Renal cell carcinoma (RCC) infiltrating lymphocytes (TILs) express killer cell immunoglobulinlike receptors (KIRs) that inhibit the antitumor CD8+ T-cell lysis. In the present study, to better examine the functional consequences of KIR engagement on cytotoxic T lymphocyte (CTL)/tumor interaction, we have investigated the influence of KIR CD158a on early steps of T-cell activation. We show that coengagement of T-cell receptor (TCR) and CD158a by tumor cells inhibited tyrosine phosphorylation of early signaling proteins ZAP-70 and LAT, lipid raft coalescence, and TCR/CD3 accumulation at the CTL/tumor cell interface. In addition, the guanine exchange factor Vav was not phosphorylated, and no actin cytoskeleton rearrangement was observed. Our data indicate a role of KIR CD158a in the dynamic events induced by TCR triggering, preventing CTL membrane reorganization, and subsequent completion of CTL activation program. Accordingly, the expression of CD158 by TILs may favor tumor cell escape to the immune response.
Introduction
In humans, inhibitory natural killer (NK) receptors for HLA-I molecules include killer cell immunoglobulinlike receptors (KIRs), the CD94/NKG2A heterodimer, and immunoglobulinlike transcript 2 (ILT-2)/leukocyte immunoglobulinlike receptor 1 (LIR-1). Although KIRs recognize specific polymorphisms on the classical HLA-A, -B, and -C molecules, CD94/NKG2A recognizes nonclassical HLA-E molecules assembled with a peptide from the leader sequence of HLA-I molecules.1,2 ILT-2/LIR-1 expressed by NK, T, and myeloid cells recognizes a broad range of cellular HLA-I molecules3 as well as viral class I–like molecule, UL-18.4 All these inhibitory receptors have in common one or more immunoreceptor tyrosine-based inhibition motifs (ITIMs) in their cytoplasmic tails. On tyrosine phosphorylation, ITIMs recruit tyrosine phosphatases Src homology 2 domain-containing protein 1 (SHP-1) and/or SHP-2 that can dephosphorylate molecules involved in immuno-tyrosine–based activation motif (ITAM)–induced signaling pathways.5,6 KIR2D CD158 receptors possessing 2 immunoglobulin domains are recognized by specific monoclonal antibodies (mAbs) and distinguish HLA-C alleles.7
In the peripheral blood of healthy individuals, low percentages of KIR+ T cells express a memory CD28−, CD45RA−, CD45RO+ phenotype and a restricted T-cell receptor (TCR) repertoire.8,9 These cells may expand in response to chronic antigenic stimulation to self-antigen and most likely represent a precise subset of the memory T-cell pool.10,11 It has been reported that inhibitory NK receptors expressed by CD8+ cells were able to counterbalance TCR-mediated activation by inhibitory signals that are transduced on specific binding to HLA-I molecules.8,12 13
A few studies have reported that CD94 and KIRs are also expressed by CD8+ T cells infiltrating tumors.14-16 We previously reported that renal tumors contained particular CD8+ infiltrating T cells that express an inhibitory NK receptor from the KIR family (CD158), specific for HLA-C molecules. In vitro, these KIR+ cytotoxic T lymphocytes (CTLs) correspond to potential highly lytic effectors, but their functional activity toward tumor cells is strongly inhibited by the NK receptor.17 In the present study, a tumor-derived CD158a+ CTL was stimulated by tumor cells expressing or not the KIR ligand to investigate the influence of the KIR engagement in the early biochemical and morphologic events leading to cell activation. This system constitutes an accurate model to analyze the interplay between TCR/major histocompatibility complex (MHC) and KIR/MHC interactions during physiologic CTL/target activation.
The recognition of MHC/peptide complex by TCRs results in the activation of protein tyrosine kinases (PTKs).18 Among them, ZAP-70 phosphorylates the adaptor molecule linker for activation of T cells (LAT) that plays a key role in linking TCRs to phospholipase Cγ1 and Ras pathways.19 LAT is also implicated in actin cytoskeleton remodeling required for the TCR-induced membrane spreading.20 TCR-mediated signaling and reorganization of the actin cytoskeleton are further connected through intracellular signaling proteins such as the Rho family guanosine triphosphatase (GTPase) exchanger Vav, SLP-76, and Arp2/3.21-23Consequently, the accumulated actin at the contact between T and antigen-presenting cells (APCs) was found to stabilize a continuous contact that allows the clustering of TCRs and costimulatory and signaling molecules into lipid rafts. Several groups have documented the importance of these membrane rafts in TCR-mediated signal transduction.24-26 Besides its inhibitory effect on cytotoxicity and cytokine production,27 the engagement of inhibitory receptors at the T-cell/APC interface is also likely to influence the outcome of the CTL/tumor interaction by modulating the cellular and morphologic events linked to T-cell activation and cytotoxicity. We took advantage of confocal microscopy to visualize the membrane interactions between a CD158a+ CTLs and tumor cells. In this physiologic CTL/tumor cell interaction, we have evidence that the KIR triggering interfered with early TCR signaling and membrane reorganization, preventing completion of the T-cell activation program.
Materials and methods
Culture of tumor cells and CTLs
Tumor cells derived from renal cell carcinoma (RCC) were maintained in Dulbecco modified Eagle medium/Ham F12 medium supplemented with 10% fetal calf serum (FCS) and Ultroser G (Gibco BRL, Paisley, Scotland, United Kingdom). Epstein-Barr virus (EBV)–transformed B cell lines (LCL) from the same donors were maintained in RPMI 1640 medium supplemented with 10% FCS. Tumor cells were HLA genotyped RCC-7: HLA-A2, A29, B51, B44, Cw15, and Cw16 (Cw4 supertype); RCC-6: HLA-A1, A2, B51, B8, Cw7, and Cw14 (Cw3 supertype); and RCC-5: HLA-A9, A32, B7, B49, and Cw7.
4D4 CD158+ CTL clone was obtained from direct cloning of tumor-infiltrating lymphocytes from RCC (patient 7) was expanded on allogeneic feeder cells in RPMI1640 medium supplemented with 10% human serum and 30 U/mL interleukin 2 (IL-2; Roussel Uclaff, Romainville, France). Briefly, 3 × 103 T cells/well plated with 7 × 104 irradiated allogeneic peripheral blood mononuclear cells (PBMCs) and 104irradiated allogeneic lymphoblastoid cell line (LCL; LAZ509) were grown for 14 days. Expression of CD158a by 4D4 CTL was stable over time in culture.
Cytotoxicity assay
The cytolytic activity of 4D4 CD158a+ T-cell clone against tumor cells was measured in a 4-hour chromium 51 (51Cr)–release assay as previously described.17 In some experiments, mAbs EB6 (anti-CD158a, immunoglobulin G1 [IgG1]), B1.23.2 (anti–HLA-B or -C, IgG2a), L243 (anti–HLA-DR, IgG2a), or UCHT1 (anti-CD3, IgG1) were added at saturating concentrations at the beginning of the cytolytic assay.
Measurement of intracellular calcium concentration by flow cytometry
Target cells were labeled with PKH26 fluorescent cell linker (5.10−6 M dye; Sigma, Saint Quentin, Fallavier, France), washed 3 times, and rested for 1 hour at 37°C. 4D4 CTL clones (5.106/mL) were labeled with 4 μM Fluo 3-am (Sigma) for 45 minutes at 37°C, washed twice with serum rich medium, and resuspended in HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid)–buffered medium. Mixed cells (5 × 104 CTLs and 105 target cells/400 μL) were centrifuged 1 minute at 1500 rpm, incubated 1 minute at 37°C, gently resuspended, and immediately analyzed on Flow cytofluorimeter (FACS-Calibur; Becton Dickinson, Le Pont de Claix, France). In blocking conditions, target cells were incubated with saturating concentration of anti–HLA-B/C or irrelevant anti–HLA-DR mAbs before stimulation. Ca++release was evaluated by measuring green fluorescence of Fluo3 emission at 526 nm. The concentration of intracellular calcium was calculated by using the formula [Ca++]i nM = Kd (F − Fmin)/(Fmax − F) as previously reported,28 whereKd represents the dissociation constant for Ca++-bound Fluo-3 and is of 400 nM. Fmax and Fmin were evaluated by adding a calcium ionophore (ionomycin 10 μg/mL; Sigma) followed by MnCl2 (2 mM; Sigma) to Fluo-3–loaded CTLs.
Receptor clustering and raft redistribution by confocal microscopy
Lipid raft aggregation on CTLs stimulated by mAb-coated beads has been described.26 Beads (polystyrene latex microspheres; Polysciences) were coated with 10 μg/mL anti-CD3ε (UCHT1, IgG1; Becton Dickinson); anti-CD3ε + anti-CD158a (10 μg/mL), or anti-HLA-DR (10 μg/mL) and used at 2:1 ratio over T cells. 4D4 CTL clones labeled with fluorescent B subunit of cholera toxin (8 μg/mL; Sigma), fluorescein isothiocyanate (FITC)–CT-B (that binds to GM1 glycosphingolipids in the lipid rafts) was incubated with mAb-coated beads for 15 minutes at 37°C. T-cell–bead complexes were settled onto poly-l-lysine–coated slides. After fixation, CTL/beads were saturated with mouse IgG1 antibodies and permeabilized for 10 minutes (HEPES-buffered phosphate-buffered saline [PBS] containing 3% bovine serum albumen [BSA] and 0.1% saponin) before staining with anti-Ptyr mAb (4G10, IgG2b; Euromedex, Souffelweyersheim, France) followed by goat antimouse IgG2b-Cy5 (Caltag Laboratories, Burlingame, CA).
For CTL/tumor cell interaction analysis, CTLs were mixed to PKH26-labeled tumor cells, centrifuged 2 minutes at 1500 rpm, settled onto slides after gentle resuspension, and incubated at 37°C for 10 minutes. After fixation, CTLs were labeled with anti-Ptyr or anti-CD3 mAb (UCHT1, IgG1) followed by the labeled-isotypic secondary antibodies. Alternatively, CTL/RCC conjugates were stained with Phalloidin Texas-red (Molecular Probes, Eugene OR) to detect polymerized F-actin. The samples were conserved in mounting medium (Vectashield; Biovalley, Conches, France) at +4°C and analyzed by laser scanning confocal microscopy, a Leica TCS Confocal System (Wetzler, Germany). Cells were considered to have clustered receptors if the staining pattern was crescent shaped and if receptors were polarized to one side of the cell.29 A total of 100 conjugates per slide were analyzed to evaluate percentages.
Immunoblot and immunoprecipitation analysis
4D4 CTL clones (5 × 105 to 106 per sample for immunoprecipitation and 2 × 105 per sample for whole cell extracts) were stimulated with target cells for 3 minutes at 37°C. CTL stimulations were performed at 7:1 effector-target (E/T) ratio for tumor cells and 5:1 ratio for LCLs. Stimulated cells were rapidly pelleted and lysed for 20 minutes on ice in 1% NP-40 lysis buffer containing 20 mM Tris (tris(hydroxymethyl)aminomethane)–HCl (pH 7.5), 150 mM NaCl, 1 mM EGTA (ethyleneglycoltetraacetic acid) and 1% n-Dodecyl beta-D-maltoside in the presence of protease and phosphatase inhibitors: 10 μg/mL leupeptin, 10 mg/mL aprotinin, 1 mM Pefabloc-sc, 50 mM NaF, 10 mM Na4P2O7, and 1 mM NaVO4. Insoluble material was removed by centrifugation for 10 minutes at 14 000 rpm at +4°C. Postnuclear lysate was kept to analyze the protein tyrosine phosphorylated profile, and the remaining material was immunoprecipitated.
Cell lysates were subjected to immunoprecipitation for 2 hours with anti-LAT (rabbit antihuman p36 serum directed against 219-233 residues), anti–ZAP-70 (IB60, rabbit polyclonal antibody directed against 266-344 residues) preadsorbed to protein A–Sepharose (O. Acuto, Paris, France), or anti-Vav (mixed H211 and C14 rabbit polyclonal Abs) (Santa Cruz Biotechnology, CA). Immunocomplexes were washed twice in 20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1% NP-40 and twice in 20 mM Tris-HCl, pH 7.5, 150 mM NaCl and boiled in sodium dodecyl sulfate (SDS) sample buffer before gel electrophoresis.
Whole cell lysates and immunoprecipitated proteins were separated on SDS-polyacrylamide gels and blotted onto nitrocellulose membranes. Separated proteins were probed initially with mAb 4G10 (anti-Ptyr) and after stripping (0.1 M glycine HCL pH 2.3) with specific antibodies: anti-LAT (L. Samelson, National Institutes of Health, Bethesda, MD); anti–ZAP-70 (IB60); anti–Vav-30 mAb (J. Griffin, Boston, MA) and M14, rabbit antihuman SLP-76 (O. Acuto, Paris, France). Proteins were revealed by peroxidase-conjugated goat antimouse or peroxidase-conjugated protein A and developed with the enhanced chemiluminescence detection system (Amersham Pharmacia Biotech, Orsay, France). Densitometric analysis was performed using Bioprofil Bio 1D Windows application V99.04 (Scanalytics, Fairfax, Vancouver, BC, Canada). Values indicate the ratio of tyrosine phosphorylation to protein signal.
Results
Inhibition of the cytotoxic activity and Ca++ response of CTLs by CD158a NK receptor
4D4 CTL clone expressing a VB13.1 TCR and a unique CD158a receptor (specific for HLA-Cw4 supertype alleles) was derived from tumor-infiltrating lymphocytes of a renal tumor. This tumor contained 2% of CD158a+ CD8+ T cells, and VB13.1+ T cells corresponded to 16% of the CD158a+ T cells (data not shown). This CTL is HLA-A2 restricted and recognized an antigen expressed by tumor cells of different origins (renal, melanoma, breast) as well as normal renal cells and EBV-transformed B cells. To analyze the respective role of TCR/MHC and KIR/MHC engagement on CTL activation, 4D4 CTL clone was stimulated by autologous (RCC-7) renal tumor cells or allogeneic renal tumor cell lines (RCC-6 and RCC-5) displaying different combinations of HLA-A and -C molecules. 4D4 CTL efficiently lysed HLA-A2+HLA-Cw3 supertype tumor cell line (RCC-6). In contrast, autologous HLA-A2+ HLA-Cw4 cell line (RCC-7) was lysed by 4D4 CTL only when the CD158a/HLA-Cw4 interaction was blocked with anti–HLA-B/C mAb. The HLA-A2− cell line (RCC-5) was not recognized even in the presence of blocking mAb (Figure 1A, left panel). EBV-transformed B cells (LCL) derived from the same RCC patients were lysed with a similar pattern as tumor targets indicating that 4D4 CTL recognizes a self-antigen (Figure 1A, right panel). In addition, the lysis of patient 6 cell lines was markedly inhibited in the presence of anti-CD3 or anti–HLA-A2 mAbs, evidence that the lytic activity of 4D4 CTL is TCR mediated and restricted by HLA-A2 molecules (data not shown).
Cytotoxicity and intracellular Ca++ release of 4D4 CTL clone are inhibited by the CD158a receptor.
(A) Cytotoxicity of CD158a+ CTL clone 4D4 was assessed at a 3:1 E/T ratio against RCC and EBV-B–transformed cell lines (LCL) derived from 3 patients: autologous RCC-7 (HLA-A2, Cw4), allogeneic RCC-6 (HLA-A2, Cw3), and RCC-5 (HLA-A9, Cw3). Cytotoxicity was determined in absence (medium) or presence of anti–HLA-B/C or control anti–HLA-DR mAbs (1 of 5 experiments is shown). Error bars indicate standard deviation. (B) Flow cytometry analysis of [Ca++]i release by Fluo3-am–loaded CTL stimulated for 1 minute at 37°C with PKH26-labeled target cells. Percentages correspond to CTL conjugates with calcium release of total CTLs. Target cell profile of LCL-7 alone is represented (left panel). (C) [Ca++]imeasurements were calculated from mean fluorescence values (see “Materials and methods”). Determination of [Ca++]i release by CTLs stimulated by RCC cells (left panel), LCL-6 or LCL-7, coated or not with mAbs (right panel).
Cytotoxicity and intracellular Ca++ release of 4D4 CTL clone are inhibited by the CD158a receptor.
(A) Cytotoxicity of CD158a+ CTL clone 4D4 was assessed at a 3:1 E/T ratio against RCC and EBV-B–transformed cell lines (LCL) derived from 3 patients: autologous RCC-7 (HLA-A2, Cw4), allogeneic RCC-6 (HLA-A2, Cw3), and RCC-5 (HLA-A9, Cw3). Cytotoxicity was determined in absence (medium) or presence of anti–HLA-B/C or control anti–HLA-DR mAbs (1 of 5 experiments is shown). Error bars indicate standard deviation. (B) Flow cytometry analysis of [Ca++]i release by Fluo3-am–loaded CTL stimulated for 1 minute at 37°C with PKH26-labeled target cells. Percentages correspond to CTL conjugates with calcium release of total CTLs. Target cell profile of LCL-7 alone is represented (left panel). (C) [Ca++]imeasurements were calculated from mean fluorescence values (see “Materials and methods”). Determination of [Ca++]i release by CTLs stimulated by RCC cells (left panel), LCL-6 or LCL-7, coated or not with mAbs (right panel).
The influence of the KIR receptor was assessed on the intracellular calcium [Ca++]i release, one of the earliest events of CTL activation. Figure 1B shows the percentages of T cells releasing intracellular Ca++ following stimulation by LCL. Quantitatively, tumor cells RCC-6 triggered high [Ca++]i release by CTLs (> 600 mM) when compared with stimulation by RCC-7 that engaged KIR and by a nonrelevant target (RCC-5; Figure 1C, left panel). In addition, preincubation of LCL-7 with blocking anti–HLA-B/C mAb restored a considerable [Ca++]i release by CTL comparable to the one detected in response to LCL-6 (Figure 1C, right panel). Thus, the difference of susceptibility of patients 7 and 6 cell lines to specific CTL was exclusively accounted for by CD158a receptor engagement.
Lack of lipid rafts polarization on the CD3-activated CTL by CD158a NK receptor
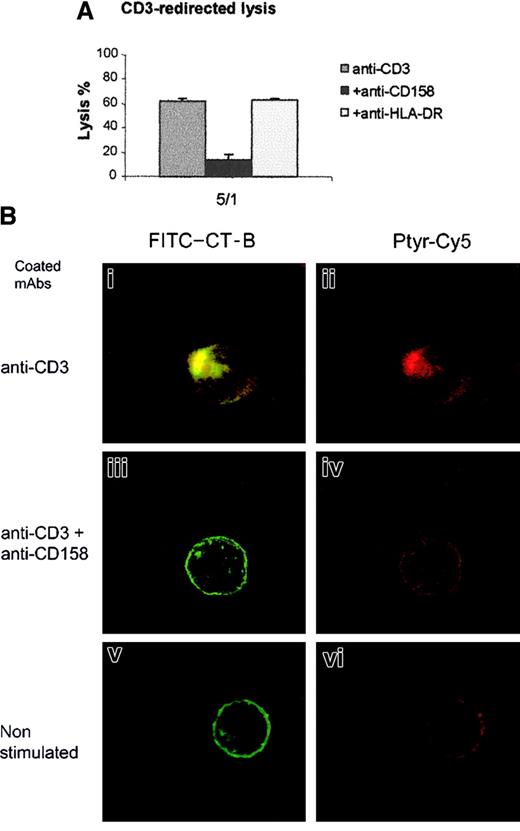
Data depicted in Figure 2A indicate that triggering of KIR by specific mAbs dramatically abrogated the CD3-redirected lysis of murine FcR+ P815 cells. Thus, TCRs and KIRs were triggered on CTLs by microbeads coated with anti-CD3ε or anti-CD3ε + anti-CD158a mAbs, and membrane reorganization was analyzed with the use of confocal microscopy (Figure 2B). Stimulation by anti-CD3ε–coated beads (beads exhibit light red autofluorescence) induced reorganization of membrane lipid rafts visualized on FITC–CT-B–labeled CTLs by formation of fluorescent patches and protrusion of the CTLs at the contact site between cells and beads when compared with unstimulated CTLs (Figure 2Bi and v). In addition, labeling with anti-Ptyr mAb revealed a bright polarized red and green overlapping staining, indicating an increased accumulation of phosphorylated proteins at the cell/bead contact compared with light diffuse staining in control cells (Figure 2Bii and vi). Cotriggering of the TCRs and KIRs on CTLs did not induce redistribution and clustering of FITC-CT-B–labeled rafts, and staining with anti-Ptyr mAb revealed a weak and diffuse staining (Figure 2Biii-iv), suggesting that KIR engagement affects TCR-mediated tyrosine phosphorylation. When CTLs were stimulated by beads coated with anti-CD3ε + anti–HLA-DR, similar results as those observed with anti-CD3ε alone were obtained, whereas anti-CD158–coated beads had no effect on CTLs (data not shown). In 3 independent experiments the numbers of CTLs that formed fluorescent patches at the cell/bead contact were counted and revealed that the triggering of KIRs markedly decreased the percentages of CTLs aggregating lipid rafts (5% versus 32%). This finding strongly indicates that KIRs play a role in the prevention of lipid raft polarization.
KIR triggering prevents CD3-redirected lysis and CD3-induced membrane raft reorganization.
(A) Cytotoxic activity of 4D4 clone was assessed against anti-CD3–coated P815 murine cells. CTL 4D4 was added at a ratio of 5:1 to target in absence (anti-CD3) or in presence of 10 μg/mL of anti-CD158a (+ anti-CD158) or anti–HLA-DR mAbs (+ anti–HLA-DR). (B) Membrane reorganization of CTL clone 4D4 was analyzed by confocal microscopy after stimulation for 15 minutes at 37°C with mAb-coated beads. CTL membrane was labeled with fluorescent B subunit of Cholera Toxin (FITC–CT-B, green), and after cell permeabilization CTLs were labeled with anti-Ptyr mAb revealed by Cy5-labeled goat antimouse IgG (red). An overlay compacted image of serial optical sections is shown on left side panels (i,iii,v), the green color has been removed on right panel (ii,iv,vi; original magnification × 63).
KIR triggering prevents CD3-redirected lysis and CD3-induced membrane raft reorganization.
(A) Cytotoxic activity of 4D4 clone was assessed against anti-CD3–coated P815 murine cells. CTL 4D4 was added at a ratio of 5:1 to target in absence (anti-CD3) or in presence of 10 μg/mL of anti-CD158a (+ anti-CD158) or anti–HLA-DR mAbs (+ anti–HLA-DR). (B) Membrane reorganization of CTL clone 4D4 was analyzed by confocal microscopy after stimulation for 15 minutes at 37°C with mAb-coated beads. CTL membrane was labeled with fluorescent B subunit of Cholera Toxin (FITC–CT-B, green), and after cell permeabilization CTLs were labeled with anti-Ptyr mAb revealed by Cy5-labeled goat antimouse IgG (red). An overlay compacted image of serial optical sections is shown on left side panels (i,iii,v), the green color has been removed on right panel (ii,iv,vi; original magnification × 63).
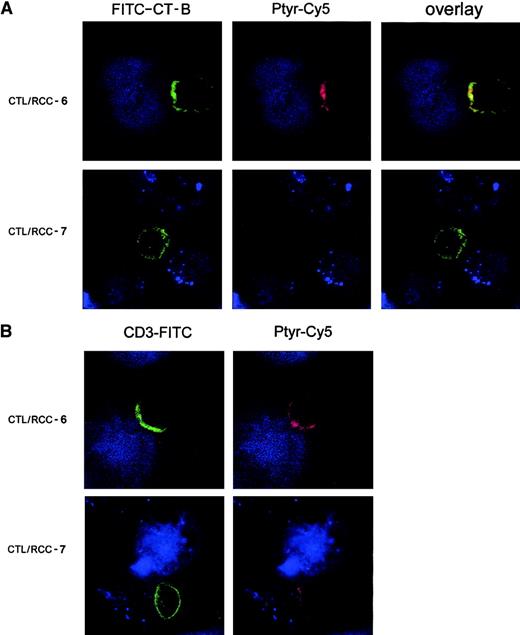
Prevention of lipid raft polarization and CD3 clustering on CTLs by tumor cells cotriggering TCRs and KIRs
With the use of fluorescence imaging, we investigated the effect of simultaneous cotriggering of TCR and KIR receptors on the membrane redistribution events at the contact zone between CTL and RCC targets. FITC–CT-B–labeled CTLs were stimulated with RCC-7 or RCC-6 targets and stained with anti-Ptyr mAbs (Ptyr-Cy5, red) after heteroconjugate fixation. Major differences between CTL/RCC-7 and CTL/RCC-6 conjugates were observed at this level of resolution. Figure3A shows a typical image of interface between CTL and RCC-6 tumor cell with strong lipid raft concentration at the contact zone with the tumor cell that colocalizes in the overlay picture with the dense red anti-Ptyr staining (Figure 3A, upper panels). The lower panels correspond to the contact zone of CTL/RCC-7 conjugates characterized by the absence of accumulation of fluorescent lipid rafts (green) and undetectable level of phosphorylation (Figure3A, lower panels). Quantitative analysis revealed that only 10% of the CTL/RCC-7 conjugates showed a tight polarization of the fluorescence at contact site, whereas fluorescent patches were evident in 32% of CTL/RCC-6 conjugates. In addition, in Figure 3B double staining with anti-CD3ε mAb and anti-Ptyr revealed a zone of dense CD3ε staining at the contact with RCC-6 tumor cells that overlapped with regions of plasma membrane containing the highest Ptyr accumulation. TCR/CD3 accumulation was observed in aggregated lipid rafts (data not shown). In CTL/RCC-7 conjugates (Figure 3B, lower panel), anti-CD3ε staining displayed a ringlike pattern and was not associated with PTK activation. These results demonstrate that CD158a engagement by its specific ligand HLA-Cw4 on RCC-7 markedly impaired lipid raft coalescence, TCR/CD3 capping, and early TCR signaling through PTK activation during CTL/tumor cell contact.
KIR engagement prevents lipid raft polarization and signaling protein phosphorylation.
Confocal analysis of serial optical sections collected at 0.3-μm intervals. (A) 4D4 CTL clone labeled by FITC–CT-B (green) was stimulated with PKH26-labeled tumor cells (blue). 4D4 CTL clone was conjugated either with RCC-6 (upper panels) or RCC-7 (lower panels) and incubated 5 minutes at 37°C. After fixation, cells were stained with anti-Ptyr mAb (red). This picture represents an overlay and the side-side picture of the overlay showing labeling lipid rafts (FITC–CT-B) or tyrosine-phosphorylated proteins (Ptyr-Cy5) as indicated. (B) Stimulated 4D4 CTL clone was stimulated as in (A) and then stained with anti-CD3 mAb (green) and anti-Ptyr mAb (red). Original magnification × 63.
KIR engagement prevents lipid raft polarization and signaling protein phosphorylation.
Confocal analysis of serial optical sections collected at 0.3-μm intervals. (A) 4D4 CTL clone labeled by FITC–CT-B (green) was stimulated with PKH26-labeled tumor cells (blue). 4D4 CTL clone was conjugated either with RCC-6 (upper panels) or RCC-7 (lower panels) and incubated 5 minutes at 37°C. After fixation, cells were stained with anti-Ptyr mAb (red). This picture represents an overlay and the side-side picture of the overlay showing labeling lipid rafts (FITC–CT-B) or tyrosine-phosphorylated proteins (Ptyr-Cy5) as indicated. (B) Stimulated 4D4 CTL clone was stimulated as in (A) and then stained with anti-CD3 mAb (green) and anti-Ptyr mAb (red). Original magnification × 63.
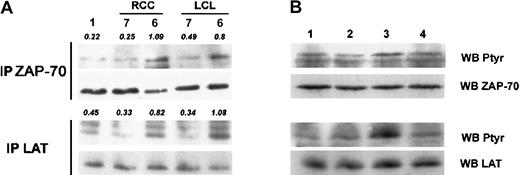
Decreased phosphorylation of TCR-induced tyrosine kinase ZAP-70 and LAT on KIR engagement
KIRs are rapidly phosphorylated on tyrosine residue of ITIM and recruits phosphatase SHP-1 that is responsible for the KIR inhibitory effect on TCR-mediated signaling.30 31 In the present model, immunoprecipitation of the PTK ZAP-70 showed that a low amount of tyrosine phosphorylation of this PTK was observed following CTL stimulation by RCC and LCL-7 target cells that engage the KIR when compared with stimulation with RCC and LCL-6 cells (Figure4A, upper panel). Although less obvious, immunoprecipitation of LAT, the ZAP-70 substrate, also showed that KIR engagement by patient 7 cell lines reduced LAT phosphorylation. As indicated by densitometric values, there was a 2.48-fold lower LAT phosphorylation in response to RCC-7 stimulation (Figure 4A, lower panel). In addition, preventing triggering of KIRs during CTL stimulation by masking HLA-C molecules with anti–HLA-B/C mAb (Figure4B, lane 3) on RCC-7 targets cells resulted in a markedly restored phosphorylation on tyrosine residues of ZAP-70 and LAT proteins. These results provide evidence that KIR triggering decreases early TCR-mediated signaling in the tumor-specific 4D4 CTL clone.
Inhibitory effect of KIR on TCR-mediated ZAP-70 and LAT tyrosine phosphorylation.
(A) Postnuclear lysates of nonstimulated CTLs (lane 1) or stimulated by LCLs or RCC targets were immunoprecipitated with polyclonal anti–ZAP-70 (top panel) or anti–LAT antibody (bottom panel) and analyzed for tyrosine phosphorylation (left panels). Densitometric values are indicated in italics. (B) Same conditions of immunoprecipitation as in (A) of CTLs (lane 1) stimulated by RCC-7 (lane 2), masking of KIR ligand with anti–HLA-B/C (lane 3), or control anti–HLA-DR mAbs (lane 4) before stimulation. Bottom panels (WB ZAP-70 and WB LAT) show membrane reprobing with corresponding antibodies specific for precipitated proteins.
Inhibitory effect of KIR on TCR-mediated ZAP-70 and LAT tyrosine phosphorylation.
(A) Postnuclear lysates of nonstimulated CTLs (lane 1) or stimulated by LCLs or RCC targets were immunoprecipitated with polyclonal anti–ZAP-70 (top panel) or anti–LAT antibody (bottom panel) and analyzed for tyrosine phosphorylation (left panels). Densitometric values are indicated in italics. (B) Same conditions of immunoprecipitation as in (A) of CTLs (lane 1) stimulated by RCC-7 (lane 2), masking of KIR ligand with anti–HLA-B/C (lane 3), or control anti–HLA-DR mAbs (lane 4) before stimulation. Bottom panels (WB ZAP-70 and WB LAT) show membrane reprobing with corresponding antibodies specific for precipitated proteins.
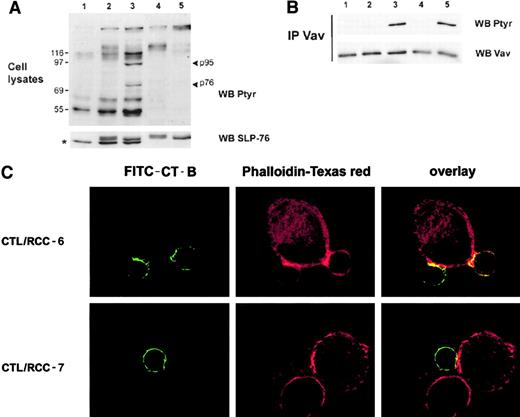
Failure of TCR-induced Vav phosphorylation and actin cytoskeleton redistribution on KIR ligation
Protein tyrosine phosphorylation patterns of 4D4 CTL clone after stimulation by the previous RCC targets revealed marked differences in CTLs stimulated with targets expressing the KIR ligand (RCC-7, Figure5, lane 2) compared with the stimulation with targets engaging TCR only (RCC-6, lane 3). Compared with unstimulated CTLs (lane 1) and to background tyrosine phosphorylated proteins in RCC-7 and RCC-6 tumor cells (lanes 4 and 5), stimulation of CTLs with the susceptible RCC-6 targets (lane 3) resulted in phosphorylation of proteins around 95 kDa and 76 kDa, respectively, that were not detected in CTLs stimulated by RCC-7 (lane 2). Immunoblotting with specific antibodies indicated that these proteins presumably corresponded to Vav and SLP-76 proteins, respectively (Figure 5). Indeed, immunoprecipitation of Vav shows that triggering of KIRs by RCC-7 targets totally prevents Vav phosphorylation compared with RCC-6 targets that do not engage the KIRs (Figure 5B, lanes 2 and 5). Interestingly, preventing KIR triggering by specific mAbs on RCC-7 tumor cells restored the tyrosine phosphorylation of Vav (Figure 5 B, lane 3). In addition, confocal microscopy analyses further indicate that actin cytoskeleton rearrangement in CTLs was greatly affected by KIR engagement. Figure 5C shows that in CTL/RCC-6 conjugates, polymerized F-actin (red staining) was accumulated at the same zone that polarized lipid rafts (green) (Figure 5C, upper panels). In contrast, a homogeneous distribution of membrane rafts and no marked actin polymerization were observed in CTLs stimulated by RCC-7 targets (Figure 5C, lower panels).
KIR engagement prevents Vav tyrosine phosphorylation and the reorganization of actin cytoskeleton.
(A) Tyrosine phosphorylated protein patterns were analyzed by immunoblot from total cell lysates of nonstimulated 4D4 CTLs (lane 1) or incubated 3 minutes at 37°C with RCC-7 (lane 2) or RCC-6 cell lines (lane 3). Ptyr-protein profile of RCC-7 (lane 4) and RCC-6 (lane 5) alone are shown. The lower panel shows membrane reprobing with mAb against SLP-76; * indicates SLP-76 band, the upper band is nonspecific detection of tumor cell proteins. (B) Postnuclear lysates of nonstimulated CTLs (lane 1) or stimulated by RCC-7 targets were immunoprecipitated with polyclonal anti-Vav antibodies and analyzed for tyrosine phosphorylation. CTL was stimulated by RCC-7 alone (lane 2), or in presence of anti–HLA-B/C (lane 3) or control anti–HLA-DR mAbs (lane 4); alternatively CTL was stimulated by RCC-6 (lane 5). Lower panels show membrane reprobing with anti-Vav30 mAb. (C) Confocal analysis was performed as indicated above. 4D4 CTL clone labeled by FITC–CT-B (green) was stimulated with RCC-6 (upper panels) or RCC-7 (lower panels) for 10 minutes at 37°C. After permeabilization, fixed cells were stained with Phalloidin-Texas red (red). This picture represents an overlay and the side-side picture of the overlay showing labeled lipid rafts (FITC–CT-B) or Phalloidin-Texas red as indicated.
KIR engagement prevents Vav tyrosine phosphorylation and the reorganization of actin cytoskeleton.
(A) Tyrosine phosphorylated protein patterns were analyzed by immunoblot from total cell lysates of nonstimulated 4D4 CTLs (lane 1) or incubated 3 minutes at 37°C with RCC-7 (lane 2) or RCC-6 cell lines (lane 3). Ptyr-protein profile of RCC-7 (lane 4) and RCC-6 (lane 5) alone are shown. The lower panel shows membrane reprobing with mAb against SLP-76; * indicates SLP-76 band, the upper band is nonspecific detection of tumor cell proteins. (B) Postnuclear lysates of nonstimulated CTLs (lane 1) or stimulated by RCC-7 targets were immunoprecipitated with polyclonal anti-Vav antibodies and analyzed for tyrosine phosphorylation. CTL was stimulated by RCC-7 alone (lane 2), or in presence of anti–HLA-B/C (lane 3) or control anti–HLA-DR mAbs (lane 4); alternatively CTL was stimulated by RCC-6 (lane 5). Lower panels show membrane reprobing with anti-Vav30 mAb. (C) Confocal analysis was performed as indicated above. 4D4 CTL clone labeled by FITC–CT-B (green) was stimulated with RCC-6 (upper panels) or RCC-7 (lower panels) for 10 minutes at 37°C. After permeabilization, fixed cells were stained with Phalloidin-Texas red (red). This picture represents an overlay and the side-side picture of the overlay showing labeled lipid rafts (FITC–CT-B) or Phalloidin-Texas red as indicated.
Discussion
RCC is considered as potentially immunogeneic tumors on the basis of the rare but documented spontaneous regressions reported and in light of the clinical responses obtained in some patients treated with cytokines. Although RCC is infiltrated by numerous T cells, confirming that the immune system may control the growth of these tumors, this tumor model is also characterized by the existence of various immune defects that result in the global functional alteration of the cytotoxic T-effector cells.32-34 It is known that human solid tumors mostly express tissue-specific antigens or self-antigens that may induce local tolerance, and cytotoxic T cells infiltrating RCC may correspond to antigen-experienced CTLs. In accordance with this hypothesis, we have previously shown that clonal KIR+ CTLs, bearing a memory-activated phenotype, infiltrated RCC.17These KIR+ CTLs that recognize tissue-specific antigens and/or self-antigens represent potentially lytic T cells, but their activity against tumor targets is inhibited by the KIRs.17 35 In the present study, we took advantage of a tumor-derived 4D4 CTL clone, expressing a unique CD158a receptor (specific for HLA-Cw4 supertype) that recognizes an HLA-A2–restricted self-antigen, to determine the influence of such receptor on the early and late steps of CTL activation. The CTL clone 4D4 efficiently lysed allogeneic HLA-A2+, Cw3+ patient 6 targets, whereas lysis and intracellular Ca++ mobilization toward autologous HLA-A2+, Cw4+ patient 7 targets were abrogated following specific CD158a triggering.
It is established that KIR receptors counteract the signaling initiated by the TCR/CD3 receptor in CTLs, and the underlining mechanism involves, at least in part, the recruitment of SHP-1 following phosphorylation of the KIR ITIMs by Src family kinases. SHP-1 extinguishes signaling on neighboring ITAMs and efficiently inhibits CD3 phosphorylation,30 suggesting a sequential aspect in the response to TCR and KIR coengagement. Our data show that when the 4D4 CTL clone was stimulated by antigen-positive targets lacking the KIR ligand (patient 6 targets), ZAP-70 and its substrate LAT, 2 proteins known to be essential for the propagation of TCR-mediated signal, were tyrosine phosphorylated.19 In contrast, after 4D4 CTL stimulation by patient 7 cells that trigger the CD158a receptor, we observed a reduced level of ZAP-70 and LAT phosphorylation. As shown for cytotoxicity and calcium response, preventing triggering of KIRs by masking of HLA-C molecules on patient 7 targets restored the phosphorylation of these 2 essential signaling proteins, indicating that KIRs efficiently down-regulated initial TCR-mediated signaling. Analysis of morphologic events by confocal microscopy studies indicate that TCR-induced signaling takes place in the membrane rafts and further illustrate that TCR and CD158 coengagement abrogates TCR/CD3 and Ptyr accumulation at the CTL/target contact zone. Following CTL/target-specific conjugate, phosphorylation of KIRs on CTL 4D4 recruits SHP-1 (data not shown) may inhibit the signaling by the few TCRs engaged. On the basis of these results, we may assume that TCRs and KIRs act concomitantly with early signaling of TCRs, involving the rapid phosphorylation of ITIM on KIRs and further down-regulation of TCR signaling. Data on early clustering of the TCR complex,36 as well as on the binding kinetics and spatial position of KIRs,37 emphasize the inhibitory role of KIRs on the early consequences of TCR engagement with its MHC/peptide ligand interrupting the activation program.
It is clearly established that LAT, predominantly localized into lipid rafts, plays a central role in signal transduction on TCR engagement. Indeed, TCR-mediated tyrosine phosphorylation of LAT results in the formation of a structural scaffold of downstream signaling proteins such as Vav, PLCγ, and SLP-76, critical for the propagation of the signal19,38,39 and for the dynamic actin polymerization.20 Furthermore, it was shown that Vav, the guanine nucleotide exchange factor (GEF) for Rac/Cdc42 small GTPases, regulates actin cytoskeleton reorganization, the association of actin to CD3ζ chain and TCR/CD3 capping.21,22,40 In our model, TCR and KIR coengagement by tumor cells decreased LAT activation and completely abrogated the tyrosine phosphorylation of Vav that was, however, restored when KIR triggering was prevented by specific mAbs. Tyrosine phosphorylation of Vav has been correlated with its GEF activity for Rho GTPases.41 Thus, the inhibitory effect of KIR triggering on Vav phosphorylation correlated with the absence of actin cytoskeleton redistribution toward patient 7 targets. Our data suggest that Vav activation is a crucial event in the control of CTL activation by KIRs because it links TCR-signaling–dependent cytoskeleton rearrangement to lipid raft clustering.42
The dynamic reorganization of membrane proteins, also termed immunologic synapse, is initiated by coalescence of lipid rafts and correlates with the sustained activation of T cells.43,44In cytotoxic T-cell effectors, the immunologic synapse brings the T-cell secretory apparatus into close contact with the target for a polarized release of cytokines and cytotoxic mediators.45,46 Our data show that KIR engagement by targets was effective in inhibiting TCR-mediated lipid raft coalescence, TCR/CD3 and phosphotyrosine protein accumulation at the contact site, as the underlining mechanism for abrogation of CTL-mediated killing. Our findings are supported by a recent report of Dietrich et al47 outlining the existence of a cross talk between ILT-2 and TCRs with a reduction of both TCR signaling and TCR-induced reorganization of the actin cytoskeleton at the interface of the APC–T cell after ILT-2 ligation.47
Taken together, the present studies emphasize that, although KIR+ CTL effectively recognized renal tumor cells, KIR engagement induced an alteration of early TCR/CD3 signaling and prevented the membrane raft reorganization, resulting in target protection from lysis. KIRs have been proposed as a regulator of homeostasis of memory T-cell subsets, involved in their in vivo survival.11 In the context of an immune response to tumor, KIRs may be involved in the local functional alteration of self-specific CTLs. In renal tumors, KIR+ CTL reflect the presence of an ongoing antitumor immune response of memory T cells induced in response to chronic antigen stimulation by tumor cells. Although the mechanisms controlling the induction of such inhibitory receptors on CTLs are not known, their influence on tumor-specific CTL functions is remarkable, and the expression of KIRs by TILs represent a powerful mechanism that contributes to antigen-specific CD8+ dysfunction. In that context, an in vivo up-regulation of CD94− NKG2A by antiviral CTLs responsible for their functional alteration and virus-induced oncogenesis was recently evidenced.48 In renal tumors, the presence of KIR+ CTL highlights a particular regulation of cytotoxic effectors and may partly explain the beneficial effect currently not clearly understood of the allogeneic hematopoietic stem cell transplantation in RCC patients.49 50 The existence of such CTLs should be taken into account in any immunotherapeutic protocols in patients with RCC.
We thank Dr Frédéric Triebel for providing CTL clones, Prof Alessandro Moretta for helpful discussions and for the kind gift of anti-KIR mAbs, Yann Lécluse for flow cytometry analysis, and Dr Salvatore Valitutti for technical advice on confocal analysis and for helpful discussions.
Prepublished online as Blood First Edition Paper, June 21, 2002; DOI 10.1182/blood-2002-02-0643.
Supported by grants from INSERM, the Association pour la Recherche sur le Cancer (grants 2038 and 5253) (N.G. and A.G.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Anne Caignard, INSERM 487, Institut Gustave Roussy, PR1, 39, rue Camille Desmoulins, 94805 Villejuif Cedex 94, France; e-mail: caignard@igr.fr.

![Fig. 1. Cytotoxicity and intracellular Ca++ release of 4D4 CTL clone are inhibited by the CD158a receptor. / (A) Cytotoxicity of CD158a+ CTL clone 4D4 was assessed at a 3:1 E/T ratio against RCC and EBV-B–transformed cell lines (LCL) derived from 3 patients: autologous RCC-7 (HLA-A2, Cw4), allogeneic RCC-6 (HLA-A2, Cw3), and RCC-5 (HLA-A9, Cw3). Cytotoxicity was determined in absence (medium) or presence of anti–HLA-B/C or control anti–HLA-DR mAbs (1 of 5 experiments is shown). Error bars indicate standard deviation. (B) Flow cytometry analysis of [Ca++]i release by Fluo3-am–loaded CTL stimulated for 1 minute at 37°C with PKH26-labeled target cells. Percentages correspond to CTL conjugates with calcium release of total CTLs. Target cell profile of LCL-7 alone is represented (left panel). (C) [Ca++]imeasurements were calculated from mean fluorescence values (see “Materials and methods”). Determination of [Ca++]i release by CTLs stimulated by RCC cells (left panel), LCL-6 or LCL-7, coated or not with mAbs (right panel).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/8/10.1182_blood-2002-02-0643/4/m_h82023240001.jpeg?Expires=1767780617&Signature=q-hsYnaR52TTi~lkRv52p~OfIqzqEcFERcmYvU8hNNPrbBMVpW1VmkS2K~k8QPakYm7lqIZ6lm1MSarim8FPOfZ1bb4dk6bz0x6l~Nqa1NQm649LCqtgTCTnAFYMp9dQsYYoEOwThmV4Xf4KLzM4NHcj6Nc77ycdVGK-MB-uXmaLagh32f6nyoe339u-xuy75t24XmoM6ndutWou3RJchwaEQuF3tV0pwbglArmvxpt1VKS-l5G89zbk2Hd9eU3tWl8sitNi9A7pMZw4vigxFAsOEF1lkLQyV-wIYc945omtSi6ZftxGRt45jNzPz7HU-gVYRjYXDmwHJu6IH7pLFQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal