Abstract
Infection of susceptible mouse strains with Plasmodium berghei ANKA (PbA) is a valuable experimental model of cerebral malaria (CM). Two major pathologic features of CM are the intravascular sequestration of infected erythrocytes and leukocytes inside brain microvessels. We have recently shown that only the CD8+ T-cell subset of these brain-sequestered leukocytes is critical for progression to CM. Chemokine receptor–5 (CCR5) is an important regulator of leukocyte trafficking in the brain in response to fungal and viral infection. Therefore, we investigated whether CCR5 plays a role in the pathogenesis of experimental CM. Approximately 70% to 85% of wild-type and CCR5+/- mice infected with PbA developed CM, whereas only about 20% of PbA-infected CCR5-deficient mice exhibited the characteristic neurologic signs of CM. The brains of wild-type mice with CM showed significant increases in CCR5+ leukocytes, particularly CCR5+ CD8+ T cells, as well as increases in T-helper 1 (Th1) cytokine production. The few PbA-infected CCR5-deficient mice that developed CM exhibited a similar increase in CD8+ T cells. Significant leukocyte accumulation in the brain and Th1 cytokine production did not occur in PbA-infected CCR5-deficient mice that did not develop CM. Moreover, experiments using bone marrow (BM)–chimeric mice showed that a reduced but significant proportion of deficient mice grafted with CCR5+ BM develop CM, indicating that CCR5 expression on a radiation-resistant brain cell population is necessary for CM to occur. Taken together, these results suggest that CCR5 is an important factor in the development of experimental CM.
Introduction
With more than 300 million new cases and approximately 2 million deaths annually, malaria represents the most important worldwide parasitic disease. The most severe complication of this disease is cerebral malaria (CM), which involves the cytoadherence of parasitized erythrocytes (PEs) to the cerebral microvasculature.1 However, the sequestration of infected erythrocytes, though necessary for CM pathogenesis, does not alone explain the progression to severe disease,2 as some investigators have observed cerebral PE sequestration in non-CM cases.3 Histopathologic studies have revealed the presence of mononuclear cells in brain capillaries of CM patients4, 5, 6, 7 (Terrie Taylor, personal communication). However, the role of these cells in CM pathogenesis remains unclear.
Infection of susceptible mouse strains with Plasmodium berghei ANKA (PbA) provides an experimental model of CM that shares some characteristics with the human disease. PEs together with leukocytes are sequestered in brain capillaries in PbA-infected mice developing CM.8, 9, 10, 11, 12 We have recently demonstrated that leukocyte accumulation in the brain strongly correlates with CM development in susceptible mice and that CD8+ T cells are critical for both the neurologic defects and the ensuing death of animals with CM.13
Chemokines and chemokine receptors are well-known regulators of leukocyte trafficking, and it is possible that some of these molecules are involved in the pathogenesis of experimental CM. A good candidate for a role in experimental CM is chemokine receptor–5 (CCR5). Like other chemokine receptors, CCR5 is a member of the 7-transmembrane, G protein–coupled receptor superfamily. CCR5 binds to multiple chemokines, including CCR ligand–3 (CCL3) (macrophage inflammatory protein–1α [MIP-1α], CCL4 (MIP-1β) and CCL5 (RANTES; regulated on activation, normal T-cell expressed and secreted),14 and it is expressed on a variety of leukocytes, including monocytes, T-helper 1 (Th1) cells, cytotoxic T cells, and immature dendritic cells.15,16 CCR5 is also expressed on certain nonhematopoietic cell types, most notably endothelial cells of the brain microvasculature.17,18 CCR5-bearing cells are among the infiltrating leukocytes in a variety of inflammatory processes, including lymphocytes in hepatitis C–infected liver,19 mononuclear cells in rheumatoid arthritis20 or infiltrated in the graft during acute and chronic transplant rejection,21 and mononuclear phagocytes accumulated in the central nervous system of patients with multiple sclerosis.22 Infection models with CCR5-deficient mice demonstrated that CCR5 is a key regulator of neuroinflammatory responses. CCR5-deficient mice infected with Cryptococcus neoformans or with mouse hepatitis virus exhibit major defects in leukocyte recruitment to the brain.23,24 However, it is not clear whether these defects in the neuromigratory behavior of leukocytes in CCR5-deficient mice reflect the absence of CCR5 from leukocytes or from the brain endothelial cells or from both cell types. Nevertheless, these results prompted us to investigate whether CCR5 might contribute to the pathogenesis of experimental CM. Our results clearly show that CCR5 is involved in murine CM.
Materials and methods
Mice
All experimental mice were on a mixed C57BL/6J × 129/Ola genetic background and included wild-type animals (WT), CCR5-deficient animals (B6129F2/J-Cmkbr5tm1Kuz) (CCR5 knock-out [CCR5 KO]), and CCR5+/– (F1) animals from a cross of WT × CCR5 KO mice. All mice were bred under specific pathogen-free conditions and were used between 8 and 12 weeks of age. In each experiment, mice were matched for age and sex. The production of the CCR5 KO mice has been previously described.23
Parasites and pathology
The blood stage of a cloned line of PbA8 was maintained as a stabilate (parasitized erythrocytes [PEs] at 107/mL in Alsever solution) in liquid nitrogen. Batches were prepared through in vivo passage in C57BL/6J mice, and they were free of any infectious agents. Mice were infected intraperitoneally with 106 PEs. Animals were monitored for the neurologic signs of CM, including paralysis, deviation of the head, ataxia, convulsions, and coma. CM incidence was calculated as the percentage of infected mice that developed CM.
Parasitemia and hematologic parameters
Parasitemia was determined daily by means of blood smears stained with Giemsa and a count of the number of PEs in at least 1000 erythrocytes. Values from parasitemia were then transformed by means of the formula x′= log (x + 1) as described.25 Hemoglobin (Hb) concentration was determined every 2 days as previously described.26 Briefly, 2 μL tail-vein blood was diluted in 500 μL Drabkin solution (Sigma, St Quentin L'Arbresle, France), and Hb was assayed in 96-well microtiter plates (Costar, Cambridge, MA) in a volume of 100 μL by measuring the absorption at 405 nm (OD405nm) in an enzyme-linked immunosorbent assay reader (Victor 1420, Wallac, Turku, Finland). Values were converted to milligrams per millimeter by means of a standard curve of human Hb (Sigma) dissolved in Drabkin solution.
Histology and immunostaining
One of the 2 hemispheres of brain from mice with or without CM was fixed in Carnoy solution (ethanol–chloroform–acetic acid, 6:3:1) for 24 hours and stored in butanol. Paraffin sections (5 μm) were cut from the midbrain region of 2 animals per group and stained with hematoxylin and eosin. For immunohistochemistry, brains from mice with or without CM were frozen in isopentane, cooled in liquid nitrogen, and stored at –80°C. Cryosections (6 μm) were cut and fixed in acetone (Prolabo, Fontenay sous Bois, France) for 10 minutes. Sections were incubated overnight at room temperature with biotinylated hamster antimouse intercellular adhesion molecule–1 (ICAM-1) antibody (clone 3E2; Pharmingen, San Diego, CA) diluted in phoshate-buffered saline (PBS). After washing, the sections were treated for 30 minutes with extravidin–alkaline phosphatase (Sigma). Phosphatase activity was detected by addition of the alkaline phosphatase substrate chromogen Fuchsin (DAKO, Carpinteria, CA). The sections were then counterstained with hematoxylin.
Purification of whole brain–sequestered leukocytes
Mice were killed and perfused intracardially with PBS to remove circulating red blood cells and leukocytes from the brain. The brains were then removed, and leukocytes were isolated by means of a previously described protocol.13 Briefly, brains were removed and crushed in HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) buffer: 100 mM NaCl, 2 mM KCl, 0.3 mM Na2HPO4.12H2O, 0.01 M HEPES (Sigma), and 100 000 IU penicillin/streptomycin (Life Technologies, Paisley, Scotland), and containing 0.05% collagenase (Boehringer Mannheim, Meylan, France) and 2 U/mL DNAse (Sigma). The tissue extract was then centrifuged at 400g for 5 minutes. The pelleted cells were further purified on a 30% Percoll gradient (Pharmacia Biotech, Uppsala, Sweden). Residual red blood cells were removed by hypotonic shock by means of ammonium chloride–potassium (ACK) lysis buffer. Brain-sequestered leukocytes (BSLs) were resuspended in fluorescence-activated cell sorter (FACS) buffer (PBS containing 1% fetal calf serum [FCS] and 0.01% NaN3) and counted.
Immunolabeling and flow cytometry analysis
BSLs were identified by their size (forward scatter [FSC]) and granulosity (side scatter [SSC]), as previously described.13 BSL subsets were further phenotyped by cytofluorimetry. Macrophages were identified as F4/80+ by means of biotinylated rat antimouse F4/80 (clone C1:A3-1; Tebu, Le Perray-en-Yvelines, France). Neutrophils were identified as F4/80– and Gr-1+ by means of rat antimouse Gr-1 conjugated to fluorescein isothiocyanate (FITC) (clone RB6-8C5; Pharmingen). T cells were identified by their small size and with the use of the following antibodies: hamster antimouse CD3 conjugated to phycoerythrin (clone 17A2; Pharmingen), rat antimouse CD8α conjugated to FITC (clone 53-6.7; Pharmingen), rat antimouse CD8α conjugated to quantum red (QR) (clone 53-6.7; Sigma), rat antimouse CD4 conjugated to QR (clone H129-19; Sigma), rat antimouse CD4 conjugated to phycoerythrin (clone H129-19; Pharmingen), and rat antimouse CCR5.27 Antibodies were diluted at the appropriate concentration in FACS buffer. Ultravidin conjugated to phycoerythrin (Leinco Technologies, St Louis, MO) and goat antirat immunoglobulin G (IgG) conjugated to FITC (Polysciences,Warrington, PA) were used as secondary reagents. For each sample, 5000 cells were screened. Data were collected by means of a FACScalibur flow cytometer and analyzed by means of Cell Quest software (Becton Dickinson, Le Pont de Claix, France).
In vivo leukocyte subset depletion
Rat antimouse CD8 (clone 2.43; ATCC [Manassas, VA] TIB 210), rat antimouse CD4 (clone GK1.5; ATCC TIB 207), or anti–polymorphonuclear cells (anti-PMNs)28 were purified from ascites or supernatant after ammonium sulfate precipitation. We injected 1 mg anti-CD8, anti-CD4, or anti-PMN monoclonal antibodies (mAbs) intraperitoneally at day 6 after parasite injection, and just before the onset of CM. More than 98% of blood CD8+ or CD4+ T cells were depleted by this procedure as verified by FACS analysis with the use of anti-CD4 (clone H129-19; Sigma) and anti-CD8 (clone 53-6.7; Pharmingen) mAbs that recognized epitopes different from those recognized by the depleting mAbs. Depletion of blood neutrophils was more than 80% as verified by FACS analysis with the use of anti–Gr-1 mAb. Purified rat IgG (Sigma) was used as a negative control. Macrophages were depleted at day 5 after PbA injection by intravenous injection of 0.2 mL PBS containing approximately 1 mg dichloromethylenediphosphonate (Cl2-MDP) encapsulated in liposomes.29 More than 90% of blood F4/80+ cells were depleted as verified by FACS analysis 2 days later.
Adoptive bone marrow reconstitution
Chimeric mice were prepared by irradiating animals twice (3 hours apart) with 5.5 Gy and then engrafting them with 106 fresh bone marrow (BM) cells injected intravenously. Mice were maintained on antibiotics for 4 weeks after BM cell injection. CCR5-deficient mice received either KO or WT BM cells, and WT mice received either KO or WT BM cells. To test for successful adoptive bone marrow cell transfer, CCR5 expression on peripheral blood leukocytes (PBLs) from reconstituted mice was determined 10 weeks after reconstitution by FACS analysis. Mice were infected with 106 PEs 10 weeks after receiving BM cells.
Splenocyte culture
Naive mice were infected with PbA, and spleens were harvested from animals with CM (at the day of the neurologic symptoms) or without CM (at day 10 after infection). Splenocytes were dissociated in RPMI glutamax medium (Life Technologies) supplemented with 10% FCS, 50 U/mL penicillin-streptomycin (Life Technologies), 50 μM β-2-mercaptoethanol (Sigma), and 10 mM HEPES (complete RPMI medium). Red blood cells were lyzed by osmotic shock, and leukocytes were cultured at 107 cells per well in complete RPMI medium in a 24-well plate (Costar). The supernatants were removed 48 hours after the beginning of culture and stored at –20°C.
TNF bioassay
Sera from naive and from infected mice with or without CM were harvested and kept at –20°C until analyzed. Filtered sera or splenocyte culture supernatants were assayed as previously described.30 Murine WEHI 164.13 cells (3 × 104 cells per well) were placed in 96-well microtiter plates in 50 μL complete RPMI medium supplemented with 40 mM LiCl and 2 μg/mL actinomycin D (Sigma). We added 50 μL serum samples, supernatants, or dilutions of recombinant murine tumor necrosis factor–α (TNF-α) (Genzyme, Paris, France) in duplicate into the wells. The plates were incubated overnight at 37°C in 5% CO2 atmosphere. Cell viability was determined by methylthiozolyl tetrazolium (MTT) reduction31 by dissolving crystals in ethanol/dimethyl sulfoxide (DMSO) (vol/vol) and measuring absorbance at 560 nm with a reference wavelength of 630 nm. The TNF-α detection limit was 0.3 U/mL.
Enzyme-linked immunosorbent assay (ELISA) detection of IFN-γ and IL-10
The wells of 96-well plates (Nunc, Napperville, IL) were coated with anti–interferon-γ (anti–IFN-γ) (clone R4-6A2; Pharmingen) or anti–interleukin 10 (anti–IL-10) (clone JES5-2A5; Pharmingen) mAbs, and the plates were incubated overnight at 4°C. Supernatants or sera were added and incubated overnight at 4°C. A second biotinylated antibody against IFN-γ (clone XMG1.2) or IL-10 (clone SXC-1) (both clones from Pharmingen) was added to the appropriate wells, and the plates were incubated for 1 hour at room temperature (RT). This was followed by addition of extravidin–alkaline phosphatase for 1 hour at RT. Phosphatase activity was measured by means of 4-methylumbelliferyl phosphate (Sigma) as substrate and reading fluorescence at 360/460 nm with the use of a spectrophotometer (Victor 1420; Wallac). Dilutions of known quantities of IFN-γ or IL-10 were used for the standard curve. Detection limits were 50 pg/mL for IFN-γ and 80 pg/mL for IL-10.
Statistical analysis
Differences in CM incidence were analyzed by means of the Fisher exact test, and differences between multiple groups were analyzed for statistical significance by means of the one-way ANOVA followed by the Tukey multiple comparison test and with P < .05 as the level of significance.
Results
CCR5-deficient mice infected with PbA have increased resistance to CM
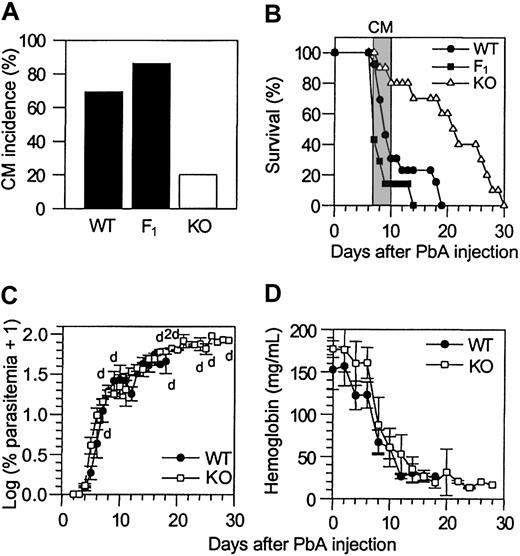
As shown in Figure 1, approximately 70% of WT mice and 85% of F1 mice infected with PbA exhibited CM-specific neurologic signs within 7 to 10 days after infection and died within the next 24 hours. In sharp contrast, only about 20% of PbA-infected CCR5-deficient mice exhibited these neurologic signs and died. In the animals from all 3 groups (WT, F1, and CCR5 KO) that did not develop CM (NCM), the infection progressed and the mice died within the third or fourth week after infection as a result of hyperparasitemia (Figure 1C) and anemia (Figure 1D). Histologic analysis of brain sections from infected WT and CCR5-deficient mice with and without CM revealed the presence of infected red blood cells in both sample sets, but characteristic petechial hemorrhages were observed only in CM brains (data not shown). In contrast, the capillaries of infected NCM WT and CCR5-deficient mice were normal with no evidence of hemorrhage (data not shown). ICAM-1, which is involved in leukocyte sequestration in the brain during CM,31 was expressed equally on large and small vessels of the brains of WT and CCR5-deficient mice with CM, whereas in NCM WT and CCR5-deficient mice, ICAM expression was restricted to large vessels in the brain (data not shown).
Increased resistance to CM in CCR5-deficient mice infected with PbA. (A-B) CM incidence occurring between day 7 and day 10 (A) and survival time (B) of WT (n = 13), F1 (n = 7), and KO (n = 10) mice infected with PbA (106 PEs). Neurologic signs first appear late on days 7 through 10 (B, shaded area), with death occurring in less than 24 hours after their onset. On day 10, as calculated by Fisher exact test, P < .05 between WT (or F1) and KO mice. This experiment is representative of 3 experiments. (C-D) Parasitemia (C) and hemoglobin levels (D) of PbA-infected WT (n = 5) and KO (n = 5) mice. Mortality is indicated on the first graph at the top (WT mice) and at the bottom (KO mice) as the number of dead mice (d) on that day. F1 mice have parasitemia and hemoglobin level profiles similar to these observed for the WT mice. Error bars represent mean (SE) of 5 mice.
Increased resistance to CM in CCR5-deficient mice infected with PbA. (A-B) CM incidence occurring between day 7 and day 10 (A) and survival time (B) of WT (n = 13), F1 (n = 7), and KO (n = 10) mice infected with PbA (106 PEs). Neurologic signs first appear late on days 7 through 10 (B, shaded area), with death occurring in less than 24 hours after their onset. On day 10, as calculated by Fisher exact test, P < .05 between WT (or F1) and KO mice. This experiment is representative of 3 experiments. (C-D) Parasitemia (C) and hemoglobin levels (D) of PbA-infected WT (n = 5) and KO (n = 5) mice. Mortality is indicated on the first graph at the top (WT mice) and at the bottom (KO mice) as the number of dead mice (d) on that day. F1 mice have parasitemia and hemoglobin level profiles similar to these observed for the WT mice. Error bars represent mean (SE) of 5 mice.
Leukocyte trafficking to the brain is reduced in CM-resistant CCR5-deficient mice
The total number of brain-sequestered leukocytes (BSLs) in PbA-infected WT and CCR5-deficient animals that did not develop CM were only slightly increased relative to the number of these cells in naive WT and CCR5-deficient mice, respectively. There were no significant differences in the accumulation of leukocyte subsets, including macrophages, neutrophils, and CD4+ and CD8+ T cells (Table 1). In contrast, PbA-infected WT and CCR5-deficient mice with CM exhibited significant accumulations of BSLs relative to naive and PbA-infected NCM mice. Neutrophils and CD4+ and CD8+ T cells were increased in number in WT mice compared with NCM WT mice. Only CD8+ T cells were significantly increased in number in CCR5-deficient mice with CM compared with NCM CCR5-deficient mice.
Quantification and characterization of brain-sequestered leukocytes of naive, CM, NCM, WT, and KO mice
. | . | . | . | T lymphocytes . | . | |
|---|---|---|---|---|---|---|
| Mice* . | Leukocytes† . | Macrophages . | Neutrophils . | CD3+ CD4+ . | CD3+ CD8+ . | |
| WT | ||||||
| Naive | 138.1 ± 17.8 | 66.4 ± 11.6 | 14.7 ± 5.1 | 6.9 ± 3.05 | 6.3 ± 2.5 | |
| NCM | 180.5 ± 36.9 | 136.1 ± 30.5 | 28.5 ± 5.2 | 4.5 ± 0.9 | 12.4 ± 4.9 | |
| CM | 697 ± 73‡§ | 278.8 ± 27.8‡ | 240 ± 38.2‡§ | 41.1 ± 10.2‡§ | 190.3 ± 64.1‡§ | |
| KO | ||||||
| Naive | 231.3 ± 37.5 | 99.6 ± 22.9 | 37.5 ± 10.5 | 4.7 ± 1 | 3.5 ± 0.5 | |
| NCM | 310.8 ± 48.6 | 190.1 ± 42.5 | 82.7 ± 17.5 | 10 ± 5.3 | 4.5 ± 1.9 | |
| CM | 619.1 ± 87.8∥¶ | 239.5 ± 66.6∥ | 183.2 ± 43.7∥ | 33.6 ± 5∥ | 181.1 ± 31.3∥¶ | |
. | . | . | . | T lymphocytes . | . | |
|---|---|---|---|---|---|---|
| Mice* . | Leukocytes† . | Macrophages . | Neutrophils . | CD3+ CD4+ . | CD3+ CD8+ . | |
| WT | ||||||
| Naive | 138.1 ± 17.8 | 66.4 ± 11.6 | 14.7 ± 5.1 | 6.9 ± 3.05 | 6.3 ± 2.5 | |
| NCM | 180.5 ± 36.9 | 136.1 ± 30.5 | 28.5 ± 5.2 | 4.5 ± 0.9 | 12.4 ± 4.9 | |
| CM | 697 ± 73‡§ | 278.8 ± 27.8‡ | 240 ± 38.2‡§ | 41.1 ± 10.2‡§ | 190.3 ± 64.1‡§ | |
| KO | ||||||
| Naive | 231.3 ± 37.5 | 99.6 ± 22.9 | 37.5 ± 10.5 | 4.7 ± 1 | 3.5 ± 0.5 | |
| NCM | 310.8 ± 48.6 | 190.1 ± 42.5 | 82.7 ± 17.5 | 10 ± 5.3 | 4.5 ± 1.9 | |
| CM | 619.1 ± 87.8∥¶ | 239.5 ± 66.6∥ | 183.2 ± 43.7∥ | 33.6 ± 5∥ | 181.1 ± 31.3∥¶ | |
PbA-infected mice were killed between day 7 and day 10 after infection, when mice displayed neurologic symptoms. Mice without CM were killed at day 10.
Results are mean ± SEM (× 103) of 3 to 9 mice per group.
P < .05 versus naive WT mice.
P < .05 versus NCM WT mice.
P < .05 versus naive KO mice.
P < .05 versus NCM KO mice.
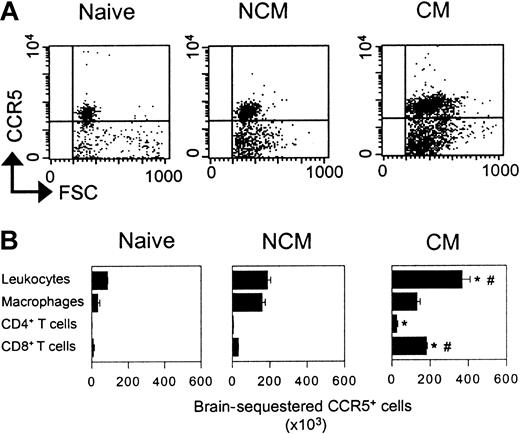
Distribution of CCR5+ leukocytes in naive and PbA-infected WT mice with and without CM
Using a recently described rat antimouse CCR5 monoclonal antibody,27 we characterized the CCR5+ cells in total BSLs from naive WT mice and PbA-infected WT mice with and without CM. This antibody recognizes CCR5 on peripheral blood mononuclear cells from WT mice but does not react on such cells obtained from CCR5-deficient animals (data not shown). As shown in Figure 2, populations of BSLs isolated from naive WT, WT CM, and WT NCM mice all contain a substantial proportion of CCR5+ cells, and these cells are significantly more abundant in the WT CM animals (Figure 2A). In naive WT mice, approximately 50% of the BSLs are CCR5+ (Figure 2B). Many of these cells are also F4/80+, and a small fraction are CD8+. The majority of CCR5+ cells in the BSLs isolated from PbA-infected WT mice without CM are also F4/80+, and most of the remaining cells are CD8+. As with the BSLs from WT NCM mice, most of the BSLs in WT CM mice are CCR5+, but in striking contrast, the predominant subset of CCR5+ cells in the BSLs from WT CM mice are also CD8+ (Figure 2B). Most of the remaining cells are F4/80+, but a significant fraction are also CD4+.We and others have previously demonstrated that CD8+ cells are critical for the development of CM in PbA-infected WT mice.13,32,33 Depleting CD8+ cells by treatment of WT or CCR5-deficient mice with anti-CD8 antibodies completely abrogated development of CM in both strains, confirming our earlier results (data not shown).
Expression of CCR5 on BSLs. (A) Dot plots representing size and CCR5 expression on whole BSLs from WT mice (naive, NCM, and CM). (B) Number of sequestered leukocyte subsets expressing CCR5 from the whole brain of WT mice (naive, NCM, and CM). *P < .05 versus naive mice. #P < .05 versus NCM mice. Error bars represent mean (SE) of 5 to 10 mice per group.
Expression of CCR5 on BSLs. (A) Dot plots representing size and CCR5 expression on whole BSLs from WT mice (naive, NCM, and CM). (B) Number of sequestered leukocyte subsets expressing CCR5 from the whole brain of WT mice (naive, NCM, and CM). *P < .05 versus naive mice. #P < .05 versus NCM mice. Error bars represent mean (SE) of 5 to 10 mice per group.
Development of CM in CCR5 KO or WT mice reconstituted with bone marrow from KO or WT mice
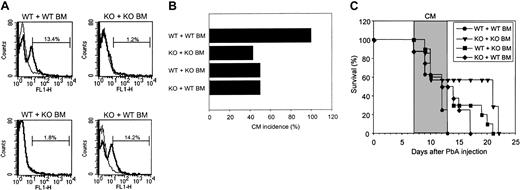
To evaluate whether the presence of CCR5 on leukocytes, on brain cells, or on both was essential for CM development, we generated chimeric mice by adoptive bone marrow transplantation after total body irradiation. After total reconstitution (10 weeks), as verified by CCR5 expression in PBLs (Figure 3A), mice were infected by PbA. As shown in Figure 3, all WT mice reconstituted with WT bone marrow cells (WT plus WT BM) developed CM (Figure 3B) and died between day 9 and day 13 (Figure 3C). These mice took longer to die from CM than normal infected WT mice (Figure 1). This was associated with a longer delay between the time of the appearance of neurologic symptoms (day 7 to 8) and death by CM. Only 50% of WT mice reconstituted with KO bone marrow cells (WT plus KO BM) developed CM. Forty-three percent of CCR5-deficient mice reconstituted with KO and 50% of CCR5-deficient mice reconstituted with WT mice bone marrow cells developed CM. It should be noted that in the KO mice group reconstituted with KO BM, a slightly higher proportion of mice died of CM than in the normal KO mice. However, when the WT plus WT BM group is compared with the KO plus KO BM group, there is a significant difference in CM incidence, confirming a role for CCR5 in CM. No differences in parasitemia among the different experimental groups were observed (data not shown), confirming data in Figure 1 on the absence of influence of CCR5 on parasite development. Taken together, these results demonstrate that the presence of CCR5 on both hematopoietic and nonhematopoietic cells is necessary for CM development.
Relationship of CM to CCR5 expression on leukocytes and a radio-resistant brain cell. CCR5 expression on leukocytes and a radio-resistant cell in the brain is essential for CM. (A) Histograms of CCR5 expression on peripheral blood mononuclear cells of WT mice engrafted with WT BM, KO mice engrafted with KO BM, WT mice engrafted with KO BM, and KO mice engrafted with WT BM. Expression of CCR5 was assessed on peripheral blood leukocytes 10 weeks after BM engraftment for all reconstituted mice (7 to 10 per group). These histograms are representative of one mouse per group. Control is shown as a thin line. (B-C) CM incidence occurring between day 7 and day 13 (B) and survival time (C) of WT mice engrafted with WT BM (n = 8), KO mice engrafted with KO BM (n = 8), WT mice engrafted with KO BM (n = 10), and KO mice engrafted with WT BM (n = 8) and all infected with PbA (106 PE) 10 weeks after reconstitution. Neurologic signs first appear late on days 7 to 10, with death occurring 2 to 3 days after their onset (shaded area). On day 13, as calculated by Fisher exact test, P < .05 between WT mice engrafted with WT BM and KO mice engrafted with KO BM.
Relationship of CM to CCR5 expression on leukocytes and a radio-resistant brain cell. CCR5 expression on leukocytes and a radio-resistant cell in the brain is essential for CM. (A) Histograms of CCR5 expression on peripheral blood mononuclear cells of WT mice engrafted with WT BM, KO mice engrafted with KO BM, WT mice engrafted with KO BM, and KO mice engrafted with WT BM. Expression of CCR5 was assessed on peripheral blood leukocytes 10 weeks after BM engraftment for all reconstituted mice (7 to 10 per group). These histograms are representative of one mouse per group. Control is shown as a thin line. (B-C) CM incidence occurring between day 7 and day 13 (B) and survival time (C) of WT mice engrafted with WT BM (n = 8), KO mice engrafted with KO BM (n = 8), WT mice engrafted with KO BM (n = 10), and KO mice engrafted with WT BM (n = 8) and all infected with PbA (106 PE) 10 weeks after reconstitution. Neurologic signs first appear late on days 7 to 10, with death occurring 2 to 3 days after their onset (shaded area). On day 13, as calculated by Fisher exact test, P < .05 between WT mice engrafted with WT BM and KO mice engrafted with KO BM.
Cytokine production
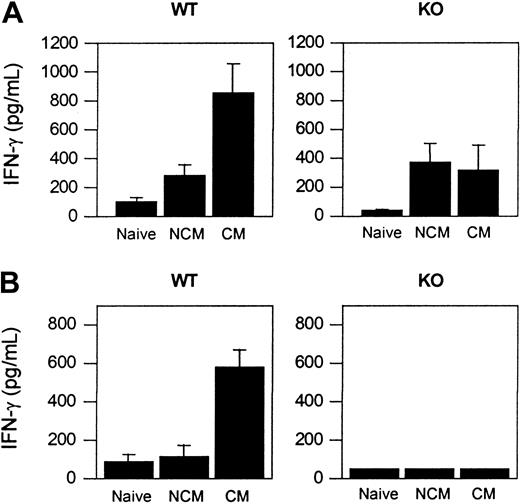
IFN-γ and TNF-α are thought to be involved in the pathogenesis of CM.34,35 Therefore, we investigated whether the absence of CCR5 affected production of these 2 Th1 cytokines during infection with PbA. Levels of IFN-γ, TNF-α, and IL-10 were measured in cultured splenocytes and in the serum from naive WT and CCR5-deficient mice and from PbA-infected WT and CCR5-deficient mice with and without CM.
Cultured splenocytes from WT mice with CM spontaneously produced more IFN-γ than splenocytes from WT NCM (P < .05) or naive WT mice (P < .01). Cultured splenocytes from PbA-infected CCR5-deficient mice with or without CM spontaneously produced higher levels of IFN-γ than splenocytes from naive CCR5-deficient animals, but these IFN-γ levels were lower than the amount of IFN-γ detected in splenocytes from WT CM mice (Figure 4A). A higher level of IFN-γ was detected in the sera of WT CM mice compared with WT NCM animals (P < .001) or naive WT mice (P < .001) (Figure 4B). In contrast, IFN-γ was not detected in the sera obtained from any of the 3 experimental groups of CCR5-deficient animals: naive, CM, or NCM.
IFN-γ production in naive and PbA-infected WT and CCR5-deficient mice. (A) Spontaneous cytokine release from splenocytes. Mean (SE) of 3 to 5 mice per group. (B) Cytokine in the serum. Mean (SE) of 4 to 12 mice per group.
IFN-γ production in naive and PbA-infected WT and CCR5-deficient mice. (A) Spontaneous cytokine release from splenocytes. Mean (SE) of 3 to 5 mice per group. (B) Cytokine in the serum. Mean (SE) of 4 to 12 mice per group.
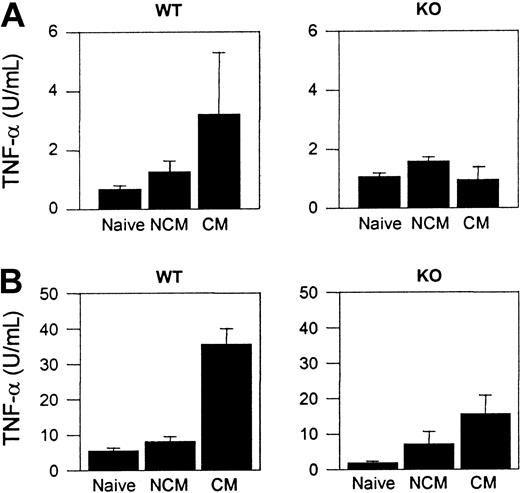
Splenocytes from WT CM mice spontaneously produced more TNF-α than splenocytes from naive WT mice or WT NCM animals (Figure 5A). No significant differences in TNF-α concentration were observed between the different groups of CCR5-deficient mice (Figure 5A). TNF-α was detected in the sera from both WT and CCR5-deficient mice that developed CM, but the levels were significantly lower in the CCR5-deficient CM animals (P < .01). Serum TNF-α was not detected in naive WT, WT NCM, and CCR5-deficient mice (Figure 5B)
TNF-α production in naive and PbA-infected WT and CCR5-deficient mice. (A) Spontaneous cytokine release from splenocytes. Mean (SE) of 3 to 5 mice per group. (B) Cytokine in the serum. Mean (SE) of 4 to 12 mice per group.
TNF-α production in naive and PbA-infected WT and CCR5-deficient mice. (A) Spontaneous cytokine release from splenocytes. Mean (SE) of 3 to 5 mice per group. (B) Cytokine in the serum. Mean (SE) of 4 to 12 mice per group.
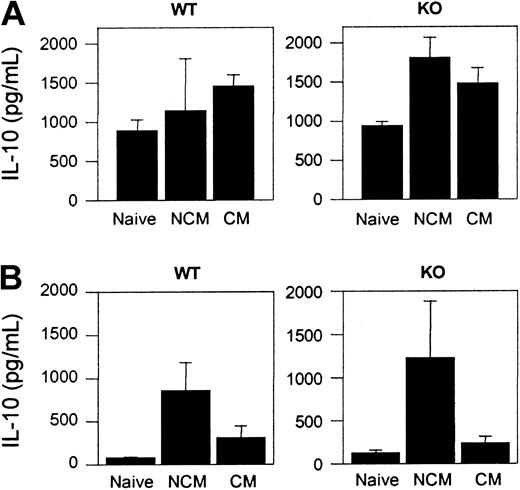
There was detectable production of IL-10 by splenocytes from PbA-infected WT and CCR5-deficient mice regardless of whether or not they had CM (Figure 6A). Serum IL-10 levels were significantly higher in WT and CCR5-deficient NCM mice than in naive WT and CCR5-deficient animals (P < .01) (Figure 6B).
IL-10 production in naive and PbA-infected WT and CCR5-deficient mice. (A) Spontaneous cytokine release from splenocytes. Mean (SE) of 3 to 5 mice per group. (B) Cytokine in the serum. Mean (SE) of 4 to 12 mice per group.
IL-10 production in naive and PbA-infected WT and CCR5-deficient mice. (A) Spontaneous cytokine release from splenocytes. Mean (SE) of 3 to 5 mice per group. (B) Cytokine in the serum. Mean (SE) of 4 to 12 mice per group.
Discussion
The results presented here strongly support the idea that CCR5 is important for progression to CM in mice after infection with P berghei ANKA. We show here that expression on both brain-sequestered leukocytes and nonhematopoietic cells, possibly endothelial cells, astrocytes, and microglial cells in the brain, is required for CM to develop in the majority of the infected animals.
We have previously demonstrated that leukocyte sequestration in the brain of PbA-infected mice correlates with the development of CM and that CD8+ T cells in the BSLs are essential for the neurologic signs and ensuing death of mice with CM,13 confirming previous indirect evidence that these cells are a key effector in the severity of malaria.32,33,36 We have now shown that a substantial number of CD8+ T cells in the BSLs of WT CM mice are CCR5+ (Figure 2B) and that in the majority of KO mice (those that do not develop CM), there was a defective trafficking of leukocytes, and in particular of CD8+ T cells, to the brain. A similar defect in leukocyte trafficking to the brain has been shown to occur in CCR5-deficient mice after disseminated Cryptococcus neoformans infection or intracranial infection with mouse hepatitis virus.23,24 In CCR5-deficient mice infected with mouse hepatitis virus, there was a reduction in acute CD8+ T-cell accumulation in the brain that correlated with reduced early virus clearance.24 In our model of experimental CM, infection of mice with PbA may lead to the activation of leukocytes and consequent CCR5 expression on their surface. Endothelial cells interacting with PEs through membrane contacts or after ingesting parasite antigens37 may be induced to produce and present specific chemokines, including ligands for CCR5, such as CCL3, CCL4, and CCL5, (MIP-1α, MIP-1β, and RANTES, respectively),38, 39, 40 which would serve to lure the CCR5-bearing leukocytes to the brain.41 Then, adhesion molecules expressed on activated brain endothelial cells (ECs) could interact with their ligands expressed on the migrating leukocytes. We have proposed that brain-recruited effector CD8+ T cells destroy ECs, thus leading to the breakdown of the blood-brain barrier and the subsequent hemorrhages and perturbation of neuronal communication.13
Reconstitution experiments of irradiated WT or KO mice with bone marrow from WT or KO mice indicated that CCR5 expression on radio-resistant cells of nonhematopietic origin was involved in CM. Brain endothelial cells, microglia, and astrocytes are likely to be involved since they express CCR5 on their surface.18,42,43 The binding of chemokines to CCR5 on these cells can serve to further activate them and enhance breakdown of the blood-brain barrier.
There is a role for cytokines in this process as well. The absence of CCR5 from PbA-infected animals resulted in a reduced Th1 cytokine production. This production was reported to up-regulate ICAM-1 on the surface of ECs.44 Low ICAM-1 expression may contribute to the absence of BSLs in the brains of CCR5-deficient mice that do not develop CM. A defect in production of inflammatory cytokines (both IFN-γ and TNF-α) was also observed in CCR5-deficient mice with CM where leukocyte recruitment was normal. However, IL-10 production in these animals was reduced, and we have previously shown that administration of IL-10 was able to protect susceptible mice from CM.45 KO mice with CM had levels of TNF-α similar to those of KO mice without CM. This suggests that TNF-α is not involved CM pathogenesis. This is an agreement with recent results showing that TNF-α deficient mice are fully susceptible to CM.46 Reduced cytokine production (mainly of IFN-γ) has also been observed in Leishmania donovani– infected CCR5-deficient mice, which led to a delay in controlling the infection in these animals.47
In the few cases of PbA-infected CCR5-deficient mice that developed CM, the absence of CCR5 function may have been compensated for by one or more additional chemokine receptors, such as CCR1, CCR2, and CCR3. CCR1 and CCR3 bind some of the same ligands that bind to CCR5 (CCR1 binds to CCL3 and CCL5; CCR3 binds to CCL5),14 and CCR2 regulates the trafficking of several of the same leukocyte subsets as CCR5.48 In addition, CCR1 and CCR2, like CCR5, are expressed on brain endothelial cells.49,50 If chemokine receptors on brain endothelial cells are involved in CM development, CCR1, CCR2, or other molecules might provide sufficient functional redundancy to promote CM in the absence of CCR5. Consistent with hypothesis, we have observed in a preliminary experiment that more than 90% of brain-sequestered leukocytes express CCR2+ (E.B. and L.R., unpublished results).
Recently, a number of polymorphisms and mutations in human CCR5 genes that alter, sometimes drastically, CCR5 functional activity or expression levels, have been identified.51,52 For example, individuals homozygous for the delta32 mutation are naturally deficient in CCR5. Because there is growing evidence that leukocytes may be involved in human CM4, 5, 6, 7 (Terrie Taylor, personal communication), it will be of interest to determine whether natural CCR5 deficiency correlates with protection against CM. The results could suggest new therapeutic strategies to decrease the mortality associated with this disease.
Prepublished online as Blood First Edition Paper, January 30, 2003; DOI 10.1182/blood-2002-05-1493.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank F. Gonnet and E. Souil for their help in histology; Nico Van Rooijen for the kind gift of Cl2-MDP; and Dr Georges Snounou for critical reading of the manuscript.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal