Abstract
We have studied the membrane proteins of band 3 anion exchanger (AE1)–deficient mouse and human red blood cells. It has been shown previously that proteins of the band 3 complex are reduced or absent in these cells. In this study we show that proteins of the Rh complex are also greatly reduced (Rh-associated glycoprotein, Rh polypeptides, CD47, glycophorin B) or absent (LW). These observations suggest that the Rh complex is associated with the band 3 complex in healthy RBCs. Mouse band 3(−/−) RBCs differed from the human band 3–deficient RBCs in that they retained CD47. Aquaporin 1 was reduced, and its glycosylation was altered in mouse and human band 3–deficient RBCs. Proteins of the glycophorin C complex, and other proteins with independent cytoskeletal interactions, were present in normal or increased amounts. To obtain direct evidence for the association of the band 3 and the Rh protein complexes in the RBC, we examined whether Rh complex proteins were coimmunoprecipitated with band 3 from membranes. RhAG and Rh were found to be efficiently coimmunoprecipitated with band 3 from deoxycholate-solubilized membranes. Results suggest that band 3 forms the core of a macrocomplex of integral and peripheral RBC membrane proteins. The presence of these proteins in a single structural macrocomplex makes it likely that they have linked functional or regulatory roles. We speculate that this macrocomplex may function as an integrated CO2/O2 gas exchange unit (metabolon) in the erythrocyte.
Introduction
The band 3 anion exchanger (AE1) is the major integral protein of the RBC membrane. This multifunctional protein has 3 domains, a membrane-spanning domain that carries out chloride/bicarbonate exchange, a short C-terminal cytoplasmic domain, and a large N-terminal cytoplasmic domain (reviewed by Tanner1,2). The C-terminal cytoplasmic domain of band 3 binds carbonic anhydrase II (CAII), forming a metabolon that channels HCO at the cytoplasmic face of band 3 and facilitates its interaction with CAII and band 3.3 The N-terminal cytoplasmic domain of band 3 (reviewed by Zhang et al4) binds glycolytic enzymes, hemoglobin (Hb), and hemichromes that may induce the aggregation of band 3 and cell turnover. However, a major function of the N-terminal domain of band 3 is to anchor the RBC membrane to the underlying cytoskeleton (reviewed by Lux and Palek5). Band 3 exists in the RBC membrane as dimers (60%) and tetramers (40%). The tetrameric form binds ankyrin6 and protein 4.2,7 and it is the major attachment site of the RBC membrane to the cytoskeleton. A second site of attachment through the glycophorin C complex (glycophorin C [GPC], protein 4.1, and p55) is thought to be of lesser importance.8 Thus, the band 3 complex (band 3, GPA, protein 4.2, ankyrin, CAII, glycolytic enzymes, Hb and so on) is important for organizing the structure of the RBC membrane and its attachment to the cytoskeleton.
Rh proteins form another major integral protein complex in the RBC membrane. The existence of the Rh protein complex (Rh-associated glycoprotein [RhAG], Rh polypeptides, glycophorin B (GPB), CD47, LW) was suggested by the absence or deficiency of these proteins in human RBCs with the Rhnull phenotype (reviewed by Cartron9). RhAG is sequence-related to the Rh polypeptides but is N-glycosylated.10,11 RhAG and the Rh polypeptides are predicted to contain 12 membrane spans and are thought to form heterotetramers comprising 2 RhAG and 2 Rh polypeptides.12Rh-like proteins have a broad species distribution, but their function remains controversial. RhAG and Rh have a distant sequence relationship with the Amt/MEP family of ammonium/methylammonium transporters in micro-organisms and plants.13 Recent evidence suggests the Amt/MEP proteins are channels that allow the facilitated diffusion of NH3 and methylamine as neutral species across membranes.14,15 RhAG (and RhCG, the mammalian nonerythroid homolog of RhAG) have been reported to carry out efflux transport of ammonium ions from complementation studies using these proteins with yeast mutants defective in all 3 endogenous MEP transporters.16 However, other workers14 have pointed out inconsistencies in the interpretation of these results and suggest they can be explained by the transport of the neutral ammonia species. It has also been reported that RhAG enhances ammonium/methylammonium ion influx into Xenopusoocytes.17 These investigators suggest that RhAG mediates an NH/H+ antiport,17 but their data do not appear to rule out facilitated diffusion of neutral NH3 by RhAG. However, at this point it is unclear whether ammonia/ammonium is the primary or only substrate for the Rh proteins or whether the Rh proteins are relatively nonspecific channels for neutral small molecules because a recent study18 suggests that an Rh ortholog present in the green alga Chlamydomonas reinhardtii (which has much closer sequence similarity to RhAG than to the Amt NH3 transporters in the same organism) acts as a gas channel for CO2. These authors propose that the Rh proteins also function as gas channels for CO2 in RBCs.14 18
CD47 (integrin-associated protein [IAP])19-21 is a 5-spanning membrane protein with a large, highly glycosylated, extracellular immunoglobulin domain. It has broad tissue distribution (reviewed by Brown and Frazier22) and associates with integrins in many cell types, where it has a role in cell signaling and activation. However, the function of CD47 in mature RBCs is unclear because RBCs lack integrins. The LW glycoprotein (ICAM-4) is a cell adhesion molecule that binds the integrinVLA-4 on hemopoietic cells and αV integrins on nonhematopoietic cells.23 GPB is a highly glycosylated, single-spanning membrane protein homologous to glycophorin A (GPA). Persons with Rhnull syndrome have hemolytic anemia, stomatocytosis, and spherocytosis,9indicating the presence of a cytoskeleton-associated defect in the RBCs.
Serologic studies on Southeast Asian ovalocytosis RBCs (which are heterozygous for a mutant band 3 containing a 9-amino acid deletion1,2) showed that these cells had depressed blood group Rh antigen expression24 and gave the first indication of an interaction between the Rh complex and band 3. Later studies using transfected K562 cell lines also support the presence of associations between band 3 and Rh complexes.25,26 Human protein 4.2(−/−) RBCs have been shown to have a marked deficiency of CD47 and altered glycosylation of RhAG, indicating that the band 3 and Rh complexes may be linked by protein 4.2 binding to CD47.27 These links through band 3 could provide connections between the Rh complex and the cytoskeleton.
We have, therefore, examined the proteins of the Rh complex in mouse band 3(−/−) RBCs and in human RBCs almost completely lacking band 3. We show that the absence of band 3 results in major deficiencies in the proteins of the Rh complex, confirming that these 2 abundant integral protein complexes are associated in the membrane. The data suggest that band 3 forms the central element of a macrocomplex of integral and peripheral proteins in the RBC membrane. We speculate that this macrocomplex may have a central role in RBC CO2/O2 gas exchange.
Materials and methods
The band 3(−/−) knockout mice28 and the band 3–deficient human patient, homozygous for the band 3 Coimbra (Val488Met) mutation, have been described.29 Since the latter report, the patient underwent splenectomy at the age of 3.5 years and is now largely transfusion independent (2 transfusions in a 2-year period). She has no major complications, and no thromboembolic events have been observed. Her liver is enlarged and her RBC indices are: RBC count, 2.4 × 109/L; Hb level, 83 g/L; hematocrit (HCT), 25%; mean corpuscular volume (MCV), 103 fL; mean corpuscular hemoglobin (MCH) level, 35 pg; mean corpuscular hemoglobin concentration (MCHC), 34 g/dL; red cell distribution width (RDW), 21.2%; reticulocyte level, 22.7%. Nephrocalcinosis remains stable without impairment of glomerular filtration. She has a daily supplement of oral NaHCO3 (8 mEq/kg). The patient's Rh serotype is RhCCDee, and her genotype is R1R1 (CDeCDe). Informed consent was sought and given in accordance with the Declaration of Helsinki for the studies undertaken. Blood samples were shipped chilled, and RBC membranes were prepared 1 to 2 days after the sample was taken.
RBC membrane protein analysis
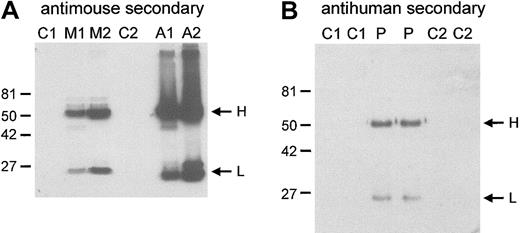
Preparation of RBC membranes, sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), Coomassie blue staining, and Western blotting of membrane proteins were made as described30,31 with the following modifications. Because band 3(−/−) mouse RBCs are prone to aggregation and membrane preparation from these cells is difficult,28 the cells were centrifuged (1 minute, 13 000 rpm), the plasma was removed, and the cells were immediately lysed in ice-cold 5 mM phosphate buffer. A fraction of the membranes formed an insoluble aggregate and was removed, and the remaining membranes were washed 5 times in ice-cold 5 mM phosphate buffer. The aggregate fraction was homogenized and analyzed separately. Immunoblotting with horseradish peroxidase (HRP)–conjugated goat antimouse secondary antibody (DAKO, Ely, United Kingdom) showed the presence of mouse immunoglobulin in the band 3(−/−) mouse membrane preparation and in the aggregates (Figure1A). This immunoglobulin accounts for some of the additional major bands visible in the protein-stained gels of the band 3(−/−) membranes that are absent in the healthy membranes (Figure 2). Coimbra RBC membranes also contained a small amount of human immunoglobulin (Figure 1B) but did not aggregate during RBC lysis. The presence of autoimmune mouse immunoglobulin on the band 3(−/−) mouse RBCs was not noted in previous reports.28,32 33 The epitopes for these antibodies have yet to be defined.
Autoimmune antibodies bound to the RBC membranes.
RBC membranes were prepared as described in “Materials and methods.” Aggregates that formed during the band 3(−/−) mouse membrane preparations were homogenized (see “Materials and methods”). Proteins were separated on 10% SDS-PAGE gels and were immunoblotted with HRP-conjugated, antimouse (A), or antihuman (B) secondary antibody. H and L indicate the positions of the immunoglobulin H and L chains. Mouse RBC membranes are shown in panel A. Loading: C1, C2, controls 1 and 2, respectively; M1, M2, band 3(−/−) mice 1 and 2, respectively; A1, A2, aggregates 1 and 2 from band 3(−/−) mice 1 and 2 preparations, respectively. C1 were C57BL/6 strain; M1, M2, and C2 were B6.129 strain. (B) Human RBC membranes. Loading: C1, C2, controls 1 and 2, respectively. P indicates proband. Molecular weight markers (kDa) are indicated to the left of the blots.
Autoimmune antibodies bound to the RBC membranes.
RBC membranes were prepared as described in “Materials and methods.” Aggregates that formed during the band 3(−/−) mouse membrane preparations were homogenized (see “Materials and methods”). Proteins were separated on 10% SDS-PAGE gels and were immunoblotted with HRP-conjugated, antimouse (A), or antihuman (B) secondary antibody. H and L indicate the positions of the immunoglobulin H and L chains. Mouse RBC membranes are shown in panel A. Loading: C1, C2, controls 1 and 2, respectively; M1, M2, band 3(−/−) mice 1 and 2, respectively; A1, A2, aggregates 1 and 2 from band 3(−/−) mice 1 and 2 preparations, respectively. C1 were C57BL/6 strain; M1, M2, and C2 were B6.129 strain. (B) Human RBC membranes. Loading: C1, C2, controls 1 and 2, respectively. P indicates proband. Molecular weight markers (kDa) are indicated to the left of the blots.
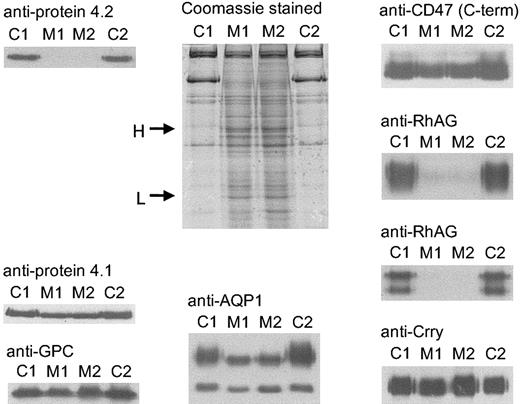
Coomassie and immunostaining of proteins from band 3(−/−) mouse RBC membranes.
RBC membranes were separated on 10% or 12% Laemmli gels and immunoblotted using polyclonal antibodies as shown. Loading: C1, C2, controls 1 and 2, respectively; M1, M2, band 3(−/−) mice 1 and 2, respectively (C1 was C57BL/6 strain; M1, M2, and C2 were B6.129 strain). H and L on the protein-stained gel indicate the immunoglobulin H and L chains in the band 3(−/−) membranes. C-term indicates C-terminal.
Coomassie and immunostaining of proteins from band 3(−/−) mouse RBC membranes.
RBC membranes were separated on 10% or 12% Laemmli gels and immunoblotted using polyclonal antibodies as shown. Loading: C1, C2, controls 1 and 2, respectively; M1, M2, band 3(−/−) mice 1 and 2, respectively (C1 was C57BL/6 strain; M1, M2, and C2 were B6.129 strain). H and L on the protein-stained gel indicate the immunoglobulin H and L chains in the band 3(−/−) membranes. C-term indicates C-terminal.
Protein concentration of RBC membranes was estimated using the Bradford assay, and equal amounts (5 μg) of protein were loaded per track of each gel. Quantification of the band 3(−/−) mouse RBC membranes was complicated by the immunoglobulin bound to the membranes. We therefore loaded amounts of control and band 3(−/−) mouse samples so that the RBC membrane proteins were present at similar concentrations (10 μg band 3(−/−) and 5 μg control mouse ghosts per track). Individual membrane proteins were quantified by scanning densitometry of Coomassie blue–stained or immunoblotted SDS-PAGE gels. Mouse monoclonal antibodies (mAbs) are listed in Tables 1and 2 and were available in-house, with the exception of anti-spectrin34and anti–β-actin (Abcam, Cambridge, United Kingdom). Rabbit polyclonal anti-Rh, anti-p55, anti-4.1, anti-4.2 and anti-CD47,27 anti-aquaporin,35 anti–Glut 1,36 anti-GPC,37 rat polyclonal anti-Crry,38 and sheep polyclonal anti–band 339 were used.
Quantification of RBC membrane proteins in the band 3(−/−) mouse by immunoblotting
| Protein . | Antibody . | Band 3(−/−) mouse, % of control* . |
|---|---|---|
| Protein 4.2 | Polyclonal | 0, 0 |
| RhAG | Polyclonal | 9, 6 |
| Rh polypeptides | Polyclonal | 1, 0.06 |
| CD47 | C-terminal polyclonal | 84, 83 |
| Glycophorin C | Polyclonal | 100, 100 |
| Protein 4.1 | Polyclonal | 100 (± 2) |
| Aquaporin, total | Polyclonal | 69, 79 |
| Crry | Polyclonal | 143, 139 |
| Protein . | Antibody . | Band 3(−/−) mouse, % of control* . |
|---|---|---|
| Protein 4.2 | Polyclonal | 0, 0 |
| RhAG | Polyclonal | 9, 6 |
| Rh polypeptides | Polyclonal | 1, 0.06 |
| CD47 | C-terminal polyclonal | 84, 83 |
| Glycophorin C | Polyclonal | 100, 100 |
| Protein 4.1 | Polyclonal | 100 (± 2) |
| Aquaporin, total | Polyclonal | 69, 79 |
| Crry | Polyclonal | 143, 139 |
Values obtained from the RBCs of 2 different band 3(−/−) animals. Amounts of protein are expressed as the percentage of control after normalization to the amount of protein 4.1 in the 2 membrane preparations.
Quantification of the membrane proteins of band 3 Coimbra RBCs by immunoblotting and Coomassie blue staining
| Protein . | Antibody . | Band 3 Coimbra, % of controls* . | n . |
|---|---|---|---|
| Band 3 | N-terminal BRIC170 | 2, 4 | 2 |
| Band 3 | C-terminal BRIC155 | 1, 2 | 2 |
| Protein 4.2 | Polyclonal | 0 | 2 |
| GPA, total | BRIC163 | 29 (± 5) | 6 |
| RhAG | LA1818 | 7 (± 1) | 4 |
| Rh polypeptides | Polyclonal | 21 (± 3) | 6 |
| CD47 | BRIC125 | 3 (± 1) | 6 |
| CD47 | C-terminal polyclonal | 2 (± 1) | 6 |
| GPB, total | R1.3 | 56 (± 3) | 6 |
| LW | BS56 | 5, 9 | 2 |
| Glycophorin C | BRIC4 | 100 (± 7) | 8 |
| Protein 4.1 | Coomassie | 94 (± 6) | 4 |
| Protein 4.1 | Polyclonal | 101 (± 7) | 4 |
| p55 | Polyclonal | 108, 115 | 2 |
| Spectrin | Coomassie | 64 (± 1) | 4 |
| α-spectrin | Monoclonal | 65, 61 | 2 |
| β-spectrin | Monoclonal | 58, 67 | 2 |
| β-actin | Monoclonal | 71 (± 3) | 4 |
| AQP1, total | Polyclonal | 59 (± 3) | 4 |
| GLUT 1, total | Polyclonal | 182 (± 10) | 4 |
| Lu | BRIC221 | 169 (± 35) | 4 |
| LFA-3, CD58 | BRIC5 | 315, 325 | 2 |
| DAF, CD55 | BRIC128 | 163 (± 15) | 4 |
| CD44 | BRIC235 | 113, 118 | 2 |
| Protein . | Antibody . | Band 3 Coimbra, % of controls* . | n . |
|---|---|---|---|
| Band 3 | N-terminal BRIC170 | 2, 4 | 2 |
| Band 3 | C-terminal BRIC155 | 1, 2 | 2 |
| Protein 4.2 | Polyclonal | 0 | 2 |
| GPA, total | BRIC163 | 29 (± 5) | 6 |
| RhAG | LA1818 | 7 (± 1) | 4 |
| Rh polypeptides | Polyclonal | 21 (± 3) | 6 |
| CD47 | BRIC125 | 3 (± 1) | 6 |
| CD47 | C-terminal polyclonal | 2 (± 1) | 6 |
| GPB, total | R1.3 | 56 (± 3) | 6 |
| LW | BS56 | 5, 9 | 2 |
| Glycophorin C | BRIC4 | 100 (± 7) | 8 |
| Protein 4.1 | Coomassie | 94 (± 6) | 4 |
| Protein 4.1 | Polyclonal | 101 (± 7) | 4 |
| p55 | Polyclonal | 108, 115 | 2 |
| Spectrin | Coomassie | 64 (± 1) | 4 |
| α-spectrin | Monoclonal | 65, 61 | 2 |
| β-spectrin | Monoclonal | 58, 67 | 2 |
| β-actin | Monoclonal | 71 (± 3) | 4 |
| AQP1, total | Polyclonal | 59 (± 3) | 4 |
| GLUT 1, total | Polyclonal | 182 (± 10) | 4 |
| Lu | BRIC221 | 169 (± 35) | 4 |
| LFA-3, CD58 | BRIC5 | 315, 325 | 2 |
| DAF, CD55 | BRIC128 | 163 (± 15) | 4 |
| CD44 | BRIC235 | 113, 118 | 2 |
Values given as the mean (± SD) of n replicates. Amount of protein expressed as the percentage of the control after normalization to the amount of GPC in the band 3 Coimbra membrane preparation.
For flow cytometry, RBCs were washed 3 times with phosphate-buffered saline (PBS), pH 7.5, supplemented with 1% bovine serum albumin, and 5 × 106 cells were incubated with appropriate mAb. Unlabeled mAbs were used as neat culture supernatants, except that purified antibody was used for Brad 3 (20 μg/mL) and BS46 (50 μg/mL). Some antibodies were directly labeled with fluorescein isothiocyanate (FITC) and were used at 1:50 dilution (BRIC6 and BRIC256) or 1:30 dilution (BRIC69, LA1818, and Brad 3). Binding of unlabeled mAbs was detected using FITC-conjugated goat antimouse or goat antihuman (Fab′)2 fragments (DAKO, Glostrup, Denmark). Samples were analyzed using a FACScalibur instrument and CellQuest software (Becton Dickinson, Mountain View, CA), and the mean fluorescence intensity FL1 was used as a measure of antibody binding.
Coimmunoprecipitation of band 3 and Rh proteins from human RBC membranes
RBC membrane proteins were solubilized in DC-IP buffer (1% deoxycholate, 150 mM NaCl, 50 mM Tris-HCl, pH 8) and centrifuged for 30 minutes at 55 000gav. The solution was incubated with protein G-Sepharose, which had been preloaded with mouse monoclonal anti–band 3 (BRIC169), for 1.5 hours at room temperature. Beads were washed 4 times with DC-IP buffer and once with DC-IP buffer without NaCl. Immunoprecipitated protein was eluted from the beads with reducing gel sample buffer containing 20% SDS and 8 M urea and was separated by SDS-PAGE. Separated protein was immunoblotted using rabbit polyclonal antibodies (as above) and a sheep anti–band 3.39
Results
Analysis of band 3(−/−) mouse RBC membranes by SDS-PAGE and immunoblotting
RBC membranes were prepared as described in “Materials and methods.” A fraction of the membranes formed an insoluble aggregate that was removed, homogenized, and analyzed separately. RBC membrane proteins were quantified, and the results were normalized to GPC and protein 4.1 in the samples (Table 1). Protein 4.1 was chosen for normalization because the proteins in the GPC complex are thought to be independent of the band 3 complex. Band 3(−/−) membranes had no protein 4.2 (Table 1; Figure 2), as previously reported.28 32
Band 3(−/−) mouse RBC membranes contained little or no Rh polypeptide (approximately 0.5% of controls) and much reduced RhAG (approximately 7.5% of controls), indicating that the Rh/RhAG proteins are highly dependent on the presence of band 3. However, CD47 was only slightly reduced in the mouse band 3(−/−) RBC membranes (approximately 85% of controls) (Table 1; Figure 2). No protein 4.2, RhAG, Rh polypeptide, or CD47 was detectable by immunoblotting in the aggregate fraction of the band 3(−/−) mouse RBC membrane (data not shown).
We also analyzed 2 other proteins, not known to be associated with the band 3 complex. The total amount of aquaporin 1 (AQP1) was slightly reduced (approximately 75% of controls), and the N-glycosylated fraction of AQP1 in the band 3(−/−) cells migrated as a sharp band on SDS-PAGE gels, suggesting that it lacked the complex glycosylation present in healthy membranes (Table 1; Figure 2). The amount of the complement inhibitor, Crry, was slightly increased in band 3(−/−) mouse RBCs (approximately 140% of controls) (Table 1; Figure2).
Human Coimbra RBC membranes contain traces of band 3 Coimbra
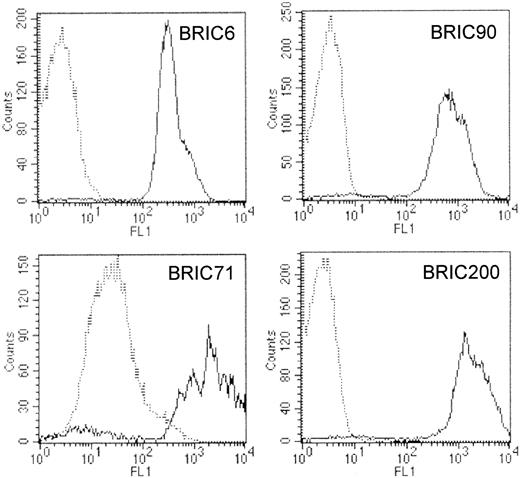
The patient homozygous for the band 3 Coimbra mutation was reported to completely lack RBC band 3.29 We analyzed RBC membrane proteins from this patient using a blood sample drawn 6 months after transfusion. Immunoblotting of membranes with monoclonal antibodies directed at the N-terminal or C-terminal regions of band 3 (BRIC170 and BRIC155) suggested that these cells contain traces of band 3 (approximately 2% of controls) (Table 2; Figure3A). Because this result was at variance with the original data on the RBCs taken from cord blood,29 flow cytometry using 4 different anti–band 3 mAbs was used to determine whether this band 3 originated from residual transfused RBCs. Any residual healthy RBCs would have normal levels of band 3 and would be clearly distinguished on flow cytometry by the much larger amount of anti–band 3 bound. Results (Table 3; Figure4) showed only one population of RBCs was present in the band 3 Coimbra sample. Event counts for the Coimbra samples indicated fewer than 1 residual transfused healthy cell in 20 000 band 3 Coimbra RBCs, using 3 different anti–band 3 antibodies (BRIC6, BRIC90, and BRIC200). These 3 different anti–band 3 antibodies reacted with band 3 Coimbra cells at levels similar to those of the negative control antibody (Table 3). However one anti–band 3 antibody (BRIC71) showed low reactivity that was greater than the negative control antibody (Table 3). Taken together with the immunoblotting results, this suggests that a small amount of the mutant band 3 is present in the band 3 Coimbra RBC membranes. The mutant band 3 retains the BRIC71 epitope but is misfolded so that it no longer displays the epitopes for the other 3 anti–band 3 mAbs used in the flow cytometry. All 4 mAb epitopes are located on the third extracellular loop of band 3, and all except the BRIC71 epitope are sensitive to chymotrypsin cleavage of RBCs,40 indicating that the BRIC71 epitope is presented by a different part of the loop. These results confirm that the band 3 remaining in the blood sample originates from the patient's own RBCs and not from residual transfused healthy RBCs.
Coomassie and immunostaining of proteins from Coimbra RBC membranes.
RBC membranes were separated on 10% or 12% Laemmli gels and were immunoblotted using antibodies as shown. Loading: C1, C2, controls 1 and 2, respectively. P indicates proband. (A) Proteins of the band 3 complex. Immunoblotting used polyclonal antibodies against protein 4.2 and monoclonal antibodies: BRIC170 (N-terminal band 3), BRIC155 (C-terminal band 3), and BRIC163 GPA. (B) Proteins of the glycophorin C (GPC) complex, plus aquaporin 1 (AQP1) and cytoskeletal proteins spectrin and actin. Immunoblotting used polyclonal antibodies against protein 4.1, p55, and AQP1 and monoclonal antibodies: BRIC4 (GPC), anti-spectrin, anti–β-actin (Abcam, Cambridge, United Kingdom). (C) Proteins of the Rh complex. Immunoblotting used polyclonal antibodies against the Rh polypeptides and the C-terminal region of CD47, and monoclonal antibodies LA1818 (RhAG), BRIC125 (N-terminal region of CD47), R1.3 (GPB), and BS56 (LW). (D) Other RBC membrane proteins. Immunoblotting used polyclonal antibodies against the glucose transporter (GLUT 1) and monoclonal antibodies BRIC221 (Lu), BRIC128 (DAF), BRIC5 (LFA-3), and BRIC235 (lymphocyte homing receptor [CD44]).
Coomassie and immunostaining of proteins from Coimbra RBC membranes.
RBC membranes were separated on 10% or 12% Laemmli gels and were immunoblotted using antibodies as shown. Loading: C1, C2, controls 1 and 2, respectively. P indicates proband. (A) Proteins of the band 3 complex. Immunoblotting used polyclonal antibodies against protein 4.2 and monoclonal antibodies: BRIC170 (N-terminal band 3), BRIC155 (C-terminal band 3), and BRIC163 GPA. (B) Proteins of the glycophorin C (GPC) complex, plus aquaporin 1 (AQP1) and cytoskeletal proteins spectrin and actin. Immunoblotting used polyclonal antibodies against protein 4.1, p55, and AQP1 and monoclonal antibodies: BRIC4 (GPC), anti-spectrin, anti–β-actin (Abcam, Cambridge, United Kingdom). (C) Proteins of the Rh complex. Immunoblotting used polyclonal antibodies against the Rh polypeptides and the C-terminal region of CD47, and monoclonal antibodies LA1818 (RhAG), BRIC125 (N-terminal region of CD47), R1.3 (GPB), and BS56 (LW). (D) Other RBC membrane proteins. Immunoblotting used polyclonal antibodies against the glucose transporter (GLUT 1) and monoclonal antibodies BRIC221 (Lu), BRIC128 (DAF), BRIC5 (LFA-3), and BRIC235 (lymphocyte homing receptor [CD44]).
Flow cytometric analysis of the band 3 Coimbra RBCs
| Monoclonal antibody . | Antigen . | Control, FL1 units . | Band 3 Coimbra, FL1 units . |
|---|---|---|---|
| BRIC169 | Mouse negative control | 2.36 ± 0.08 | 2.49 |
| BRIC6 | Band 3 | 334 ± 23 | 2.67 |
| BRIC71 | Band 3 | 1035 ± 525 | 26.2 |
| BRIC90 | Band 3 | 672 ± 89 | 3.02 |
| BRIC200 | Band 3 | 1379 ± 330 | 2.42 |
| B6 H12.2 | CD47 | 1081 ± 100 | 159 |
| R10 | GPA | 1595 ± 107 | 3240 |
| BRIC256 | GPA | 840 ± 59 | 614 |
| BRIC14 | Wrb | 1991 ± 631 | 1670 |
| BRIC201 | Wrb | 92.5 ± 13.6 | 2.37 |
| BRIC4 | GPC | 888 ± 59 | 846 |
| BS 46 | LW | 30.9 ± 8.9 | 9.54 |
| BRIC110 | Daf | 29.3 ± 3.6 | 38.0 |
| BRIC68 | Kell | 140 ± 10 | 152 |
| BRIC69 | Rh polypeptides | 548 ± 49 | 181 |
| LA1818 | RhAG | 1820 ± 72 | 267 |
| Monoclonal antibody . | Antigen . | Control, FL1 units . | Band 3 Coimbra, FL1 units . |
|---|---|---|---|
| BRIC169 | Mouse negative control | 2.36 ± 0.08 | 2.49 |
| BRIC6 | Band 3 | 334 ± 23 | 2.67 |
| BRIC71 | Band 3 | 1035 ± 525 | 26.2 |
| BRIC90 | Band 3 | 672 ± 89 | 3.02 |
| BRIC200 | Band 3 | 1379 ± 330 | 2.42 |
| B6 H12.2 | CD47 | 1081 ± 100 | 159 |
| R10 | GPA | 1595 ± 107 | 3240 |
| BRIC256 | GPA | 840 ± 59 | 614 |
| BRIC14 | Wrb | 1991 ± 631 | 1670 |
| BRIC201 | Wrb | 92.5 ± 13.6 | 2.37 |
| BRIC4 | GPC | 888 ± 59 | 846 |
| BS 46 | LW | 30.9 ± 8.9 | 9.54 |
| BRIC110 | Daf | 29.3 ± 3.6 | 38.0 |
| BRIC68 | Kell | 140 ± 10 | 152 |
| BRIC69 | Rh polypeptides | 548 ± 49 | 181 |
| LA1818 | RhAG | 1820 ± 72 | 267 |
FL1 units are mean fluorescence intensity obtained on flow cytometry. Control values are the mean (± SD) of 3 control RBC samples.
Flow cytometric analysis of band 3 Coimbra RBCs.
Cells were analyzed by flow cytometry using murine monoclonal anti–band 3 antibodies that were directly labeled with FITC (BRIC6) or were detected using FITC-labeled goat antimouse (Fab′)2fragments (BRIC71, BRIC90, BRIC200). In each histogram, dotted lines represent results obtained with Coimbra RBCs, and solid lines represent results obtained with the positive control. All 4 antibodies detected a single population of RBCs in the band 3 Coimbra sample. With BRIC6, BRIC90, and BRIC200, fluorescence intensity (FL1) was similar to that of the negative control antibody (BRIC169, directed against an intracellular band 3 epitope) and lower than the fluorescence intensity obtained with positive control cells, demonstrating that the epitopes recognized by these antibodies were absent on band 3 Coimbra RBCs. In contrast, band 3 Coimbra cells incubated with BRIC71 yielded a fluorescence intensity, FL1, that was also lower than that obtained with positive control cells but approximately 10-fold higher than that obtained with BRIC169, demonstrating that the epitope recognized by BRIC71 was expressed at a low level on all the band 3 Coimbra cells.
Flow cytometric analysis of band 3 Coimbra RBCs.
Cells were analyzed by flow cytometry using murine monoclonal anti–band 3 antibodies that were directly labeled with FITC (BRIC6) or were detected using FITC-labeled goat antimouse (Fab′)2fragments (BRIC71, BRIC90, BRIC200). In each histogram, dotted lines represent results obtained with Coimbra RBCs, and solid lines represent results obtained with the positive control. All 4 antibodies detected a single population of RBCs in the band 3 Coimbra sample. With BRIC6, BRIC90, and BRIC200, fluorescence intensity (FL1) was similar to that of the negative control antibody (BRIC169, directed against an intracellular band 3 epitope) and lower than the fluorescence intensity obtained with positive control cells, demonstrating that the epitopes recognized by these antibodies were absent on band 3 Coimbra RBCs. In contrast, band 3 Coimbra cells incubated with BRIC71 yielded a fluorescence intensity, FL1, that was also lower than that obtained with positive control cells but approximately 10-fold higher than that obtained with BRIC169, demonstrating that the epitope recognized by BRIC71 was expressed at a low level on all the band 3 Coimbra cells.
Analysis of band 3 Coimbra RBC membranes
Other membrane proteins in the band 3 Coimbra RBCs were also analyzed. Proteins associated with the GPC complex (GPC, protein 4.1, p55) were present in equivalent amounts in band 3 Coimbra and healthy RBCs (Table 2; Figure 3B). Flow cytometry using an anti-GPC mAb (BRIC4) also showed the same amount of GPC was present in control and band 3 Coimbra RBCs (Table 3). Therefore, as with the mouse membranes, we chose to use the proteins of the GPC complex to normalize the quantitative SDS-gel data (Table 2). Protein 4.2 was undetectable, and GPA was reduced (approximately 29% of controls) in band 3 Coimbra RBCs (Table 2; Figure 3A) in agreement with the previous report.29
All proteins of the Rh complex were markedly reduced in the band 3 Coimbra RBC membranes, RhAG (approximately 7% of controls), Rh polypeptides (approximately 21% of controls), CD47 (approximately 2.5% of controls), LW (approximately 7% of controls), and GPB (approximately 56% of controls) (Table 2; Figure 3C). The amount of spectrin and actin in band 3 Coimbra membranes, assessed from either protein-stained or immunoblotted SDS-PAGE gels, was also reduced (approximately 63% and 71% of controls respectively) (Table 2; Figure 3A-B). AQP1 was reduced (approximately 59% of controls) (Table 2; Figure 3B), and the N-glycosylated fraction of AQP1 migrated with a faster mobility on SDS-PAGE gels than in healthy cells, suggesting that it was hypoglycosylated (Figure 3B). The amount of glucose transporter (GLUT 1) was increased in band 3 Coimbra RBCs (approximately 182% of controls), as were the GPI-linked protein, decay-accelerating factor ([DAF] CD55) (approximately 163% of controls), Lutheran protein (Lu) (approximately 169% of controls), and lymphocyte function–associated antigen 3 ([LFA-3] CD58) (approximately 320% of controls) (Table 2; Figure 3D). The lymphocyte homing receptor, CD44 (approximately 115% of controls), was present at close to normal amounts (Table 2; Figure 2D).
Flow cytometric analysis of band 3 Coimbra RBCs
Flow cytometry was performed on band 3 Coimbra RBCs using a range of mAbs and in general gave results that support the SDS-PAGE and immunoblotting data (Table 3; Figure 4). However, there were some exceptions. The main differences between the flow cytometry and SDS-PAGE data were with anti-GPA mAbs. Surprisingly, flow cytometric analysis of the variant RBCs (Table 3) showed more reactivity with 2 anti-GPA monoclonals (BRIC256 and R10) than would be expected from the reduced amount of GPA in these cells indicated by the immunoblotting data (Table 2). GPA associates with band 3 in healthy RBCs, and these results probably reflect an increased accessibility of GPA epitopes or altered glycosylation of GPA in band 3 Coimbra RBCs. The deficiency of nearly all the major integral membrane proteins in the band 3 Coimbra RBCs drastically changes the surface organization of the cells and, therefore, the accessibility of epitopes to antibodies. The R10 epitope is closer to the GPA N-terminus than the BRIC256 epitope,41 and the greater reactivity of R10 compared with BRIC256 may reflect a more accessible location of the R10 epitope in the variant RBCs. Given that flow cytometry is performed on intact RBCs and antibody binding depends on the presentation and steric accessibility of the antigenic sites, flow cytometry results from the healthy and band 3–deficient cells cannot be directly compared because of the very different surface organizations of the 2 cell types. This effect, combined with the intrinsic nonlinearity of the fluorescence signal, interferes with quantitative analysis by flow cytometry. Immunoblotting data more accurately reflect the quantitative amounts of the proteins present in the band 3 Coimbra RBC membranes. Unexpectedly, one Wrb mAb (BRIC14) was as reactive with band 3 Coimbra RBCs as with control RBCs, though the other (BRIC201) did not react with the variant RBCs (Table 3). The Wrb antigen depends on an interaction between band 3 and GPA.42 We presume that the small amount of band 3 in band 3 Coimbra RBCs is sufficient to form some BRIC14 epitope, and the amount of this epitope is overestimated by flow cytometry because of its increased accessibility in the variant cells (as discussed above for the R10 and BRIC256 epitopes). Flow cytometry also showed that the band 3 Coimbra RBCs were more reactive with anti-CD47 than would be expected from the immunoblotting data, as previously observed with CD47-deficient RBCs.27
Coimmunoprecipitation of band 3 and Rh components from RBC membranes
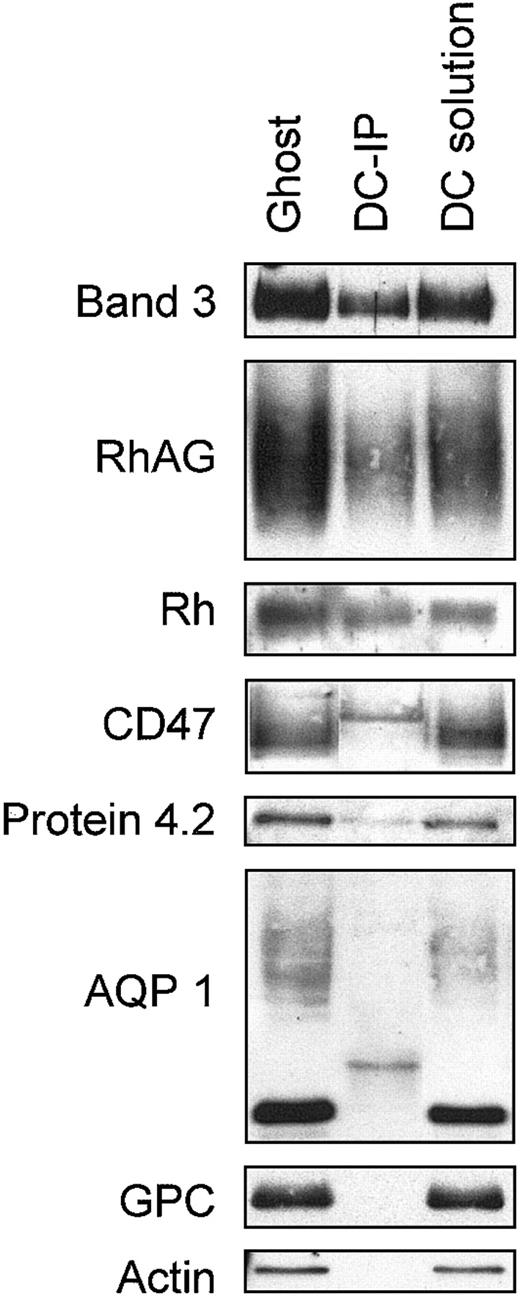
We sought direct evidence for the association of band 3 with the Rh complex in mature RBC membranes from coimmunoprecipitation studies using healthy human membranes. Band 3 was immunoprecipitated with the mouse monoclonal anti–band 3 BRIC169 from membranes that were completely solubilized using sodium deoxycholate. Immunoprecipitated material was separated by SDS-PAGE, and the protein components associated with band 3 were examined by immunoblotting using rabbit polyclonal antibodies or a sheep polyclonal anti–band 3. All the components examined were efficiently solubilized by sodium deoxycholate (Figure 5). RhAG and Rh were present in the band 3 immunoprecipitate, relative to band 3, in amounts similar to those present in the original membranes (Figure 5), but little CD47 appeared to be present in the band 3 immunoprecipitate. However, rabbit polyclonal anti-CD47 bound an additional sharp band (probably the immunoprecipitated mouse H-chains) in the region of the CD47 band that made it difficult to determine whether CD47 was completely absent. There was also poor recovery of protein 4.2 in the band 3 immunoprecipitate from the deoxycholate-solubilized membranes (Figure5). Band 4.2 is known to be associated with band 3,4 and the low recovery likely results from the dissociation of protein 4.2 from band 3 by deoxycholate treatment. This probably also accounts for the poor recovery of CD47 in the band 3 immunoprecipitate because band 4.2 appears to be a major site of association of CD47 in the membrane.27 We were unable to determine whether LW was present in the immunoprecipitate because we lacked a suitable nonmurine anti-LW. As expected, no GPC or actin was detected in the immunoprecipitate (Figure 5), confirming that specific coimmunoprecipitation of Rh and RhAG with band 3 was obtained. In addition, no AQP1 was detected in the band 3 immunoprecipitate (Figure5). Results show that band 3 is associated with the core proteins of the Rh complex (RhAG and Rh) in the RBC membrane but is not associated with AQP1. Parallel experiments were carried out using Triton X-100 instead of sodium deoxycholate (data not shown), but efficient solubilization of the membrane components required the additional presence of high salt concentrations (0.5 M KCl). We recovered less RhAG in band 3 immunoprecipitates from Triton-solubilized membranes than deoxycholate-solubilized membranes. Band 3 association with the Rh proteins may be destabilized by the high salt concentration used, or Triton X-100 may not be a suitable detergent for maintaining this association.
Coimmunoprecipitation of band 3 and Rh proteins from human RBC membranes.
RBC membrane proteins were solubilized in a deoxycholate-containing buffer and were incubated with protein G-Sepharose, which had been preloaded with mouse monoclonal anti–band 3 (BRIC169), as described in “Materials and methods.” Immunoprecipitated protein was separated by SDS-PAGE and immunoblotted using rabbit polyclonal antibodies (“Materials and methods”) and a sheep anti–band 3.39Loading: ghost, RBC membrane protein; DC-IP, band 3 immunoprecipitate from deoxycholate-solubilized membrane proteins; DC solution, total deoxycholate-solubilized membrane proteins. Rabbit antibodies bound 2 bands in the DC-IP samples, at molecular weights of approximately 25 kDa and approximately 50 kDa (see panels AQP 1 and CD47, respectively), which probably results from cross-reactivity with the L and H chains of the large amount of mouse immunoglobulin in the immunoprecipitates.
Coimmunoprecipitation of band 3 and Rh proteins from human RBC membranes.
RBC membrane proteins were solubilized in a deoxycholate-containing buffer and were incubated with protein G-Sepharose, which had been preloaded with mouse monoclonal anti–band 3 (BRIC169), as described in “Materials and methods.” Immunoprecipitated protein was separated by SDS-PAGE and immunoblotted using rabbit polyclonal antibodies (“Materials and methods”) and a sheep anti–band 3.39Loading: ghost, RBC membrane protein; DC-IP, band 3 immunoprecipitate from deoxycholate-solubilized membrane proteins; DC solution, total deoxycholate-solubilized membrane proteins. Rabbit antibodies bound 2 bands in the DC-IP samples, at molecular weights of approximately 25 kDa and approximately 50 kDa (see panels AQP 1 and CD47, respectively), which probably results from cross-reactivity with the L and H chains of the large amount of mouse immunoglobulin in the immunoprecipitates.
Discussion
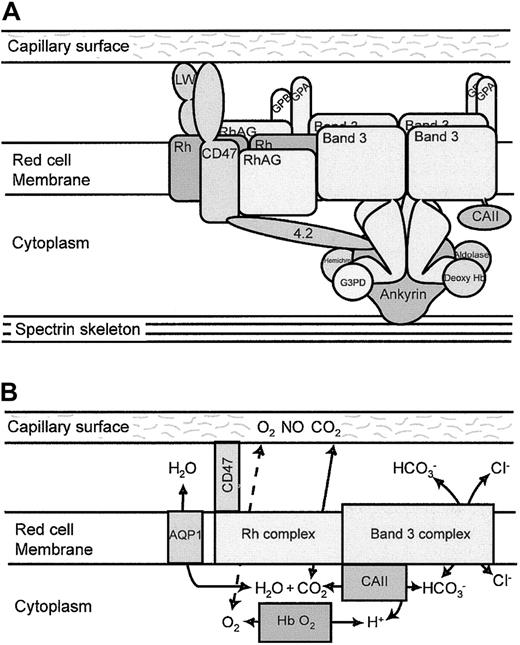
The most significant finding of this study is that the band 3 and the Rh complexes are associated in the RBC membrane in a single complex, which we term the band 3 macrocomplex. This is demonstrated by the major deficiencies in the proteins of the Rh complex when band 3 is absent from human or mouse RBCs and by the coimmunoprecipitation of band 3 and Rh complex proteins (RhAG and Rh) from human RBC membranes, and it confirms earlier indirect evidence for this association.24-27 The number of tetrameric Rh complexes (105 per cell43) is similar to the number of band 3 tetramers that linked the cytoskeleton to the membrane. This, together with observations that the Rh antigens are linked to the RBC skeleton, suggests that the band 3 macrocomplex is formed around the ankyrin-associated tetrameric fraction of band 3 that binds the spectrin-actin skeleton.27 A schematic illustration of the organization of this macrocomplex is illustrated in Figure6A.
Band 3 macrocomplex and proposed gas exchange metabolon.
(A) Schematic diagram of the band 3 macrocomplex showing probable interactions of the proteins that form the band 3 macrocomplex. Tetramers of band 3 are attached to the spectrin cytoskeleton through ankyrin. The acidic N-terminal region of the cytoplasmic domain of band 3 binds deoxy-hemoglobin, hemichromes, glyceraldehyde-3-phosphate dehydrogenase (G3PD), and aldolase.4 The short C-terminal cytoplasmic tail of band 3 binds CAII.3 The GPA dimer is close to the membrane domain of band 3. Protein 4.2 binds ankyrin, band 3, and CD47 (in humans), providing one link between the Rh complex and the band 3 complex.27 CD47 and LW are closely associated with the Rh tetramers9 and may be involved in adhesive interactions with the capillary surface.22,23 The Rh tetramer associates directly with band 3. GPB associates with the Rh tetramers,9 and GPA and GPB may form heterotetramers, providing another link between the Rh complex and the band 3 complex. (B) Proposed gas exchange metabolon in the RBC membrane. The model suggests that CO2 passes from the capillary endothelial cell to the RBC through the Rh proteins. CAII converts CO2and H2O to HCO and a proton, and HCO passes out of the RBC through band 3 in exchange for a chloride ion. The removal of HCO leaves a proton that promotes local acidification in the vicinity of band 3 and the release of oxygen from oxy-hemoglobin by the Bohr effect. O2 may then leave the RBC through the Rh gas channel and pass into the endothelial cells. The channeling of substrate through this metabolon reduces substrate loss by diffusion from the system. In the pulmonary microcapillaries, the system would be reversed. Water movements involved in the hydration/dehydration of CO2 may be mediated by AQP1,55 though AQP1 is not directly associated with the band 3 macrocomplex.
Band 3 macrocomplex and proposed gas exchange metabolon.
(A) Schematic diagram of the band 3 macrocomplex showing probable interactions of the proteins that form the band 3 macrocomplex. Tetramers of band 3 are attached to the spectrin cytoskeleton through ankyrin. The acidic N-terminal region of the cytoplasmic domain of band 3 binds deoxy-hemoglobin, hemichromes, glyceraldehyde-3-phosphate dehydrogenase (G3PD), and aldolase.4 The short C-terminal cytoplasmic tail of band 3 binds CAII.3 The GPA dimer is close to the membrane domain of band 3. Protein 4.2 binds ankyrin, band 3, and CD47 (in humans), providing one link between the Rh complex and the band 3 complex.27 CD47 and LW are closely associated with the Rh tetramers9 and may be involved in adhesive interactions with the capillary surface.22,23 The Rh tetramer associates directly with band 3. GPB associates with the Rh tetramers,9 and GPA and GPB may form heterotetramers, providing another link between the Rh complex and the band 3 complex. (B) Proposed gas exchange metabolon in the RBC membrane. The model suggests that CO2 passes from the capillary endothelial cell to the RBC through the Rh proteins. CAII converts CO2and H2O to HCO and a proton, and HCO passes out of the RBC through band 3 in exchange for a chloride ion. The removal of HCO leaves a proton that promotes local acidification in the vicinity of band 3 and the release of oxygen from oxy-hemoglobin by the Bohr effect. O2 may then leave the RBC through the Rh gas channel and pass into the endothelial cells. The channeling of substrate through this metabolon reduces substrate loss by diffusion from the system. In the pulmonary microcapillaries, the system would be reversed. Water movements involved in the hydration/dehydration of CO2 may be mediated by AQP1,55 though AQP1 is not directly associated with the band 3 macrocomplex.
A major difference between mouse and human band 3–deficient RBCs is that the mouse cells retain almost normal levels of CD47, whereas CD47 is almost completely absent from the human cells. A similar difference is shown by mouse and human protein 4.2(−/−) RBCs. Mouse 4.2(−/−) RBCs have normal amounts of CD47,44 whereas CD47 is almost totally absent from human 4.2(−/−) RBCs.27 These observations indicate that the major interacting site for CD47 in mature human RBCs is protein 4.2, but CD47 has a different site of high affinity binding in mouse RBCs.
Other less marked differences between the mouse and human band 3–deficient RBCs most likely reflect the dissimilar natures of the defects that lead to band 3 deficiency in the 2 cell types. Disruption of the band 3 gene in band 3(−/−) mouse RBCs results in the absence of any band 3 mRNA or translated band 3 protein. In contrast, the human band 3 Coimbra allele gives rise to mRNA with normal stability45 and yields a misfolded protein that is incorporated inefficiently into the RBC membrane. The presence of significant amounts of GPA in the human cells (29% normal) but the complete absence of GPA in mouse band 3(−/−) RBCs33suggests that band 3 Coimbra remains able to facilitate the movement of GPA to the cell surface. Because almost no band 3 Coimbra reaches the RBC surface, the band 3 Coimbra (and also normal band 3) probably exerts this effect on GPA targeting in an intracellular compartment, not by stabilizing GPA incorporated into the surface membrane.
Another difference between the mouse and human band 3–deficient cells is that although both show a similar marked reduction in RhAG protein, the mouse cells contain almost no Rh polypeptide, whereas the human cells contain reduced but still significant amounts (21% normal) of Rh polypeptide. The fact that mice have only the RhCcEe gene, whereas humans have both the RhCcEe and the RhDgenes, combined with the fact that band 3 is thought to interact more tightly with RhCcEe than with RhD polypeptides,26 may partly explain this difference, but the difference may also result from the presence of residual band 3 Coimbra in the cells. Surprisingly, there is more Rh polypeptide than RhAG in the band 3 Coimbra RBCs, suggesting that the Rh polypeptide can exist independently of RhAG in these membranes.
Aquaporin 1 (AQP1) was altered in mouse and human band 3–deficient RBCs. In healthy RBCs, AQP1 migrates as 2 separate bands because only one of the subunits in the aquaporin tetramer is N-glycosylated.46 The total amount of AQP1 was reduced in the band 3–deficient cells, and the N-glycosylated aquaporin band in human and mouse band 3–deficient RBCs was noticeably sharper than in healthy cells, indicating altered AQP1 N-glycan processing. Both effects suggest that in healthy RBCs the intracellular biosynthetic pathways of band 3 and AQP1 are connected and that AQP1 movement to the cell surface is linked with band 3 in some way. One possible mechanism for this interaction is suggested by the observation that the C-termini of both proteins can bind the PDZ domain of the homo-oligomeric protein PICK1.47 PICK1 is involved in the targeting and clustering of synaptic proteins (reviewed by Deken et al48). AQP1 and band 3 may become associated during biosynthesis by the binding of both proteins to PICK1 (or a PDZ protein with similar specificity because it is unknown whether PICK1 is present in erythroid precursors). However, this association probably only occurs during the biosynthesis of the 2 proteins in erythroid precursors because our coimmunoprecipitation results suggest band 3 and AQP1 do not interact in the mature RBC membrane. This is consistent with observations that the lateral mobility of AQP1 in the RBC membrane is not affected by antibody-induced immobilization of band 3 or GPA.49
We used the proteins of the GPC complex (GPC, protein 4.1, and p55) to normalize the levels of membrane proteins between the variant cells and control RBCs. One group of proteins was present at significantly higher levels in the band 3 Coimbra RBCs than the proteins of the GPC complex. These were GLUT1, the cell adhesion protein Lu, and the GPI-anchored proteins LFA-3 (CD58) and DAF (CD55). Cell adhesion protein CD44 was also slightly increased. The presence of large amounts of bound immunoglobulin on the mouse membranes (see “Materials and methods”) precluded the use of murine mAbs to identify all the corresponding membrane proteins in the mouse band 3(−/−) membranes, but the level of the complement regulatory protein Crry was also increased in the band 3(−/−) mouse RBCs. Hereditary spherocytosis (HS) associated with band 3 deficiency results from the blebbing of membrane vesicles, causing the cells to become spherical. Proteins within the membrane areas lost as vesicles are depleted, but proteins that have interactions independent of the band 3 macrocomplex remain. The GPC complex (GPC, protein 4.1, p55) has independent cytoskeletal interactions,50 as do CD44 and Lu.51,52 DAF, Crry, and LFA-3 are all known to partition into lipid rafts.53 Although the proteins associated with lipid rafts were found to be significantly enriched in the band 3–deficient membranes, the mechanism for this enrichment is unclear. A recent study has shown a differential distribution of raft-associated components in calcium-induced exovesicles released from healthy human RBCs54 and has suggested the existence of at least 2 different types of raft domains. It remains to be established whether different types of raft domains are selectively enriched in the band 3–deficient cells.
The presence of all these components within one structural macrocomplex makes it likely that the individual components have linked functional or regulatory roles, and we can speculate on the possible nature of this function. CAII and band 3 are part of a metabolon at the cytoplasmic surface of the RBC membrane that accepts CO2 on entry into the cell and forms bicarbonate, which is then channeled through band 3 to leave the cell in exchange for chloride.3 H2O may be supplied from outside the cell through AQP1.55 The efflux of bicarbonate through band 3–mediated chloride–bicarbonate exchange acidifies the cell and causes the release of O2 from hemoglobin by the Bohr effect. This provides the link between coordinated CO2 uptake and O2 release by the RBC, which is essential to meet the respiratory demands of tissues and which is reversed in the pulmonary capillary system. Because the protons produced by the export of HCO from the cell are released in the immediate neighborhood of band 3, O2 release by the Bohr effect occurs most rapidly from hemoglobin in the same region.
It has been suggested that the Rh proteins function as CO2 channels in RBCs.14,18 Data showing that CO2 transport into RBCs is inhibited by more than 90% by treatment with the band 3–specific inhibitor 4,4′-di-isothiocyanato-stilbene-2,2′disulfonate (DIDS) also supports the view that CO2 transport is mediated by a protein closely associated with band 3.56 We speculate that the Rh proteins might be relatively nonspecific channels for neutral small molecules and might act as gas channels for O2 and CO2 given that they are optimally located to channel CO2 to and from CAII, and O2 to and from hemoglobin in the local area around band 3. We hypothesize that the band 3 macrocomplex acts as an O2/CO2 gas exchange metabolon (Figure 5B), extending the model of a metabolon between band 3 and CAII that has been suggested by others.3 55 Membrane localization and channeling offered by this metabolon would provide the short paths for O2, CO2, H+, and HCO movements needed for efficient O2/CO2 exchange in the capillaries, minimizing loss through the long diffusive paths for O2 and H+ that would occur if these processes were not physically associated.
Why should the RBC require the accelerated gas exchange provided by this putative metabolon? The movement of RBCs through the tissue and lung capillaries is rapid (transit time, 0.3-1 second in humans, depending on cardiac output). Cells in the tissue surrounding the microcapillary endothelium signal their requirement for O2from RBCs by their release of CO2. To satisfy this, O2 must be released from RBCs in the vicinity of the cells that signal their need for it. Thus, RBC CO2 uptake and O2 release must be complete within the brief time required for the RBC to move past a single cell of the capillary endothelium. Slower RBC gas exchange would result in the inappropriate and potentially detrimental delivery of O2 to cells further along the capillary that might not have any requirement for O2. This would be especially important during vigorous physical exercise, when capillary transit time is decreased but CO2 production and O2 demand in muscle tissues are increased.
Although there is constant movement of RBCs within the microcapillaries, intimate contact between the surfaces of RBCs and endothelial cells (to facilitate gas exchange between the 2) can be maintained over longer periods by tank-treading of the RBC membrane, which is known to occur during the movement of RBCs in narrow vessels.57 This interfacial contact may also be facilitated by transient adhesive interactions between RBC and the endothelium and local regulation of the RBC skeleton, both of which could be mediated by RBC adhesion proteins, such as CD4727and LW.
Our understanding of the properties of the RBC is based mainly on in vitro studies, under conditions in which the cell is relatively inert. Further studies that focus on the properties of RBCs during their movement through the microcirculation are likely to illuminate unexpected features of the RBC membrane and to rationalize the reasons for the complex organization of the membrane proteins and the skeleton in this “simple” cell.
We thank the family studied here for their kind cooperation. We thank P. Agre for anti-AQP1, S. A. Baldwin for anti-GLUT1, V. M. Holers for anti-Crry, D. Shotton for anti-spectrin, J. Poole and I. Skidmore for serology testing, P. G. Martin for Rh genotyping, and J. S. Smythe for helpful advice on FACS analysis.
Prepublished online as Blood First Edition Paper, January 16, 2003; DOI 10.1182/blood-2002-09-2824.
Supported in part by grants from the Wellcome Trust, the Institut National de la Santé et de la Recherche Médicale (Unité 473) alone or jointly with the Association Française contre les Myopathies (project no. 4MR09F), and the National Institutes of Health (grants HL64885, DK56267, DK26263, DK32094, and HL31579) and by the Director, Office of Health and Environment Research Division, US Department of Energy (contract DE-AC03-76SF00098).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Michael J. A. Tanner, Department of Biochemistry, School of Medical Sciences, University of Bristol, Bristol, BS8 1TD, United Kingdom; e-mail:m.tanner@bristol.ac.uk.

![Fig. 3. Coomassie and immunostaining of proteins from Coimbra RBC membranes. / RBC membranes were separated on 10% or 12% Laemmli gels and were immunoblotted using antibodies as shown. Loading: C1, C2, controls 1 and 2, respectively. P indicates proband. (A) Proteins of the band 3 complex. Immunoblotting used polyclonal antibodies against protein 4.2 and monoclonal antibodies: BRIC170 (N-terminal band 3), BRIC155 (C-terminal band 3), and BRIC163 GPA. (B) Proteins of the glycophorin C (GPC) complex, plus aquaporin 1 (AQP1) and cytoskeletal proteins spectrin and actin. Immunoblotting used polyclonal antibodies against protein 4.1, p55, and AQP1 and monoclonal antibodies: BRIC4 (GPC), anti-spectrin, anti–β-actin (Abcam, Cambridge, United Kingdom). (C) Proteins of the Rh complex. Immunoblotting used polyclonal antibodies against the Rh polypeptides and the C-terminal region of CD47, and monoclonal antibodies LA1818 (RhAG), BRIC125 (N-terminal region of CD47), R1.3 (GPB), and BS56 (LW). (D) Other RBC membrane proteins. Immunoblotting used polyclonal antibodies against the glucose transporter (GLUT 1) and monoclonal antibodies BRIC221 (Lu), BRIC128 (DAF), BRIC5 (LFA-3), and BRIC235 (lymphocyte homing receptor [CD44]).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/10/10.1182_blood-2002-09-2824/4/m_h81034307003.jpeg?Expires=1767752136&Signature=w-~j~fX0ZSTH267RpkxnhAMpMoV39ReRTDfalimbbNcJm~1C4ozM8A-Nz2TUQFWamMSdJS-21sqvMasdFzA6iSkWNHirYpE6OVJTtsnguQ-CLctmskeX1QVnXvOtPFa6uObASroB0pkGqQg4ShgcoFOLUzSeZpGTH3cC82CMfdk5c9Fz~SG8PfttOODD0ieUVMC8iVQ26xv8uuYmpDd2~CiYQgJhpRPlARg6dcDjlIxd5KycuAogeZiG9K1DZ8LK45BLkkfqhRRzsCEo9GjUqvCewNTCnJ8mvMMEqwUAxe3cYVS~kd7fWysRON-QYC-sPoDq77KIDnaEISB5MIsTbw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal