Abstract
This paper addresses the capacity of naive, effector, and memory CD4 T cells to control growth of a major histocompatibility complex (MHC) class II—positive B-cell lymphoma in vivo. To assess the role of T cells on their own without contributions by B cells, antibodies, or natural killer (NK) cells, we generated pure effector or memory CD4 T cells in Rag–/–gc–/– mice deficient in endogenous lymphocytes and NK cells. Lymphoma cells expressing a model antigen were injected into mice with T cells of cognate specificity that were either naive or in effector or resting memory state. Naive T cells were unable to prevent tumor growth, probably due to delay of efficient cross-presentation by dendritic cells. However, both effector and memory T cells, dependent on the amount of antigen available, controlled the tumor for a considerable period of time without the need for dendritic cell stimulation. Nevertheless, the tumor eventually grew uncontrolled in all cases. This was not because of a defect in T-cell homing to the tumor site or loss of MHC class II or costimulatory molecules by the tumor, but reflected mutual paralysis of T-cell responsiveness and antigen processing by tumor cells.
Introduction
As most tumor cells express major histocompatibility complex (MHC) class I but not class II molecules, it has been assumed that the predominant tumoricidal effector mechanism involved in rejecting tumors is direct cytotoxic killing by CD8 T cells, and in many models CD8 T cells have been effective in eliminating tumors in the absence of CD4 T cells.1, 2, 3 However, CD4 T-cell help can play a role in the development of tumor immunity in both the priming phase, for development and maintenance of cytotoxic T lymphocytes,4,5 and during the effector phase of an antitumor immune response, particularly against MHC class II—negative tumors.6, 7, 8, 9, 10, 11, 12, 13, 14 This has been demonstrated by depleting vaccinated mice of CD4 T cells before tumor challenge and showing that they were unable to reject the tumor6,12 and by adoptive transfer of CD4 T cells into tumor-bearing mice, which could then mediate tumor rejection.9 Nevertheless, the mechanism by which CD4 T cells mediate antitumor immunity is relatively poorly understood. As most tumor cells do not express MHC class II molecules and CD4 T cells can promote rejection of MHC class II—negative tumors, much of their action has been attributed to effector mechanisms such as the production of cytokines crucial for tumor rejection.12,15, 16, 17 A key cytokine associated with the antitumor effects of CD4 T cells in vivo is interferon-γ (IFN-γ).9,18 IFN-γ can up-regulate MHC expression,18,19 inhibit tumor cell growth,20,21 activate innate effector cells involved in tumor rejection (such as macrophages),22, 23, 24, 25 induce angiogenesis inhibitors, 20,26, 27, 28 and bring about inhibition of tumor-induced angiogenesis.29,30 However, because of poor immunogenicity and strong immunosuppression, the induction of therapeutic T-cell responses to tumors has been difficult to achieve. Many strategies have been devised to try to optimize conditions that may lead to productive antitumor immune responses. These have included transfecting tumor cells with costimulatory molecules,31,32 using dendritic cell (DC)—based vaccine therapy,33, 34, 35, 36, 37, 38, 39, 40, 41, 42 and transferring cytokines or cytokine genes into the hosts.43,44 Although successful induction of CD4 immune responses resulting in tumor rejection has been widely reported, it is less clear whether such immune responses are able to protect against re-emerging tumor cells once the acute response has terminated and T cells have differentiated to resting memory cells.
The aim of this study was to assess the contribution of CD4 T cells in different activation states in controlling tumor growth. For this, we made use of an experimental system previously described by us that allows the in vivo generation of pure effector or memory CD4 T cells from naive T-cell precursors.45 Naive CD4 T cells from the T-cell receptor transgenic strain (TCR tg Rag1–/–) A18, recognizing the complement component C5 in the context of H-2Ek,46 were cotransferred with syngeneic bone marrow—derived DCs into common cytokine γ chain (Rag–/–gc–/–)—deficient allogeneic hosts. Due to the absence of natural killer (NK) cells in these hosts, no rejection of the allogeneic T cells occurs; the allogeneic MHC itself has been shown to have no influence on A18 T cells. The hosts are either C5 deficient, in which case the DCs are pulsed with C5 peptide, or they contain C5 in their blood circulation. In the latter case the cotransferred DCs internalize, process, and present C5 from the host. Cotransfer of naive T cells and DCs leads to virtually synchronous activation of all T cells, and any remaining nonactivated T cells fail to survive beyond 2 weeks due to the absence of syngeneic MHC molecules that are needed to transmit survival signals for naive T cells. The disappearance of DCs about 3 to 4 weeks after transfer terminates antigenic stimulation, and all remaining T cells are of memory phenotype, whereas reinjection of DCs at later time points reactivates these memory cells to effector cells.45
Using mice that contained either naive, effector, or memory T cells in the absence of any other components of the immune system, such as CD8 T cells, B cells, NK cells, or antibodies, we asked under what circumstances rejection of a class II—positive B-cell lymphoma, expressing C5 as model antigen, could be achieved. Our results show that CD4 T cells in effector or memory stage, in contrast to naive T cells, can to some extent prevent tumor growth without the involvement of other components of the immune system. However, eventually the tumor will grow unchecked due to mutual paralysis of T-cell responsiveness and antigen processing by tumor cells.
Materials and methods
Mice
The A18 TCR (C5–, H-2a) transgenic strain on a Rag1–/– background, Rag–/– gc–/– C5+ (H-2b) or C5– (H-2q or H-2b) and Rag–/– (H-2a) mice were bred at the animal facilities of the National Institute for Medical Research (NIMR) in accordance with the established guidelines.
Generation of effector and memory CD4 T cells
Mice containing a pure memory CD4 T-cell population were generated by the adoptive transfer model described previously.45 Briefly, single cell suspensions were made from spleen and lymph nodes of the naive A18 Rag–/– TCR transgenic strain.46 These were transferred by intravenous injection together with syngeneic DCs in a 1:1 ratio into gc–/–Rag–/–C5+. For generation of memory CD4 T cells in C5-negative hosts, DCs were incubated with 1 μM A18 peptide and 100 μg lipopolysaccharide (LPS) (Sigma, Poole, Great Britain) for 2 hours at 37°C prior to transfer. Animals were left for at least 4 weeks before use in experiments. Effector CD4 T cells were generated by reactivating resting memory cells in vivo by intravenous injection of peptide-pulsed syngeneic DCs 8 days prior to analysis.
Cell lines and culture
The C5-specific T-cell hybridoma A18 carries the T-cell receptor used for generation of the transgenic strain and recognizes C5 peptide 105-121 in the context of H-2Ek.47 LK35 lymphoma cells are a fusion product of the B-cell lymphoma A20 with H-2k splenic B cells, so that it expresses H-2k,d (ATCC cat. no. HB 98). Cell lines and cultures were maintained in Iscoves modified Dulbecco medium (IMDM, Gibco-BRL, Paisley, Scotland) supplemented with 5% heat-inactivated fetal calf serum (FCS), penicillin (100 U/mL), streptomycin (100 μ/mL), mercaptoethonal (5 × 10–5 M), and L-glutamine (2 × 10–3 M) (all from Sigma). C5-transfected LK35 cells48 were maintained as above and supplemented with 1 mg/mL G418. The IL-2—dependent T-cell line, CTLL (ATCC cat. no. TIB 214) was grown in interleukin-2 (IL-2)—supplemented media. Dendritic cells were generated ex vivo from bone marrow as previously described49 and cultured overnight before use with 10 μg/mL LPS (Sigma) to ensure that the DCs were mature.
Tumor inoculation
Animals were inoculated subcutaneously with 5 × 105 C5-transfected LK35 tumor cells per mouse onto the back. Tumor volume was monitored, and mice were culled when the tumor volume reached 1 cm3.
Flow cytometry and cell sorting
Analytic flow cytometry was carried out using a FACScalibur (Becton Dickinson, Mountain View, CA) and the data processed using Cellquest software (Becton Dickinson). The following antibodies were used for phenotypic analysis of T cells: biotinylated anti-CD44 mAb (BD Pharmingen, Mountain View, CA), phycoerythrin (PE)—conjugated anti-CD4, fluorescein isothiocyanate (FITC)—conjugated anti-H57 and APC-conjugated anti-CD8 mAbs (all BD Pharmingen). For analysis of tumor cells: FITC-conjugated anti—MHC class II mAb (clone 14.4.4; ATCC catalog no. HB-32;50 ), PE-conjugated anti—B7-2 mAb and biotinylated anti—B7-1 mAb (both BD Pharmingen). Cy7-PE—conjugated streptavidin (BD Pharmingen) was used as a second step.
For single cell sorting, ex vivo tumor cells or C5-transfected LK35 tumor cells were incubated at 1 × 106 cells/50 μL in phosphate-buffered saline (PBS) 2% FCS with FITC-conjugated anti—MHC class II mAb (clone 14.4.4, as above) for 20 to 30 minutes on ice. Cells were washed, filtered, and resuspended at 5 × 106 cells/mL in IMDM 5% FCS for single cell sorting into round-bottom 96-well plates using a Cytomation MoFlo (Fort Collins, CO) equipped with a Cyclone unit.
Analysis of C5 message by RT-PCR
For generation of cDNA, ex vivo tumor cells were lysed and total RNA was extracted from the lysate in the presence of RNAse inhibitors according to the RNeasy RNA isolation kit manual (Qiagen Ltd, United Kingdom). Single strand cDNA was synthesized and amplified using the RNA PCR Core Kit (Perkin Elmer, Roche Molecular Systems). Expression of the C5 cDNA was detected by reverse transcriptase—polymerase chain reaction (RT-PCR) using C5 specific primers (forward 5′ GGGAAGGATCCACATTAAG 3′ and reverse 5′ TAATGGGATCCTGTATGGGA3′) under standard PCR conditions. This PCR is cDNA specific because the distance between binding sites of these primers precludes amplification of genomic sequences.
Functional T-cell activation tests
Single cell suspensions were made from the spleen of tumor-bearing mice or from mice with memory or effector CD4 T cells. After filtering through 70 μM cell strainers (BD Falcon, Bedford, MA), cells were cultured in 96-well U-bottom plates (BD Falcon) at 1 to 3 × 103 CD4 T cells per well. Tumor cells were added in a serial dilution, starting at 1 × 105 per well. A18 peptide was added at a final concentration of 0.1 μM. In some experiments, the loss of antigen from tumor cells was assessed using the A18 hybridoma (5 × 104 cells/well). For tumor-processing experiments exogenous C5 protein (0-5 μg/mL) or peptide (0-10 μM) was added to the cultures. Cells were cultured in 5% FCS IMDM for 48 hours at 37°C. After this time, 50 μL supernatant was used to assess T-cell activation using the IL-2—dependent cell line CTLL. CTLLs were added at 5 × 103 per well in 96-well flat-bottom plates (Costar, Corning, Corning, NY) and incubated for 24 hours, after which time proliferation was assessed by an alamar blue—based assay51 or by [3H] thymidine incorporation over 18 hours.
Intracellular staining for IFN-γ
Splenocytes or single cell suspensions of in vivo—derived tumors were cultured overnight with 50 ng/mL PdBu and 50 ng/mL ionomycin (both Sigma) or with C5 peptide-pulsed DCs. For the last 4 hours of culture 10 μg/mL brefeldin A (Sigma) also was added. For intracellular staining of IFN-γ, cells were first stained with FITC-conjugated anti-CD4 mAb (BD Pharmingen) and fixed for 20 minutes on ice in PBS 1% paraformaldehyde, followed by incubation in PBS 0.1% NP40 for 3 minutes on ice. After washing, cells were incubated with PE-conjugated anti—IFN-γ Ab or the appropriate PE-conjugated isotype control (both BD Pharmingen) for 30 minutes on ice. Cells were analyzed by flow cytometry using a FACscalibur (Becton Dickinson).
Results
Tumor model
The tumor model used in this study was LK35, a fusion product of the B-cell lymphoma A20 (from BALB/c) with H-2k splenic B cells expressing H-2k,d MHC class I and II molecules and transfected to express the model antigen C5.48 Initial experiments assessing the dose requirements of tumor cells inoculated into naive A18 TCR tg Rag–/– mice indicated that doses from 1.25 × 105 to 1 × 106 cells resulted in tumor growth starting from 15 to 30 days after inoculation (data not shown). We chose to inoculate animals subcutaneously with 5 × 105 tumor cells, which resulted in onset of tumor growth around 15 to 20 days.
Role of NK cells and naive T cells in tumor rejection
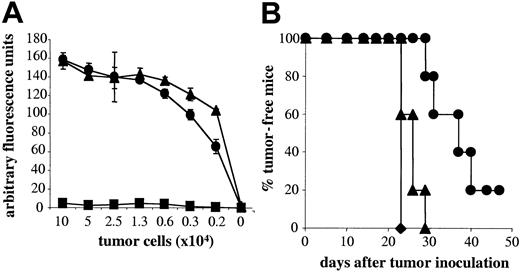
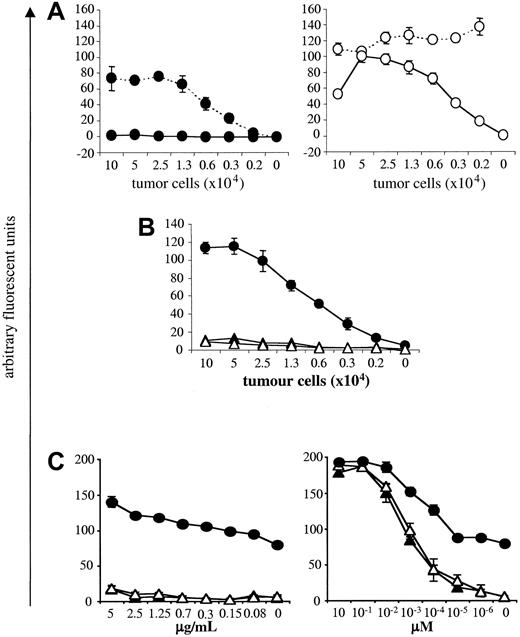
NK cells play a role in mediating tumor rejection. As naive A18 TCR tg Rag–/– mice have a large population of NK cells, we first investigated to what degree NK cells, in the absence of T cells, contribute to tumor rejection in our system. Tumor elimination was compared in gc–/–Rag–/– mice, which have neither T cells nor NK cells and Rag–/– mice, which lack T cells but have NK cells. Figure 1A shows that by day 10 after tumor inoculation, all mice lacking both T cells and NK cells developed tumors and had to be culled by day 15 due to tumor volume (Figure 1B). A small proportion of animals with NK cells remained tumor-free up to around day 20 after inoculation (Figure 1A), but on average, animals had to be culled on day 17 because their tumor volume exceeded 1 cm3 (Figure 1B). Thus, NK cells on their own do not play a significant role in mediating tumor rejection in our system. This observation allowed us to assess the effect of naive CD4 T cells on tumor growth. As seen in Figure 1A, naive CD4 T cells cannot control tumor growth significantly better than mice without T cells, although tumors in mice with naive A18 T cells took slightly longer to reach the maximum size of 1 cm3 (Figure 1B). This could be due to the fact that tumor cells that are not professional antigen-presenting cells cannot directly activate naive T cells. Figure 1C shows that in vitro, activation of naive CD4 T cells by C5-transfected tumor cells is marginal. In contrast, addition of activated DCs, which would be able to cross-present tumor cells, induced a strong response from naive T cells in vitro. It is therefore likely that cross-presentation of transfected LK35 in vivo is suboptimal and thus cannot trigger an efficient antitumor response in time to prevent outgrowth of tumor unless deliberate strategies, as detailed in the “Introduction,” are used to enhance T-cell priming.
NK cells and naive CD4 T cells are weak in mediating tumor rejection. Percentage of tumor-free mice (A) or tumor volume (B) is shown for mice with neither T cells nor NK cells (gc–/–Rag–/–; ▪), or lacking T cells but having NK cells (Rag–/–; •), or mice with naive transgenic C5-specific T cells (A18 TCR transgenic mice; ▴) following subcutaneous inoculation with 5 × 105 C5-transfected LK35 tumor cells. Symbols represent individual animals in groups of 5 mice (A) or mean data for groups of 5 mice (B). (C) IL-2 secretion by naive CD4 A18 TCR transgenic T cells after stimulation with C5-transfected LK35 tumor cells with 1 × 104 DCs (○) or without DCs (•). Mean cpm of [3H] thymidine incorporation by triplicate cultures of IL-2—dependent CTLL cells are shown.
NK cells and naive CD4 T cells are weak in mediating tumor rejection. Percentage of tumor-free mice (A) or tumor volume (B) is shown for mice with neither T cells nor NK cells (gc–/–Rag–/–; ▪), or lacking T cells but having NK cells (Rag–/–; •), or mice with naive transgenic C5-specific T cells (A18 TCR transgenic mice; ▴) following subcutaneous inoculation with 5 × 105 C5-transfected LK35 tumor cells. Symbols represent individual animals in groups of 5 mice (A) or mean data for groups of 5 mice (B). (C) IL-2 secretion by naive CD4 A18 TCR transgenic T cells after stimulation with C5-transfected LK35 tumor cells with 1 × 104 DCs (○) or without DCs (•). Mean cpm of [3H] thymidine incorporation by triplicate cultures of IL-2—dependent CTLL cells are shown.
Memory cells can be reactivated by tumor cells in vitro but fail to control the tumor in vivo
In contrast to naive T cells, memory T cells are expected to recognize antigen on a wider range of antigen-presenting cells. To assess whether CD4 memory T cells can directly recognize antigen presented by tumor cells, we first tested their capacity to be activated in vitro. Memory CD4 T cells, isolated from gc–/–Rag–/– mice that had received transfers of naive A18 and DCs at least 4 weeks before, do not contain any syngeneic APCs anymore. Therefore, activation by C5-transfected tumor cells could not be ascribed to cross-presentation but would reflect direct recognition of the tumor itself. As shown in Figure 2A, memory CD4 T cells responded to C5-transfected tumor cells to a similar degree as to tumor cells pulsed with C5 peptide, but did not respond to the untransfected parental cell line. This suggests that CD4 memory T cells have the potential to be reactivated by C5-transfected tumor cells alone, without additional signals from professional APCs.
Memory cells can be reactivated by tumor cells in vitro but do not eradicate tumors in C5–hosts without DC reactivation. (A) IL-2 secretion by memory CD4 A18 T cells after stimulation with untransfected (▪), C5-transfected (•), or C5 peptide-pulsed (▴) LK35 tumor cells. The figure shows T-cell activation as assessed by proliferation of triplicate cultures of IL-2—dependent CTLL cells using an alamar blue—based assay. (B) Percentage of tumor-free mice following inoculation with C5-transfected LK35 tumor cells (5 × 105) for groups of mice lacking T cells (•), with DC-reactivated effector CD4 T cells (•), or with memory CD4 T cells (▴). Symbols represent individual data for groups of 5 mice. All memory or effector T cells were derived from mice that had been injected with naive A18 TCR transgenic T cells and syngeneic DCs.
Memory cells can be reactivated by tumor cells in vitro but do not eradicate tumors in C5–hosts without DC reactivation. (A) IL-2 secretion by memory CD4 A18 T cells after stimulation with untransfected (▪), C5-transfected (•), or C5 peptide-pulsed (▴) LK35 tumor cells. The figure shows T-cell activation as assessed by proliferation of triplicate cultures of IL-2—dependent CTLL cells using an alamar blue—based assay. (B) Percentage of tumor-free mice following inoculation with C5-transfected LK35 tumor cells (5 × 105) for groups of mice lacking T cells (•), with DC-reactivated effector CD4 T cells (•), or with memory CD4 T cells (▴). Symbols represent individual data for groups of 5 mice. All memory or effector T cells were derived from mice that had been injected with naive A18 TCR transgenic T cells and syngeneic DCs.
To address the capacity of resting memory CD4 T cells to be reactivated directly by tumor cells in vivo, mice with memory CD4 T cells were inoculated subcutaneously with 5 × 105 tumor cells. In parallel, tumor cells also were injected into mice containing CD4 T cells in effector stage, created by reinjection of antigen-pulsed DCs into mice containing memory cells. As shown in Figure 2B, mice with resting memory CD4 T cells were not effective in controlling tumor growth and developed tumors almost as fast as animals lacking T cells. In contrast, memory CD4 T cells reactivated to effector cells could control tumor growth for up to 50 days. This suggests that in vivo, direct activation of resting CD4 memory T cells by tumor cells is not sufficient to control tumor growth. However, effector cells, despite their ability to control tumor growth for longer time periods than resting memory cells, were nevertheless unable to control tumor growth indefinitely.
Do tumor cells lose antigenicity?
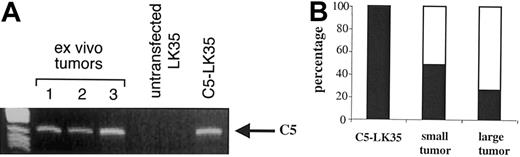
One reason for the failure of tumor eradication could be escape of tumor cells that no longer expressed recognizable C5 so that they would be ignored by the CD4 T cells. RT-PCR analysis for C5 expression established that ex vivo tumors were still positive for C5 as assessed by this method (Figure 3A). However, RT-PCR does not allow quantitative conclusions on the single cell level. For this reason the ex vivo tumors were also sorted as single cells and then assessed for their ability to activate the C5-specific A18 hybridoma as a readout of physiologically relevant antigen expression. All single cell clones (99%) from C5-transfected LK35 tumor cells maintained in vitro activated the C5-specific hybridoma (Figure 3B). However, only 26% to 50% of ex vivo tumors isolated from effector mice 35 days after inoculation expressed physiologically recognizable antigen, depending on tumor size. This suggests that immune pressure resulted in the loss of tumor antigenicity.
Loss of antigen-expressing tumor cells. (A) RT-PCR for C5 in ex vivo tumors (lanes 1-3) untransfected LK35, and C5-transfected LK35 tumor cells cultured under selection in vitro. (B) Percentage of antigen-positive (▪) and -negative (□) single cell clones from C5-transfected LK35 tumor cells maintained in vitro and ex vivo tumors (small and large). Single cell clones were assessed for their ability to activate the C5-specific A18 hybridoma to IL-2 secretion, measured by proliferation of IL-2—dependent CTLL cells using an alamar blue—based assay.
Loss of antigen-expressing tumor cells. (A) RT-PCR for C5 in ex vivo tumors (lanes 1-3) untransfected LK35, and C5-transfected LK35 tumor cells cultured under selection in vitro. (B) Percentage of antigen-positive (▪) and -negative (□) single cell clones from C5-transfected LK35 tumor cells maintained in vitro and ex vivo tumors (small and large). Single cell clones were assessed for their ability to activate the C5-specific A18 hybridoma to IL-2 secretion, measured by proliferation of IL-2—dependent CTLL cells using an alamar blue—based assay.
High antigen levels in C5+ hosts improve control of tumor growth by memory T cells
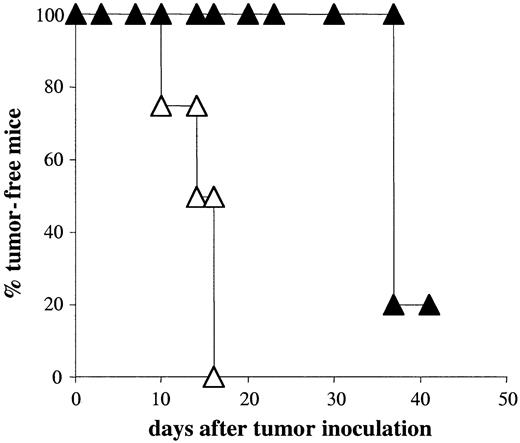
To circumvent loss of antigen by tumor cells, we used adoptive hosts with endogenous C5 expression to generate memory T cells. These mice contain a high dose of C5 (50 μg/mL) in their circulation that is efficiently internalized, processed, and presented by LK35 tumor cells.48 Endogenous C5 is only presented to A18 T cells by DCs during the first 3 weeks after adoptive transfer when syngeneic, coinjected DCs are still present. Host allogeneic APCs cannot present circulating C5, and therefore the only source of T-cell activation in hosts containing resting A18 memory T cells could be tumor cells. C5+ gc–/–Rag–/– hosts containing resting memory CD4 T cells but no DCs that could present C5 were injected subcutaneously with 5 × 105 tumor cells. As shown in Figure 4, memory CD4 T cells were now able to control tumor growth up to 3 weeks longer than naive CD4 T cells. This suggests that memory CD4 T cells in mice containing a higher dose of antigen accessible to the tumor are able to recognize the tumor directly and delay its growth. However, these mice eventually succumbed to tumor growth around the same time as mice containing effector T cells in C5-negative hosts. This could not be explained by loss of transfected C5 expression, since all tumor cells would have had access to endogenous C5 protein.
Memory CD4 T cells can delay tumor growth in C5+ hosts. Percentage of tumor-free mice following inoculation of naive A18 TCR transgenic mice (▵) and C5+ mice with memory CD4 T cells (▴) with 5 × 105 C5-transfected LK35 tumor cells. Symbols represent individual data for groups of 5 mice. Memory T cells were derived from mice that had been injected with naive A18 TCR transgenic T cells and syngeneic DCs.
Memory CD4 T cells can delay tumor growth in C5+ hosts. Percentage of tumor-free mice following inoculation of naive A18 TCR transgenic mice (▵) and C5+ mice with memory CD4 T cells (▴) with 5 × 105 C5-transfected LK35 tumor cells. Symbols represent individual data for groups of 5 mice. Memory T cells were derived from mice that had been injected with naive A18 TCR transgenic T cells and syngeneic DCs.
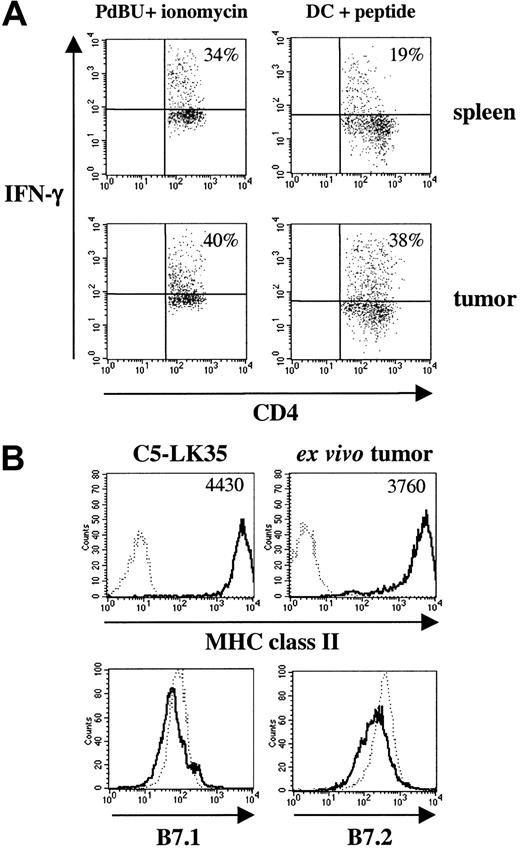
To understand why the tumor suddenly and rapidly grows after being controlled for this period of time, it was of interest to address problems that are often associated with poor tumor eradication in vivo. These include 1) defective lymphocyte homing to the site of the tumor, 2) decreased or loss of expression of MHC molecules on the tumor cells, and 3) absence or loss of costimulatory molecules on the tumor cells. Figure 5A shows representative analysis of spleen (top panels) and tumor (bottom panels) taken at day 39 from tumor-bearing mice and clearly indicates that CD4 T cells can be detected both within the spleen and tumor of these mice, suggesting that there is no defect in lymphocyte homing to the tumor site. In addition, these cells are able to produce IFN-γ, a cytokine associated with tumor eradication by CD4 T cells in vivo,9,18 when restimulated with PdBU and ionomycin (top and bottom left panels). Restimulation with DCs and peptide stimulation showed a lower proportion of IFN-γ—producing cells in the spleen (top right panel), whereas a similar proportion of cells present in the tumor as in PdBU-stimulated cells produced IFN-γ (bottom right panel). Furthermore, levels of MHC class II molecules and costimulatory molecules (B7.1 and B7.2) on the surface of ex vivo tumor cells were comparable to the original tumor cells (Figure 5B). Therefore, loss or decreased expression of these molecules is also not an explanation for the failure of CD4 T cells to control tumor growth.
Assessment of lymphocyte homing or IFN-γ production by memory CD4 T cells from tumor-bearing mice. (A) Spleen cells (top row) and single cell suspensions of in vivo growing tumors (bottom row) were stained with anti—CD4-FITC and intracellularly with anti—IFN-γ-PE. Cells were restimulated with PdBu and ionomycin (left panels) or DCs and C5 peptide (right panels) overnight. Brefeldin A was added for the last 4 hours of culture. The dot plots show staining on gated CD4 T cells. Numbers refer to percentages of IFN-γ—positive cells. (B) MHC class II and costimulatory molecule expression on tumor cells. C5-transfected LK35 tumor cells or ex vivo tumor cells were stained with anti—MHC class II—FITC (clone 14.4.4; top row). Background staining is shown with a dotted line; mean fluorescence values are shown in upper righthand corners. C5-transfected LK35 tumor cells (...) and ex vivo tumor cells (—) also were stained with anti—B7.1-PE or anti—B7.2-PE (bottom row). Memory T cells were derived from mice that had been injected with naive A18 TCR transgenic T cells and syngeneic DCs.
Assessment of lymphocyte homing or IFN-γ production by memory CD4 T cells from tumor-bearing mice. (A) Spleen cells (top row) and single cell suspensions of in vivo growing tumors (bottom row) were stained with anti—CD4-FITC and intracellularly with anti—IFN-γ-PE. Cells were restimulated with PdBu and ionomycin (left panels) or DCs and C5 peptide (right panels) overnight. Brefeldin A was added for the last 4 hours of culture. The dot plots show staining on gated CD4 T cells. Numbers refer to percentages of IFN-γ—positive cells. (B) MHC class II and costimulatory molecule expression on tumor cells. C5-transfected LK35 tumor cells or ex vivo tumor cells were stained with anti—MHC class II—FITC (clone 14.4.4; top row). Background staining is shown with a dotted line; mean fluorescence values are shown in upper righthand corners. C5-transfected LK35 tumor cells (...) and ex vivo tumor cells (—) also were stained with anti—B7.1-PE or anti—B7.2-PE (bottom row). Memory T cells were derived from mice that had been injected with naive A18 TCR transgenic T cells and syngeneic DCs.
Mutual deactivation of memory/effector T cells and tumor cells
The reduced proportion of IFN-γ producing memory cells in the spleens of mice with growing tumors suggested a possible impairment of their immune reactivity. Indeed, CD4 T cells isolated from spleens of these mice display decreased responsiveness to stimulation in vitro. In response to both the in vitro maintained tumor cell line (Figure 6A left) and even peptide-pulsed tumor cells (Figure 6A right), CD4 T cells from tumor-bearing mice were unable to respond to the same degree as memory CD4 T cells isolated from mice that had not been given tumor cells. This suggests that interaction of these memory cells with the tumor had inactivated them in some way. However, the tumor itself contained a high proportion of CD4 T cells that appeared to be capable of IFN-γ production not only after recall with PdBU and ionomycin, which would not reflect their physiologic activation state, but also after antigenic stimulation. Yet, rapid tumor growth at this stage suggested that this activation was not taking place in vivo. In addition, it was curious that ex vivo tumors were no longer able to stimulate memory cells (Figure 6B), even though they should be capable of internalizing, processing, and presenting endogenous C5 present in the circulation of the host. When we examined ex vivo tumor cells for their capacity to process and present C5 protein to A18 hybridoma cells in vitro, it became clear that tumor cells from mice with memory CD4 T cells had lost the capacity to present C5 protein (Figure 6C left), whereas they still presented C5 peptide (Figure 6C right). On the other hand, tumor cells isolated from mice without T cells retained their processing and presentation capacity. This strongly suggests that CD4 T cells recognizing tumors in vivo had exerted pressure for tumor escape by virtue of loss of some crucial step in the processing of C5 protein.
Mutual deactivation of memory/effector T cells and tumor cells. (A) Memory cells from tumor-bearing mice are desensitized to activation in vitro: IL-2 secretion by memory CD4 A18 T cells from tumor-bearing mice (—) or from mice not inoculated with tumor (- - -) following stimulation with C5-transfected LK35 tumor cells (left panel; •) or C5 peptide-pulsed tumor cells (right panel; ○). (B) Ex vivo tumor cells from mice with T cells cannot stimulate T-cell responses in vitro: IL-2 secretion by A18 T cells (memory T cells isolated from mice not inoculated with tumor) following stimulation with: ex vivo tumors (▴ and ▵ representing tumor cells from 2 different donors), compared with C5-transfected LK35 tumor cells cultured in vitro (•). (C) Ex vivo tumor cells fail to process C5 protein: IL-2 secretion by the C5-specific A18 hybridoma following stimulation with ex vivo tumors, from mice with T cells (▴ and ▵ from 2 different mice) or lacking T cells (•). Exogenous C5 protein (left panel) or peptide (right panel) was added to the cultures to assess the processing capacity of the tumor cells. All figures show T-cell activation as assessed by proliferation of triplicate cultures of IL-2—dependent CTLL cells using an alamar blue—based assay. Tumors were removed at day 34 after inoculation. All memory or effector cells were derived from mice that had been injected with naive A18 TCR transgenic T cells and syngeneic DCs.
Mutual deactivation of memory/effector T cells and tumor cells. (A) Memory cells from tumor-bearing mice are desensitized to activation in vitro: IL-2 secretion by memory CD4 A18 T cells from tumor-bearing mice (—) or from mice not inoculated with tumor (- - -) following stimulation with C5-transfected LK35 tumor cells (left panel; •) or C5 peptide-pulsed tumor cells (right panel; ○). (B) Ex vivo tumor cells from mice with T cells cannot stimulate T-cell responses in vitro: IL-2 secretion by A18 T cells (memory T cells isolated from mice not inoculated with tumor) following stimulation with: ex vivo tumors (▴ and ▵ representing tumor cells from 2 different donors), compared with C5-transfected LK35 tumor cells cultured in vitro (•). (C) Ex vivo tumor cells fail to process C5 protein: IL-2 secretion by the C5-specific A18 hybridoma following stimulation with ex vivo tumors, from mice with T cells (▴ and ▵ from 2 different mice) or lacking T cells (•). Exogenous C5 protein (left panel) or peptide (right panel) was added to the cultures to assess the processing capacity of the tumor cells. All figures show T-cell activation as assessed by proliferation of triplicate cultures of IL-2—dependent CTLL cells using an alamar blue—based assay. Tumors were removed at day 34 after inoculation. All memory or effector cells were derived from mice that had been injected with naive A18 TCR transgenic T cells and syngeneic DCs.
Discussion
In this study we have focused on the ability of T cells at different stages of activation to recognize and control a B-cell lymphoma in order to determine under what circumstances T cells on their own can prevent re-emergence of tumors. Successful initiation of antitumor immune responses by a number of manipulations, such as chemokine gene transfer,52 cell-based53 and immunostimulatory (ISS) DNA-based vaccines,54,55 DC-based immunotherapy,36,38,56, 57, 58, 59, 60 and immunomodulatory drugs61 strongly harnesses the help of the innate immune system. In agreement with other studies,62,63 we found that naive T cells were not able to control the tumor on their own and required specific activation by professional APCs such as DCs. Single cell suspensions of tumor cells can reach secondary lymphoid organs and induce an immune response, whereas solid peripheral tumors are immunologically ignored.64 Although evidence in vivo and in vitro suggests that the LK35 tumor cells reach lymphoid organs and the model tumor antigen is cross-presented by DCs, this event may not be optimized to allow efficient and timely activation of naive T cells.
However, even if a tumor is seemingly eradicated by an effective primary immune response, this does not necessarily guarantee its long-term control. Tumor cells may re-emerge without alerting the innate immune system so that the only line of defense would be resting memory T cells differentiated from the original antitumor response. We mimicked this scenario by a reductionist strategy to assess the role of memory/effector CD4 T cells on their own without involvement of CD8 T cells or B cells and minimal involvement of the innate immune system and asked the question whether these cells, once differentiated to effector or memory cells, can be relied upon to prevent re-emergence of a tumor. The results clearly indicate that while memory and reactivated effector cells offer partial protection in the absence of the innate immune system, it is not strong enough to prevent eventual outgrowth of tumor. On the one hand, antigen dose seems to be an important variable, and in our system the dose of antigen achieved by transfection of the tumor was clearly suboptimal for in vivo recognition, although in vitro activation could be demonstrated easily. In addition, loss of antigen in vivo by immune selection would limit immune recognition. This might in fact mimic the situation encountered when very few re-emerging tumor cells are present. Loss of antigen expression following induction of an immune response is a well-known complication of tumor immune surveillance65 but could be bypassed in our system because of the use of adoptive hosts that expressed high concentrations of the model antigen C5 in their blood circulation. Clearly, the increased dose of antigen made a difference, because CD4 memory cells could control the tumor in C5+ mice but not C5– mice at least for a period of 30-40 days. Since these mice would not have contained any syngeneic DCs anymore, their activation could only have been effected by the tumor cells themselves. In C5– mice, however, a boost with DCs was necessary to drive resting memory cells to effector cells before partial control of the tumor could be achieved.
While these data show that, given sufficient amounts of antigen, tumor cells can indeed reactivate resting memory cells, neither these, nor effector cells generated by DC activation, could maintain protection against tumor growth in the long term. The development of anergy in T cells recognizing tumors has been well documented,66, 67, 68, 69, 70, 71 but it has not been shown previously that this would apply also to T cells that had differentiated to memory cells following optimal activation by professional APCs. Thus, tumor cells seem to be capable of paralyzing T-cell responses at any stage of their differentiation. Surprisingly, the negative regulation seemed to be reciprocal because at a certain stage tumors grew although they contained T cells with the capacity to secrete IFN-γ. However, the defect at the level of protein processing detected by in vitro analysis would have prevented antigen recognition and thereby reactivation for IFN-γ secretion by these T cells in vivo. The mechanisms responsible for induction of T-cell anergy as well as the block in protein processing by the tumor cells have not been the focus of our study, but cytokines such as IL-10 that have been shown to interfere with antigen processing by down-regulating endo/lysosomal proteases72 are likely candidates.
Our data indicate that T cells on their own would not be sufficient to protect against the emergence of tumors, even if they become activated and manage to delay their growth. It remains to be determined whether harnessing the innate immune system with all its components may, in addition to its role in facilitating initiation of antitumor responses, also prevent the mutual deactivation of T cells and tumor cells.
Prepublished online as Blood First Edition Paper, January 23, 2003; DOI 10.1182/blood-2002-10-3030.
Supported by grant no. 0040 from the Leukaemia Research Fund, United Kingdom.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank Anne O'Garra for critical comments on the manuscript and Trisha Norton, Rachel Simmons, and Keith Williams for maintaining the mouse colony. We also thank J. Disanto for the Rag–/–gc–/– mice.

![Figure 1. NK cells and naive CD4 T cells are weak in mediating tumor rejection. Percentage of tumor-free mice (A) or tumor volume (B) is shown for mice with neither T cells nor NK cells (gc–/–Rag–/–; ▪), or lacking T cells but having NK cells (Rag–/–; •), or mice with naive transgenic C5-specific T cells (A18 TCR transgenic mice; ▴) following subcutaneous inoculation with 5 × 105 C5-transfected LK35 tumor cells. Symbols represent individual animals in groups of 5 mice (A) or mean data for groups of 5 mice (B). (C) IL-2 secretion by naive CD4 A18 TCR transgenic T cells after stimulation with C5-transfected LK35 tumor cells with 1 × 104 DCs (○) or without DCs (•). Mean cpm of [3H] thymidine incorporation by triplicate cultures of IL-2—dependent CTLL cells are shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/11/10.1182_blood-2002-10-3030/5/m_h81134387001.jpeg?Expires=1765930091&Signature=ZcW1KEvbPU453sesqCLUPAMQAA8XeA7KjNacG6LyENrkHzVUsk05mRB-LYcw5veRwzHCzX51hUDf2nWLDnTHdB4bdljdpr9owu4ju9kDpQjAVTJDzxVqK5mpQlmWEPeMxk2fLT8olRskwHT4~9DNDHKvAc99rUodDAAiV85pt9jWWl99-SpfSh~OdGD4jfg6nJvpaXrPNnhyoern5mSUt3irGmR4WZrvb9ycKMK6SD9DhXByF1apICu343a7EQ6I98uAZN70z5nlU2s0pApBnK64mcVsQkkENsuUUdSy10evMcpQPUfydeF9pXl6kyyVmpX9iYWPcjql5y7xSo2SBw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal