Neutrophil apoptosis represents a major mechanism involved in the resolution of acute inflammation. In contrast to the effect of hypoxia observed in many other cell types, oxygen deprivation, as we have shown, causes a profound but reversible delay in the rate of constitutive apoptosis in human neutrophils when aged in vitro. This effect was mimicked by exposing cells to 2 structurally unrelated iron-chelating agents, desferrioxamine (DFO) and hydroxypyridines (CP-94), and it appeared specific for hypoxia in that no modulation of apoptosis was observed with mitochondrial electron transport inhibitors, glucose deprivation, or heat shock. The involvement of chelatable iron in the oxygen-sensing mechanism was confirmed by the abolition of the DFO and CP-94 survival effect by Fe2+ ions. Although hypoxia inducible factor-1α (HIF-1α) mRNA was identified in freshly isolated neutrophils, HIF-1α protein was only detected in neutrophils incubated under hypoxic conditions or in the presence of DFO. Moreover, studies with cyclohexamide demonstrated that the survival effect of hypoxia was fully dependent on continuing protein synthesis. These results indicate that the neutrophil has a ferroprotein oxygen-sensing mechanism identical to that for erythropoietin regulation and results in HIF-1α up-regulation and profound but reversible inhibition of neutrophil apoptosis. This finding may have important implications for the resolution of granulocytic inflammation at sites of low-oxygen tension.
Introduction
Neutrophils are key effector cells of the innate immune system and play a vital role in host defense against gram-negative bacteria. However, excessive or sustained activation of these cells can result in significant tissue damage, with injury resulting from the release of histotoxic granule contents, reactive-oxygen intermediates, and other proinflammatory mediators.1 Persistent accumulation of primed and activated neutrophils is a cardinal feature of a number of lung diseases, including acute lung injury, bronchiectasis, and chronic obstructive airways disease, and it is associated with disease progression and destruction of lung tissue.2 Similarly, in myocardial infarction, neutrophils have been implicated in extending the area of primary myocardial injury by releasing protease-rich granule contents into adjacent viable tissue,3 and, in rheumatoid arthritis, neutrophil-derived reactive-oxygen species and granule enzymes have been demonstrated in synovial fluid and again implicated in disease pathogenesis.4,5 Understanding the mechanisms that modulate neutrophil influx, activation, and longevity is therefore of major pathophysiologic importance to a number of organ systems. The latter 2 events are regulated in large part by the capacity of senescent neutrophils to undergo spontaneous apoptosis, which leads to inhibition of secretory function and to prompt ingestion and removal by inflammatory macrophages.6 7
Apoptosis is a constitutive event in neutrophils and is proposed to be a major mechanism underlying the resolution of granulocyte inflammation.8-11 Neutrophil apoptosis and subsequent macrophage clearance have also been identified in a number of in vivo settings, including pulmonary inflammation in neonates,12resolution of experimental pneumonia,13 and resolution of oleic-acid–induced acute lung injury.14 Interleukin-10 (IL-10) has also been shown to enhance the resolution of lipopolysaccharide (LPS)-induced pulmonary inflammation in rats by promoting granulocyte apoptosis,13 again lending support to the importance of neutrophil apoptosis and clearance in the resolution of inflammation.
It is now recognized that the rate of neutrophil apoptosis can be profoundly influenced by certain inflammatory mediators including granulocyte macrophage–colony-stimulating factor (GM-CSF), G-CSF, IL-1β, IL-2, IL-6, LPS and interferon-γ (IFN-γ).2,15Such data highlight the potential importance of the local environment to the modulation of neutrophil apoptosis in vivo. Furthermore, the rate of neutrophil apoptosis can be accelerated by exposing these cells to stress-inducing stimuli. Hence, UV irradiation, hyperosmolar and oxidizing conditions, and phagocytosis of oil-red particles16 have all been shown to stimulate neutrophil apoptosis.17,18 One surprising exception to this role is the ability of extreme hypoxia to inhibit apoptotic cell death in these cells.19,20 This contrasts with the major proapoptotic effect of hypoxia observed in other cell types, including cultured neurons,21 human adenocarcinoma HT 29 cells,22 and certain oncogenically transformed cells.23
Tissue injury and inflammation can result in a significant decrease in local oxygen tension, and it is essential that the normal mechanisms of tissue protection and repair not be compromised by such conditions. Clearly, the importance of neutrophil apoptosis in the resolution of inflammation dictates that any ability of hypoxia to block this process could have important adverse effects on the clearance of these cells in vivo. Although the mean alveolar PO2 in healthy adults is 13.6 kPa, mean PO2 values of 3.5 kPa have been reported in rheumatoid synovium,24 with even lower values recorded in wound tissues (range, 0.8-1.5 kPa).25 26
To enable cells to respond to changes in oxygen tension, mechanisms for cellular oxygen sensing must be present. Studies looking at the 3′ hypoxic response element of the EPO gene led to the identification of a transcriptional activator, hypoxia inducible factor-1 (HIF-1).27 HIF-1 is a heterodimeric member of the basic helix-loop-helix Per-Arnt-Sim homology (PAS) proteins and is composed of an HIF-1β (or aryl hydrocarbon nuclear translocator [ARNT]) subunit, which is constitutively expressed, and an HIF-1α subunit whose expression and transcriptional activity are tightly regulated by the ambient oxygen concentration.28 This regulator has been identified in many mammalian cell types29 and in most human tissues,30 and it appears to play a key role in oxygen homeostasis. HIF-1α contains an oxygen-dependent degradation domain (ODDD) through which it interacts with von Hippel-Lindau tumor-suppressor protein and subsequently undergoes proteasomal breakdown.31 This process is dependent on prolyl hydroxylation of HIF-1α by the prolyl hydroxylase domain (PHD)–containing proteins 1, 2, and 332; these proteins have an absolute requirement for oxygen and hence represent the proximal sensing mechanism by which the existence of hypoxia is detected by a cell.
In view of the importance of neutrophil apoptosis to the resolution of inflammation and our previous observation that hypoxia can inhibit this event, we wanted to explore the cellular events underlying this process and the associated oxygen-sensing mechanisms. We demonstrate that the apoptotic threshold in neutrophils is acutely but reversibly regulated by the ambient PO2 and that the hypoxia-mediated survival effect can be mimicked by the iron chelators DFO and CP-94, suggesting the involvement of a similar ferroprotein-sensing mechanism as described in other tissues. Of note, prolonged hypoxia (24 hours or longer) appears to induce a more refractory survival effect. The specificity of this effect is demonstrated in part by the inability of mitochondrial electron chain inhibitors, glucose deprivation, or heat shock to influence the rate of neutrophil apoptosis. Furthermore, the antiapoptotic effect of hypoxia is shown to be dependent on continuing protein synthesis, associated with the up-regulation of HIF-1α protein levels, and independent of IL-8 release.
Materials and methods
Neutrophil isolation and culture
Human neutrophils were purified from the peripheral blood of healthy human volunteers by dextran sedimentation followed by centrifugation through discontinuous plasma-Percoll gradients.7,33 Cell purity, assessed using air-dried cytocentrifuge preparations fixed in methanol and stained with May-Grünwald-Giemsa, was routinely greater than 95% neutrophils with less than 0.1% mononuclear cell contamination. The above neutrophil isolation method results in minimal cell priming or activation, as judged by low levels (less than 5%) of basal shape change and formyl-methionyleucylphenylalanine (fMLP)–stimulated superoxide anion generation.34,35 Viability was assessed by the capacity of cells to exclude trypan blue and was greater than 99% under all experimental conditions tested. Purified cells were washed sequentially in platelet-poor plasma and phosphate-buffered saline (PBS) without and with CaCl2 and MgCl2, and they were suspended at 5 × 106 cells/mL in Iscove modified Dulbecco medium (IMDM) supplemented with 10% autologous serum, 50 U/mL streptomycin, and 50 U/mL penicillin (supplemented IMDM). Human peripheral blood monocytes were isolated using the same plasma-Percoll gradient system detailed above, with cells harvested from the upper platelet-poor plasma/42% Percoll interface.7 Approval was obtained from the Cambridge local research ethics committee for these studies. Informed consent was provided according to the Declaration of Helsinki.
Neutrophils were routinely cultured in supplemented IMDM in the presence or absence of test agents (1 μM-10 mM desferrioxamine [DFO], 3 mM FeCl2, 0.1-10 μg/mL cyclohexamide, 1 mM azide, 1 mM potassium cyanide, 100 ng/mL rotenone, 100 μM cobaltous chloride, 0.1 μg/mL gliotoxin, 200 U/mL tumor necrosis factor-α (TNF-α), 100 μM ZVAD-fmk, or 25 μM Boc-D-fmk) at 6.75 × 105 cell/150 μL in flat-bottomed, 96-well Falcon flexible plates (Becton Dickinson, Franklin Lakes, NJ) in a humidified 5% CO2/air or 5% CO2/nitrogen atmosphere at 37°C. The latter incubations were undertaken using an MK3 anaerobic incubator (Don Whitley Scientific, Yorkshire, United Kingdom) that reduced the PO2 of the culture medium (measured using an automated blood gas analyser, model ABL-330; Radiometer, Copenhagen, Denmark) to 3.3 ± 0.3 kPa by 60 minutes. To generate truly anaerobic conditions for certain experiments, 5% CO2/5% H2/90% nitrogen was used in a MACS 500 Don Whitley H2 catalyst–dependent anaerobic incubator, which reduced the PO2 of the cultured medium to 0.2 ± 0.17 kPa. These values were independently verified using an oxygen electrode (World Precision Instruments, Sarasota, FL); the PO2 of the medium was maintained at these levels throughout the subsequent incubation period (data not shown).
To assess the effects of heat shock on the rate of neutrophil apoptosis, cells were incubated at either 42°C or 4°C for 1 hour before incubation at 37°C under normoxic or hypoxic conditions.36 37 For glucose deprivation, cells were incubated in IMDM without D-glucose or sodium pyruvate (Gibco Life Technologies, Paisley, Scotland).
Assessment of neutrophil apoptosis
After gentle resuspension, neutrophils were harvested and cytocentrifuged, and the resultant slide preparations were fixed and stained as detailed above. Cell morphology was examined under oil-immersion light microscopy using a 100× objective, and apoptotic neutrophils were defined as cells containing darkly stained pyknotic nuclei.7 For each condition examined, slides were prepared from triplicate incubations, and more than 500 neutrophils covering at least 5 randomly selected high-power fields were counted with the observer masked to the assay conditions. Apoptosis was also assessed by 2 independent flow cytometry–based methods using fluorescein isocyanate–labeled recombinant human annexin V38 and propidium iodide staining.39 40
ELISA measurement of secreted IL-8
IL-8 released into culture supernatants was measured by enzyme-linked immunosorbent assay (ELISA). Following 6- and 20-hour incubations at 5 × 106/mL under normoxia, hypoxia, or anoxia, cells were removed by centrifugation (352g, 10 minutes), and supernatants were collected. Microlon 96-well ELISA plates (L Greiner, Stonehouse, Gloucester, United Kingdom) were coated overnight at 4°C with 50 μL mouse monoclonal antihuman IL-8 at 2 μg/mL. Following 3 washes with PBS + 0.05% Tween, plates were blocked with 100 μL of 5% fetal calf serum (FCS)/0.05% Tween/PBS for 1.5 hours, washed once with PBS + 0.05% Tween, and incubated overnight at 4°C with 50 μL recombinant human IL-8 standards at 0 to 100 000 pg/mL and 50 μL supernatant samples, each in duplicate. Plates were subsequently washed 3 times as described above, incubated for 3 hours at 37°C with 50 μL biotinylated antihuman IL-8 polyclonal antibody at 0.125 μg/mL, washed 3 more times, incubated with 50 μL extraavidin alkaline phosphatase conjugate at 1:400 for 3 hours at 37°C, and washed twice with PBS + 0.05% Tween and once with distilled water. ELISA reactions were developed in the dark at 37°C with 50 μL/well of 1 mg/mL p-nitrophenyl phosphate in diethanolamine buffer. Plates were read on a Bio-Rad 550 microplate reader (Bio-Rad Laboratories, Hercules, CA) at 405 lambda and were analyzed with MPM version 1.57 software (Bio-Rad).
Identification of HIF-1α mRNA and protein in cell lysates
To obtain total cellular RNA, freshly isolated neutrophils or monocytes (30 × 106 cells) were washed twice in PBS (without CaCl2 and MgCl2), and the resultant cell pellet was lysed by incubation in 6 mL TRIzol reagent (5 minutes, 25°C). Following the addition of 1.2 mL chloroform, the samples were vortex-mixed for 2 to 3 minutes and centrifuged (12 000g, 4°C, 15 minutes), and the RNA was recovered and precipitated from the upper aqueous phase by the addition of an equal volume of propan-2-ol. After incubation for 10 minutes at room temperature or overnight at −20°C, the RNA was pelleted (12 000g, 4°C, 10 minutes), washed in 6 mL 75% (vol/vol) ethanol, and recentrifuged (7500g, 4°C, 5 minutes). The final pellets were air dried for 20 to 30 minutes, redissolved in a minimum amount of 0.5% sodium dodecyl sulfate (SDS), and stored at −80°C before analysis. RNA content was quantified using an RNA/DNA calculator (Pharmacia Biotech, Herts, United Kingdom).
Total cellular RNA was extracted from HeLa cells as a positive control for endothelial PAS domain protein 1 (EPAS) using the above protocol. HeLa cells were grown in Dulbecco modified Eagle medium (DMEM) with 10% fetal calf serum, 1% glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. Cells were used when they were 100% confluent and serially passaged.
For the RNA protection assays, 40 μg total neutrophil RNA was subjected to parallel hybridization with 32P-labeled riboprobes for HIF-1α and EPAS-1. These were generated using SP6 or T7 RNA polymerase. The templates used yielded protected riboprobes of distinct sizes: 221 base pair (bp) for EPAS-1 (nucleotides 2542 to 2762; GenBank accession no. U81984) and 268 bp for HIF-1α (nucleotides 760 to 1028; GenBank accession no. U22431). Hybridization was performed at 60°C in 80% formamide, 40 mM PIPES (piperazine-N,N′-bis(2-ethane sulfonic acid; pH 6.4), 400 mM NaCl, and 1 mM EDTA (ethylenediaminetetraacetic acid) overnight, and RNase digestion was performed at 20°C for 30 minutes. Protected fragments were then subjected to denaturing polyacrylamide gel electrophoresis (PAGE) using 8% gels.
For detection of HIF-1α protein, neutrophils and monocytes were resuspended at a cell density of 5 × 106/mL in supplemented IMDM that had been incubated overnight in normoxic (21% O2) or hypoxic (0% O2) conditions. Cells were cultured in a final volume of 2 mL in 6-well Falcon plates and were incubated for the time period specified in either a hypoxic or a normoxic (±1 mM DFO) environment. Neutrophils were then harvested rapidly into a large volume of ice-cold PBS and were collected by centrifugation (7500g, 4°C), 1 minute). RNA was extracted as previously detailed. The protein-containing supernatant was transferred to clean tubes and incubated for 10 minutes at room temperature with propan-2-ol (1.5 mL/1 mL TRIzol used for the initial lysis). Resultant protein pellets (12 000g, 4°C) were washed 3 times in 0.3 M guanidine hydrochloride in 95% ethanol (2 mL/1 mL TRIzol, resuspended and incubated in 100% ethanol for 20 minutes at 25°C), and vacuum dried and dissolved in 50 μL 1% SDS (50°C, 30 minutes). Finally, the samples were centrifuged (10 000g, 4°C, 10 minutes), and the resultant supernatants were stored at −80°C. Protein concentrations were assessed using a bicinchoninic acid protein assay (Pierce, Rockford, IL).
For immunoblotting, proteins were resolved in SDS/6% polyacrylamide gels and transferred to Immobilon P (Millipore, Bedford, MA) overnight in 10 mM glycine/10% methanol/0.05% SDS. Membranes were blocked with PBS/5% fat-free dried milk/0.1% Tween 20. For HIF-1α detection, monoclonal antibody (mAb) 28b was used at a final concentration of 4 μg/mL. This antibody was raised against a glutathione-S-transferase (GST) fusion containing amino acids 329 to 530 of human HIF-1α. For EPAS-1, 190b supernatant was diluted at a 1:4 ratio. Detection was with horseradish peroxidase (HRP)–conjugated goat antimouse immunoglobulin (DAKO, Ely, United Kingdom), used at 1:2000 dilution, and enhanced chemiluminescence (SuperSignal Ultra; Pierce and Warriner, Chester, United Kingdom). After analysis, membranes were stained with Ponceau S to verify equal protein loading and transfer.
Semiquantitative RT-PCR for adrenomedullin
Semiquantitative reverse transcription–polymerase chain reaction (RT-PCR) was set up using an Access RT-PCR system from Promega (Southampton, United Kingdom). Primer sequences were designed using the published mRNA sequences in GenBank. Sequences were (all 5′ to 3′): adrenomedullin forward, AAGAAGTGGAATAAGTGGGCT; adrenomedullin reverse, TGGCTTAGAAGACACCAGAGT; β-actin forward, ATGAAGTGTGACGTTGACATCCG; β-actin reverse, GCTTGCTGATCCACATCTGCTG; glyceraldehyde-3-phosphate dehydrogenase (GAPDH) forward, AGAACATCATCCCTGCCTC; and GAPDH reverse, GCCAAATTCGTTGTCATACC. Each primer set produced a single band of the expected size in RT-PCR reactions (410 bp adrenomedullin, 232 bp β-actin, 346 bp GAPDH). Neutrophil RNA was extracted as detailed above following 3-hour incubation in normoxia, hypoxia, and anoxia. Semiquantitative RT-PCR used an initial RT step at 48°C followed by 95°C for 5 minutes. PCR was then performed over 20 cycles using the following conditions for all 3 PCR products: denaturation at 95°C for 60 seconds, annealing at 54°C for 90 seconds, and extension at 72°C for 50 seconds. At the end of this protocol, a final elongation step of 72°C for 7 minutes was performed. All reactions contained 2 mM MgSO4 and used 100 ng RNA for a 10-μL reaction volume. PCR products were fractionated on a Tris borate EDTA/2% agarose gel. Gel images were captured using a Stratagene Eagle Eye II system (Stratagene Europe, Amsterdam, Netherlands), and band intensities were calculated using the Scion Image program (Scion, Frederick, MD). Adrenomedullin expression was corrected for GAPDH and β-actin.
Statistical analysis
Results are expressed as mean ± SEM of (n) number of independent experiments; these were all conducted using neutrophils from separate donors, with each condition performed in triplicate. Statistical analysis was performed using analysis of variance, with comparisons between groups made using the Newman-Keuls procedure. Differences were considered significant when P < .05.
Results
Effects of hypoxia on neutrophil apoptosis in vitro
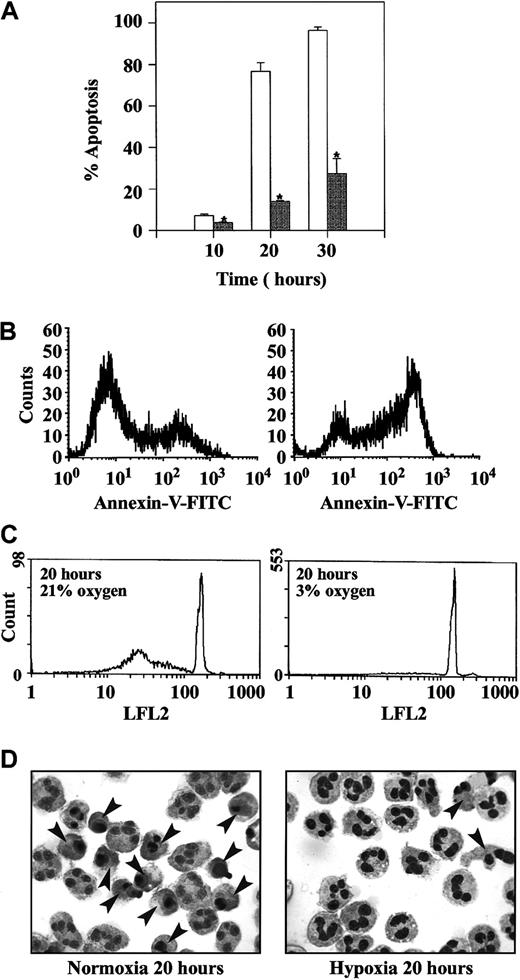
Incubation of isolated human peripheral blood neutrophils under hypoxic conditions (PO2 3.3 ± 0.3 kPa) caused a profound inhibition of neutrophil apoptosis. This effect was verified using 3 independent methods to assess apoptosis—neutrophil morphology (Figure1A,D), externalization of plasma membrane phosphatidylserine (annexin V binding; Figure 1B), and DNA fragmentation (propidium iodide staining; Figure 1C). Cell viability (assessed by trypan blue exclusion) was more than 95% at all time points examined (data not shown). Previous experimental work has demonstrated that the inhibition of neutrophil apoptosis under hypoxic conditions is concentration dependent, with a threshold effect observed at a PO2 of 8.8 ± 0.5 kPa.19
Inhibition of neutrophil apoptosis by hypoxia.
Freshly isolated human peripheral blood neutrophils were incubated at 5 × 106 cells/mL in atmospheres containing 21% (open bars) or 3% oxygen (gray bars). At the time points indicated, cells were recovered and apoptosis was assessed (A) by morphologic examination of cytocentrifuge preparations, (B) by flow cytometry of annexin V binding, or (C) by flow cytometry following propidium iodide staining. Data in panel A represent the mean ± SEM of 3 separate experiments each performed in triplicate (*P < .05, compared with time-matched controls incubated in 21% oxygen). Data in panels B and C are representative flow-cytometry histograms; identical data were obtained in 4 additional independent experiments. (D) Classic neutrophil appearances following in vitro aging under normoxia and hypoxia are shown, and apoptotic cells are highlighted by arrowheads.
Inhibition of neutrophil apoptosis by hypoxia.
Freshly isolated human peripheral blood neutrophils were incubated at 5 × 106 cells/mL in atmospheres containing 21% (open bars) or 3% oxygen (gray bars). At the time points indicated, cells were recovered and apoptosis was assessed (A) by morphologic examination of cytocentrifuge preparations, (B) by flow cytometry of annexin V binding, or (C) by flow cytometry following propidium iodide staining. Data in panel A represent the mean ± SEM of 3 separate experiments each performed in triplicate (*P < .05, compared with time-matched controls incubated in 21% oxygen). Data in panels B and C are representative flow-cytometry histograms; identical data were obtained in 4 additional independent experiments. (D) Classic neutrophil appearances following in vitro aging under normoxia and hypoxia are shown, and apoptotic cells are highlighted by arrowheads.
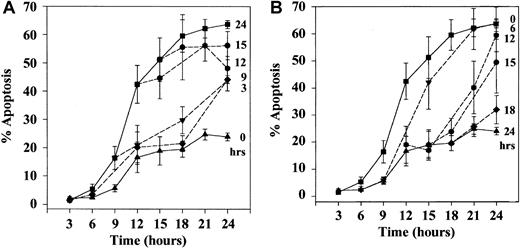
Detailed kinetic analysis of the effects of hypoxia on neutrophil apoptosis demonstrated that hypoxia could delay the rate of constitutive apoptosis even when introduced at a late stage (eg, after 15 hours of normoxia when more than 50% of the cells had already undergone apoptosis) (Figure 2A). Similarly, when neutrophils were incubated initially under hypoxic conditions and then transferred back to a normoxic environment, the cells regained their ability to undergo apoptosis. This occurred at a rate and to an extent identical to that observed in cells incubated for the entire period under normoxic conditions (Figure 2B). This ability of so-called hypoxic neutrophils to regain their ability to undergo apoptosis at a normal rate when reoxygenated was, however, lost when the initial period of hypoxic exposure was extended to 20 hours (Figure3).
Effect of delayed hypoxic incubation and reoxygenation on the kinetics of neutrophil apoptosis.
(A) Neutrophils were incubated at 5 × 106 cells/mL in 21% oxygen for the time periods indicated at right before they were transferred to an incubator containing 3% oxygen. Top and bottom curves represent the time courses for the onset of apoptosis in neutrophils cultured throughout in 21% or 3% oxygen, respectively. Percentage apoptosis was assessed morphologically from cytocentrifuge preparations. (B) Effect of reoxygenation was examined by incubating neutrophils in 3% oxygen for increasing periods of time (indicated at right) before they were transferred to an incubator containing 21% oxygen. Top and bottom curves represent the kinetics for the onset of apoptosis for neutrophils incubated throughout in 21% oxygen or 3% oxygen, respectively. In panels A and B, data represent the mean ± SEM of 4 separate experiments, each performed in triplicate.
Effect of delayed hypoxic incubation and reoxygenation on the kinetics of neutrophil apoptosis.
(A) Neutrophils were incubated at 5 × 106 cells/mL in 21% oxygen for the time periods indicated at right before they were transferred to an incubator containing 3% oxygen. Top and bottom curves represent the time courses for the onset of apoptosis in neutrophils cultured throughout in 21% or 3% oxygen, respectively. Percentage apoptosis was assessed morphologically from cytocentrifuge preparations. (B) Effect of reoxygenation was examined by incubating neutrophils in 3% oxygen for increasing periods of time (indicated at right) before they were transferred to an incubator containing 21% oxygen. Top and bottom curves represent the kinetics for the onset of apoptosis for neutrophils incubated throughout in 21% oxygen or 3% oxygen, respectively. In panels A and B, data represent the mean ± SEM of 4 separate experiments, each performed in triplicate.
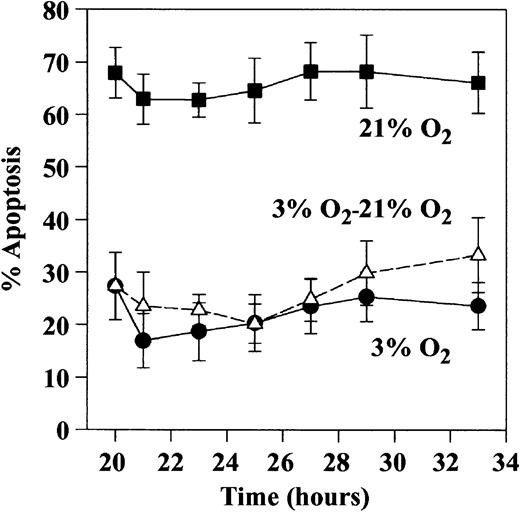
Ability of prolonged hypoxic exposure to impair the subsequent rate of neutrophil apoptosis following reoxygenation.
Neutrophils were incubated for 20 to 30 hours in 21% (▪) or 3% (●) oxygen, and apoptosis was assessed morphologically on cytocentrifuge preparations. In addition, the extent of apoptosis was assessed in cells initially incubated in 3% oxygen for 20 hours before transfer to 21% oxygen (▵). Data are expressed as mean ± SEM of 5 separate experiments, each performed in triplicate.
Ability of prolonged hypoxic exposure to impair the subsequent rate of neutrophil apoptosis following reoxygenation.
Neutrophils were incubated for 20 to 30 hours in 21% (▪) or 3% (●) oxygen, and apoptosis was assessed morphologically on cytocentrifuge preparations. In addition, the extent of apoptosis was assessed in cells initially incubated in 3% oxygen for 20 hours before transfer to 21% oxygen (▵). Data are expressed as mean ± SEM of 5 separate experiments, each performed in triplicate.
Inability of mitochondrial inhibitors, glucose deprivation, or heat shock to inhibit neutrophil apoptosis
Subsequent experiments were undertaken to determine the specificity of the survival effect of hypoxia in neutrophils and particularly to determine whether such an effect could be observed when neutrophils were subjected to other forms of stress, namely heat shock, glucose deprivation, or inhibition of mitochondrial oxidative metabolism.
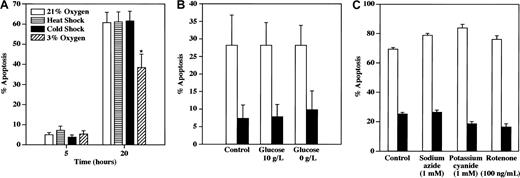
In contrast to the marked survival effect of hypoxia, heat shock at either 42°C or 4°C did not influence the rate of constitutive apoptosis at early (5 hours) or late (20 hours) times (Figure4A). Western blot and flow immunocytometric analysis of Hsp 70 expression in neutrophils demonstrated no alteration in the level of Hsp 70 expression at 4 and 20 hours under normoxic or hypoxic conditions (data not shown).
Inability of heat and cold shock, glucose deprivation, and mitochondrial inhibitors to modulate the rate of neutrophil apoptosis in vitro.
(A) Neutrophils (5 × 106/mL) were incubated at 42°C (heat shock) or 4°C (cold shock) for 1 hour before culture at 37°C under normoxic (21% oxygen) conditions for 5 or 20 hours. Control cells were preincubated for 1 hour at 37°C before culture under normoxic (open bar) or hypoxic (3% oxygen; diagonally striped bar) conditions. Cells were recovered at the time points indicated, and apoptosis was assessed morphologically. Data represent mean ± SEM of 7 independent experiments, each performed in triplicate (*P < .05 compared with time-matched controls). (B) Neutrophils were cultured under normoxic (21% oxygen; open bars) or hypoxic (3% oxygen; filled bars) conditions in IMDM containing either 4.5 g/L glucose (control), 10 g/L glucose, no glucose, or sodium pyruvate. In all conditions IMDM was supplemented with 10% dialyzed (glucose-free) fetal calf serum. Cells were recovered at 20 hours, and apoptosis was assessed morphologically. Data represent the mean ± SEM of 4 independent experiments, each performed in triplicate. (C) Neutrophils were cultured under normoxic (21% oxygen) (open bars) or hypoxic (3% oxygen) (filled bars) conditions for 20 hours in the presence or absence of sodium azide (1 mM), potassium cyanide (1 mM), or rotenone (100 ng/mL), as indicated. Apoptosis was assessed morphologically. Data represent the mean ± SEM of triplicate incubations from 1 of 2 representative experiments.
Inability of heat and cold shock, glucose deprivation, and mitochondrial inhibitors to modulate the rate of neutrophil apoptosis in vitro.
(A) Neutrophils (5 × 106/mL) were incubated at 42°C (heat shock) or 4°C (cold shock) for 1 hour before culture at 37°C under normoxic (21% oxygen) conditions for 5 or 20 hours. Control cells were preincubated for 1 hour at 37°C before culture under normoxic (open bar) or hypoxic (3% oxygen; diagonally striped bar) conditions. Cells were recovered at the time points indicated, and apoptosis was assessed morphologically. Data represent mean ± SEM of 7 independent experiments, each performed in triplicate (*P < .05 compared with time-matched controls). (B) Neutrophils were cultured under normoxic (21% oxygen; open bars) or hypoxic (3% oxygen; filled bars) conditions in IMDM containing either 4.5 g/L glucose (control), 10 g/L glucose, no glucose, or sodium pyruvate. In all conditions IMDM was supplemented with 10% dialyzed (glucose-free) fetal calf serum. Cells were recovered at 20 hours, and apoptosis was assessed morphologically. Data represent the mean ± SEM of 4 independent experiments, each performed in triplicate. (C) Neutrophils were cultured under normoxic (21% oxygen) (open bars) or hypoxic (3% oxygen) (filled bars) conditions for 20 hours in the presence or absence of sodium azide (1 mM), potassium cyanide (1 mM), or rotenone (100 ng/mL), as indicated. Apoptosis was assessed morphologically. Data represent the mean ± SEM of triplicate incubations from 1 of 2 representative experiments.
Although glucose deprivation has been linked to the inhibition of apoptosis resistance in hemopoietic and breast cancer cells41 and it has been demonstrated that high glucose levels induce apoptosis in FRTL5 and endothelial cells,42 43 neither glucose removal nor glucose supplementation influenced the extent of neutrophil apoptosis at 20 hours under normoxic or hypoxic conditions (Figure 4B). The relatively low levels of apoptosis observed in this particular set of experiments reflected the need to replace normal 10% autologous serum with dialyzed (glucose-free) fetal calf serum (data not shown).
Finally, neutrophils were incubated under normoxic (21% oxygen) or hypoxic (3 kPa oxygen) conditions in the presence and absence of the mitochondrial complex I inhibitor, rotenone (100 ng/mL), or the complex IV inhibitors, sodium azide (1 mM) and potassium cyanide (1 mM). These inhibitors were used at a concentration previously demonstrated to modulate TNF-α–induced apoptosis in murine fibrosarcoma cells.44 As shown in Figure 4C, these agents were unable to mimic or abrogate the survival effect of hypoxia even after prolonged coincubation (20 hours), suggesting that the ability of hypoxia to inhibit neutrophil apoptosis is not a consequence of compromised oxidative metabolism or a decline in cellular adenosine triphosphate (ATP) levels. Such data would concur with our previous nuclear magnetic resonance (NMR) spectroscopy findings indicating that the ATP/adenosine diphosphate (ADP) ratio in the neutrophil does not change as the cell undergoes apoptosis.45
Protective effect of hypoxia on neutrophil apoptosis is protein synthesis dependent
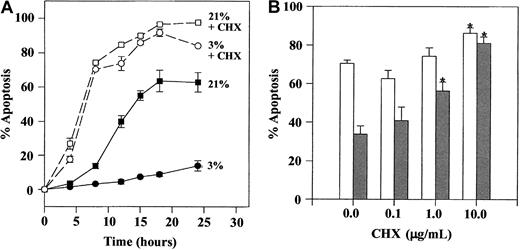
To investigate whether the inhibition of neutrophil apoptosis by hypoxia was protein synthesis dependent, the effect of cycloheximide (CHX) was studied. Initial time-course studies indicated that although the survival effect of hypoxia was completely lost in the presence of 50 μM CHX, this concentration of CHX also caused a major increase in the extent of apoptosis between 4 and 24 hours.46 In view of this, we tested the effect of lower concentrations of CHX (0.1-10 μg/mL), which, although they continue to interfere with protein synthesis,47 do not affect the basal rate of neutrophil apoptosis. Figure 5B demonstrates that CHX, even at these much lower concentrations (1-10 μg/mL), was able to inhibit the survival effect of hypoxia.
Effect of cycloheximide on hypoxia-mediated inhibition of neutrophil apoptosis.
Isolated neutrophils were cultured at 5 × 106/mL in the presence or absence of 50 μg/mL (A) or 0.1 to 10 μg/mL (B) of CHX in atmospheres containing 21% or 3% oxygen. Cells were recovered at the time points indicated, and apoptosis was assessed morphologically. (A) Time-course data (CHX 50 μg/mL) showing mean ± SEM of triplicate incubations from a single experiment representative of 2. (B) Concentration-response data at 20 hours (open bars, cells incubated in 21% oxygen; gray bars, cells incubated at 3% oxygen). Data represent mean ± SEM of 6 separate experiments.
Effect of cycloheximide on hypoxia-mediated inhibition of neutrophil apoptosis.
Isolated neutrophils were cultured at 5 × 106/mL in the presence or absence of 50 μg/mL (A) or 0.1 to 10 μg/mL (B) of CHX in atmospheres containing 21% or 3% oxygen. Cells were recovered at the time points indicated, and apoptosis was assessed morphologically. (A) Time-course data (CHX 50 μg/mL) showing mean ± SEM of triplicate incubations from a single experiment representative of 2. (B) Concentration-response data at 20 hours (open bars, cells incubated in 21% oxygen; gray bars, cells incubated at 3% oxygen). Data represent mean ± SEM of 6 separate experiments.
Hypoxia does not modulate neutrophil release of IL-8
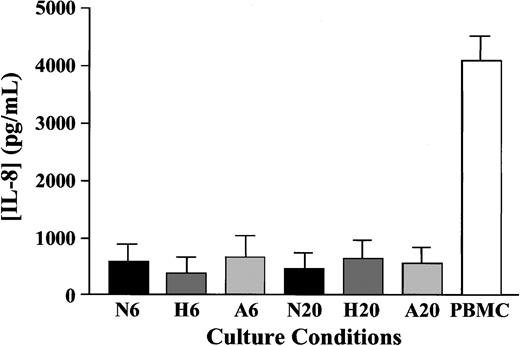
To assess whether the hypoxic regulation of IL-8 release from neutrophils could in part explain the survival effect, IL-8 release into culture supernatants was measured using ELISA. Although IL-8 was detectable at all time points, there was no significant difference in IL-8 levels in cells incubated under normoxic, hypoxic, or anoxic conditions at either 6 or 20 hours (Figure6).
Effect of oxygen tension on neutrophil release of IL-8.
Neutrophils (5 × 106/mL) were cultured in normoxia (N; 21%), hypoxia (H; 3%), or anoxia (A; 0%) for 6 or 20 hours as indicated, and supernatants were collected and stored at −20°C. IL-8 release was subsequently analyzed by ELISA. Phytohemagglutinin-stimulated (10 μg/mL) peripheral blood mononuclear cell (PBMC) supernatants were used as positive controls. Neutrophil data represent mean of 9 separate experiments, each performed in duplicate. PBMC control data represent mean ± SEM of 3 separate experiments.
Effect of oxygen tension on neutrophil release of IL-8.
Neutrophils (5 × 106/mL) were cultured in normoxia (N; 21%), hypoxia (H; 3%), or anoxia (A; 0%) for 6 or 20 hours as indicated, and supernatants were collected and stored at −20°C. IL-8 release was subsequently analyzed by ELISA. Phytohemagglutinin-stimulated (10 μg/mL) peripheral blood mononuclear cell (PBMC) supernatants were used as positive controls. Neutrophil data represent mean of 9 separate experiments, each performed in duplicate. PBMC control data represent mean ± SEM of 3 separate experiments.
Effect of the iron chelators DFO and CP-94 on neutrophil apoptosis
To pursue the hypothesis that HIF-1 may be involved in the inhibition of neutrophil apoptosis by hypoxia, we examined the effect of 2 structurally distinct iron chelators, DFO and 1,2-diethyl-3-hydroxypyridine-4-1 (CP-94), on the rate of neutrophil apoptosis. These agents have been shown to mimic the cellular and biochemical effects of hypoxia in other cell types and to induce HIF-1 activation. DFO and CP-94 caused a concentration-dependent inhibition of neutrophil apoptosis and, at maximally effective (nontoxic) concentrations, were able to inhibit apoptosis to an extent similar to that observed under extreme hypoxia (Figure7A). The survival effect of DFO, CP-94, and hypoxia were also nonadditive (data not shown), suggesting a common mechanism of action. Confirmation that the functional effects of DFO and CP-94 were caused by their iron-chelating properties was obtained by demonstrating that inclusion of a molar excess of iron (Fe2+) could fully block the survival effect of these agents (Figure 7B).
Effect of the iron chelators DFO and CP-94 on neutrophil apoptosis.
(A) Neutrophils were cultured at 5 × 106 cells/mL under normoxic (21% oxygen) conditions for 20 hours in the presence or absence of increasing concentrations of DFO or CP-94. (B) Cells were incubated again under normoxic conditions for 20 hours in the absence or presence of CP-94 (300 μM), DFO (300 μM), and FeCl2(3 mM) as indicated. Apoptosis was assessed morphologically. In panels A and B, data represent the mean ± SEM of 3 separate experiments, each performed in triplicate, with values in panel A expressed as a percentage of the matched-vehicle control values.
Effect of the iron chelators DFO and CP-94 on neutrophil apoptosis.
(A) Neutrophils were cultured at 5 × 106 cells/mL under normoxic (21% oxygen) conditions for 20 hours in the presence or absence of increasing concentrations of DFO or CP-94. (B) Cells were incubated again under normoxic conditions for 20 hours in the absence or presence of CP-94 (300 μM), DFO (300 μM), and FeCl2(3 mM) as indicated. Apoptosis was assessed morphologically. In panels A and B, data represent the mean ± SEM of 3 separate experiments, each performed in triplicate, with values in panel A expressed as a percentage of the matched-vehicle control values.
Expression of HIF-1 in human neutrophils
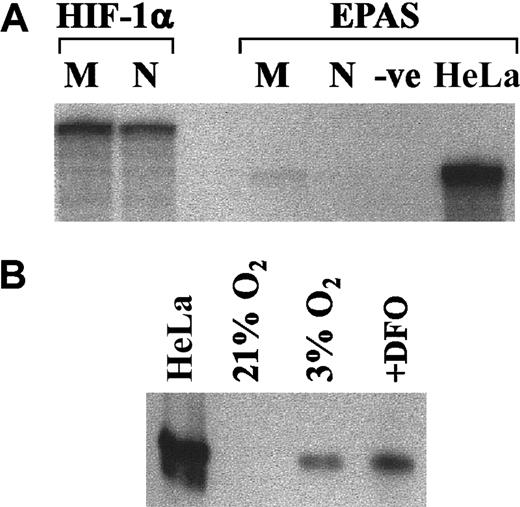
To explore the potential involvement of HIF-1 in hypoxic signaling in the neutrophil, we sought evidence for HIF-1 mRNA and protein expression. RNase protection assays were performed using total RNA extracted from freshly isolated neutrophils and monocytes to identify mRNA for HIF-1 and endothelial PAS domain protein 1 (EPAS-1). EPAS-1 shares 48% sequence homology with HIF-1α.48 HeLa cell RNA was used as a positive control for the EPAS RNase protection assays. Figure8A demonstrates the presence of HIF-1α mRNA in freshly isolated monocytes and neutrophils; this is in contrast to EPAS, which was only identified in the HeLa cells. To demonstrate whether hypoxia could modulate HIF-1α protein levels, neutrophils were incubated for 3 hours in hypoxic conditions, ensuring that these cells were initially resuspended in medium that had been pre-equilibrated at 0% oxygen overnight. Thereafter, whole-cell lysates were prepared using a TRIzol-based lysis method and were analyzed for HIF-1α by Western blotting. As shown in Figure 8B, HIF-1α was not detectable in the control cells but was found in cells incubated under hypoxic conditions or in the presence of DFO.
Expression of HIF-1 in human neutrophils.
(A) Ribonuclease protection assay of total RNA from monocytes (M) and neutrophils (N) was performed for EPAS-1 and HIF-1α, as detailed in “Materials and methods,” with HeLa cell RNA used as a positive control. A representative blot from 2 separate experiments is shown. (B) Neutrophils (5 × 106/mL) were incubated for 3 hours in normoxic (21% oxygen) (± 1 mM DFO) or hypoxic (3% oxygen) environments, and whole-cell lysates were prepared. Hypoxia-treated HeLa cells were used as positive controls. Proteins were separated by SDS-PAGE and probed using an HIF-1α antibody (mAb 28b). Immunoreactive bands were imaged by enhanced chemiluminescence. A representative blot from 2 separate experiments is shown.
Expression of HIF-1 in human neutrophils.
(A) Ribonuclease protection assay of total RNA from monocytes (M) and neutrophils (N) was performed for EPAS-1 and HIF-1α, as detailed in “Materials and methods,” with HeLa cell RNA used as a positive control. A representative blot from 2 separate experiments is shown. (B) Neutrophils (5 × 106/mL) were incubated for 3 hours in normoxic (21% oxygen) (± 1 mM DFO) or hypoxic (3% oxygen) environments, and whole-cell lysates were prepared. Hypoxia-treated HeLa cells were used as positive controls. Proteins were separated by SDS-PAGE and probed using an HIF-1α antibody (mAb 28b). Immunoreactive bands were imaged by enhanced chemiluminescence. A representative blot from 2 separate experiments is shown.
Effect of hypoxia on adrenomedullin transcription
To demonstrate that a HIF-1α–dependent gene was transcriptionally regulated by hypoxia in neutrophils, we examined the effect of hypoxia and anoxia on adrenomedullin expression using semiquantitative RT-PCR. After 3 hours of incubation, adrenomedullin RNA expression—corrected for β-actin expression—was increased by 28% in hypoxia and 20% in anoxia compared with normoxic controls. Furthermore, when corrected for GAPDH expression, adrenomedullin RNA was increased by 50% and 62% in hypoxia and anoxia, respectively (data not shown).
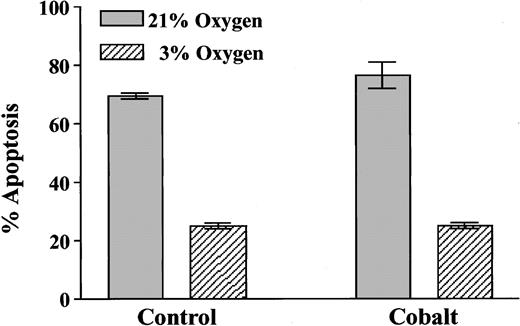
Effect of cobalt on the extent of neutrophil apoptosis
In contrast to the ability of cobalt ions to replicate the effect of hypoxia on erythropoietin expression in Hep3B cells,49 50 100 μM cobaltous chloride caused no mimicry of the hypoxic survival effect in neutrophils cultured for 20 hours and no additional survival advantage in the hypoxic cells (Figure9).
Effect of cobaltous chloride on neutrophil apoptosis.
Neutrophils (5 × 106/mL) were incubated in the presence or absence of cobaltous chloride (100 μM) for 20 hours in atmospheres containing 21% or 3% oxygen. Apoptosis was assessed morphologically, and data represent mean ± SD of triplicate incubations from 1 of 2 representative experiments.
Effect of cobaltous chloride on neutrophil apoptosis.
Neutrophils (5 × 106/mL) were incubated in the presence or absence of cobaltous chloride (100 μM) for 20 hours in atmospheres containing 21% or 3% oxygen. Apoptosis was assessed morphologically, and data represent mean ± SD of triplicate incubations from 1 of 2 representative experiments.
Constitutive neutrophil apoptosis is not blocked by the caspase inhibitors ZVAD-fmk and Boc-D-fmk
To address whether the survival effect of hypoxia might reflect an inhibition of caspase activity, we initially examined whether the inhibition of caspase activity could prevent constitutive neutrophil apoptosis. This was performed by incubating cells in the presence of 100 μM ZVAD-fmk or 25 μM Boc-D-fmk. These agents, although they abolished the proapoptotic effect of TNF-α and gliotoxin (from 95% to 9% at 2 hours; P = <.0001) (Figure10A), had no effect on the extent of constitutive apoptosis at 6 and 20 hours (Figure 10B,C, respectively).
Effect of caspase inhibition on neutrophil apoptosis.
(A) Neutrophils (5 × 106/mL) were cultured for 2 hours in the presence or absence of gliotoxin and TNF-α with or without 100 μM ZVAD-fmk. Data represent 6 replicates from one experiment. (B,C) Neutrophils (5 × 106/mL) were cultured under normoxic (21%), hypoxic (3%), or anoxic (0%) conditions for 6 hours (B) or 20 hours (C) in DMEM containing 10% autologous serum (Monofeed) in the presence or absence of 100 μM ZVAD-fmk or 25 μM Boc-D-fmk. Control, untreated, and 1 μL/mL dimethyl sulfoxide (vehicle)–treated neutrophils were also analyzed. Data represent the mean ± SEM of 3 experiments, each performed in triplicate.
Effect of caspase inhibition on neutrophil apoptosis.
(A) Neutrophils (5 × 106/mL) were cultured for 2 hours in the presence or absence of gliotoxin and TNF-α with or without 100 μM ZVAD-fmk. Data represent 6 replicates from one experiment. (B,C) Neutrophils (5 × 106/mL) were cultured under normoxic (21%), hypoxic (3%), or anoxic (0%) conditions for 6 hours (B) or 20 hours (C) in DMEM containing 10% autologous serum (Monofeed) in the presence or absence of 100 μM ZVAD-fmk or 25 μM Boc-D-fmk. Control, untreated, and 1 μL/mL dimethyl sulfoxide (vehicle)–treated neutrophils were also analyzed. Data represent the mean ± SEM of 3 experiments, each performed in triplicate.
Discussion
These experiments indicate that neutrophils have a ferroprotein oxygen sensor that is able to induce a profound and transcription-dependent inhibition of neutrophil apoptosis. This effect seems to be specific for hypoxia and to display a striking resemblance to the oxygen-dependent regulation of erythropoietin and angiogenic growth-factor expression observed in other cell types.51Hence, the ability of hypoxia to inhibit neutrophil apoptosis could be mimicked by exposure of the cells to 2 discrete iron-chelating agents, DFO and CP-94, in a manner that was blocked by the inclusion of an excess of Fe2+ ions, and it was associated with the induction of HIF-1α protein. Moreover, it has been shown that this effect is not mimicked by antioxidants.19
Detailed kinetic analysis revealed that aging neutrophils could also respond to hypoxia by delaying apoptosis and that reoxygenation caused a prompt reversal of the survival effect. After 20 hours, however, neutrophils were unable to regain their normal apoptotic potential, implying that a more long-term resetting of the cell's apoptotic threshold/steady state occurred with prolonged exposure of neutrophils to low-oxygen tension.
The effect of hypoxia on neutrophil apoptosis appears distinct from the effect of other stress-inducing stimuli. Hence, UV irradiation, sphingosine treatment, and incubation of neutrophils under hyperosmolar conditions17 18 all result in p38 mitogen-activated protein kinase (MAPK) activation, and specific inhibition of this pathway fully protects against the proapoptotic effects of these stimuli. The antiapoptotic effect of hypoxia, however, is not modulated by the specific p38 MAPK inhibitor SB 203580 (K.M., E.R.C., unpublished observations, January 1998) and is not influenced by glucose deprivation. Moreover, the release of antiapoptotic cytokines, including IL-8, seemed unlikely because we showed no significant difference in IL-8 release between the different oxygen tensions and found similar results for GM-CSF, IL-6, IL-1β, and TNF-α (S.R.W., E.R.C., unpublished observations, December 2001).
Although up-regulation of heat-shock proteins, especially Hsp 70 and Hsp 27, have been shown to increase the resistance of certain cells to cytotoxic drug or Fas-induced apoptosis52 and although Hsp 70 is constitutively expressed in neutrophils,37 the rate of neutrophil apoptosis was not sensitive to heat-shock treatment in normoxia or under reduced-oxygen conditions. This argues against a role for conventional heat-shock proteins in the inhibition of neutrophil apoptosis by hypoxia and again lends support to a prosurvival effect of hypoxia mediated through an independent pathway involving specific oxygen sensors and, potentially, HIF-1α.
In normoxia, HIF-1α is rapidly destroyed by ubiquitin proteasome pathways53-55; indeed, in neutrophils we demonstrated the presence of HIF protein only under hypoxic conditions, where this regulated proteolysis is suppressed.56 In addition, the up-regulation of adrenomedullin RNA in neutrophils by hypoxia supports the ability of HIF-1 to act as a transcriptional regulator in these cells because adrenomedullin has 8 recognized HIF-1α–binding sites in its promoter region alone.57
HIF-1α proteolysis is dependent on an interaction with the von Hippel-Lindau tumor-suppressor protein, which recruits the functional E3 ligase complex31 and is itself regulated by the enzymatic hydroxylation of prolyl residues within the LXXLAP amino acid motif of HIF-1α.32,58-60 Recent studies withCaenorhabditis elegans have revealed a novel prolyl hydroxylase egl-9, which has 3 mammalian homologues—prolyl hydroxylase domain-containing proteins (PHDs) 1, 2, and 3.32 All these human enzymes require iron, oxoglutarate, and oxygen as cofactors, and each hydroxylates human HIF-1α at Pro-564.32
PHD1 has been shown to modify HIF-1α in an oxygen-concentration–dependent manner and, as such, provides a mechanism for the ability of oxygen to regulate HIF-1α stability but no direct link to the regulation of apoptosis. PHD2, however, has been identified as a human homolog of rat SM-20,61,62 a protein that localizes to the mitochondria and regulates caspase-dependent apoptosis in nerve growth factor-dependent neurons. In addition, PHD3 has been shown to have significant sequence homology to SM-20.32,63,64 This offers the possibility that these novel oxygen- and iron-dependent regulators of HIF-1α could be linked directly to pathways that regulate apoptosis with HIF-1α serving other functions. The inability of cobalt to mimic the hypoxic inhibition of neutrophil apoptosis does not exclude a role for HIF because cobalt acts by substitution at the catalytic center of 2 oxoglutarate32 and, therefore, provides no additional mechanistic information.
The role of caspase activity in the constitutive apoptosis of hematopoietic cells remains uncertain. Hence, although caspase activation is identified in temperature-regulated65 and TNF-α–induced66,67 neutrophil apoptosis, we show no effect of caspase blockade on constitutive neutrophil apoptosis. This would make the negative regulation of caspase activity, as recently demonstrated for caspase 9,68 an unlikely target for hypoxia in neutrophils. Our demonstration that iron chelators mimic the effect of hypoxia on neutrophil apoptotic thresholds suggests that the same mechanism of 2-oxoglutarate–dependent oxygenase function found to regulate HIF hydroxylation and proteolysis may be operating in this response, either through HIF itself or through another hydroxylation target.
In summary, we have shown that hypoxia causes a profound but reversible inhibition of neutrophil apoptosis. This effect was specific for hypoxia and mediated by a ferroprotein-sensing mechanism characteristic of PHD-regulated events in other cell types. Although hypoxia also caused a marked increase in HIF-1α levels, the precise role of this protein in regulating apoptosis signaling in neutrophils remains to be determined. These findings have important implications for the clearance of neutrophils at sites of inflammation and injury.
We thank P. J. Ratcliffe and J. M. Gleadle (University of Oxford) for contributions to this work and for critical appraisal of the manuscript.
Prepublished online as Blood First Edition Paper, June 28, 2002; DOI 10.1182/blood-2002-02-0454.
Supported by a Raymond and Beverly Sackler Studentship (S.R.W.), the MRC (S.R.W.), Wellcome Trust (E.R.C.), Faculty of Medicine, University of Edinburgh (K.I.M.), Chest, Heart and Stroke Association (Scotland) (E.R.C.), Papworth Hospital NHS Trust (E.R.C.), and British Lung Foundation (E.R.C.).
K.I.M. and S.R.W. are joint first authors.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Edwin R. Chilvers, Respiratory Medicine Division, Department of Medicine, Level 5, Box 157, Addenbrooke's Hospital, Hills Road, Cambridge, CB2 2QQ, United Kingdom; e-mail:erc24@hermes.cam.ac.uk.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal