Abstract
The role of iron overload as cause of liver dysfunction has never been studied in detail in patients without concomitant hepatotropic infections who receive multiple transfusions. We therefore investigated the relationship between the extent of hepatocellular injury as reflected by serum levels of aminotransferases (alanine aminotransferase [ALT] and aspartate aminotransferase [AST]) and several iron status indices in 39 anti–hepatitis C virus-negative (HCV−) nonthalassemic patients with transfusional iron overload owing to acquired anemias. In 12 patients, we monitored aminotransferase levels and indices of iron status during iron chelation treatment. Before treatment, elevated aminotransferase activity was seen only at liver iron concentrations more than 300 μM/g. During treatment all aminotransferase values were normal if the liver iron concentration returned below 350 μM/g. At the start of treatment, ALT (R2 = 0.64, P = .006) and AST activity (R2 = 0.57,P = .01) were closely related to urinary iron excretion, reflecting the size of the chelatable or the labile iron pool. During treatment, a comparable pattern was seen and the urinary iron excretion was also directly related to the liver iron concentration at concentrations above approximately 400 μM/g. All elevated ALT values were associated with a urinary iron excretion more than 15 mg/24 h. In conclusion, our data suggest the existence of a critical liver iron concentration range, above which hepatocellular injury is seen. The extent of the injury seems to be determined mainly by the size of the chelatable or labile iron pool, supporting the concept of the labile iron pool as the compartment directly involved in iron toxicity. Our findings may be helpful in establishing criteria for safety from complications of transfusional iron overload.
Introduction
Liver disease associated with blood transfusion is caused by hepatotropic infections or hepatic siderosis.1Both factors may act either synergistically or independently in promoting chronic liver disease, inducing cellular damage through similar oxidative pathways.1 Even after the introduction of hepatitis C virus (HCV) screening of blood prior to transfusion, primary HCV infection is still the main cause of hepatotropic infections, and thus an important cause of morbidity among patients with β-thalassemia who receive multiple transfusions.2The high prevalence of HCV infection in β-thalassemic patients may explain why the role of hepatic iron overload without coexisting evidence of infection with major hepatotropic viruses in transfusion-associated liver disease has not been studied in detail in the vast majority of studies on the consequences of transfusional iron overload. Moreover, many of these studies were carried out before the discovery of HCV.3-5
HCV infection is an uncommon cause of liver dysfunction in multiply transfused patients in Denmark where HCV prevalence is low.6 This afforded us a unique opportunity to study the relationship between transfusional iron overload and liver disease without coexisting liver disease caused by hepatotropic virus infection. Remarkably few studies have focused on this issue in nonthalassemic patients. Some studies have explored liver dysfunction after bone marrow transplantation for hematologic malignancies,7-11 but transfusional iron overload as the sole cause of liver dysfunction is seen only in a minor fraction of these patients.11 One small study examined liver dysfunction in 15 adults with transfusional iron overload caused by refractory anemias.12 Serologic tests for hepatitis B virus infection were negative, but this study was also conducted before the discovery of HCV.
It is now generally accepted that primary as well as secondary iron overload can have prefibrogenic effects on the liver by giving rise to a sustained condition of oxidative stress.13 Although hepatic iron excess is characterized by a low degree of inflammation and hepatocellular necrosis, this low necrogenic activity may initiate and promote progressive fibrosclerosis, eventually leading to cirrhosis.13 The relationship between the magnitude of iron overload and the degree of hepatocellular injury has never been studied in detail for the reasons mentioned above. Hence, it remains unknown if hepatocellular injury depends on liver iron concentration, as is the case in patients with hereditary hemochromatosis, who do not seem to develop liver fibrosis or cirrhosis at liver iron concentration levels below a certain critical threshold.14
This study seeks to fill this knowledge gap by investigating the relationship between the extent of hepatocellular injury reflected in the aminotransferase serum activities and the magnitude of transfusional iron overload as assessed by several indices of body iron status (liver iron concentration, serum ferritin, urinary iron excretion, serum iron saturation of transferrin) in adult nonthalassemic, anti-HCV− patients with acquired transfusion-dependent anemias prior to and during iron chelation with desferrioxamine (DFO).
Patients, materials, and methods
Patients
The present study, approved by the local ethics committee, is based on 41 nonthalassemic adults with transfusional iron overload caused by acquired anemias. The patients had given written, informed consent to participate in the evaluation of the consequences of transfusional iron overload. The investigational techniques included noninvasive determination of the liver iron concentration by magnetic resonance imaging (MRI), routine liver tests, measurement of serologic indices of body iron status, serologic tests for hepatitis B and C, investigation for mutations within the HFE gene, and an MRI-based evaluation of the myocardial iron content. The data on the myocardial iron content have already been published.15
All patients were tested for viral hepatitis B and C infection (see “Serologic tests”). One patient had HCV antibodies and another patient had biopsy-verified alcoholic liver disease before developing transfusional iron overload. Both patients were excluded from the study, leaving 39 eligible patients. All patients (14 women, 25 men) had regular blood transfusions. All but 3 patients had exclusively received erythrocyte suspensions screened for anti-HCV, hepatitis B surface antigen (HBsAg), and anti–HIV-1 and -2. None had received iron chelation treatment before entry into the study and none had had venesections. Twenty-seven of the patients had a confirmed diagnosis of myelodysplastic syndrome (MDS) of various stages. Three patients had aplastic anemia, 1 had red cell aplasia, 4 had acute myeloid leukemia in complete remission after autologous bone marrow transplantation, and 1 each had chronic hemolysis, chronic myeloid leukemia, chronic lymphatic leukemia, and myelofibrosis. Thirty-seven of 39 patients were investigated for the Cys282Tyr mutation and the His63Asp variant within the HFE gene causing hereditary hemochromatosis. We did not find patients who were homozygous for the Cys282Tyr mutation or compound-heterozygous for the Cys282Tyr mutation and the His63Asp variant, excluding cases of hereditary hemochromatosis caused by mutation within the HFE gene, but not excluding rare cases caused by other known or still unknown mutations. We did, however, observe 5 patients heterozygous for His63Asp and 2 for Cys282Tyr.
Twelve anti-HCV− patients without clinical evidence of liver disease unrelated to transfusional iron overload started iron chelation with DFO after entering the study. These patients were followed by repeated determination of the liver iron concentration, routine liver tests, and serologic indices of body iron status, MRI-based evaluation of myocardial iron, and evaluation of the left ventricular ejection fraction by multigated cardiac scintigraphy. Data on ventricular function16 and preliminary data on the changes of the iron status during iron chelation (serum ferritin, liver iron concentration)17 have been published. The follow-up period lasted from 12 to 48 months. Serologic screening of the patients for anti-HCV and HBsAg during follow-up revealed no evidence of hepatitis B or C virus infection. Patient 9 had already been on iron chelation for 9 months when the first MRI examination was performed. Characteristics of individual patients are given in Table1.
Clinical characteristics of 12 patients at start of iron chelation
| Patient no. . | Diagnosis . | Sex . | Age, y . | Liver iron, μM/g . | Ferritin, μg/L . | TfS, % . | Blood transfusions . | |
|---|---|---|---|---|---|---|---|---|
| U* . | U/mo . | |||||||
| 1 | MDS, RARS | M | 58 | 501 | 3600 | 88 | 132 | 8.1 |
| 2 | MDS, RA | F | 67 | 521 | 4340 | 79 | 77 | 3.8 |
| 3 | MDS, RA | F | 32 | 405 | 1740 | 84 | 44 | 3.0 |
| 4 | MDS, RAEB | F | 63 | 558 | 1780 | 94 | 116 | 4.7 |
| 5 | MDS, RA+ERD | F | 47 | 403 | 2510 | 75 | 105 | 4.8 |
| 6 | MDS, RA | F | 64 | 540 | 5770 | 83 | 88 | 5.7 |
| 7 | MDS, RA | F | 46 | 558 | 3800 | 88 | 75 | 3.2 |
| 8 | MDS, RA | F | 41 | 623 | 8715 | 100 | 254 | 2.9 |
| 9† | DBS | M | 37 | 388 | 2380 | 108 | 420 | 3.1 |
| 10 | AML, CR | M | 34 | 468 | 2200 | 62 | 109 | 3.0 |
| 11 | CH | F | 41 | 479 | 1280 | 66 | 70 | 1.0 |
| 12 | MDS, RARS | M | 69 | 491 | 4790 | 102 | 90 | 4.0 |
| Patient no. . | Diagnosis . | Sex . | Age, y . | Liver iron, μM/g . | Ferritin, μg/L . | TfS, % . | Blood transfusions . | |
|---|---|---|---|---|---|---|---|---|
| U* . | U/mo . | |||||||
| 1 | MDS, RARS | M | 58 | 501 | 3600 | 88 | 132 | 8.1 |
| 2 | MDS, RA | F | 67 | 521 | 4340 | 79 | 77 | 3.8 |
| 3 | MDS, RA | F | 32 | 405 | 1740 | 84 | 44 | 3.0 |
| 4 | MDS, RAEB | F | 63 | 558 | 1780 | 94 | 116 | 4.7 |
| 5 | MDS, RA+ERD | F | 47 | 403 | 2510 | 75 | 105 | 4.8 |
| 6 | MDS, RA | F | 64 | 540 | 5770 | 83 | 88 | 5.7 |
| 7 | MDS, RA | F | 46 | 558 | 3800 | 88 | 75 | 3.2 |
| 8 | MDS, RA | F | 41 | 623 | 8715 | 100 | 254 | 2.9 |
| 9† | DBS | M | 37 | 388 | 2380 | 108 | 420 | 3.1 |
| 10 | AML, CR | M | 34 | 468 | 2200 | 62 | 109 | 3.0 |
| 11 | CH | F | 41 | 479 | 1280 | 66 | 70 | 1.0 |
| 12 | MDS, RARS | M | 69 | 491 | 4790 | 102 | 90 | 4.0 |
TfS indicates iron saturation of serum transferrin; MDS, myelodysplastic syndromes (classified according to French-American-British criteria); RARS, refractory anemia with ringed sideroblasts; RA, refractory anemia; RAEB, refractory anemia with excess of blasts; ERD, end-stage renal disease; DBS, Diamond-Blackfan syndrome; AML CR, acute myeloid leukemia in complete remission; and CH, chronic hemolysis of unknown etiology.
Total number of transfused blood units.
Patient 9 had been on iron chelation for 9 months when entering the study.
Serologic tests
All patients were tested for anti-HCV antibodies using a microparticle enzyme immunoassay (MEIA), detecting antibodies to HCV-encoded antigens recombinant HCr43, c200, c100-3, and NS5 (AxSYM HCV version 3.0, from Abbott Laboratories, Wiesbaden, Germany). All patients were tested for viral hepatitis B infection with an MEIA for HBsAg (AxSYM HBsAg [V2]), hepatitis B surface antibody (HbsAb; AxSYM AUSB), HBcAb (AxSYM Core and AxSYM Core-M) all from Abbott Laboratories.
Investigations for the Cys282Tyr mutation and the His63Asp variant within the HFE gene were performed by polymerase chain reaction (PCR)–based restriction fragment length analysis (RFLA).18
The serum ferritin concentration was assessed by enzyme-immunometric assay (Amerlite, Ferritin assay, monoclonal; Amersham, Cardiff, United Kingdom). The reference range was 10 to 120 μg/L for women and 15 to 300 μg/L for men. The serum transferrin (Tf) concentration (reference range, 23-40 μM/L) was determined by nephelometric measurement of immune complexes using rabbit antibodies against human Tf. The serum iron concentration (reference range, 11-31 μM/L) was measured by a spectrophotometric method.
Liver dysfunction was evaluated by alanine aminotransferase (ALT) and aspartate aminotransferase (AST) using a standard autoanalyzer-based method. The upper normal limits for both enzymes were 40 U/L.
Iron chelation therapy
DFO was administered subcutaneously in the abdominal wall by 12-hour infusions, 5 days a week with a computer-assisted infusion pump (CADD II; SIMS Deltec, St Paul, MN) in 5 patients and in 7 patients by twice daily subcutaneous bolus injections according to Jensen et al.17 The daily DFO dose was 2 g daily (2.5 g daily in patient 1). Vitamin C supplementation (200 mg once a day) was started 6 to 18 months after start of DFO therapy.
Urinary iron excretion
Iron excretion was determined by atomic absorption spectrophotometry19 based on 24-hour collections. Follow-up values represent means of three 24-hour collections, performed on successive days. In patients 3 and 12 urine samples were not available.
Estimation of liver iron
MRI studies were performed using a Philips Gyroscan (Best, The Netherlands), S 15-HP, operating at 1.5 T. The images were obtained using the following electrocardiographic-gated spin echo sequence: echo time = 25 ms, repetition time equal to the heart rate (500-960 ms). The field of view was 450 mm2 matrix = 256 × 256 pixels. Oblique images (15-25 slices, with a slice thickness of 9 mm) were recorded, visualizing the right liver lobe and posterior vertebral muscles in the same slice. The signal intensity (SI) for hepatic tissue and the posterior vertebral muscle were determined by use of operator-defined regions of interest, which were always more than 50 pixels. We then calculated the liver-to-muscle SI ratio and corrected for variable values of the repetition time as previously described.20 The MRI system was calibrated daily by testing the system resonance frequency and the signal-to-noise ratio using a performance phantom. Validation experiments20 have shown a close linear inverse and semilogarithmic relationship (R2 = 0.98; P < .0001) between the liver-to-muscle SI ratio and the liver iron concentration by chemical analysis of liver biopsies within the range of 5 to 650 μM Fe/g (dry weight). This method established a reference range for the liver iron concentration of 1 to 15 (mean ± 2 SD) μM/g. The day-to-day variation was 2.9 ± 2.7 μM/g (healthy controls).
Data analysis
Data are given as percentages or as means ± SD for continuous variables with symmetric distributions if not otherwise indicated. Data with skewed distributions are given as median. Linear relationships between variables were investigated by linear regression analysis (least square method). If appropriate, logarithmic transformation of the data was performed before analysis. The model quality was assessed by calculation of R2.
Relationships between variables monitored during iron chelation (including repeated measurements on the same individual) were investigated by a locally weighted scatter plot smoother (lowess), producing lowess curves based on least squares fits. All statistical tests were 2-tailed. A significance level of P < .05 was used. All statistics were done by using Stat View 5.0 software for Macintosh (1992-1998 SAS Institute, Cary, NC).
Results
Aminotransferase activities and iron status prior to iron chelation
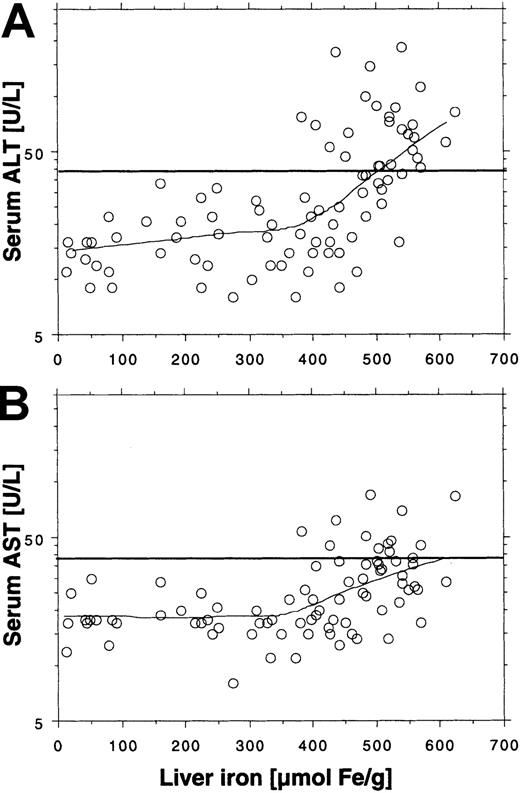
We found elevated ALT activity in 21 (54%) of 39 patients and elevated AST activity in 11 (28%) of 39 patients, which suggests increased hepatocellular cell injury induced by transfused iron. Serum ALT activity was generally higher than serum AST activity. Aminotransferase levels appeared dependent on liver iron concentration (Figure 1). Elevated ALT and AST values were seen only at liver iron concentrations above 300 μM/g and 400 μM/g, respectively. An elevated ALT was, however, only found in 21 (75%) of 28 patients and an elevated AST in 11 (39%) of 28 patients with these high liver iron levels. Elevated aminotransferase levels were not seen until the iron saturation was more than 75%. Twenty-one (68%) of 31 patients exceeding this saturation had elevated ALT and 11 of 31 patients (36%) had elevated AST. The logarithmically expressed serum ferritin concentration showed a close positive and linear relationship with the logarithmically expressed ALT serum activity (R2 = 0.55; P < .0001), and all patients with serum ferritin levels more than 2500 μg/L had elevated ALT values. The relationship between the logarithmically expressed serum ferritin and the logarithmically expressed AST serum activity was even closer (R2 = 0.62;P < .0001). We found no correlation between the number of blood units given and the aminotransferase serum activities.
Relationship between aminotransferase serum levels and several indices of iron status in 39 nonthalassemic adult patients with transfusional iron overload.
Horizontal lines represent upper reference limit for ALT (40 U/L) and ALT (40 U/L) serum activity. The serum ferritin panels also show regression of ALT and AST to the serum ferritin concentration. For further details, see “Patients, materials, and methods.”
Relationship between aminotransferase serum levels and several indices of iron status in 39 nonthalassemic adult patients with transfusional iron overload.
Horizontal lines represent upper reference limit for ALT (40 U/L) and ALT (40 U/L) serum activity. The serum ferritin panels also show regression of ALT and AST to the serum ferritin concentration. For further details, see “Patients, materials, and methods.”
Urinary iron excretion at start of iron chelation
Twelve patients underwent treatment with DFO. All patients had a liver iron concentration exceeding 400 μM/g (range, 403-623 μM/g) at start of treatment (Table 1), except patient 9 who had been on iron chelation for 9 months on entering the study. Serum ferritin levels ranged from 1280 to 8715 μg/L, indicating heavy iron overload. In 10 of the 12 patients we measured the urinary iron excretion at start of treatment after administration of 2 g DFO given as continuous, subcutaneous 12-hour infusion by pump. The log-transformed urinary iron excretion rose steeply with the liver iron concentration between 400 and 600 μM/g, and was concomitantly closely, positively correlated with the log-transformed ALT activity (R2 = 0.64, P = .006) and with the AST activity (R2 = 0.57,P = .01). The log-transformed urinary iron excretion also correlated positively with the log-transformed serum ferritin concentration, but at a lower level of significance (R2 = 0.50, P = .02), and with the iron saturation of transferrin for saturations more than 75% (R2 = 0.64, P = .005). However, iron saturation was not significantly related to the ALT activity (R2 = 0.38, P = .05).
Aminotransferase activities during DFO treatment
Eight of the 12 patients had elevated ALT at start of treatment. Follow-up data (Figure 2A) show that the ALT activity and the serum ferritin concentration fell progressively and to the same degree during treatment (Figure 2A,B). ALT had reached normal levels after 12 months in all but 2 patients (nos. 1 and 6), and the serum ferritin had fallen below 4000 μg/L in all except patient 1. The 2 patients who had not obtained normal ALT levels had the largest blood transfusion requirement, and patient 1 also had the largest urinary iron excretion (Figure 2D). Whereas the ferritin curves suggest effective iron chelation in all other patients, the repeated follow-up of the liver iron concentration by MRI revealed conflicting results. Thus, treatment had induced highly effective iron depletion in only 7 of the 12 patients (high responders). In the other patients (low responders), the liver iron concentration decreased slowly in 3 patients (nos. 3, 4, and 8) and remained largely unchanged in 2 patients (nos. 1 and 6). These 2 had the highest transfusion requirements of all patients in the follow-up period. In high responders the liver iron concentration was below 200 μM/g after 21 months of iron chelation, whereas low responders still had liver iron concentrations exceeding 400 μM/g, except for one patient.
Follow-up data on the ALT and indices of iron status in 12 patients with transfusional iron overload on iron chelation with DFO.
Individual patients may be identified within the different panels by use of a single symbol and color for each patient. Urinary iron excretion was not measured in patients 3 and 12. The horizontal line in panel A represent the upper reference limit for ALT serum activity (40 U/L).
Follow-up data on the ALT and indices of iron status in 12 patients with transfusional iron overload on iron chelation with DFO.
Individual patients may be identified within the different panels by use of a single symbol and color for each patient. Urinary iron excretion was not measured in patients 3 and 12. The horizontal line in panel A represent the upper reference limit for ALT serum activity (40 U/L).
Aminotransferase activities and iron status during iron chelation
The log-transformed aminotransferase activity levels were similarly related to the liver iron concentration prior to and during treatment (Figure 3A,B). Thus, elevated ALT or AST was only observed if liver iron concentration exceeded 350 μM/g. As was the case before treatment, not all patients exceeding the threshold had elevated aminotransferase activity. Fifty-five liver iron values were more than 350 μM/g, but only 26 (44%) corresponding ALT values and only 13 (24%) corresponding AST values were elevated. This finding suggests that other factors may affect the degree of hepatocellular injury. We therefore investigated whether the degree of hepatocellular injury could be related to the urinary iron excretion, which reflects the amount of chelatable iron, including non–transferrin-bound iron (NTBI), a component of plasma iron assumed to be potentially toxic.21 Figure4A shows a plot of the log-transformed ALT activity by the log-transformed urinary iron excretion. The lowess curve suggests that the ALT activity is not related to the urinary iron excretion below an iron excretion of around 10 mg/24 h. But at higher iron excretions we observed a proportional increase in log-transformed ALT with the log-transformed urinary iron excretion, which was similar to the findings at start of treatment. The urinary iron excretion also depended on the liver iron concentration, because it increased in proportion to liver iron concentration at values above approximately 400 μM/g (Figure 4C). Moreover, all elevated ALT values were associated with a urinary iron excretion of at least 15 mg/24 h (Figure4C). The plot of the serum ferritin concentration to the liver iron concentration (Figure 4D) also showed an increase in the slope of the regression curve for liver iron concentrations more than 400 μM/g, but it was moderate compared with the rise in urinary iron excretion and the variation was larger. The findings suggest that urinary iron is more directly related to the liver iron concentration than to the serum ferritin concentration, as long as the liver iron concentration exceeds 350 μMl/g. Similar to the findings at the start of treatment, the serum ferritin values (> 1000 μg/L) were closely related to the log-transformed ALT activity (Figure 4B) and to the AST activity.
Relationship between ALT and AST and liver iron concentration during iron chelation by lowess regression (tension = 66).
Data are obtained from Figure 2, panels A and C, and corresponding AST values (not shown in Figure 2). The total number of observations is 86 in panel A and 83 in panel B. Horizontal lines represent upper reference limits for ALT and AST (40 U/L).
Relationship between ALT and AST and liver iron concentration during iron chelation by lowess regression (tension = 66).
Data are obtained from Figure 2, panels A and C, and corresponding AST values (not shown in Figure 2). The total number of observations is 86 in panel A and 83 in panel B. Horizontal lines represent upper reference limits for ALT and AST (40 U/L).
Relationships between the ALT and indices of iron status and interrelationships during iron chelation by lowess regression (tension = 66).
The data are obtained from Figure 2, panels A to D. The total number of observations is 66 in panel A, 68 in panel B, and 86 in panels C and D. Lines in panels A-B show regression of ALT to the urinary iron excretion and the serum ferritin concentration; lines in panels C-D show regression of urinary iron excretion and serum ferritin concentration to the liver iron concentration.
Relationships between the ALT and indices of iron status and interrelationships during iron chelation by lowess regression (tension = 66).
The data are obtained from Figure 2, panels A to D. The total number of observations is 66 in panel A, 68 in panel B, and 86 in panels C and D. Lines in panels A-B show regression of ALT to the urinary iron excretion and the serum ferritin concentration; lines in panels C-D show regression of urinary iron excretion and serum ferritin concentration to the liver iron concentration.
Discussion
To our knowledge, this is the first study describing the relationship between liver function and liver iron concentration in patients with transfusional iron overload without serologic evidence of coexisting infection with major hepatotropic viruses excluding factors able to contribute to the extent of hepatocellular injury induced by iron. Causes of hepatocellular injury other than transfusional iron overload cannot, however, be excluded. Thus, patterns of ALT serum levels commonly seen in posttransfusion hepatitis in thalassemic patients have been demonstrated in patients without known hepatotropic infection.22 Alcohol ingestion is another well-known cause of elevated aminotransferase activity, and we excluded one patient from the study due to alcoholic liver disease. In none of the other patients was alcohol abuse suspected.
Our investigations show that aminotransferase serum activity in patients with transfusional iron overload caused by acquired anemias depends on the liver iron concentration and is directly related to the log-transformed urinary iron excretion. We observed increased prechelation hepatocellular injury reflected in elevated aminotransferase activities only when liver iron concentrations exceeded 300 to 400 μM/g. During iron chelation the aminotransferase activities became normal when levels fell below 350 μM/g. These observations strongly suggest the existence of a critical liver iron concentration level or range of 300 to 400 μM/g that determines whether transfusional iron deposited within the liver induces hepatocellular injury or not. A critical liver iron concentration for hepatocellular injury is consistent with the critical liver iron concentration of 400 μM/g for development of hepatic fibrosis in hereditary hemochromatosis as suggested by Basset et al,14and, indeed this concentration is close to the critical level reported here.
We assessed the extent of hepatocellular injury by determining the aminotransferase serum activity, which is mainly caused by leakage of the enzymes from cytoplasmic and mitochondrial compartments of injured hepatocytes to the plasma.23 A normal aminotransferase activity, however, does not necessarily ensure absence of developing tissue damage caused by fibrogenesis because the fibrogenic process induced by iron is associated with little cell damage and inflammation, often with borderline or even normal transaminase levels.13 Iron may induce fibrogenesis without pre-existing hepatocellular injury.24 A recent study in patients with hereditary hemochromatosis showed that only 77% of the homozygous patients with severe fibrosis or cirrhosis had elevated aminotransferase levels.25 Unfortunately, we did not have the possibility of studying liver histology in our patients because many were pancytopenic, increasing the risk of side effects of liver biopsy. Fibrogenesis caused by pre-existing hepatocellular injury may, however, be identified by elevated aminotransferase levels.
Measurements of urinary iron excretion at the start of treatment clearly showed that the extent of hepatocellular injury was most closely related to urinary iron excretion, but also significantly related to serum ferritin concentration. The corresponding follow-up data during iron chelation showed a similar pattern. The close relationship between the ALT activity and serum ferritin probably reflects a parallel leakage of aminotransferase and ferritin from the injured cells to the plasma in addition to an increased ferritin synthesis. A significant, positive correlation between aminotransferase serum activities and the serum ferritin concentration was also shown in an earlier study on β-thalassemia patients with transfusional iron overload by Worwood et al,26 but the correlation was clearly weaker (R2 = 0.17,P < .001) than in our study (R2 = 0.55, P < .0001). This difference may be due to the high number of transfusion-associated infections with hepatotropic viruses in the patients of Worwood et al. Thus, HBsAb was present in most of the serum samples which indicates previous hepatitis B, and, moreover, the study was performed before the discovery of HCV infection. The study of Wonke et al27demonstrated that the serum ferritin concentration is roughly doubled by ongoing chronic necrosis and inflammatory changes in patients with transfusional iron overload and chronic hepatitis C, showing again the relationship between iron overload and the degree of the resulting hepatocellular injury must be studied in patients without hepatotropic infections. Recently, Prati et al22 published data on 171 anti-HCV− thalassemic patients demonstrating a significant association between serum ferritin values above 3000 μg/L and elevated ALT values in the same patients. Similarly, in our study we found elevated ALT in all patients with ferritin levels above 2500 μg/L both before and during iron chelation.
The close correlation between aminotransferase activity and urinary iron excretion demonstrated at start of treatment and the finding of a comparable pattern during iron chelation suggests that the extent of hepatocellular injury is mainly determined by the size of the chelatable iron pool, as reflected by the urinary iron excreted. The observation that ALT was elevated only when the critical liver iron concentration was exceeded and the urinary iron excretion was above approximately 15 mg/24 h suggests that the chelatable iron pool only expands beyond the critical size when the liver iron concentration exceeds a critical concentration. The cytosolic, labile iron pool (LIP), classically referred to as the chelatable iron pool, contains the metabolically active forms of intracellular iron.28The pool also contains forms of iron loosely associated with macromolecular complexes and possibly also the low-molecular-weight non–transferrin-bound plasma iron fraction (NTPI).29,30Efficient regulatory mechanisms prevent fluctuations in LIP, but massive iron loading may result in uncontrollable expansion of the pool, which fails to be matched by the sequestrating capacity of cellular ferritin.31 The critical liver iron concentration, as suggested by our data, may reflect a critical iron load within the hepatocyte that exceeds the iron sequestrating capacity of the mentioned regulatory system. A liver iron concentration exceeding the critical level may therefore allow the chelatable iron pool to expand uncontrollably proportional to further hepatic iron accumulation. This may cause a proportional increase in hepatocellular injury and leakage of aminotransferase enzymes and ferritin to the plasma. The observed normalization of the aminotransferase serum activity observed when the liver iron concentration drops below the critical level may, on the other hand, reflect a restoration of the regulatory system controlling the size of the chelatable iron pool. Other defense mechanisms may, however, also be at play such as a cellular protective system like the variety of antioxidant cellular defenses counteracting the effect of oxyradical production.32
The close relationship between serum ferritin concentration and ALT activity demonstrated in our study has implications for the usefulness of the serum ferritin concentration as a marker of effective iron chelation therapy. Decreasing ferritin values may not reflect a proportional decrease in the body iron store but rather reflect improved liver function caused by a minor reduction of the liver iron concentration just below the critical range for hepatocellular injury. Despite this limitation, the ferritin test has been shown to reflect clinical outcome in patients with β-thalassemia major,33,34 suggesting that monitoring liver function, or possibly monitoring urinary iron excretion, may also be a useful prognostic marker in patients with transfusional iron overload receiving DFO treatment. The most accurate evaluation of the efficacy of iron chelation is, however, still achieved by repeated determination of the liver iron concentration.35
In conclusion, hepatocellular injury as reflected by elevated aminotransferase serum activity induced by transfusional iron overload in adult patients with transfusional iron overload caused by acquired anemias, and without serologic evidence of infection with major hepatotropic viruses, is dependent on the liver iron concentration. Our data suggest the existence of a critical liver iron concentration range above which hepatocellular injury is seen. The extent of the hepatocellular injury seems mainly to be determined by the size of the chelatable iron pool, as evaluated by the urinary iron excretion. Our findings support the concept of LIP as the compartment directly involved in iron toxicity. The findings may be helpful in establishing criteria for safety from complications of transfusional siderosis.
Prepublished online as Blood First Edition Paper, August 22, 2002; DOI 10.1182/blood-2002-06-1704.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Peter D. Jensen, Department of Hematology, Aarhus University Hospital, Amtssygehus, Tage Hansensgade 2, DK-8000 Aarhus C, Denmark; e-mail: d222615@inet.uni2.dk.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal