagosomal space.
Introduction
The Nramp2 gene,1 also known as divalent metal transporter 1 (DMT1),2divalent cation transporter 1 (DCT1),3 and SLC11A2, was initially identified by cross-hybridization to theNramp1 gene.1 Naturally occurring or experimentally induced mutations at Nramp1(natural-resistance–associated macrophage protein 1) cause susceptibility to infection by several intracellular parasites, including Salmonella, Mycobacterium, andLeishmania.4 Nramp1 is expressed exclusively in circulating phagocytes, where it localizes to the late endosomal/lysosomal compartment.5,6 On phagocytosis, it is recruited to the phagosomal membrane,5,6 where its function is important for efficient antimicrobial activity against intracellular pathogens.7,8 We have obtained evidence suggesting that Nramp1 functions as a pH-dependent manganese efflux pump at the phagosomal membrane.9 Nramp1 and Nramp2/DMT1 are closely related and have similar predicted secondary structural features that together define a new family of membrane proteins structurally and functionally conserved from bacteria to humans.10 11
Nramp2 mRNA expression is ubiquitous and is highest in the brain, proximal intestine, kidney, bone marrow, and reticulocytes in particular.1,3,12 Functional studies in Xenopusoocytes have demonstrated that Nramp2/DMT1 (DCT1) is a pH-dependent divalent cation transporter that has a broad substrate specificity, including Fe2+, Mn2+, Zn2+, Cd2+.3 Likewise, expression studies in transfected HEK29313 and CaCo-2 human cells14 have shown that Nramp2/DMT1 can also transport Fe2+ in mammalian cells. Studies in transfected Chinese hamster ovary (CHO) cells have shown that Nramp2/DMT1 can transport iron at the plasma membrane into the intracellular, calcein-accessible, so-called labile iron pool.15 The Nramp2 gene is mutated (G185R) in mk mice and Belgrade(b) rats, both of which display a severe iron deficiency associated with impaired intestinal iron uptake, impaired iron acquisition by peripheral tissues, and severe microcytic anemia.16,17 Nramp2/DMT1 protein is expressed at the brush border of the duodenum epithelium, where it is dramatically induced on deprivation of dietary iron.18 19 Together, these observations have established that Nramp2/DMT1 is the major, transferrin-independent, intestinal iron uptake system of mammals.
In nonintestinal tissues, including primary cells such as reticulocytes12,20,21 and transfected cell lines,20 Nramp2/DMT1 protein has been found to colocalize with fluorescently labeled transferrin (transferrin–fluorescein isothiocyanate [FITC]) in recycling endosomes. These observations, together with studies in mk mice and b rats showing that the defect in iron acquisition in peripheral tissues in these animals cannot be corrected by oral or intravenous administration of iron, have strongly suggested that Nramp2/DMT1 may play a role in the transport of transferrin iron across the membrane of acidified endosomes.12,22 However, in Hep-2 cells overexpressing a GFP-Nramp2 fusion, this protein is found associated with late endosomes and lysosomes and is poorly associated with early endosomal markers such as the transferrin receptor and EEA1.23 Studies with phagosomes purified from the macrophage cell line J774A have suggested that a portion of the Nramp2/DMT1 protein may become associated with latex beads containing phagosomes in macrophages.20
In a preliminary screen of a number of cell lines derived from different anatomic sites, Nramp2/DMT1 protein expression was detected in a Sertoli cell line, TM4.20 Sertoli cells are nurse cells of the testis that play a key role in the normal development of spermatogenic cells24 and participate in a peculiar iron transport system unique to maturing germ cells.25 They also are phagocytic and are responsible for the removal of apoptotic degenerating germ cells in the testis.26 27 Here, we have used immunofluorescence, confocal microscopy, and density-gradient centrifugation to localize Nramp2/DMT1 protein in 2 independently derived Sertoli cell lines and RAW murine macrophages before and after phagocytosis of latex particles, erythrocytes, and spermatozoids.
Materials and methods
Cell culture and reagents
The mouse Sertoli cell line TM4 was obtained from the American Type Culture Collection (ATCC, Manassas, VA) and was cultured under conditions recommended by the supplier. The mouse 15P-1 cell line (kind gift of F. Cuzin, Nice, France) and the macrophage cell line RAW264.7 were cultured in Dulbecco modified Eagle medium supplemented with 10% heat-inactivated fetal bovine serum (FBS) and glutamine (1 mM). WEHI 231 (B lymphocyte) and Chinese hamster ovary (CHO) cell lines were grown in α-MEM supplemented with 10% FBS and glutamine. CHO and RAW cells transfected with a c-Myc–tagged wild-type Nramp2 or Nramp1 expression plasmid were generated and used as previously described.20 All media and media supplements were purchased from Gibco BRL (Burlington, ON, Canada). Rabbit polyclonal anti-Nramp2/DMT1 was obtained as previously described,20 mouse monoclonal anti–c-Myc clone 9E10 was purchased from Babco (Cumberland, VA), goat anti-EEA1 and rabbit anti-Rab7 were from Santa Cruz Biotechnology (Santa Cruz, CA), and sheep red blood cells (RBCs) and rabbit antisheep RBC antibody were from ICN-Cappel (Aurora, OH). Fluorescein isothiocyanate (FITC)–conjugated antirat and antigoat, Cy3 or rhodamine antimouse and antirabbit, and horseradish peroxidase (HRP)–conjugated antirabbit, antimouse, and antigoat were from Jackson ImmunoResearch Laboratories (West Grove, PA).
Immunofluorescence
TM4 cells were grown on poly-L-lysine (Sigma Chemical, St Louis, MO)–coated glass coverslips for 48 hours and were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) for 30 minutes at 4°C. Immunofluorescence was performed as previously described5 with the following modifications: incubation with the primary antibody was for 5 hours at room temperature for rabbit polyclonal anti-Nramp2/DMT1 antiserum (1:100), rat anti-Lamp1 monoclonal antibody (1:400), mouse anti–c-Myc monoclonal antibody (1:1000), and goat anti-EEA1 (1:200), followed by a 1-hour incubation with secondary antibodies, antirabbit conjugated to rhodamine (1:300), Cy3 (1:1500), or antirat or antigoat conjugated to FITC (1:100). Colocalization studies were performed using a Zeiss laser confocal microscope fitted with a ×63 objective (Thornwood, NY). Phagosomes were formed by incubating the cells with 3-μm latex beads (Sigma Chemical), diluted 1:300 in complete culture medium, for 1 hour at 37°C in 5% CO2.
Phagosome fractionation
Phagosomes were isolated as described previously20on a sucrose density gradient. Phagosomes were extracted from the 10% to 25% sucrose interface, washed with PBS containing protease inhibitors, and recovered by a final centrifugation at 40 000g at 4°C (SW41; Beckman, Mississauga, ON, Canada). The final pellets were resuspended in 2× Laemmli sample buffer. Phagosomes prepared by this protocol have previously been shown to be largely free of endoplasmic reticulum and Golgi vesicles (BiP, galactosyl transferase),7,28 though they show some reactivity to endoplasmin and Calnexin.29
Immunoblotting
Crude membrane and phagosomal fractions were prepared as described above and in an earlier study.20 To verify the protein content of each phagosomal fraction, increasing amounts of an internal standard of total membrane proteins were run in parallel and were compared by Coomassie blue staining of the gel after sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). In addition, for each phagosomal fraction, OD590 was measured to load similar amounts of phagosomal fractions in kinetic studies. Similar transfer of proteins to the membrane was determined by staining the blots with Ponceau S red (Sigma Chemical). Incubation of the blots with antibody and detection of specific immune complexes by enhanced chemiluminescence (ECL) was as previously described.5
Spermatogenic cell preparation and phagocytosis assays
Spermatogenic cells were recovered from the testes of 3-month-old male mice as described elsewhere.27Spermatozoids were adjusted at a concentration of 25 × 106/mL in PBS, pH 8, labeled for 30 minutes at room temperature with 0.5 mg/mL biotin (NHS-LS-Biotin; Pierce, Rockford, IL) and then passaged several times through a 24-gauge syringe to induce cellular damage. They were then added at an approximate ratio of 10:1 to TM4 cells plated on poly-L-lysine–coated coverslips, followed by further incubation for 1 hour at 37°C to allow phagocytosis. TM4 cells were washed extensively with PBS before they were fixed with paraformaldehyde; then they were stained with avidin-FITC (Sigma Chemical) and for Nramp2/DMT1 protein, as described above.
Sheep RBCs were opsonized with rabbit anti-sheep RBC antibody at 1:50 for 1 hour at room temperature and washed 3 times with PBS. Approximately 50 RBCs were added per macrophage in complete media for 1 hour at 37°C, 5% CO2, to induce erythrophagocytosis. Nonphagocytosed RBCs were lysed by hypotonic shock as previously described.30 Cells where subsequently processed for phagosomal fractionation as described above.
Results
Subcellular localization of Nramp2/DMT1 in macrophages and Sertoli cells
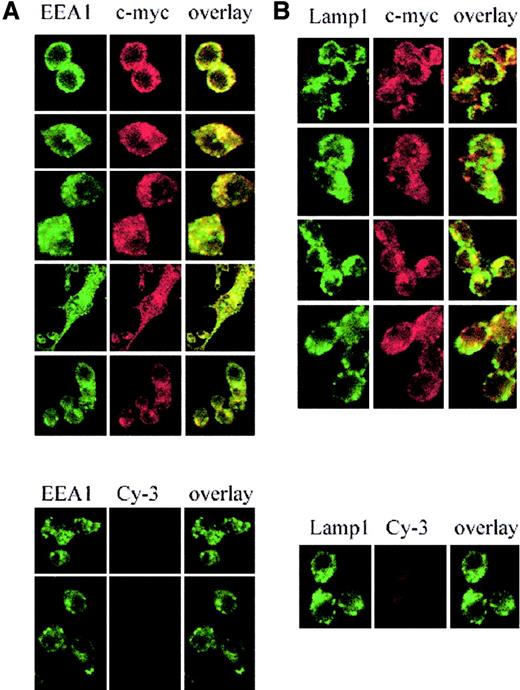
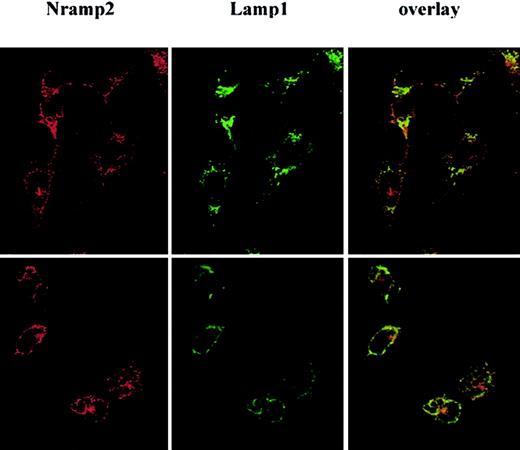
Nramp2/DMT1 localization was analyzed by immunofluorescence in RAW macrophages expressing a transfected c-Myc–tagged version of Nramp2/DMT1. In these cells, c-Myc reactivity showed a network of fine punctate intracellular staining throughout the cytoplasm (Figure1A-B, upper panels, red staining) that very similar to that seen for the endosomal marker EEA1 (early endosomal antigen; Figure 1A, left panel, green staining). Superimposition of the 2 images (overlay, yellow staining) showed extensive overlap suggesting colocalization of Nramp2/DMT1 and EEA1 in RAW cells. Immunofluorescence with an antibody against the late endosomal/lysosomal marker Lamp1 (Figure 1B, green staining) also produced a punctated intracellular staining that was distinct (large intracellular vesicles) from that seen for Nramp2/DMT1 and EEA1. Superimposition of the Lamp1 and Nramp2/DMT1 (c-Myc) staining (overlay, yellow staining) showed a small number of structures that appeared positive for both markers, suggesting some overlap. Staining with the secondary antibody alone (Cy-3) was used as a control for the specificity of the c-Myc staining (lower panels). Similar colocalization results were obtained when the distribution of endogenous Nramp2/DMT1 protein was compared with EEA1 (data not shown), transferrin,20 and Lamp1 (Nramp2/DMT1, Lamp1 in Figure2, upper panel) in the Sertoli cell line TM4.
Subcellular localization of Nramp2/DMT1 in transfected RAW macrophages.
Immunofluorescence detection of a c-Myc–tagged Nramp2 protein expressed in RAW macrophages using a mouse monoclonal anti–c-Myc antibody (9E10) and a Cy3-labeled secondary antimouse antibody (red staining). The recycling endosome marker EEA1 was detected using a goat antiserum revealed with Alexa 488–coupled secondary antibody (green staining, left panel A). The late endosomal/lysosomal marker, Lamp1, was detected with a specific rat monoclonal antibody and revealed with FITC-conjugated antirat antibody (green staining, right panel B). Cells were examined under a × 63 objective by confocal microscopy. Green staining was overlaid with the red staining (yellow shows colocalization).
Subcellular localization of Nramp2/DMT1 in transfected RAW macrophages.
Immunofluorescence detection of a c-Myc–tagged Nramp2 protein expressed in RAW macrophages using a mouse monoclonal anti–c-Myc antibody (9E10) and a Cy3-labeled secondary antimouse antibody (red staining). The recycling endosome marker EEA1 was detected using a goat antiserum revealed with Alexa 488–coupled secondary antibody (green staining, left panel A). The late endosomal/lysosomal marker, Lamp1, was detected with a specific rat monoclonal antibody and revealed with FITC-conjugated antirat antibody (green staining, right panel B). Cells were examined under a × 63 objective by confocal microscopy. Green staining was overlaid with the red staining (yellow shows colocalization).
Subcellular localization of Nramp2/DMT1 and Lamp1 in Sertoli cells after phagocytosis of latex particles.
TM4 cells were fixed and stained with anti-Lamp1 followed by an FITC-conjugated secondary antibody (green) and anti-Nramp2/DMT1 with rhodamine conjugated secondary antibody (red), either before (upper panel) or 1 hour after phagocytosis of latex beads (lower panel). Cells were examined under a ×63 objective by confocal microscopy. Rhodamine (red, Nramp2/DMT1) and FITC (green, Lamp1) staining were overlaid (yellow shows overlap).
Subcellular localization of Nramp2/DMT1 and Lamp1 in Sertoli cells after phagocytosis of latex particles.
TM4 cells were fixed and stained with anti-Lamp1 followed by an FITC-conjugated secondary antibody (green) and anti-Nramp2/DMT1 with rhodamine conjugated secondary antibody (red), either before (upper panel) or 1 hour after phagocytosis of latex beads (lower panel). Cells were examined under a ×63 objective by confocal microscopy. Rhodamine (red, Nramp2/DMT1) and FITC (green, Lamp1) staining were overlaid (yellow shows overlap).
Colocalization of Nramp2/DMT1 and Lamp1 around latex particles internalized by macrophages and Sertoli cells
Previous studies have suggested that Nramp2/DMT1 may be targeted to the phagosome in macrophages.20 This was further investigated in the Sertoli cell line TM4, after phagocytosis of latex beads by these cells (Figure 2). In control TM4 cells exposed to medium (upper panel), both Nramp2/DMT1 (rhodamine conjugate; red stain) and Lamp1 (FITC conjugate; green stain) showed punctated intracellular staining, with some degree of overlap detected on superimposition of the 2 images (yellow stain). In TM4 cells with phagocytosed latex beads (lower panel), Nramp2/DMT1 and Lamp1 staining could be observed at the periphery of internalized beads, and superimposition of the 2 images suggested that indeed both proteins may be present at that site. A similar apparent phagosome recruitment of Nramp2/DMT1 was detected in c-Myc–Nramp2 RAW–transfected cells (data not shown).20
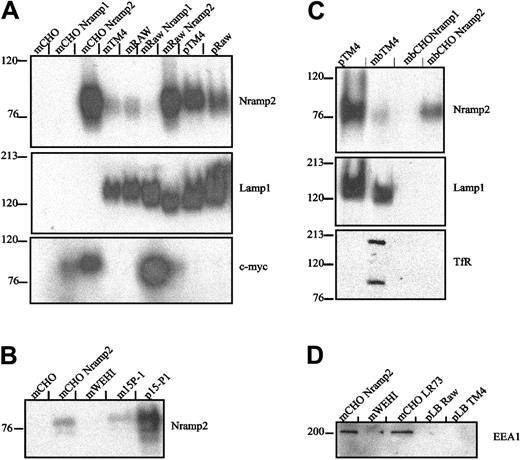
The apparent colocalization of Lamp1 and Nramp2/DMT1 at the periphery of latex phagosomes in TM4 and RAW cells may reflect association of the 2 proteins with the phagosome or it may be an optical artifact caused by the displacement of independent vesicular compartments individually positive for either protein. To distinguish between these possibilities, latex bead-containing phagosomes were purified from TM4 and RAW cells by sucrose gradient centrifugation.20 Equal amounts of protein from total membrane fractions (m) or purified phagosomal fractions (p) were analyzed by SDS-PAGE and immunoblotting (Figure3A). Nramp2/DMT1 was detected at high levels in the total membrane fraction of control CHO and RAW cells transfected and overexpressing a recombinant, c-Myc–tagged Nramp2/DMT1 protein (upper panel; mCHONramp2 and mRAWNramp2, positive controls). Normal CHO cells (mCHO) and CHO cells transfected with the Nramp1 homolog (mCHONramp1) were negative for Nramp2/DMT1 expression (negative controls). Endogenous Nramp2/DMT1 protein was also detected with an anti-Nramp2/DMT1 antibody in membrane fractions from either TM4 or RAW cells and from RAW cells transfected and overexpressing a c-Myc–tagged Nramp1 (Figure 3A). Importantly, endogenous Nramp2/DMT1 was greatly enriched in latex phagosomes purified from either TM4 or RAW cells by a factor of at least 8-fold when compared to total membrane fractions. Latex phagosomes formed under these conditions in TM4 and RAW cells were also positive for Lamp1 (middle panel). To rule out possible cell line-specific effects, latex phagosomes formed in another phagocytic murine Sertoli cell line, 15-P1, were similarly analyzed and found to be enriched for Nramp2/DMT1 protein (Figure 3B).
Western blot analysis of Nramp2/DMT1 association with latex particle–containing phagosomes formed in Sertoli cells and macrophages.
(A) Latex bead–containing phagosomes were purified from cell homogenates of TM4 Sertoli cells and from RAW macrophages. Crude membrane extracts (m) from control CHO (CHO LR73), TM4, and RAW cells or from stable c-Myc–Nramp1 or c-Myc–Nramp2 CHO or RAW transfectants22 or from latex bead–containing phagosomes (p) isolated from either TM4 (pTM4) or from RAW cells (pRAW) were separated by SDS-PAGE. Immunoblots were sequentially analyzed with a rabbit antimouse Nramp2/DMT1 antiserum (upper panel), a rat anti-Lamp1 (middle panel), and a mouse anti–c-Myc (lower panel) monoclonal antibody and revealed by ECL. (B) Crude membrane proteins (m) from control CHO and WEHI cells, c-Myc–Nramp2 CHO transfectants, murine Sertoli cell line 15-P1, or latex bead phagosomes isolated from 15P-1 (p15P-1) were analyzed by immunoblotting with a rabbit anti-Nramp2/DMT1 antiserum. (C) Latex bead phagosomes (p) from TM4 cells, together with membrane fractions (m) from control TM4, and CHO cells and from c-Myc–Nramp2 CHO transfectants were analyzed by immunoblotting with antibodies directed against Nramp2/DMT1, Lamp1, and the transferrin receptor (TfR). (D) Crude membrane proteins (m) from CHO or WEHI cells (used as negative controls) or from c-Myc–Nramp2 CHO transfectants (used as positive controls) or from latex bead–containing phagosomes isolated from RAW (pRAW) of TM4 cells (pTM4) were analyzed with an anti-EEA1 antibody.
Western blot analysis of Nramp2/DMT1 association with latex particle–containing phagosomes formed in Sertoli cells and macrophages.
(A) Latex bead–containing phagosomes were purified from cell homogenates of TM4 Sertoli cells and from RAW macrophages. Crude membrane extracts (m) from control CHO (CHO LR73), TM4, and RAW cells or from stable c-Myc–Nramp1 or c-Myc–Nramp2 CHO or RAW transfectants22 or from latex bead–containing phagosomes (p) isolated from either TM4 (pTM4) or from RAW cells (pRAW) were separated by SDS-PAGE. Immunoblots were sequentially analyzed with a rabbit antimouse Nramp2/DMT1 antiserum (upper panel), a rat anti-Lamp1 (middle panel), and a mouse anti–c-Myc (lower panel) monoclonal antibody and revealed by ECL. (B) Crude membrane proteins (m) from control CHO and WEHI cells, c-Myc–Nramp2 CHO transfectants, murine Sertoli cell line 15-P1, or latex bead phagosomes isolated from 15P-1 (p15P-1) were analyzed by immunoblotting with a rabbit anti-Nramp2/DMT1 antiserum. (C) Latex bead phagosomes (p) from TM4 cells, together with membrane fractions (m) from control TM4, and CHO cells and from c-Myc–Nramp2 CHO transfectants were analyzed by immunoblotting with antibodies directed against Nramp2/DMT1, Lamp1, and the transferrin receptor (TfR). (D) Crude membrane proteins (m) from CHO or WEHI cells (used as negative controls) or from c-Myc–Nramp2 CHO transfectants (used as positive controls) or from latex bead–containing phagosomes isolated from RAW (pRAW) of TM4 cells (pTM4) were analyzed with an anti-EEA1 antibody.
To verify that the apparent enrichment of Nramp2/DMT1 in TM4 and RAW phagosomes was not caused by contamination by endosomal or lysosomal fractions during phagosome isolation, mixing experiments were performed. Homogenates from CHO cells overexpressing either c-Myc–Nramp1 or c-Myc–Nramp2 were mixed with cell homogenates of TM4 and RAW cells that had phagocytosed latex beads before phagosome isolation and immunoblotting. Analysis of the same blots (Figure 3A, lower panel) with an anti–c-Myc antibody shows c-Myc reactivity only in membrane fractions from CHO or RAW c-Myc–Nramp1 and c-Myc–Nramp2 transfectants, whereas both phagosome fractions from TM4 (pTM4) and RAW (pRAW) remained negative, arguing against contamination of the phagosome fractions by endosomes, lysosomes, or both. Additional immunoblots with markers of early endosomal compartments (transferrin receptor, EEA1) or late endosomal, lysosomal (Rab7, cathepsin D) identified expression of the tranferrin receptor (plasma membrane and endosomes) in the total membrane fractions but not in Nramp2/DMT1- and Lamp1-positive phagosomal fractions of TM4 cells (Figure 3C, lower panel). In addition, the endosomal marker EEA1 was only detected in membrane from WEHI cells, CHO cells, and c-Myc–Nramp1 transfectants, whereas latex phagosomes formed in RAW or TM4 cells were negative for this marker, supporting the absence of early endosome contaminants in phagosome preparations (Figure 3D). Finally, latex beads containing phagosomal fractions of RAW and TM4 cells were enriched for the late lysosomal markers Rab7 and cathepsin D (see below). These results confirm the association of Nramp2/DMT1 protein with the latex phagosomes formed in macrophage and Sertoli cell lines.
Nramp2/DMT1 associates with phagocytosed erythrocytes in RAW macrophages
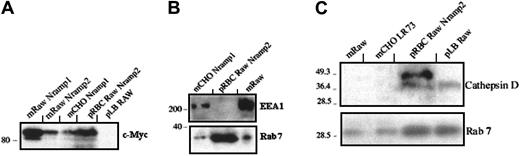
Erythrocytes (RBCs) are a main source of iron in the body, accounting for 75% of total body iron stores, and effete RBCs are phagocytosed by macrophages. A possible contribution of Nramp2/DMT1 to this recycling process remains unknown and was analyzed. Sheep RBCs were opsonized with a rabbit antibody and were fed to c-Myc–Nramp2 RAW transfectants. After phagocytosis, phagosome fractionation and analysis by immunoblotting were performed. Tracking the c-Myc–tagged protein in c-Myc–Nramp2/DMT1 RAW transfectants, as opposed to following endogenous Nramp2/DMT1, was carried out to avoid problems of cross-reactivity of the secondary antibody (rabbit antimouse Nramp2/DMT1) and to avoid possible interference from endogenous Nramp2/DMT1 possibly expressed in erythroid cells used in the assay.12 Results in Figure4A indicate that c-Myc–Nramp2/DMT1 was enriched in RBC phagosomes formed in RAW transfectants but was absent from Latex phagosomes formed in untransfected RAW or in control RBCs opsonized with the antirabbit antibody (data not shown). As for latex phagosomes formed in RAW and TM4 cells, RBC phagosomes formed in c-Myc–Nramp2 RAW transfectants were negative for the endosomal marker EEA1 (Figure 4B). However, they were positive for Rab 7, a small GTPase protein involved in late endosomal fusion and associated with late-stage phagosomes31 (Figure 4B-C). Rab 7 was also associated with latex phagosomes from RAW cells (Figure 4C). The lysosomal hydrolase cathepsin D, another matrix marker of fully mature phagolysosomes, was also associated with latex bead- and RBC-containing phagosomes from RAW and c-Myc–Nramp2 RAW transfectants (Figure 4C). These results demonstrate that Nramp2/DMT1 is associated with the membrane of fully mature EEA1- and transferrin receptor-negative, Rab7- and cathepsin D-positive erythrocytes or latex beads containing phagosomes formed in RAW and TM4 cells.
Nramp2/DMT1 is associated with erythrocyte-containing phagosomes in RAW macrophages.
(A) Crude membrane extracts (m) from control c-Myc–Nramp1 CHO, c-Myc–Nramp2/DMT1, c-Myc–Nramp1 RAW cells, RBCs containing phagosomes, or latex bead–containing phagosomes (p), prepared from, respectively, c-Myc–Nramp2 RAW and RAW cells, were analyzed for anti–c-Myc reactivity. (B) Crude membrane extracts (m) from c-Myc–Nramp1 CHO or RAW cells or RBCs containing phagosomes from c-Myc–Nramp2 RAW cells (p) were sequentially probed with goat anti-EEA1 and rabbit anti–Rab 7 antibodies. (C) Crude membrane extracts (m) from RAW or untransfected CHO cells or RBCs containing phagosomes from c-Myc–Nramp2 RAW cells and latex bead–containing phagosomes from RAW cells (p) were analyzed with rabbit anti–cathepsin D and anti–Rab 7 antibodies.
Nramp2/DMT1 is associated with erythrocyte-containing phagosomes in RAW macrophages.
(A) Crude membrane extracts (m) from control c-Myc–Nramp1 CHO, c-Myc–Nramp2/DMT1, c-Myc–Nramp1 RAW cells, RBCs containing phagosomes, or latex bead–containing phagosomes (p), prepared from, respectively, c-Myc–Nramp2 RAW and RAW cells, were analyzed for anti–c-Myc reactivity. (B) Crude membrane extracts (m) from c-Myc–Nramp1 CHO or RAW cells or RBCs containing phagosomes from c-Myc–Nramp2 RAW cells (p) were sequentially probed with goat anti-EEA1 and rabbit anti–Rab 7 antibodies. (C) Crude membrane extracts (m) from RAW or untransfected CHO cells or RBCs containing phagosomes from c-Myc–Nramp2 RAW cells and latex bead–containing phagosomes from RAW cells (p) were analyzed with rabbit anti–cathepsin D and anti–Rab 7 antibodies.
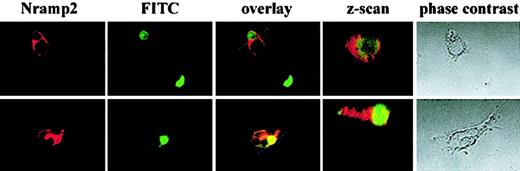
Nramp2/DMT1 associates with phagocytosed sperm cells in TM4 cells
One of the major physiological roles of Sertoli cells is the elimination of degenerating spermatozoids in the testis.24-27 Nramp2/DMT1 presence at the phagosomal membrane in Sertoli cell lines may play a role in capturing iron from degenerating spermatozoids and in recycling it in the “iron shuttle” system of these cells. To test this hypothesis, spermatogenic cell preparations were labeled by biotinylation, allowed to be phagocytosed by TM4 Sertoli cells, and washed and fixed for immunofluorescence. Fixation and double staining with avidin-FITC and anti-Nramp2/DMT1 antiserum were performed after a 1-hour phagocytosis period. Approximately 20% of the Sertoli cell population was found to contain phagocytosed sperm cells under these conditions (Figure5). In phagosomes containing intact or degenerating spermatozoids (green staining), Nramp2/DMT1 presence was enhanced in the immediate vicinity of the spermatozoid (red staining). Moreover, a Z-scan of the phagocytosed sperm clearly demonstrates that the sperm is inside the cell and is surrounded by Nramp2/DMT1 immunostaining. These data strongly suggest that Nramp2/DMT1 becomes associated with spermatozoid-containing phagosomes in TM4 Sertoli cells.
Nramp2/DMT1 localizes around phagocytosed spermatozoids in TM4 cells.
Biotinylated spermatogenic cells were fed to TM4 cells before fixation and staining with FITC-avidin and anti-Nramp2/DMT1 antiserum and a rhodamine conjugated secondary antibody. Slides were analyzed by confocal microscopy, and images were overlaid. Phase-contrast images of the cells and a z-axis centered on the phagocytosed sperm are included.
Nramp2/DMT1 localizes around phagocytosed spermatozoids in TM4 cells.
Biotinylated spermatogenic cells were fed to TM4 cells before fixation and staining with FITC-avidin and anti-Nramp2/DMT1 antiserum and a rhodamine conjugated secondary antibody. Slides were analyzed by confocal microscopy, and images were overlaid. Phase-contrast images of the cells and a z-axis centered on the phagocytosed sperm are included.
Discussion
Localization studies in primary cells and in transfected cell lines20-22 have established that Nramp2/DMT1 protein is associated with the plasma membrane and recycling endosomes, where it colocalizes with transferrin.20 This localization is in keeping with the function of this protein as a pH-dependent iron transporter at the plasma membrane and in recycling endosomes.3,12 Interestingly, in one study of transfected liver Hep-2 cells, Nramp2/DMT1 was associated with Lamp1 but did not colocalize with 2 early endosomal markers, EEA1 and TfR.23In this report, we investigated the subcellular localization of Nramp2/DMT1 in 2 murine-cultured Sertoli cell lines of the testis (TM4, 15P-1) and in RAW264.7 murine macrophages. We further examined the possible association of Nramp2/DMT1 with phagosomes formed in these cells. Nramp2/DMT1 was found primarily expressed in the early EEA1-positive compartment, with possible additional staining in the late endosomal compartment of these phagocytic cells. The Nramp2/DMT1 isoform mainly expressed in these cells is the non-iron response element (IRE)–containing isoform (F.C.-H. and P.G., unpublished data, August 2001). Importantly, we report that after phagocytosis, Nramp2/DMT1 becomes associated with the phagosome. Characterization of Nramp2/DMT1-positive phagosomes shows that they are negative for markers from the early endocytic pathway—EEA1 and the transferrin receptor (TfR)—but remain positive for markers from the late endosomal/lysosomal pathway—Lamp1, rab7, and cathepsin D. This suggests that Nramp2/DMT1 is actively recruited at the phagosomal membrane and is a feature of phagosome maturation to phagolysosome. These results are in agreement with our previous preliminary findings20 and suggest that Nramp2/DMT1 may carry out active transport at the phagosomal membrane in phagocytic cells.
Nramp2/DMT1 functions as an electrogenic divalent cation transporter of broad substrate specificity in a variety of cell types and tissues.24 The IRE-containing isoform of Nramp2/DMT1 is the major transporter responsible for dietary iron absorption at the apical pole of the enterocyte. However, Nramp2/DMT1 also plays a role in divalent metal transport in peripheral tissues, as suggested by its ubiquitous distribution,1 and also by the observation that the severe microcytic anemia of mk and b animals cannot be corrected by intravenous administration of iron.32 In peripheral tissues, Nramp2/DMT1 transports transferrin-bound iron across the membrane of acidified endosomes, after reduction to Fe(II).33 In particular, the non-IRE isoform of Nramp2/DMT1 is abundantly expressed in reticulocytes, cells that consume large amounts of iron for heme biosynthesis.12 Results obtained in this study support the proposition that Nramp2/DMT1 may also function as a divalent cation transporter to remove cations from the luminal space of phagosomes. They further suggest that, depending on the cell type, Nramp2/DMT1 may transport cations at different cellular sites, such as the plasma membrane (brush border of enterocytes; role in dietary iron absorption), acidified endosomes (peripheral tissues including reticulocytes; role in the transferrin cycle), and phagosomes (phagocytic cells). What can be the function of Nramp2/DMT1 at the phagosomal membrane of macrophages and Sertoli cells? Most living organisms have an absolute requirement for iron that is used in many metabolic activities,34 and they have developed highly sophisticated systems to scavenge and recycle iron from their environment. However, even small amounts of the soluble ferrous ion, Fe2+, are highly toxic because they catalyze the peroxidation of membrane lipids. Thus, iron homeostasis is tightly controlled by the presence of complex systems for the transport, uptake, storage, and recycling of iron.35 Sertoli cells play a unique role in iron homeostasis. Iron is essential for spermatogenesis. Indeed, hypotransferrinemic mutant mice (Tfhpx/hpx), which produce only 1% to 2% of transferrin, have a decreased number of germ cells and a greatly reduced level of spermatogenesis.36 Moreover,mk mice and belgrade rats, which express a nonfunctional, G185R mutant variant of Nramp2, also show reduced fertility with impaired spermatogenesis,37indicating the important role of iron in this process. In the mammalian testis, Sertoli cells have developed a sophisticated iron-transport system designed to recruit iron across the blood testis barrier to maturing germ cells.25 Moreover, 50% of all spermatogenic cells degenerate during the seminiferous cycle and are phagocytosed by macrophages and Sertoli cells.24 25 Thus, it is tempting to speculate that Nramp2/DMT1 function at the phagosomal membrane of Sertoli cells may be to recycle iron from phagocytosed degenerating spermatozoids back into its iron-shuttle system. Results shown in Figure 5 indeed suggest that Nramp2/DMT1 is associated with sperm cells containing phagosomes.
Divalent cation transport by Nramp2/DMT1 at the phagosomal membrane has not yet been described and has been previously thought to be the exclusive function of the phagocyte-specific Nramp1 isoform.5,9,38 Targeting of Nramp1 to the phagosomal membrane in macrophages also makes it a potentially good candidate for iron recycling from effete RBCs and for scavenging iron from the phagosomal space of phagocytosed microbes. Thus, Nramp1 and Nramp2/DMT1 may transport the same divalent cation at the phagosomal membrane. Alternatively, the 2 proteins may have different substrate specificities, and this possibility is being explored experimentally. It is tempting to speculate that for iron, which is an essential cofactor in many cellular processes and for which body levels are tightly regulated, mechanisms that help prevent or minimize excessive loss must exist where such loss may occur. In favor of this hypothesis Nramp2/DMT1 is expressed at a high level in the kidney, where it may function to reclaim excreted iron back into the organism (Gunshin et al,3 Canonne-Hergaux et al,18Ferguson et al,39 and Canonne-Hergaux and Gros40). Macrophages are the major source of iron recycling in mammals through erythrophagocytosis. Here, we show that Nramp2/DMT1 is indeed associated with the membranes of phagosomes containing RBCs. Therefore, our findings suggest that Nramp2/DMT1 may play a role in recycling iron from effete RBC-containing phagosomes to the cytoplasm.
Prepublished online as Blood First Edition Paper, May 31, 2002; DOI 10.1182/blood-2002-04-1182.
Supported by National Institutes of Health grant 1 R01 A1 35237-08 (P.G.). P. G. is supported by a salary award from the Canadian Institutes of Health Research. N. J. and F. C.-H. are supported by fellowships from Human Science Frontier Program and Milestone Medica Inc, respectively.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Philippe Gros, Department of Biochemistry, McGill University, Rm 907, 3655 Sir William Osler Dr, Montreal, QC, Canada H3G-1Y6; e-mail: gros@med.mcgill.ca.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal