Reactive oxygen species (ROS) have been increasingly recognized as important components of cell signaling in addition to their well-established roles in host defense. The formation of ROS in phagocytic and nonphagocytic cells involves membrane-localized and Rac guanosine triphosphatase (GTPase)–regulated reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase(s). We discuss here the current molecular models for Rac GTPase action in the control of the phagocytic leukocyte NADPH oxidase. As a mechanistically detailed example of Rac GTPase signaling, the NADPH oxidase provides a potential paradigm for signaling by Rho family GTPases in general.
Introduction
Phagocytic leukocytes play major roles in the innate immune response to pathogens. An important component of this response is the ability of leukocytes to generate reactive oxygen species (ROS) via a membrane-associated reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase.1,2 This multicomponent enzyme utilizes electrons derived from intracellular NADPH to generate superoxide anion, which subsequently dismutes to H2O2 and results in the formation of other ROS that are used for host defense. However, the inappropriate or excessive action of this system results in inflammatory disorders. The NADPH oxidase was the first identified and remains one of the best-characterized Rho guanosine triphosphatase (GTPase)–regulated systems. It has been shown that either Rac1 or Rac2 GTPase is required for oxidase activity in cell-free systems,3,4 with Rac2 being the predominantly active isoform in human neutrophils.5 Additional evidence has established that Rac2 is an integral and required component of the NADPH oxidase in the intact leukocyte, including the demonstration thatrac2−/− neutrophils had significantly reduced or absent superoxide production in response to various stimuli.6-8
Recently, homologs of the cytochrome b component gp91phox (see “Components and regulation of the phagocyte NADPH oxidase”) of the phagocyte NADPHoxidase, termed Nox, have been found in several tissues (reviewed by Lambeth9). These new NADPH oxidases produce low levels of oxidants that appear to be used as signals for a variety of cellular activities, including cell growth and transformation. It has been known for some time that Ras and Rac GTPases regulate signaling pathways that are critical for mitogenesis and oncogenesis. Transient expression of a constitutively activated form of Ras in NIH3T3 cells induced a significant increase in intracellular ROS that was inhibitable by expression of a dominant negative allele of either Ras or Rac1.10,11 ROS production was suppressed by treatment with diphenylene iodonium (DPI), an inhibitor of the phagocyte NADPH oxidase, suggesting that a Nox protein may be involved. Rac1 also regulates ROS production leading to reperfusion injury during reoxygenation of vascular smooth muscle.12,13 Recombinant adenoviral expression of a dominant negative Rac1 suppressed tissue damage in an in vivo model of mouse hepatic ischemia/reperfusion injury. This was also observed in mice deficient for the gp91phox of phagocytic NADPH oxidase, suggesting that the Rac mutant inhibited ROS production by a Nox system rather than by one employing gp91phox.13 Thus, it appears that ROS production in nonphagocytic cells may involve the regulation of an NADPH oxidase–like enzyme by Rac GTPase. Understanding how this system is regulated in phagocytic leukocytes is therefore likely to have important implications for understanding the regulation of ROS formation in nonphagocytic cells as well. We discuss here current models for the action of the Rac GTPase in phagocyte NADPH oxidase function.
Components and regulation of the phagocyte NADPH oxidase
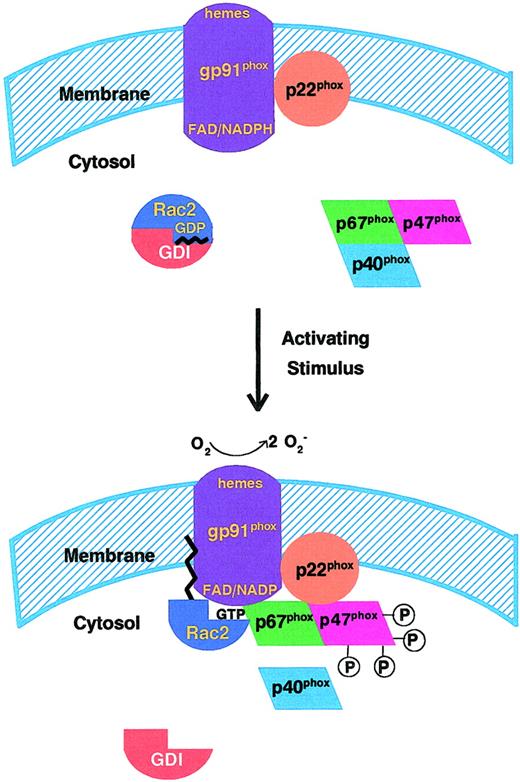
The NADPH oxidase system of neutrophils and other phagocytic leukocytes is composed of multiple membrane-associated (cytochrome b558) and cytosolic components (Rac, p67phox, p47phox, p40phox [Figure 1]). The active, fully assembled oxidase catalyzes the one electron reduction of oxygen to produce superoxide anion using NADPH as substrate. When the leukocyte is activated through the action of inflammatory mediators (soluble chemoattractants, chemokines, or phagocytic particles), the cytosolic oxidase component p47phox becomes phosphorylated on multiple sites through the action of several kinases. The phosphorylation of p47phox is thought to lead to the disruption of an inhibitory intramolecular interaction that exposes SH3 domains in p47phox for binding to proline-rich regions of other NADPH oxidase components (reviewed by Babior,1Lambeth,2 and DeLeo and Quinn14). Cytosolic p47phox exists both in a free form and in complexes with the cytosolic oxidase components, p67phox and p40phox. The phosphorylation-induced conformational change(s) in p47phox results in translocation of a p47phox/p67phox complex to the membrane, where it interacts via multiple binding sites with the integral membrane protein, flavocytochrome b558 (cytochrome b [cyt b]) to form an active enzyme complex.2,14The active oxidase also requires the translocation of Rac GTPase (see below), which occurs simultaneously, but dissociably, from the translocation of the p47phox/p67phoxcomplex.5,15 Cyt b possesses 2 subunits, gp91phox and p22phox, with the larger subunit containing an NADPH binding site, 2 heme groups, and bound flavin adenine dinucleotide (FAD).2 The formation of this minimal protein complex allows electrons to flow via a 2-step mechanism from NADPH to FAD (step 1) and then from FAD to the heme of cyt b and finally to molecular oxygen (step 2), whose reduction leads to the formation of superoxide anion. The cytosolic component p40phox 16 may play a role in regulating the response of the system to phosphatidylinositol-3-phosphate in vivo17 but is not required for NADPH oxidase activity in the cell-free system.
Activation and assembly of the phagocyte NADPH oxidase.
Upon stimulation of human neutrophils by inflammatory mediators, membrane assembly of the activated NADPH oxidase occurs, as described in “Components and regulation of the phagocyte NADPH oxidase.” The known functional components involved in activation are shown: the integral membrane cytochrome b558, consisting of the gp91phox and p22phoxsubunits; the cytosolic oxidase components p47phox, p67phox, and p40phox; and the Rac2 GTPase in complex with the regulatory protein GDI (GDP dissociation inhibitor). Multiple regulatory phosphorylations of p47phox are depicted by the attached 4 P groups; this is not meant to indicate the actual number of phosphorylation sites. Wavy line on Rac2 represents the C-terminal geranylgeranyl isoprenyl group and associated polybasic domain. Portions of this figure have been used in Diebold and Bokoch.47
Activation and assembly of the phagocyte NADPH oxidase.
Upon stimulation of human neutrophils by inflammatory mediators, membrane assembly of the activated NADPH oxidase occurs, as described in “Components and regulation of the phagocyte NADPH oxidase.” The known functional components involved in activation are shown: the integral membrane cytochrome b558, consisting of the gp91phox and p22phoxsubunits; the cytosolic oxidase components p47phox, p67phox, and p40phox; and the Rac2 GTPase in complex with the regulatory protein GDI (GDP dissociation inhibitor). Multiple regulatory phosphorylations of p47phox are depicted by the attached 4 P groups; this is not meant to indicate the actual number of phosphorylation sites. Wavy line on Rac2 represents the C-terminal geranylgeranyl isoprenyl group and associated polybasic domain. Portions of this figure have been used in Diebold and Bokoch.47
p47phox is also known to be dispensable for NADPH oxidase activity under cell-free conditions and appears to serve primarily as an adapter in the intact cell to facilitate binding of p67phox with cyt b in response to activating stimuli.18,19 It should be emphasized that this adapter function of p47phox is critical for normal NADPH oxidase activation by stimuli in intact leukocytes, as evidenced by the marked impairment in superoxide formation in chronic granulomatous disease patients lacking p47phox.1,2 In contrast to p47phox, p67phox appears to be an absolutely required component of the enzyme. An “activation domain” has been identified in p67phox (amino acid [aa] 199-210 and perhaps aa 187-193) that is required for stimulation of oxidant formation via cyt b in cell-free systems.20,21 This domain has been reported to interact directly with cyt b to regulate the transfer of electrons from NADPH to cyt b– bound flavin (step 1).22
As indicated above, Rac GTPase is also a required component of the active phagocyte NADPH oxidase (Figure 1). Upon cell activation, Rac (Rac2 in human neutrophils) dissociates from a cytosolic complex with guanosine diphosphate (GDP) dissociation inhibitor (GDI) by an as yet undetermined mechanism. GDP is exchanged for GTP through the action of a guanine nucleotide exchange factor(s) (GEF), which appears to be membrane localized23; Rac, now in its GTP-bound active form, becomes membrane associated.24 Rac supports NADPH oxidase activity only in its GTP-bound active form25 (an exception to this has been proposed26 27).
GTPases of the Rho family, including Rac2, contain 2 important regions involved in guanine nucleotide triphosphate binding and interactions with regulatory molecules and effectors. These are termed switch I (or the “effector” domain), encompassing amino acid residues about 25 to 45, and switch II, which includes residues about 58 to 77. Only switch I undergoes extensive conformational changes upon GTP binding (there is evidence that the switch II domain in Rac adopts very similar conformations in both the GDP- and GTP-bound states28). A third region of potential importance in molecular interactions of Rac is the insert helix unique to Rho family GTPases (residues 120-137). This so-called “insert domain” does not undergo nucleotide-dependent conformational change but is readily solvent accessible for protein-protein interactions.
The GTP-dependence of NADPH oxidase activation indicates that the Rac switch I domain is necessary for oxidase regulation. Consistent with this, point mutations within Rac switch I were unable to support oxidase activity.29,30 The finding that Rac binds directly to p67phox (but not p47phox) via the switch I domain provided important insight into Rac function in the oxidase.31 It was shown that the tetratricopeptide repeat (TPR) in the N-terminus of p67phox was the site of Rac binding,32 and this was confirmed upon determination of the crystal structure of the Rac-p67(TPR) complex.33 The structure revealed specific stabilizing interactions between p67phox and Rac, including amino acids A27 and G30 of Rac and the TPR domain of p67phox, and suggested the availability of the insert helix for possible additional protein interactions. Several prior investigations had suggested a requirement for the Rac insert domain in the activation of the NADPH oxidase. Peptide walking experiments indicated that blocking the insert domain of Rac abrogated NADPH oxidase activation.34Studies using insert domain deletion mutants of Rac have yielded conflicting results, however, concluding either that the insert domain was absolutely required35,36 or unnecessary37,38 for Rac oxidase activity. Similarly, it has been reported that the Rac1 insert region is either essential39 or not required40 for mitogenesis and ROS formation in fibroblasts.
Current models of Rac function in NADPH oxidase regulation
The role of Rac in controlling NADPH oxidase assembly and activity appears to involve several components. While Rac does not directly mediate the translocation of the cytosolic oxidase components p47phox and p67phox to the membrane,15 Rac activation can induce NADPH oxidase assembly,41 perhaps indirectly through the action of effector kinases such as p21-activated kinase.42 Data from cell-free systems cited above indicate a distinct requirement for Rac in the membrane-assembled oxidase as well. Currently, there are 3 major molecular models to describe how Rac GTPase directly regulates activity of the assembled NADPH oxidase complex.
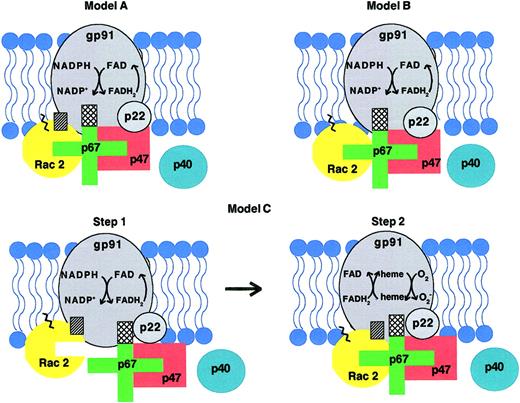
The model proposed by Lambeth et al (Figure2, model A) suggests that p67phox, containing a defined activation domain for cyt b, is the only protein influencing the rate-limiting electron transfer step (step1) of the NADPH oxidase (reviewed by Lambeth2). Rac GTPase is activated (see preceding sections) and is recruited to the plasma membrane via its C-terminal prenyl group and polybasic domain. Rac is considered to act solely as an adapter molecule that binds to p67phox through the switch I region and aids in the proper orientation of this active regulator of the system to cyt b. The Rac insert domain may bind to cyt b but, as with p47phox, this interaction serves only to position and facilitate binding of p67phox to cyt b. This aspect of the model is based on the observation that a nonprenylated Rac1 mutant lacking the insert domain decreased the affinity (median effective concentration [EC50]) of Rac for the oxidase but had no effect on the maximal rate,Vmax, of superoxide production.36 Our laboratory, on the other hand, has observed a decrease in Vmax when using a prenylated Rac2 version of this mutant.35
Comparison of proposed models of NADPH oxidase regulation by Rac GTPase.
In each of the proposed molecular models for NADPH oxidase regulation by Rac GTPase, the switch 1 region of Rac (not indicated) interacts with p67phox, the prenylated tail and polybasic domain of Rac (shown as a zigzag line) interact with the membrane, and the activation domain of p67phox (a crosshatched section) interacts with cytochrome b558. A role for p40phox remains unclear. In the model of Lambeth and colleagues (model A), Rac is thought to be recruited to the plasma membrane phospholipid bilayer via its prenylated C-terminus and polybasic domain. Indirect evidence supports the idea that the Rac insert domain (hatched section) may interact directly with cytochrome b558. Lambeth2 proposes the activation domain of p67phox is the sole regulator of the electron transfer step from NADPH to FAD. Rac and p47phox serve as adapters aiding in the interaction of p67phox with cytochrome b558. In model B proposed by Pick and colleagues,37,38 Rac is thought to interact only with plasma membrane phospholipids via its C-terminal prenyl group and polybasic domain and does not interact physically with cytochrome b558. In this model, the insert domain is not involved in protein interactions or regulation of the NADPH oxidase. As in the model of Lambeth and colleagues, p67phox is the sole regulator of electron transfer by cytochrome b558, while Rac and p47phox serve only as adapters to position p67phox for interaction with cytochrome b558. Diebold and Bokoch35 propose a 2-step model (model C) for the regulation of NADPH oxidase by Rac. In step 1, Rac translocates to the membrane and interacts with the phospholipid bilayer via its prenylated/polybasic C-terminus. In addition, Rac, via its insert domain, interacts with cytochrome b558 and contributes to the regulation of electron flow from NADPH to FAD without interacting with p67phox. p67phox is still required for electron flow to occur in step1 and regulates electron flow via its activation domain. The interaction of the insert domain of Rac with cytochrome b558 may induce a conformational change in cytochrome b558 that modulates the interaction of p67phox and cytochrome b558. In step 2, the interaction between the switch I domain of Rac and the Rac-binding domain of p67phox, probably inducing a conformational change in p67phox, is required for electrons to continue to flow from FAD to the heme groups of cytochrome b558. (The step 1 reaction only is depicted in models A and B because this is thought to be the rate-limiting step in the overall electron transfer pathway to molecular oxygen. The step 2 reaction does take place in all 3 models depicted.) Portions of this figure have been used in Diebold and Bokoch.47
Comparison of proposed models of NADPH oxidase regulation by Rac GTPase.
In each of the proposed molecular models for NADPH oxidase regulation by Rac GTPase, the switch 1 region of Rac (not indicated) interacts with p67phox, the prenylated tail and polybasic domain of Rac (shown as a zigzag line) interact with the membrane, and the activation domain of p67phox (a crosshatched section) interacts with cytochrome b558. A role for p40phox remains unclear. In the model of Lambeth and colleagues (model A), Rac is thought to be recruited to the plasma membrane phospholipid bilayer via its prenylated C-terminus and polybasic domain. Indirect evidence supports the idea that the Rac insert domain (hatched section) may interact directly with cytochrome b558. Lambeth2 proposes the activation domain of p67phox is the sole regulator of the electron transfer step from NADPH to FAD. Rac and p47phox serve as adapters aiding in the interaction of p67phox with cytochrome b558. In model B proposed by Pick and colleagues,37,38 Rac is thought to interact only with plasma membrane phospholipids via its C-terminal prenyl group and polybasic domain and does not interact physically with cytochrome b558. In this model, the insert domain is not involved in protein interactions or regulation of the NADPH oxidase. As in the model of Lambeth and colleagues, p67phox is the sole regulator of electron transfer by cytochrome b558, while Rac and p47phox serve only as adapters to position p67phox for interaction with cytochrome b558. Diebold and Bokoch35 propose a 2-step model (model C) for the regulation of NADPH oxidase by Rac. In step 1, Rac translocates to the membrane and interacts with the phospholipid bilayer via its prenylated/polybasic C-terminus. In addition, Rac, via its insert domain, interacts with cytochrome b558 and contributes to the regulation of electron flow from NADPH to FAD without interacting with p67phox. p67phox is still required for electron flow to occur in step1 and regulates electron flow via its activation domain. The interaction of the insert domain of Rac with cytochrome b558 may induce a conformational change in cytochrome b558 that modulates the interaction of p67phox and cytochrome b558. In step 2, the interaction between the switch I domain of Rac and the Rac-binding domain of p67phox, probably inducing a conformational change in p67phox, is required for electrons to continue to flow from FAD to the heme groups of cytochrome b558. (The step 1 reaction only is depicted in models A and B because this is thought to be the rate-limiting step in the overall electron transfer pathway to molecular oxygen. The step 2 reaction does take place in all 3 models depicted.) Portions of this figure have been used in Diebold and Bokoch.47
The model suggested by Pick and colleagues (Figure 2, model B) is similar to the Lambeth model in that p67phoxremains the primary regulator of the electron transfer reactions. As with the Lambeth model, the Pick model portrays Rac and p47phox as adapter molecules that aid p67phox in binding to cyt b (discussed by Gorzalczany et al43,44). However, this model opposes the view that Rac interacts directly with cyt b and that the Rac insert domain is important in oxidase regulation. Instead, they propose that Rac interacts only with membrane phospholipids via the prenylated C-terminus to “carry” p67phox into position with cyt b. This postulate is based upon observations that prenylated Rac can bind equally well to phospholipid vesicles either devoid of or containing cyt b.43 In addition, Pick's group used prenylated Rac1-p67phox chimeras in the cell-free system and reported that deletion of the insert domain of Rac1 did not affect the ability of this chimera to support superoxide production.44 (Interestingly, this group of investigators originally observed that peptides overlapping the insert domain of Rac inhibited superoxide production in the cell-free system.34) Consequently, based on these observations and that by Lambeth's group that deletion of the insert domain does not affect Vmax,36 the model of Pick et al does not support a physical or functional interaction of Rac with cyt b.
There are several observations that appear to be inconsistent with aspects of the above models. First, it is possible to dissociate the membrane translocation of Rac from that of p67phox pharmacologically,15 which is inconsistent with a required carrier function of Rac for p67phox. Additionally, an effect of Rac mediated solely through interactions with p67phox is inherently problematic due to the low affinity of this interaction as determined in vitro.31 With regard to a role of the Rac insert domain, a careful examination of the data of Gorzalczany et al44 shows that, in fact, there is a marked decrease (> 60%) in the ability of the insert domain deletion mutant to support oxidase activity at concentrations of chimera below 200 nM (ie, within the normal physiologic range of reactants). Finally, based upon effects of exogenous Rac-GTP on the activity of various Rac-p67phox chimeras, Pick has recently suggested that Rac may play an additional role in modifying the active conformation of p67phox.44
The regulatory model proposed by Diebold and Bokoch35differs in several ways from the previous 2 models (Figure 2, model C). First, it is proposed that there is differential regulation of the 2 steps involved in electron transfer from NADPH to molecular oxygen. Rac is required apart from and in addition to p67phox for the step 1 reaction. However, Rac must subsequently then interact with p67phox for the step 2 reaction to occur. This is based upon the observation that a non Rac-binding p67phox aa 178 to 184 deletion mutant could still support electron transfer from NADPH to FAD (step 1) but not from FAD to cyt b (step 2). Of note, the basis for the loss of interaction of this p67phox mutant with Rac is not clear, because these residues are not directly involved in binding interactions observed in the crystal structure.33 Our laboratory has subsequently performed these same experiments with p67phox mutated within the Rac-interacting TPR domains, with similar results (B.A.D., G.M.B., unpublished observations, June 2001). In support of this observation, the reciprocal experiment utilizing the Rac2 D38A mutant that does not bind p67phox also indicated independent roles for Rac and p67phox in step 1 but not step 2 activity. In contrast, the Rac2 insert domain was found to be critical for both the step 1 and step 2 electron transfer reactions. This 2-step model is consistent with kinetic analysis of oxidase activation45and can explain the observation that neutrophils from a patient with a gp91phox missense mutation were able to reduce FAD but could not complete the reaction to transfer electrons to molecular oxygen.46
Secondly, because Rac2 was operative in the absence of p47phox, this suggested that Rac2 must interact directly with cyt b to support the step 1 reaction. Indeed, the intensity of a fluorescent analog of GppNHp (mant-GppNHp) bound to Rac2 increased in the presence of cyt b, indicating direct interaction between Rac2 and cyt b. Use of the insert domain deletion mutant of Rac2 in place of wild-type Rac2 eliminated this interaction, indicating that this domain was critical for cyt b binding. In support of the fluorescence data, we have recently demonstrated that glutathione-S-transferase (GST)–Rac2 can specifically bind cyt b purified from neutrophils in pull-down assays (B.A.D., G.M.B., unpublished observations, April 2002). These data are consistent with previous observations that membrane translocation of Rac2 was decreased by 75% in neutrophils from cyt b–deficient CGD patients.5 Overall, these data strongly indicate that the Rac2 insert domain is necessary for both the functional and physical interaction of Rac2 with cyt b.
It is clear that the 3 models differ in how they view oxidase regulation by Rac GTPase: adapter versus active participant. It is not clear at this point how the experimental data supporting each model can be reconciled. At least part of the discrepancy between these models may be in part due to the use of prenylated Rac versus nonprenylated Rac in certain key experiments. The concentration of prenylated Rac required in the cell-free system is at least 100-fold less than nonprenylated Rac. The use of high concentrations of unprocessed GTPase may obscure relevant high-affinity protein-protein interactions that normally occur at physiologic concentrations of reactants within the plane of the membrane. Ultimately, understanding the complete mechanism(s) through which Rac controls NADPH oxidase activity will depend upon additional in vitro biochemical studies combined with intact cell studies using neutrophils and other leukocytes or leukocytic cell lines.
With regard to the regulation of ROS in nonphagocytic cells, there interestingly is little rigorous evidence supporting the existence of p67phox and p47phox in most nonleukocytic cells. This suggests that either some other protein(s) serves the normally essential regulatory function of p67phox in the nonphagocyte system or that Nox regulation does not require this oxidase component. It is therefore not clear to what extent the regulation of Nox proteins will be similar to regulation of the phagocyte NADPH oxidase. However, the model proposed by Diebold and Bokoch35 does suggest a minimal means whereby Rac might directly regulate superoxide production by Nox proteins through a mechanism similar to Rac binding and regulation of cyt b in phagocytes. In this light, understanding how Rac GTPase regulates the NADPH oxidase of phagocxytic leukocytes may provide the basic insights necessary to develop therapeutic means to intervene in ROS-related pathological disease states in both inflammatory and noninflammatory cells.
Prepublished online as Blood First Edition Paper, June 21, 2002; DOI 10.1182/blood-2002-04-1149.
References
Author notes
Gary M. Bokoch, Department of Immunology, The Scripps Research Institute, 10666 N Torrey Pines Road, IMM-14, La Jolla, CA 92037; e-mail: bokoch@scripps.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal