Despite improvements in the treatment of acute myeloid leukemia (AML), approximately 50% of children die of the disease. Clinical trials in adult patients with AML indicate that idarubicin may have superior efficacy when compared to daunorubicin in the remission-induction phases of chemotherapy. We conducted consecutive clinical trials in children with newly diagnosed AML in which daunorubicin (group 1, n = 102) or idarubicin (group 2, n = 160) was used during the remission-induction (RI) and the early consolidation phases of chemotherapy. Idarubicin was given at a dose of either 10 mg/m2 (group 2A, n = 106) or 12 mg/m2 (group 2B, n = 53). A high rate of RI was achieved for all groups (95% group 1, 90% group 2A, 94% group 2B). There were no significant differences in 5-year event-free survival (EFS) or in overall survival (OS) when the 3 groups were compared (group 1: EFS 50%, OS 56%; group 2A: EFS 50%, OS 60%; group 2B: EFS 34%, OS 50%). RI deaths resulting from treatment toxicity were low—2% for group 1 and 5% for group 2. More gastrointestinal, pulmonary, and renal toxicity but fewer infections were observed in patients receiving idarubicin (P < .001, P = .04,P = .03, respectively). Following RI chemotherapy, all patients received 3 to 4 more courses of identical chemotherapy and then underwent either autologous (n = 156) or an allogeneic bone marrow transplantation (BMT) (n = 35). OS was higher in allogeneic BMT patients than in autologous BMT patients (79% vs 63%;P = .23). We conclude that daunorubicin is as effective as idarubicin for remission-induction therapy for childhood AML and has reduced toxicity.
Introduction
Reported survival figures for childhood acute myeloid leukemia (AML) range from 31% to 60%, and the proportion of patients achieving complete remission (CR) ranges from 61% to 91%.1-9 Debate continues regarding the best chemotherapeutic agents to use in remission induction. Most of the large collaborative pediatric AML studies have used different remission-induction regimens, which perhaps explains the wide variability in remission-induction rates. The most widely used combination of chemotherapy for remission induction is anthracycline and cytarabine, often in combination with etoposide or thioguanine.10 Daunorubicin has been shown to be superior to doxorubicin in the treatment of pediatric AML, and it has a more favorable toxicity profile.11 Several large randomized studies have compared different combinations of induction agents. For example, the Paediatric Oncology Group, in a randomized study, compared the use of vincristine, cytarabine, and dexamethasone (VADx) with daunorubicin, cytarabine, and thioguanine (DAT) for remission induction. A significantly higher complete remission (CR) rate using DAT over VADx was found (82% vs 61%).4 Similarly, a randomized comparison of DAT versus daunorubicin, cytarabine, and etoposide (ADE) in the United Kingdom Medical Research Council 10th AML Trial showed no difference in the remission-induction rate—89.5% versus 93%, respectively.3 The Children's Cancer Group has used a 5-drug induction regimen consisting of dexamethasone, cytarabine, 6-thioguanine, etoposide, and daunorubicin (DCTER) to achieve a CR rate of 70% to 75%.2Timed-sequential repetition of this 4-drug regimen, with a second cycle commencing 10 days after the first, regardless of evidence of marrow recovery, led to increased event-free survival (EFS).1 2
Evidence from adult AML collaborative clinical trials showing that idarubicin may be superior to daunorubicin and to have improved CR rates, higher overall survival (OS) rates, and a similar toxicity profile prompted a review of the use of idarubicin in pediatric patients.12-14 Limited data are published on the efficacy of idarubicin as a remission-induction agent in the treatment of AML in children.15 16 For this reason, the Australian and New Zealand Children's Cancer Study Group (ANZCCSG) undertook consecutive single-arm studies comparing daunorubicin with idarubicin for remission-induction and early consolidation chemotherapy.
Patients, materials, and methods
Patients and study design
Two consecutive single-arm studies were conducted between December 1986 and May 1999 (N = 280). One hundred thirteen patients younger than 18 years with previously untreated AML were registered on the ANZCCSG AML I protocol (group 1 patients) between December 1986 and November 1992. Between January 1993 and May 1999, 167 patients were enrolled on the ANZCCSG AML 2 protocol (group 2 patients). Recruitment for both protocols was from 8 of 9 children's cancer treatment centers in Australia and New Zealand. Informed consent was obtained from parents or guardians at the local institution. A small subset of group 1 patients had been previously reported.17 18 Patients with acute promyelocytic leukemia (FAB [French-American-British] M3) were included. Patients with myelodysplasia, secondary AML, and Down-syndrome–related leukemic disorders were excluded from this analysis.
Treatment
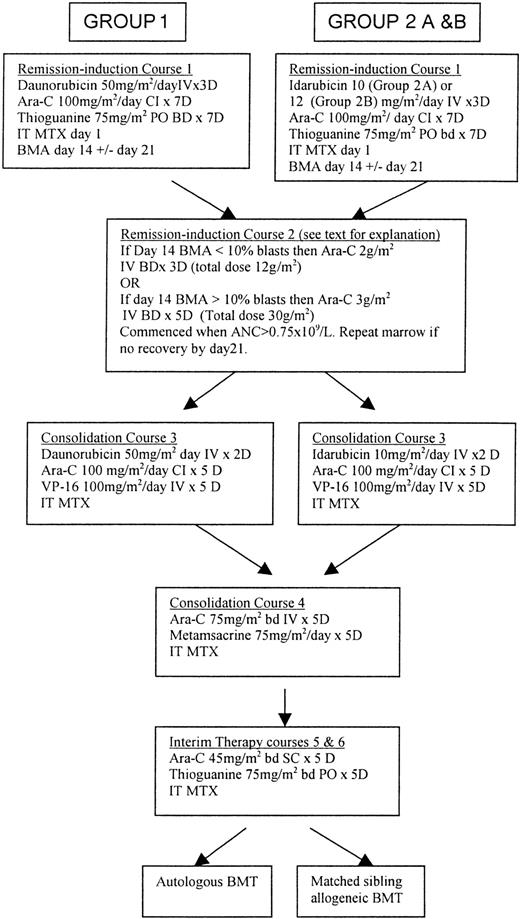
Treatment regimens for groups 1 and 2 (subgroups 2A and 2B) are shown in Figure 1. The main difference between regimens was the use of daunorubicin in remission induction (courses 1, 2) and early consolidation (course 3) in group 1 compared with the use of idarubicin in group 2. Both regimens used high-dose cytosine arabinoside (Ara-C) as course 2 of remission induction and have identical late consolidation treatments (course 4) and interim therapy (courses 5, 6). Because of concerns regarding toxicity in induction, the dose of idarubicin was reduced from 12 mg/m2to 10 mg/m2 from May 1995. Of the 160 group 2 patients available for evaluation, 103 (64.4%) received idarubicin at a dose of 10 mg/m2 per day (group 2A), whereas 53 (33.2%) patients received 12 mg/m2 per day (group 2B). Reduction in the idarubicin dose to 10 mg/m2 was made at the discretion of the treating physician. Bone marrow aspiration (BMA) was performed in all patients on day 14 to assess cytoreduction. If the marrow was hypocellular and contained less than 10% blasts, cytarabine was given at a dose of 2 g/m2 intravenously, twice daily, for 3 days when there were signs of marrow recovery. If the bone marrow was hypocellular but contained more than 10% blasts, course 2 began on day 15, with 2 g/m2 of cytarabine, twice daily, for 3 days. If the day 14 BMA showed poor cytoreduction, then cytarabine was given at a dose of 3 g/m2 intravenously, twice daily, for 5 days, beginning on day 15. If no evidence of marrow recovery was noted on peripheral blood film by day 21 following course 1, BMA was repeated. Provided a life-threatening infection was not present, course 2 was begun no later than day 21. At 21 to 28 days after the start of course 2, BMA was repeated to confirm that remission course 3 had started.
Comparison of treatment regimens used in consecutive protocols for the treatment of childhood AML.
ANC indicates absolute neutrophil count; Ara-C, cytosine arabinoside; BMA, bone marrow aspirate; bd, twice daily; CI, continuous infusion; IT, intrathecal; IV, intravenous; PO, per oral; BMT; bone marrow transplantation; MTX, methotrexate (age-dependent dose).
Comparison of treatment regimens used in consecutive protocols for the treatment of childhood AML.
ANC indicates absolute neutrophil count; Ara-C, cytosine arabinoside; BMA, bone marrow aspirate; bd, twice daily; CI, continuous infusion; IT, intrathecal; IV, intravenous; PO, per oral; BMT; bone marrow transplantation; MTX, methotrexate (age-dependent dose).
For patients in groups 1 and 2, end-consolidative therapy consisted of bone marrow transplantation (BMT). For patients with an HLA-identical sibling, allogeneic-matched sibling BMT conditioned with busulphan and cyclophosphamide (BU/CY) was recommended. Busulphan was given at a dose of 16 mg/kg and cyclophosphamide at a dose of 120 mg/kg. Patients without an HLA-identical sibling received an autograft either with Percoll-gradient–separated marrow after melphalan (180 mg/m2) conditioning or with BU/CY. A pilot study, reported previously, indicated that autologous BMT, using melphalan conditioning and Percoll-gradient cell separation, improved survival rates in childhood AML.17
For patients in group 2 with acute promyelocytic leukemia (FAB M3), all-trans retinoic acid (ATRA) was commenced on day 1 at a dose of 25 mg/m2 and continued daily until CR was achieved. Prednisone was also commenced in these patients on day 1 and continued for 14 to 21 days. Patients with AML and WBC counts greater than 10 × 109/L at diagnosis or whose WBC counts rose more than 4 × 109/L over the first 48 hours of ATRA were also started on chemotherapy according to the protocol on days 1 to 3. FAB M3 patients in group 1 were treated with chemotherapy as detailed in Figure 1, with no ATRA. All patients with FAB M3, regardless of patient group, underwent BMT as end-consolidation therapy.
Supportive care
Fever and neutropenia was treated at the discretion of treating institutions with multiagent, broad-spectrum antibiotics. Antifungal cover was added for persistent fever or clinical suspicion of fungal infection. Patients who were seronegative for cytomegalovirus (CMV) received CMV-negative blood products. Random donor platelets were given prophylactically during remission induction to maintain a platelet count greater than 20 × 109/L. Hydrocortisone eye drops were given to all patients while they were taking high-dose Ara-C. Cotrimoxazole was recommended as prophylaxis against Pneumocystis carinii. Granulocyte–colony-stimulating factor (G-CSF) became available during the group 2 study from 1994 and was given, at investigator discretion following chemotherapy, at a dose of 5 μg/kg per day. During and after BMT conditioning, children were nursed in single HEPA-filtered rooms.
Statistical methods
Observed differences in proportions were tested for statistical significance using the appropriate χ2 statistic. For small sample sizes, the Fisher exact test was used. Kaplan-Meier curves were constructed for survival data and were compared using log-rank testing. Data for all surviving patients was censored as of June 2000. First CR was defined as normocellular bone marrow containing less than 5% blast cells. Remission failure was classified as the result of induction death or failure to remit. Overall survival (OS) was defined as the time from entry to death or to end of study observation period, if alive. EFS represented time from diagnosis to first event (relapse, death, failure to remit). For BMT patients, disease-free survival (DFS) is the time from CR to first event (either relapse or death in remission). Cytogenetic risk groups were classified as at good, standard, or poor risk, in keeping with recent published data.3 19 Good risk was defined as balanced translocation between chromosomes 8 and 21 and between 15 and 17, inversion of chromosome 16, and FAB type M3. Poor cytogenetic risk was defined as monosomy of chromosome 5 or 7, deletions and abnormalities of the long arm of chromosome 5 or 3, or complex cytogenetic rearrangements with 4 or more abnormalities. Standard cytogenetic risk included all other patients.
Results
Patient characteristics
Of the 113 group 1 patients, 102 were available for evaluation. Eleven patients registered in group 1 were excluded from analysis—4 patients died before treatment was given, and 7 patients were registered in the study but did not receive protocol treatment. Of the 167 group 2 patients, 160 were available for evaluation. Seven patients were excluded from analysis—4 died before any treatment was given, and 3 were registered at diagnosis but did not receive protocol treatment. Overall, 262 patients were available for comparison. Median follow-up for survivors was 103 months (range, 49-161 months) for group 1 patients and 42 months (range, 12-85 months) for group 2 patients.
Potential prognostic factors previously reported in the literature, including age, WBC count, FAB morphological classification, and cytogenetics at diagnosis, were examined for any confounding effect on OS. Clinical and laboratory characteristics comparing group 1 (daunorubicin) with group 2 (idarubicin) and group 2A (10 mg/m2 idarubicin) with group 2B (12 mg/m2idarubicin) are shown in Table 1. Groups were well matched, and there were no major differences between them in terms of age, median WBC count at diagnosis, presence or absence of CNS disease at diagnosis, FAB morphological subclass, and cytogenetic risk groups. Five-year OS rates for each group based on potential prognostic factors are shown in Table 2.
Comparison of diagnostic characteristics
| Characteristics . | Group 1 n = 102 . | Group 2 n = 160 . | P . | Group 2A n = 106 . | Group 2B n = 53 . | P . |
|---|---|---|---|---|---|---|
| n . | n . | n . | n . | |||
| Age, y | ||||||
| 0-5 | 45 | 79 | .48 | 58 | 21 | .07 |
| 6-10 | 30 | 47 | .99 | 28 | 19 | .22 |
| 11-18 | 27 | 34 | .41 | 20 | 13 | .41 |
| Median | 6.3 | 7 | .72 | 5.1 | 8.4 | .04 |
| Range | 3 mo-17.75 y | 3 mo-15.75 y | — | — | — | — |
| WCC count at diagnosis, ×109/L | ||||||
| Median | 25.5 | 14 | .34 | 14.1 | 12 | .06 |
| Range | 1-680 | 1-484 | — | — | — | — |
| Central nervous system disease | ||||||
| No | 96 | 151 | .93 | 98 | 52 | .15 |
| Yes | 6 | 9 | .93 | 8 | 1 | .15 |
| French-American-British | ||||||
| M0 | 1 | 4 | .18 | 4 | 0 | .15 |
| M1 | 19 | 16 | .07 | 12 | 4 | .46 |
| M2 | 23 | 41 | .62 | 25 | 16 | .37 |
| M3 | 12 | 19 | .99 | 11 | 7 | .60 |
| M4 | 18 | 28 | .99 | 18 | 10 | .77 |
| M4Eo | 5 | 2 | .10 | 0 | 2 | .05 |
| M5 | 18 | 35 | .48 | 21 | 13 | .59 |
| M6 | 2 | 2 | .56 | 2 | — | .32 |
| M7 | 3 | 7 | .70 | 7 | — | .06 |
| Granulocytic sarcoma | 0 | 3 | .16 | 2 | 1 | .99 |
| Unclassifiable | 1 | 3 | .56 | 3 | 0 | .22 |
| Cytogenetics* | ||||||
| Good | 20 | 39 | .37 | 24 | 14 | .60 |
| Standard | 54 | 90 | .60 | 61 | 29 | .74 |
| Poor | 8 | 18 | .37 | 12 | 6 | .99 |
| No result | 20 | 13 | .006* | 9 | 4 | .84 |
| Characteristics . | Group 1 n = 102 . | Group 2 n = 160 . | P . | Group 2A n = 106 . | Group 2B n = 53 . | P . |
|---|---|---|---|---|---|---|
| n . | n . | n . | n . | |||
| Age, y | ||||||
| 0-5 | 45 | 79 | .48 | 58 | 21 | .07 |
| 6-10 | 30 | 47 | .99 | 28 | 19 | .22 |
| 11-18 | 27 | 34 | .41 | 20 | 13 | .41 |
| Median | 6.3 | 7 | .72 | 5.1 | 8.4 | .04 |
| Range | 3 mo-17.75 y | 3 mo-15.75 y | — | — | — | — |
| WCC count at diagnosis, ×109/L | ||||||
| Median | 25.5 | 14 | .34 | 14.1 | 12 | .06 |
| Range | 1-680 | 1-484 | — | — | — | — |
| Central nervous system disease | ||||||
| No | 96 | 151 | .93 | 98 | 52 | .15 |
| Yes | 6 | 9 | .93 | 8 | 1 | .15 |
| French-American-British | ||||||
| M0 | 1 | 4 | .18 | 4 | 0 | .15 |
| M1 | 19 | 16 | .07 | 12 | 4 | .46 |
| M2 | 23 | 41 | .62 | 25 | 16 | .37 |
| M3 | 12 | 19 | .99 | 11 | 7 | .60 |
| M4 | 18 | 28 | .99 | 18 | 10 | .77 |
| M4Eo | 5 | 2 | .10 | 0 | 2 | .05 |
| M5 | 18 | 35 | .48 | 21 | 13 | .59 |
| M6 | 2 | 2 | .56 | 2 | — | .32 |
| M7 | 3 | 7 | .70 | 7 | — | .06 |
| Granulocytic sarcoma | 0 | 3 | .16 | 2 | 1 | .99 |
| Unclassifiable | 1 | 3 | .56 | 3 | 0 | .22 |
| Cytogenetics* | ||||||
| Good | 20 | 39 | .37 | 24 | 14 | .60 |
| Standard | 54 | 90 | .60 | 61 | 29 | .74 |
| Poor | 8 | 18 | .37 | 12 | 6 | .99 |
| No result | 20 | 13 | .006* | 9 | 4 | .84 |
Group 1, daunorubicin; group 2, idarubicin; group 2A, 10 mg/m2 idarubicin; group 2B, 12 mg/m2idarubicin.
Cytogenetics: good risk is defined as balanced translocations between chromosomes 15 and 17, 8 and 21, and inversion of chromosome 16. Poor risk includes monosomy of chromosome 5, abnormalities of the long arm of chromosome 3, complex cytogenetics with 5 or more separate abnormalities. Standard risk is all others.
Comparison of diagnostic characteristics and OS rates
| Characteristics . | Group 1 OS, % . | Group 2 OS, % . | P . | Group 2A OS, % . | Group 2B OS, % . | P . |
|---|---|---|---|---|---|---|
| Age, y | ||||||
| 0-5 | 64 | 45 | .11 | 58 | 26 | .12 |
| 6-10 | 46 | 54 | .85 | 50 | 57 | .96 |
| 11-18 | 54 | 78 | .20 | 79 | 77 | .89 |
| Central nervous system disease | ||||||
| No | 55 | 54 | .66 | 59 | 49 | .45 |
| Yes | 83 | 67 | .52 | 62.5 | 100 | .51 |
| French-American-British | ||||||
| M0 | 100 | 100 | — | 100 | — | — |
| M1 | 58 | 60 | .87 | 56 | 67 | .46 |
| M2 | 48 | 46 | .87 | 56 | 41 | .54 |
| M3 | 75 | 72 | .96 | 82 | 67 | .79 |
| M4 | 32 | 68 | .19 | 61 | 80 | .31 |
| M4Eo | 80 | 0 | .02* | — | 0 | — |
| M5 | 61 | 42 | .35 | 52 | 23 | .02 |
| M6 | 0 | 0 | — | 0 | — | — |
| M7 | 100 | 71 | .34 | 71 | — | — |
| Granulocytic sarcoma | — | 100 | — | 100 | 100 | — |
| Unclassifiable | 100 | 33 | .36 | 33 | — | — |
| Cytogenetics | ||||||
| Good | 78 | 69 | .17 | 74 | 65 | .97 |
| Standard | 44 | 49 | .47 | 55 | 44 | .46 |
| Poor | 50 | 42 | .51 | 38 | 50 | .60 |
| No result | 70 | 59 | .45 | 78 | 25 | .14 |
| Characteristics . | Group 1 OS, % . | Group 2 OS, % . | P . | Group 2A OS, % . | Group 2B OS, % . | P . |
|---|---|---|---|---|---|---|
| Age, y | ||||||
| 0-5 | 64 | 45 | .11 | 58 | 26 | .12 |
| 6-10 | 46 | 54 | .85 | 50 | 57 | .96 |
| 11-18 | 54 | 78 | .20 | 79 | 77 | .89 |
| Central nervous system disease | ||||||
| No | 55 | 54 | .66 | 59 | 49 | .45 |
| Yes | 83 | 67 | .52 | 62.5 | 100 | .51 |
| French-American-British | ||||||
| M0 | 100 | 100 | — | 100 | — | — |
| M1 | 58 | 60 | .87 | 56 | 67 | .46 |
| M2 | 48 | 46 | .87 | 56 | 41 | .54 |
| M3 | 75 | 72 | .96 | 82 | 67 | .79 |
| M4 | 32 | 68 | .19 | 61 | 80 | .31 |
| M4Eo | 80 | 0 | .02* | — | 0 | — |
| M5 | 61 | 42 | .35 | 52 | 23 | .02 |
| M6 | 0 | 0 | — | 0 | — | — |
| M7 | 100 | 71 | .34 | 71 | — | — |
| Granulocytic sarcoma | — | 100 | — | 100 | 100 | — |
| Unclassifiable | 100 | 33 | .36 | 33 | — | — |
| Cytogenetics | ||||||
| Good | 78 | 69 | .17 | 74 | 65 | .97 |
| Standard | 44 | 49 | .47 | 55 | 44 | .46 |
| Poor | 50 | 42 | .51 | 38 | 50 | .60 |
| No result | 70 | 59 | .45 | 78 | 25 | .14 |
Group 1, daunorubicin; group 2, idarubicin; group 2A, 10 mg/m2 idarubicin; group 2B, 12 mg/m2idarubicin.
P < .05.
Remission induction
Higher remission-induction rates than many previously reported studies were achieved in this study—95.1% for group 1 (n = 102) and 91.2% for group 2 (n = 160;P = .11).4-7 When group 2 patients are considered in terms of the dose of idarubicin they received, there was no statistical difference in achievement of remission, with 90.6% of patients receiving 10 mg/m2 idarubicin (group 2A, n = 106) achieving CR compared to 94.3% receiving 12 mg/m2 (group 2B, n = 53; P = .41). There were 2 (2%) deaths during induction in group 1 compared with 8 (5%) deaths in group 2 (P = .21). In group 1, 1 patient died of neutropenic colitis after course 1, and 1 died as a result of hemorrhage during course 2. In group 2, 3 patients died after course 1, 4 after or during course 2, and 1 during treatment with ATRA (before chemotherapy). Five of the 8 deaths were caused by overwhelming sepsis, and the remaining 3 resulted from hemorrhage.
The median interval between course 1 and 2 was similar for groups 1 and 2 and for the different idarubicin dose groups in the group 2 patients. The dose of Ara-C received, based on the results of day 14 BMA results, was also similar between treatment groups (Table3). The 5-year EFS rate for patients receiving 12 g/m2 of Ara-C for course 2 (48%, n = 203) was not significantly different from that for patients receiving 30 g/m2 of Ara-C (38%, n = 43; P = .22). There were no significant differences in 5-year EFS rates for patients whose day 14 bone marrow examination showed a good (less than 10% blasts) response to course 1 and who were then given the lower dose of Ara-C at day 21 (48%, n = 188), compared with patients who had a poor (more than 10% blasts) response to course 1 and who received the higher dose of Ara-C at day 14 (49%, n = 56).
Remission-induction results
| . | Group 1 n = 102 . | Group 2 n = 160 . | Group 1 vs group 2 . | Group 2A (n = 106) . | Group 2B (n = 53) . | Group 2A vs group 2B . |
|---|---|---|---|---|---|---|
| . | . | |||||
| n (%) . | n (%) . | P . | n (%) . | n (%) . | P . | |
| CR | 84 (82.4) | 135 (84.4) | .67 | 87 (82.1) | 48 (90.1) | .16 |
| CR delayed | 13 (12.7) | 11 (6.9) | .11 | 9 (8.5) | 2 (3.8) | .27 |
| Total CR | 97 (95.1) | 146 (91.2) | .11 | 96 (90.6) | 50 (94.3) | .41 |
| Failure to remit | 3 (2.9) | 5 (3.1) | .93 | 4 (3.8) | 1 (1.8) | .52 |
| Deaths | 2 (2.0) | 8 (5.0) | .21 | 6 (5.6) | 1 (1.8) | .28 |
| Time between courses 1 and 2 (d) | 23.5 | 23 | .44 | 23 | 24 | .39 |
| Ara-C total dose | ||||||
| 12 g/m2 | 79 (75.5) | 127 (79.4) | .73 | 82 (77.4) | 45 (84.9) | .27 |
| 30 g/m2 | 19 (18.6) | 24 (15.0) | .44 | 19 (17.9) | 5 (9.4) | .16 |
| . | Group 1 n = 102 . | Group 2 n = 160 . | Group 1 vs group 2 . | Group 2A (n = 106) . | Group 2B (n = 53) . | Group 2A vs group 2B . |
|---|---|---|---|---|---|---|
| . | . | |||||
| n (%) . | n (%) . | P . | n (%) . | n (%) . | P . | |
| CR | 84 (82.4) | 135 (84.4) | .67 | 87 (82.1) | 48 (90.1) | .16 |
| CR delayed | 13 (12.7) | 11 (6.9) | .11 | 9 (8.5) | 2 (3.8) | .27 |
| Total CR | 97 (95.1) | 146 (91.2) | .11 | 96 (90.6) | 50 (94.3) | .41 |
| Failure to remit | 3 (2.9) | 5 (3.1) | .93 | 4 (3.8) | 1 (1.8) | .52 |
| Deaths | 2 (2.0) | 8 (5.0) | .21 | 6 (5.6) | 1 (1.8) | .28 |
| Time between courses 1 and 2 (d) | 23.5 | 23 | .44 | 23 | 24 | .39 |
| Ara-C total dose | ||||||
| 12 g/m2 | 79 (75.5) | 127 (79.4) | .73 | 82 (77.4) | 45 (84.9) | .27 |
| 30 g/m2 | 19 (18.6) | 24 (15.0) | .44 | 19 (17.9) | 5 (9.4) | .16 |
Group 1, daunorubicin; group 2, idarubicin; group 2A, 10 mg/m2 idarubicin; group 2B, 12 g/m2idarubicin.
12 g/m2 represents total dose received during course 2 with drug delivered as 3 consecutive days of 2 g/m2 twice daily intravenously; 30 g/m2 represents total dose received during course 2 with drug delivered as 5 consecutive days of 3 g/m2 twice daily intravenously.
CR delayed indicates complete remission achieved after course.
Toxicity graded according to the Children's Cancer Group toxicity and complications criteria following remission-induction chemotherapy (courses 1 and 2) is shown in Table 4. Gastrointestinal (GI), renal toxicity, and pulmonary toxicity (grades 3-4) were seen significantly more frequently in those receiving idarubicin during remission induction. Twenty-five percent of patients receiving idarubicin experienced grades 3 to 4 gastrointestinal toxicity compared with 4.9% of patient's receiving daunorubicin (P < .01). Significantly more GI toxicity (grades 3-4) was noted in patients receiving the higher dosage of idarubicin, with 43% of group 2B patients (12 mg/m2 idarubicin) compared with 16% of group 2A (10 mg/m2 idarubicin) (P < .01) experiencing toxicity. Both renal and pulmonary toxicity was noted more frequently in patients receiving idarubicin (4.4% and 9.4% respectively) compared with patients receiving daunorubicin during remission induction (0% and 3% respectively;P = .03, P = .04). Unlike GI toxicity, patients receiving the higher dose of idarubicin did not experience more renal or pulmonary toxicity. Documented infections, conversely, were seen less frequently during remission induction in the idarubicin group than in those receiving daunorubicin (P = .02). Despite this, however, the toxicity death rate was higher in group 2 patients than in group 1 patients (5% vs 2%, respectively;P = .23).
Nonhematological toxicity (grades 3-4) associated with remission-induction therapy
| . | Group 1 n = 102 . | Group 2 n = 160 . | Group 1 vs group 2 . | Group 2A (n = 106) . | Group 2B (n = 53) . | Group 2A vs group 2B . |
|---|---|---|---|---|---|---|
| . | . | |||||
| n (%) . | n (%) . | P . | n (%) . | n (%) . | P . | |
| Liver | 4 (3.9) | 12 (7.5) | .23 | 8 (7.5) | 4 (7.5) | >.99 |
| Renal | 0 (.0) | 7 (4.4) | .034-150 | 6 (5.7) | 1 (1.9) | .28 |
| CNS | 0 (.0) | 6 (3.8) | .11 | 4 (3.8) | 2 (3.8) | >.99 |
| GIT | 5 (4.9) | 40 (25) | <.014-150 | 17 (16) | 23 (43) | <.014-150 |
| Lung | 3 (3.0) | 15 (9.4) | .044-150 | 11 (10.4) | 4 (7.5) | .57 |
| Heart | 0 (0) | 4 (2.5) | .11 | 4 (3.8) | 0 (0) | .034-150 |
| Infection | 29 (28) | 26 (16.3) | .024-150 | 22 (21) | 4 (7.5) | .034-150 |
| . | Group 1 n = 102 . | Group 2 n = 160 . | Group 1 vs group 2 . | Group 2A (n = 106) . | Group 2B (n = 53) . | Group 2A vs group 2B . |
|---|---|---|---|---|---|---|
| . | . | |||||
| n (%) . | n (%) . | P . | n (%) . | n (%) . | P . | |
| Liver | 4 (3.9) | 12 (7.5) | .23 | 8 (7.5) | 4 (7.5) | >.99 |
| Renal | 0 (.0) | 7 (4.4) | .034-150 | 6 (5.7) | 1 (1.9) | .28 |
| CNS | 0 (.0) | 6 (3.8) | .11 | 4 (3.8) | 2 (3.8) | >.99 |
| GIT | 5 (4.9) | 40 (25) | <.014-150 | 17 (16) | 23 (43) | <.014-150 |
| Lung | 3 (3.0) | 15 (9.4) | .044-150 | 11 (10.4) | 4 (7.5) | .57 |
| Heart | 0 (0) | 4 (2.5) | .11 | 4 (3.8) | 0 (0) | .034-150 |
| Infection | 29 (28) | 26 (16.3) | .024-150 | 22 (21) | 4 (7.5) | .034-150 |
GIT indicates gastrointestinal system.
P < .05.
Four patients in group 1 were withdrawn from the study at the discretion of the treating physician after the commencement of remission-induction chemotherapy. One patient was withdrawn after course 1 for severe esophagitis and peptic ulcer disease. One patient, who did not achieve remission, was withdrawn after course 2 and subsequently died of disease progression. One patient, who achieved CR, was withdrawn after course 3 because of cardiac toxicity. The remaining group 1 patient withdrawn from the study also achieved CR and was withdrawn after course 3. Additional nonprotocol chemotherapy agents were used for courses 4 to 6 in this patient. One patient in group 2 was removed from the study after induction therapy and was treated with nonprotocol agents after not achieving CR. Data on all 5 patients removed from the study are included from study entry to the point of study withdrawal, where this is considered to be an event.
Overall survival, event-free survival, and relapse before bone marrow transplantation
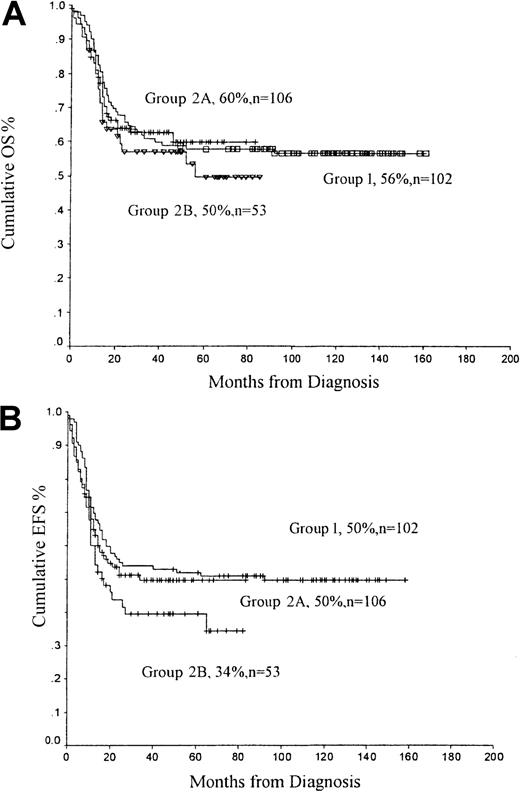
The OS rate for group 1 patients was 56%; it was 55% for group 2 patients (P = .58) (Figure2A). There was a trend for superior OS rates for patients receiving the lower dosage of idarubicin—OS 60% for group 2A (10 mg/m2) compared with 50% for group 2B patients (12 mg/m2) (P = .48). Similarly, the EFS rate was better in patients receiving the lower dose of idarubicin—50% for patients receiving 10 mg/m2 idarubicin compared with 34% for patients receiving 12 mg/m2(P = .24). When group 2 patients are considered together, the EFS rate (41%) was lower than that of patients receiving daunorubicin (group 1, EFS 50%; P = .13) (Figure2B).
OS and EFS rates from diagnosis.
(A) OS and (B) EFS from diagnosis comparing group 1 (daunorubicin), group 2A (idarubicin, 10 mg/m2), and group 2B (idarubicin, 12 mg/m2).
OS and EFS rates from diagnosis.
(A) OS and (B) EFS from diagnosis comparing group 1 (daunorubicin), group 2A (idarubicin, 10 mg/m2), and group 2B (idarubicin, 12 mg/m2).
OS rates for cytogenetic risk groups are shown in Table 2. As expected, patients with good risk cytogenetic abnormalities did better in both groups than patients with standard or poor risk cytogenetic features. OS was better than expected for the small number of patients with FAB M7 who have traditionally had a poor outcome; the OS rate for group 1 was 100% (n = 3), and for group 2A it was 71% (n = 7). Patients with FAB M3 had an OS rate of 75% for group 1 and 72% for group 2. Because this is a group of patients known to have superior survival and therefore possibly increased OS results for the group as a whole, data were reanalyzed without the inclusion of FAB M3 patients. No significant difference was noted; the OS rate was 55% for all patients, including FAB M3 patients (n = 262), compared with 52% (n = 231) for all patients, excluding those with FAB M3. Group 2 patients with FAB M5 subclass had superior OS with 10 mg/m2idarubicin (group 2A) than patients receiving 12 mg/m2(group 2B; OS 52% vs 23%, respectively; P = .02).
Fourteen (15%) group 1 patients who achieved CR had relapses before BMT. Nine of these patients went on to have a BMT in second remission. One patient did not achieve a second remission and underwent transplantation during first relapse. Thirteen of the 14 group 1 patients who did not undergo transplantation during the first CR because of relapse died of disease progression. Twenty (14%) patients in group 2 who achieved CR had relapses before BMT. Eleven of these patients underwent BMT in second remission; 4 remain alive and free of disease at the time of censoring. Nine relapsed patients in group 2 died of disease progression without achieving a second CR or without undergoing BMT. Of the patients in group 2 who had relapses before transplantation, 13 had received 10 mg/m2 idarubicin compared with 7 who had received 12 mg/m2. This represented 12% of group 2A (10 mg/m2) and 13% of group 2B patients (12 mg/m2).
Another 11 patients in group 2 who achieved CR did not progress to BMT. Three of these patients had granulocytic sarcomas without bone marrow involvement and received chemotherapy alone. Two patients in group 2 died in CR1 before BMT. Three patients did not proceed to BMT because of chronic lung disease, including 1 patient with persistent fungal infection, and 3 patients did not proceed because of parental or physician preference. Two patients in group 1 also refused BMT in CR1.
Bone marrow transplantation
Time from diagnosis to BMT was similar for both groups: a median of 6 months for group 1 (range, 4-9 months) and 5 months (range, 3-8 months) for group 2. Five-year DFS and OS rates for patients whose treatment included BMT are shown in Table5 and Figure3.
DFS and OS rates for patients who underwent BMT
| Type of BMT . | Group 1, daunorubicin (n = 78) . | Group 2, idarubicin (n = 113) . | Groups 1 and 2 combined (n = 191) . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n . | DFS, %(P) . | OS, % (P) . | n . | DFS, %(P) . | OS, % (P) . | n . | DFS, %(P) . | OS, % (P) . | |
| Autologous | 62 | 57 | 62 | 94 | 47 | 64 | 156 | 52 | 63 |
| Allogeneic | 16 | 75 (.22) | 63 (.99) | 19 | 63 (.63) | 72 (.93) | 35 | 69 (.12) | 79 (.23) |
| Type of BMT . | Group 1, daunorubicin (n = 78) . | Group 2, idarubicin (n = 113) . | Groups 1 and 2 combined (n = 191) . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n . | DFS, %(P) . | OS, % (P) . | n . | DFS, %(P) . | OS, % (P) . | n . | DFS, %(P) . | OS, % (P) . | |
| Autologous | 62 | 57 | 62 | 94 | 47 | 64 | 156 | 52 | 63 |
| Allogeneic | 16 | 75 (.22) | 63 (.99) | 19 | 63 (.63) | 72 (.93) | 35 | 69 (.12) | 79 (.23) |
Results for autologous BMT vs allogeneic BMT were determined using χ2 analysis.
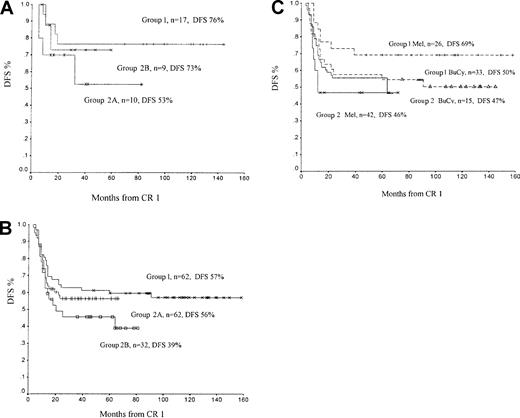
Disease-free survival curves for patients undergoing bone marrow transplantation.
(A) Disease-free survival (DFS) for patients receiving an allogenic transplant. Group 1, daunorubicin; group 2A, 10 mg/m2idarubicin; group 2B, 12 mg/m2 idarubicin. (B) DFS for patients receiving an autologous transplant. (C) DFS for patients receiving an autologous transplant comparing melphalan (Mel) conditioning with busulphan and cyclophosphamide (BU/CY). Group 1, daunorubicin; group 2, all idarubicin patients.
Disease-free survival curves for patients undergoing bone marrow transplantation.
(A) Disease-free survival (DFS) for patients receiving an allogenic transplant. Group 1, daunorubicin; group 2A, 10 mg/m2idarubicin; group 2B, 12 mg/m2 idarubicin. (B) DFS for patients receiving an autologous transplant. (C) DFS for patients receiving an autologous transplant comparing melphalan (Mel) conditioning with busulphan and cyclophosphamide (BU/CY). Group 1, daunorubicin; group 2, all idarubicin patients.
Sixteen patients in group 1 and 19 in group 2 underwent matched allogeneic BMT in CR 1 (33 matched-sibling, 1 matched-related cord blood, and 1 matched-unrelated BMT). Five-year DFS rates were 76% for group 1 and 73% for group 2B (12 mg/m2) and 53% for group 2A (10 mg/m2). With small numbers of patients in each group, however, this difference was not statically significant (group 1 vs group 2A, P = .22; group 1 vs group 2B,P = .85; group 2A vs group 2B, P = .44). The incidence of clinically significant (more than grade 2) graft-versus-host disease was not different between group 1 and group 2 (12% group 1, 25% group 2; P = .34). There were 3 deaths within the first 3 months of allogeneic BMT. Despite a difference in the 5-year DFS rates of allogeneic BMT recipients (69%, n = 35) and autologous BMT recipients (52%, n = 156), the difference was not statistically significant (P = .12; Table 5). Overall survival rates at 5 years, from time of diagnosis, were 79% for patients undergoing allogeneic BMT and 63% for patients undergoing autologous BMT (P = .23).
A total of 156 autologous BMTs were performed: 62 in group 1, 62 in group 2A, and 32 in group 2B. Patients in group 1 and group 2A had superior DFS rates than group 2B patients (Figure 3B; group 1 vs group 2A, P = .50; group 1 vs group 2B,P = .10; group 2A vs group 2B, P = .35). Autografts were conditioned using either high-dose melphalan with Percoll gradient separation or with BU/CY. Ninety-eight melphalan autografts were performed; 26 in group 1, 61 in group 2A, and 11 in group 2B. DFS overall for all melphalan autografts was 56%, and there were no transplantation-related deaths. DFS was better for group 1 (daunorubicin) patients than for group 2A and group 2B patients (group 1 vs group 2, P = .10; group 2A vs group 2B,P = .83). Of the 72 melphalan autografts performed in group 2, 65 grafts were also Percoll gradient separated, whereas all group 1 melphalan autografts were Percoll gradient separated. Thirty-four patients had relapses following melphalan autograft. Of these, 12 went on to have allogeneic BMT, and 7 (58%) were alive at the time of data censoring.
Forty-eight patients received autograft conditioned with BU/CY, 33 in group 1 and 15 in group 2, giving a combined 5-year DFS rate of 52% (Figure 3B). DFS rates for patients receiving daunorubicin (group 1) were similar to those for patients receiving idarubicin (group 2; 50% vs 47%, respectively). Most group 2 patients receiving BU/CY had received idarubicin at a dose of 12 mg/m2 (n = 14; DFS, 43%), and only 1 patient received idarubicin at 10 mg/m2(n = 1; DFS, 100%). Twenty-two patients receiving a BU/CY autograft had relapses following BMT, and only one of these patients survived in second remission following a second allogeneic BMT. Ten patients received alternative conditioning regimens, mostly using BU/CY with melphalan (n = 6). When these 10 patients are taken together, there is no significant difference in 5-year DFS compared with patients conditioned with melphalan or BU/CY (P = .46,P = .57, respectively).
Comparisons of outcomes for patients conditioned with melphalan compared with BU/CY are shown for different morphological subtype, age, presence of central nervous system disease, and cytogenetics for all patients who received autografts (Tables6 and 7). With the exception of group 1 patients between 6 and 10 years of age, who had higher DFS rates after receiving autografts conditioned with melphalan than after receiving autografts conditioned with BU/CY (P = .04), no significant difference in DFS was noted in conditioning regimens (Table 5).
Comparison of patients who underwent autograft conditioned with high-dose melphalan or busulphan and cyclophosphamide: group 1, daunorubicin
| . | Mel n = 26 . | BU/CY n = 33 . | Total DFS, % n = 62 . | Mel DFS, % . | BU/CY DFS, % . | P, Mel vs BU/CY . |
|---|---|---|---|---|---|---|
| French-American-British | ||||||
| M0 | 1 | 0 | 100 | 100 | — | — |
| M1 | 6 | 8 | 40 | 17 | 50 | .19 |
| M2 | 4 | 6 | 55 | 75 | 50 | .44 |
| M3 | 4 | 5 | 78 | 100 | 60 | .18 |
| M4 | 2 | 7 | 5 | 100 | 29 | .16 |
| M4Eo | 2 | 0 | 100 | 100 | — | — |
| M5 | 7 | 6 | 62 | 71 | 50 | .32 |
| M6 | 0 | 0 | — | — | — | — |
| M7 | 0 | 1 | 100 | — | 100 | — |
| Age, y | ||||||
| 0-5 | 9 | 15 | 64 | 78 | 60 | .33 |
| 6-10 | 11 | 8 | 50 | 73 | 25 | .046-150 |
| 11-15 | 6 | 10 | 58 | 50 | 53 | .57 |
| Central nervous system disease | ||||||
| No | 24 | 32 | 58 | 71 | 49 | .13 |
| Yes | 2 | 1 | 67 | 50 | 100 | .48 |
| Cytogenetics | ||||||
| Good | 4 | 4 | 63 | 75 | 50 | .92 |
| Poor | 6 | 1 | 67 | 67 | 100 | .54 |
| Standard | 14 | 20 | 50 | 64 | 40 | .15 |
| . | Mel n = 26 . | BU/CY n = 33 . | Total DFS, % n = 62 . | Mel DFS, % . | BU/CY DFS, % . | P, Mel vs BU/CY . |
|---|---|---|---|---|---|---|
| French-American-British | ||||||
| M0 | 1 | 0 | 100 | 100 | — | — |
| M1 | 6 | 8 | 40 | 17 | 50 | .19 |
| M2 | 4 | 6 | 55 | 75 | 50 | .44 |
| M3 | 4 | 5 | 78 | 100 | 60 | .18 |
| M4 | 2 | 7 | 5 | 100 | 29 | .16 |
| M4Eo | 2 | 0 | 100 | 100 | — | — |
| M5 | 7 | 6 | 62 | 71 | 50 | .32 |
| M6 | 0 | 0 | — | — | — | — |
| M7 | 0 | 1 | 100 | — | 100 | — |
| Age, y | ||||||
| 0-5 | 9 | 15 | 64 | 78 | 60 | .33 |
| 6-10 | 11 | 8 | 50 | 73 | 25 | .046-150 |
| 11-15 | 6 | 10 | 58 | 50 | 53 | .57 |
| Central nervous system disease | ||||||
| No | 24 | 32 | 58 | 71 | 49 | .13 |
| Yes | 2 | 1 | 67 | 50 | 100 | .48 |
| Cytogenetics | ||||||
| Good | 4 | 4 | 63 | 75 | 50 | .92 |
| Poor | 6 | 1 | 67 | 67 | 100 | .54 |
| Standard | 14 | 20 | 50 | 64 | 40 | .15 |
Cytogenetics results were available for 51 of 62 group 1 patients. Ten patients received conditioning other than Mel or BU/CY. See ”Results” for outcomes.
Mel indicates melphalan; BU/CY, busulphan and cyclophosphamide.
P < .05
Comparison of patients who underwent autograft conditioned with high-dose melphalan or busulphan and cyclophosphamide: group 2, idarubicin
| . | Mel n = 72 . | BU/CY n = 15 . | Total DFS, % n = 94 . | Mel DFS, % . | BU/CY DFS, % . | P, Mel vs BU/CY . |
|---|---|---|---|---|---|---|
| French-American-British | ||||||
| M0 | — | — | — | — | — | |
| M1 | 8 | 3 | 42 | 50 | 33 | .52 |
| M2 | 18 | 6 | 48 | 45 | 50 | .90 |
| M3 | 9 | 0 | 76 | 76 | — | — |
| M4 | 14 | 3 | 47 | 25 | 67 | .87 |
| M4Eo | 1* | — | 0 | — | — | — |
| M5 | 2 | 2 | 60 | 62 | 0 | .06 |
| M6 | 0 | 0 | 0 | 0 | — | — |
| M7 | 0 | 0 | 75 | 75 | — | — |
| Age, y | ||||||
| 0-5 | 37 | 5 | 49 | 51 | 20 | .08 |
| 6-10 | 20 | 5 | 58 | 49 | 60 | .89 |
| 11-15 | 15 | 5 | 57 | 37 | 60 | .87 |
| Central nervous system disease | ||||||
| No | 68 | 14 | 51 | 44 | 43 | .39 |
| Yes | 4 | 1 | 100 | 100 | 100 | — |
| Cytogenetics | ||||||
| Good | 18 | 5 | 54 | 52 | 80 | .18 |
| Poor | 14 | 2 | 50 | 50 | 50 | .86 |
| Standard | 34 | 7 | 52 | 55 | 29 | .06 |
| . | Mel n = 72 . | BU/CY n = 15 . | Total DFS, % n = 94 . | Mel DFS, % . | BU/CY DFS, % . | P, Mel vs BU/CY . |
|---|---|---|---|---|---|---|
| French-American-British | ||||||
| M0 | — | — | — | — | — | |
| M1 | 8 | 3 | 42 | 50 | 33 | .52 |
| M2 | 18 | 6 | 48 | 45 | 50 | .90 |
| M3 | 9 | 0 | 76 | 76 | — | — |
| M4 | 14 | 3 | 47 | 25 | 67 | .87 |
| M4Eo | 1* | — | 0 | — | — | — |
| M5 | 2 | 2 | 60 | 62 | 0 | .06 |
| M6 | 0 | 0 | 0 | 0 | — | — |
| M7 | 0 | 0 | 75 | 75 | — | — |
| Age, y | ||||||
| 0-5 | 37 | 5 | 49 | 51 | 20 | .08 |
| 6-10 | 20 | 5 | 58 | 49 | 60 | .89 |
| 11-15 | 15 | 5 | 57 | 37 | 60 | .87 |
| Central nervous system disease | ||||||
| No | 68 | 14 | 51 | 44 | 43 | .39 |
| Yes | 4 | 1 | 100 | 100 | 100 | — |
| Cytogenetics | ||||||
| Good | 18 | 5 | 54 | 52 | 80 | .18 |
| Poor | 14 | 2 | 50 | 50 | 50 | .86 |
| Standard | 34 | 7 | 52 | 55 | 29 | .06 |
Cytogenetics results were available for 86 of 94 group 2 patients. Ten patients received conditioning other than Mel or BU/CY. See “Results” for outcomes.
Discussion
Anthracyclines have been used for many years in the treatment of AML. Murine models have shown that idarubicin is more potent against leukemic cells and less cardiotoxic than daunorubicin.19When compared with daunorubicin, idarubicin has more rapid cellular uptake, longer intracellular transit time, and an active metabolite, 13-hydroxyidarubicin, with a long half-life.19 Multiple randomized studies and a recent metaanalysis of adult patients with AML have shown that idarubicin has superior efficacy than daunorubicin, particularly in achieving remission induction.14,20-23 In contrast to current findings in adults, there is a paucity of information regarding the efficacy of idarubicin in the initial treatment of childhood AML. A recently published randomized trial showed that idarubicin used in remission induction resulted in a greater reduction in AML blast cell count in the bone marrow of patients on day 15 than in patients receiving daunorubicin in remission induction.15 However, the reduction in marrow blast count did not provide an overall benefit in terms of survival rates.15 In our study we showed no benefit for idarubicin compared with daunorubicin for remission induction, EFS rates, or OS rates at 5 years. Furthermore, there was a trend for patients who received daunorubicin in remission induction to have higher DFS rates following either allogeneic or autologous BMT than the idarubicin group. Creutzig et al15 found that idarubicin when used for remission induction in childhood AML was associated with more bone marrow toxicity, with a greater number of days to neutrophil recovery, than patients treated with daunorubicin. We have shown in our study that greater renal, gastrointestinal, and pulmonary toxicity occurred in patients receiving idarubicin during remission induction. Gastrointestinal toxicity in our patient group was dose related and entailed significantly more toxicity in patients receiving the higher dosage of idarubicin. This may reflect different pharmacokinetic parameters in pediatric patients than those used in previously published studies in adults. No difference in acute cardiotoxicity was noted in our study, but long-term follow-up is needed to establish any evidence of benefit that idarubicin may provide in that regard.
Recent large collaborative group studies have reported remission-induction rates in childhood AML varying from 61% to 91%.2-5,8,24 Our results are superior to these reports, with remission-induction rates reaching 95% and 91% for patients receiving daunorubicin and idarubicin, respectively. Moreover, toxicity-related death rates in the remission-induction phase of chemotherapy in our study were low compared with those in other studies.2 When group 1 and 2 patients are combined, 79% of patients undergoing allogeneic BMT and 63% of patients undergoing autograft remain alive at 5 years. This compares favorably with larger published series.1,3 5
We used an algorithm for course 2, which stratified some patients to higher dose Ara-C if the day 14 bone marrow response demonstrated more than 10% blasts. This approach might have improved the prognosis of patients who responded poorly to course 1, but it will have to be formally assessed in a randomized trial. Although several studies in adults with AML have demonstrated that high-dose Ara-C (3 g/m2) can induce remission in patients who have not responded to conventional-dose Ara-C (100 mg/m2), a randomized comparison of different dosing levels of high-dose Ara-C has not been performed.26
In patients who underwent autologous BMT, we have shown that conditioning with melphalan alone is safe, with no BMT-related deaths and with survival rates equal to those achieved with BU/CY. Furthermore, if relapse occurred following melphalan autografting, significantly more patients benefited from further chemotherapy or allogeneic BMT than from autograft conditioned with BU/CY. In patients who underwent autograft, best results were achieved in patients who received daunorubicin for remission-induction therapy followed by autograft and melphalan. Overall survival was 20% lower for patients receiving a melphalan autograft following idarubicin for remission induction. The reason for this is unclear but may be related to greater systemic toxicity and lower dose intensity experienced by patients taking idarubicin.
Allogeneic sibling BMT for childhood AML has now been shown to achieve the best survival results in most published randomized trials, with many studies showing statistically significant differences.1,8,25 27-29 Our results are consistent with this finding; however, statistical significance was not achieved, possibly because of the small numbers of allogeneic transplantations performed. Furthermore, for patients without an HLA-matched sibling donor, we have shown that autologous BMT achieved results exceeding reported outcomes with intensive chemotherapy alone.
Consistent with other studies, our results have shown superior results for patients with favorable cytogenetic abnormalities. Patients in our studies, regardless of cytogenetic risk group, underwent allogeneic BMT if a matched sibling donor was available. Future studies examining reduction of therapy in this group with good prognosis, possibly without the need for transplantation, may be warranted. As other authors have highlighted, definitive conclusions may be difficult to make, even in large published series, because of small numbers of patients with specific cytogenetic abnormalities.30 Two FAB morphological groups are worthy of further mention. First, the 10 patients in our series with acute megakaryoblastic leukemia (AMKL) FAB M7 had an excellent outcome, with OS rates of 100% for group 1 patients and 71% for group 2 patients. A recently published single-institution study of 29 children with de novo AMKL reported an estimated 2-year EFS rate of 14%, with an advantage for those undergoing allogeneic BMT (EFS, 26%) compared with those receiving chemotherapy alone (EFS, 0%).31 Of the 10 patients in our series, 5 underwent autograft and 5 underwent allogeneic transplantation. Although our data involve small numbers of AMKL patients, an argument can be made that, in absence of a timely available allogeneic match, autologous transplantation may be superior to intensive chemotherapy as consolidation therapy in this subclass of patients. The second FAB subclass of patients worthy of discussion is the group with acute promyelocytic leukemia (FAB M3). ATRA was available for group 2 patients only because our group 1 patients were enrolled before 1992. Recent reports indicate superior survival rates in M3 patients with ATRA containing chemotherapy protocols, without the need for BMT.32 Furthermore, in our series of M3 patients, there was no survival advantage for patients in group 2 compared with group 1, possibly indicating that any benefit gained from the administration of ATRA is reduced by the toxicity associated with aggressive induction therapy and BMT.
Consistent with other studies, our data confirm that the major cause of mortality in childhood AML is recurrence of disease. Improvements in remission-induction rates, decreased treatment-related toxicity, and improved outcomes with allogeneic sibling transplantation have contributed to higher survival rates. Nevertheless, further improvements are needed. Unlike AML trials in adults, our results do not support an advantage of idarubicin over daunorubicin in the treatment of pediatric AML in terms of remission induction or overall survival rates. In addition, we found greater toxicity with idarubicin than with daunorubicin.
We thank the patients, parents and families, nursing staff, hospital medical staff, and data managers at each of the participating institutions. Particular thanks go to Genevieve Daly (Senior Paediatric Oncology Pharmacist) and Brigitte Richmond (Data Manager).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Glenn M. Marshall, Centre for Children's Cancer and Blood Disorders, Sydney Children's Hospital, High St, Randwick, 2031, Sydney, Australia; e-mail: g.marshall@unsw.edu.au.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal