Abstract
Autoantibodies against factor VIII (FVIII) are rare but can cause life-threatening bleeding requiring costly factor replacement and prolonged immunosuppression. We report 4 consecutively treated patients whose acquired FVIII inhibitors responded rapidly to immunosuppressive regimens that included rituximab, a monoclonal antibody against CD20+ B cells. Three patients had spontaneously occurring inhibitors. The fourth, a patient with mild hemophilia A, developed both an autoantibody and an alloantibody following recombinant FVIII treatment. Pretreatment FVIII activities ranged from less than 1% to 4% and inhibitor titers from 5 to 60 Bethesda units (BU). One patient with polymyalgia rheumatica who developed the inhibitor while receiving prednisone responded to single agent rituximab. The hemophilia patient had rapid resolution of the autoantibody, whereas the alloantibody persisted for months. Responses continue off treatment from more than 7 to more than 12 months. This report adds to the growing evidence that rituximab has efficacy in immune disorders resulting from autoantibody formation.
Introduction
Autoantibodies against factor VIII (FVIII) develop in less than one individual per million per year and have a reported mortality between 6% and 22%.1-3 Most cases are idiopathic; up to 50% are associated with autoimmune diseases, malignancies, drugs, or the postpartum period.1,4 Human or porcine FVIII, prothrombin complex concentrates, or recombinant human FVIIa may be required to control bleeding.2,5,6 To suppress inhibitor formation most patients receive immunosuppressive drugs,1,3,7 such as prednisone,8cyclophosphamide,9-11 azathioprine, or cyclosporine.12 13
The anti-CD20 monoclonal antibody rituximab rapidly eliminates most circulating B cells, suggesting that it could be beneficial in autoantibody-mediated diseases by targeting the autoreactive B cells.14 Early reports of responses in immune thrombocytopenia (ITP) and autoimmune hemolytic anemia (AIHA) appear to confirm this notion,14-16 prompting consideration of its use for FVIII inhibitors.
Study design
Four consecutive patients diagnosed with acquired FVIII inhibitors at our institutions received either 4 (patients 1-3) or 2 (patient 4) weekly infusions of rituximab, 375 mg/m2. Except for patient 3, each patient received a brief course of prednisone at 1 mg/kg/d that was rapidly tapered (Figure1 and 2). All patients gave informed consent. FVIII inhibitors were titered by the Bethesda assay.17 The STACLOT LA test by Diagnostica Stago (Asnieres-sur-Seine, France) was used to detect lupus anticoagulants.
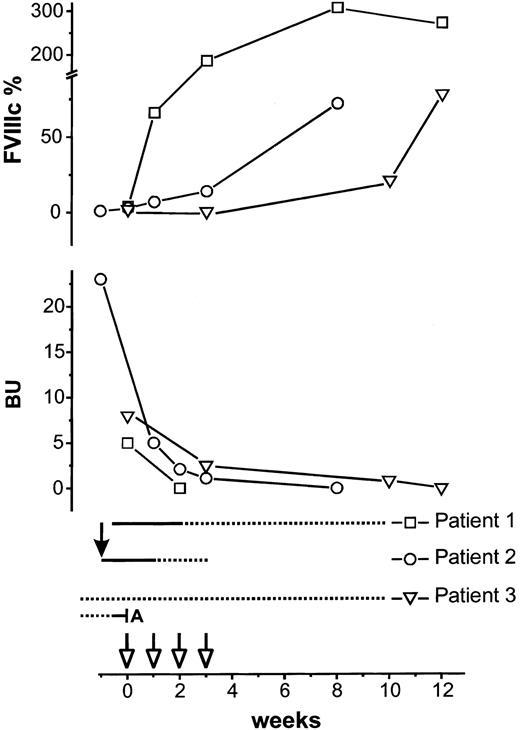
Response to treatment in 3 patients with acquired FVIII inhibitors.
Upper half of the figure shows percentages of FVIII activity (FVIIIc). Lower half shows inhibitor titer in Bethesda units. Rituximab 375 mg/m2 (open arrows) was started at week 0 and repeated weekly for 4 doses. Prednisone at 1 mg/kg/d is indicated by a solid line; broken line indicates prednisone taper. Solid arrow indicates cyclophosphamide 1 g intravenously. In patient 3, azathioprine (A) was stopped, and prednisone 30 mg every day was continued unchanged as indicated by the broken line.
Response to treatment in 3 patients with acquired FVIII inhibitors.
Upper half of the figure shows percentages of FVIII activity (FVIIIc). Lower half shows inhibitor titer in Bethesda units. Rituximab 375 mg/m2 (open arrows) was started at week 0 and repeated weekly for 4 doses. Prednisone at 1 mg/kg/d is indicated by a solid line; broken line indicates prednisone taper. Solid arrow indicates cyclophosphamide 1 g intravenously. In patient 3, azathioprine (A) was stopped, and prednisone 30 mg every day was continued unchanged as indicated by the broken line.
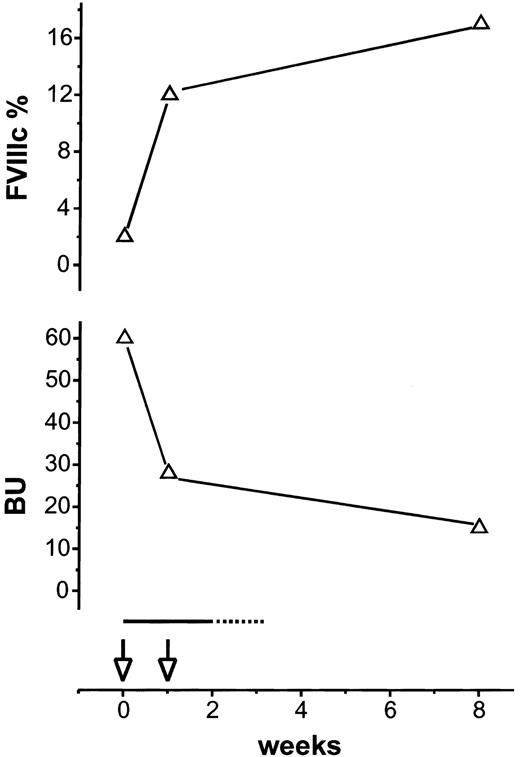
Response to treatment in a patient with mild hemophilia A and an acquired FVIII inhibitor.
Upper half of the figure shows percentage of FVIII activity (FVIIIc). Lower half shows inhibitor titer in Bethesda units. Rituximab 375 mg/m2 (open arrows) was given at week 0 and week 1. Prednisone at 1 mg/kg/d is indicated by a solid line; broken line indicates prednisone taper.
Response to treatment in a patient with mild hemophilia A and an acquired FVIII inhibitor.
Upper half of the figure shows percentage of FVIII activity (FVIIIc). Lower half shows inhibitor titer in Bethesda units. Rituximab 375 mg/m2 (open arrows) was given at week 0 and week 1. Prednisone at 1 mg/kg/d is indicated by a solid line; broken line indicates prednisone taper.
Patient 1, a 69-year-old man with chronic renal failure, presented with melena, bleeding from an arthrocentesis site, hemoglobin of 5.6 g/dL, partial thromboplastin time (PTT) of 94 sec, FVIII activity (FVIIIc) of 4%, and inhibitor titer of 5 Bethesda units (BU). He required 7 units of red cells. Bleeding resolved following treatment with recombinant human FVIII (100 U/kg loading, 10 U/kg/h maintenance), desmopressin acetate (DDAVP), and conjugated estrogen. Prednisone was started on day 1 and rituximab on day 3. Pretreatment serum electrophoresis revealed a very small monoclonal immunoglobulin G (IgG) λ paraprotein. Total γ-globulin level was 0.92 g/dL (normal range, 0.6-1.6 g/dL).
Patient 2, a 38-year-old man with ascites and lymphadenopathy of unknown etiology, PTT of 67 sec, FVIIIc less than 1%, inhibitor titer of 23 BU, and a lupus anticoagulant, developed a large hematoma at a venepuncture site and a 2 g/dL fall in hemoglobin. One week after treatment with FVIII inhibitor bypassing activity (FEIBA) 50 IU/kg four times a day, 1 g cyclophosphamide intravenously, and prednisone, FVIIIc was 3%, and treatment with rituximab was initiated because of lack of clinical improvement. FEIBA was safely discontinued 1 wk after rituximab was initiated.
Patient 3, a 79-year-old woman on prednisone and azathioprine for polymyalgia, developed spontaneous giant ecchymoses; the largest was 20 × 25 cm. Investigations revealed a PTT of 58 sec, FVIIIc of 2%, inhibitor titer of 8 BU, and a lupus anticoagulant. Azathioprine was discontinued, prednisone maintained, and weekly rituximab initiated. Within 1 week, the ecchymoses resolved.
Patient 4, a 39-year-old man with mild hemophilia A and FVIIIc level of 15% because of an Arg2150His mutation received recombinant human FVIII perioperatively. Six days after the operation, on a regimen of FEIBA and DDAVP, an inhibitor of 60 BU was detected, but FVIIIc was unchanged at 15%. Two weeks later FVIIIc fell to 2% with only a minimal increase following DDAVP prompting treatment with rituximab and prednisone.
Results and discussion
Our patients who presented with bleeding had rapid clinical improvement following initiation of immunosuppressive treatment that included rituximab. This improvement was most striking in patient 3 whose giant ecchymoses resolved rapidly following the first dose of rituximab, her sole treatment. In patients 1 to 3, FVIIIc normalized, and the inhibitor became undetectable between 3 and 12 weeks from the start of rituximab (Figure 1). Patient 1 had a normal FVIIIc within 2 weeks and resolution of the inhibitor at 3 weeks. The antigenic stimulation by the FVIII infusions patient 1 received may have had a role in his rapid response.10 In patient 4 (Figure 2), endogenous FVIIIc returned to baseline within 1 week, indicating resolution of the autoantibody, whereas antibody directed against wild-type FVIII persisted for more than 2 months. All patients remain in remission from more than 7 to more than 12 months off treatment.
Spontaneous resolution of FVIII inhibitors occurs in up to 30% of patients, most frequently when the inhibitors develop in the postpartum period.18 Because the median time to spontaneous resolution is 21 months, most patients are treated with immunosuppressive drugs to induce more rapid resolution.1,3,7 Single-agent prednisone has a reported response rate of 30%8,9 and could have accounted for the responses seen in 3 of our patients. However, patients who respond to prednisone frequently require long-term maintenance therapy to prevent relapse.8 In contrast, we were able to taper prednisone rapidly without relapse in our patients.
The combination of prednisone with cyclophosphamide is effective in 70% to 100% of patients, and inhibitors resolve within 3 to 37 weeks.10,11 The time to response in our patients treated with rituximab appears shorter, and potential complications associated with combination prednisone and cyclophosphamide therapy such as neutropenic fever, herpes zoster, myelodysplasia, and cataracts10 11 were avoided.
Inhibitor titers in our patients were of intermediate strength and comparable to those reported in many studies.3 10 Although patients with low-level inhibitors respond more easily to immunosuppressive treatment, inhibitor titer is not directly related to bleeding complications, and even patients with low titer inhibitors can have fatal bleeding.
The addition of short courses of prednisone to rituximab may have enhanced the response in our patients. A preliminary report found only partial remissions in 3 patients with FVIII inhibitors treated with rituximab alone and a complete remission in a patient who also received prednisone.19 However, the complete response of our patient 3 indicates that single-agent rituximab can suffice. Two of our patients (patients 1 and 2) may have had an underlying lymphoproliferative disorder, possibly accounting for a better response.
Rituximab was well tolerated in our study. γ-Globulin levels decreased by 16% at 3 months in patient 1 and returned to pretreatment levels by 7 months. In the context of recent reports on viral infections following rituximab,20-22 it is noteworthy that we observed no worsening of pre-existing hepatitis C in patient 4, who was on a course of ribavirin and interferon when rituximab was started.
FVIII inhibitors have been reported in several kindreds with mild hemophilia because of missense mutations, usually arising after factor replacement and causing a bleeding pattern similar to acquired hemophilia.23 Patient 4's course was similar to these previously reported experiences (Figure 2). Consistent with an alloresponse, the high titer inhibitor initially spares the endogenous FVIII, but a subsequent drop in the FVIII level indicates the formation of autoantibodies. In our patient, prednisone and rituximab led to a rise in FVIIIc back to his baseline level within a week. However, measurable antibody against wild-type FVIII persisted for more than 2 months, suggesting that rituximab eliminated autoreactive B cells more effectively than alloreactive B cells.
Rituximab has been used with varying success in other autoantibody-mediated disorders. Reported response rates range from 30% in ITP15 to 100% in childhood AIHA.16Different response rates may be due to differences in pathogenesis or expression levels of CD20 on the antibody-producing B-cell population.14 During maturation of B cells to plasma cells, CD20 expression is down-regulated. Therefore, timing may be critical for response because rituximab may not be effective if a CD20− plasma cell population has become established. The patients with ITP received rituximab a median of 15 months from diagnosis,15 whereas patients in our study were treated within weeks of diagnosis. Early reports also suggest that rituximab may have efficacy in rheumatoid arthritis24 and Wegener granulomatosis.25 Our patient's polymyalgia unfortunately did not improve following treatment with rituximab. However, the lupus anticoagulants in patients 2 and 3 and the monoclonal gammopathy in patient 1 resolved after treatment.
We conclude that rituximab appears to be an effective and safe treatment for patients with FVIII inhibitors and merits further study. Early treatment and combination with prednisone may be required for maximal benefit.
Prepublished online as Blood First Edition Paper, July 12, 2002; DOI 10.1182/blood-2002-03-0765.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Geraldine P. Schechter, Hematology Section, Room 4D113, Veterans Affairs Medical Center, 50 Irving St NW, Washington, DC 20422; e-mail: g.p.schechter@med.va.gov.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal