Abstract
Therapy of B-cell chronic lymphocytic leukemia (CLL) is currently palliative, emphasizing the need for identification of new therapies for this disease. KRN5500 is a novel agent that has a unique sensitivity pattern in the National Cancer Institute cell line screening panel, suggesting a unique mechanism of action. To assess its in vitro activity in CLL, we exposed peripheral mononuclear cells from CLL patients (n = 11) to varying concentrations of this agent. Viability of the CLL cells was reduced by 50% (LC50) at 4 hours, 24 hours, and 4 days at KRN5500 concentrations of 2.50 μM, 0.276 μM, and 0.139 μM, respectively. KRN5500 induced cellular injury via caspase-dependent apoptosis involving the intrinsic mitochondrial (caspase-9) initiating caspase and caspase-3 effector caspase; however, expression of the antiapoptotic mitochondrial membrane protein Bcl-2 was unaffected. These data demonstrate KRN5500 has significant in vitro activity against human CLL cells, thus providing support for introduction of this agent into clinical trials for patients with CLL.
Introduction
B-cell chronic lymphocytic leukemia (CLL) is the most common leukemia in the Western hemiphere.1 Outcome for advanced-stage CLL patients is poor, with an expected median survival of 3 years.2 Treatment of patients with alkylator or fludarabine therapy is not generally considered until the development of symptoms or cytopenias of advanced-stage disease.3 Neither of these therapies has been shown to be curative or to prolong survival over best supportive care interventions. Recent studies have demonstrated clinical activity with the monoclonal antibodies rituximab4,5 and Campath-1H6,7 in CLL. These therapies are not curative, thus supporting continued preclinical studies to identify new, structurally distinct agents for clinical investigation of CLL treatment.
One such agent is KRN5500, a synthetic derivative of the spicamycin class of compounds that were initially isolated from Streptomyces alanosinicus 879-MT3. The structure of this class of agents includes a variant purine nucleoside with the purine joined to a sugar unit via its amino group, forming the backbone spicamycin amino-nucleoside (SAN) joined to a glycine amino acid (SAN-gly).8 The different spicamycin compounds vary by the composition of the fatty acid chain.8,9 A variety of studies have demonstrated that KRN5500 is the most optimal structural analog of those examined and that deletion of the glycine residue or the fatty acid chain greatly compromises the activity of this agent.10, 11, 12 The fatty acid allows effective diffusion of KRN5500 into the cell where it is metabolized by a yet to be identified enzyme to the biologically active SAN-gly subunit. Indeed, cancer cell lines that are resistant to KRN5500 have adequate diffusion of KRN5500 into the cell but do not covert the prodrug to the active form.13 In the National Cancer Institute (NCI) in vitro drug screening, KRN5500 demonstrated variable activity, which ranged from marked sensitivity to leukemia (HL60TB), lymphoma (SR), colon (Colo 205), glioma (U251), ovarian (OVCAR-5), and renal (786-0) carcinoma cell lines, to zero activity in 17 cell lines at concentrations of drug exceeding 10 μM.13 Most importantly, this pattern of cytotoxicity is unique relative to all other compounds tested by the NCI, suggesting this agent has a novel mechanism of action.13 Several studies have demonstrated in vivo activity of KRN5500 in animals11,13, 14, 15 with acceptable toxicity, thus prompting investigation of this novel agent in phase 1 clinical trials.16,17
Extending these findings to CLL, we describe a preclinical evaluation of KRN5500 that suggests it has promise for future clinical trials in this disease.
Patients, materials, and methods
Patients, cell separation, and culture conditions
Approval for patient blood collection was obtained from The Ohio State University's institutional review board for these studies. Informed consent was provided according to the Declaration of Helsinki. Cells were procured from patients previously diagnosed with CLL as defined by the modified NCI criteria.18 All of the CLL patients had been without prior therapy for a minimum of 2 months. Clinical data provided in Table 1 include modified Rai stage, previous treatment, and presence of active disease, at the time of cell acquisition. Patients were considered to have active disease if they required initiation of therapy within 2 months of donating cells.
Patient characteristics and in vitro sensitivity of B-cell chronic lymphocytic leukemia cells to KRN5500
Patient . | Modified Rai stage . | Previous treatment . | 4-hour LC50 KRN5500, μM . | 24-hour LC50 KRN5500, μM . | 96-hour LC50 KRN5500, μM . |
|---|---|---|---|---|---|
| 1 | IR | None | 22.46 | 1.89 | 0.44 |
| 2 | HR | C, C + Pent + Theo | 0.21 | 0.91 | 0.09 |
| 3 | HR | Flu | 0.16 | 0.02 | 0.027 |
| 4 | HR | None | 1.12 | 0.22 | 0.26 |
| 5 | HR | C + P, Flu, 2CDA | 0.18 | 0.05 | 0.05 |
| 6 | IR | C + P | 0.45 | 0.13 | 0.123 |
| 7 | IR | None | 0.94 | 0.24 | 0.175 |
| 8 | IR | None* | 0.14 | 0.05 | 0.024 |
| 9 | IR | None* | 0.19 | 0.07 | 0.052 |
| 10 | IR | None* | 0.11 | 0.028 | 0.024 |
| 11 | IR | None | 1.55 | 0.36 | 0.260 |
Patient . | Modified Rai stage . | Previous treatment . | 4-hour LC50 KRN5500, μM . | 24-hour LC50 KRN5500, μM . | 96-hour LC50 KRN5500, μM . |
|---|---|---|---|---|---|
| 1 | IR | None | 22.46 | 1.89 | 0.44 |
| 2 | HR | C, C + Pent + Theo | 0.21 | 0.91 | 0.09 |
| 3 | HR | Flu | 0.16 | 0.02 | 0.027 |
| 4 | HR | None | 1.12 | 0.22 | 0.26 |
| 5 | HR | C + P, Flu, 2CDA | 0.18 | 0.05 | 0.05 |
| 6 | IR | C + P | 0.45 | 0.13 | 0.123 |
| 7 | IR | None | 0.94 | 0.24 | 0.175 |
| 8 | IR | None* | 0.14 | 0.05 | 0.024 |
| 9 | IR | None* | 0.19 | 0.07 | 0.052 |
| 10 | IR | None* | 0.11 | 0.028 | 0.024 |
| 11 | IR | None | 1.55 | 0.36 | 0.260 |
IR indicates intermediate risk; HR, high risk; C, chlorambucil; Pent, pentostatin; Theo, theophylline; Flu, fludarabine; P, prednisone; 2CDA, cladribine.
Active disease as required treatment within 6 months of donating cells.
CLL cells and normal mononuclear cells were isolated immediately following donation using Ficoll density gradient centrifugation (Ficoll-Paque Plus, Pharmacia Biotech, Piscataway, NJ). Isolated mononuclear cells were incubated (37°C and 5% CO2) in RPMI 1640 media supplemented with 10% heat-inactivated fetal bovine serum (FBS) (HyClone Laboratories, Logan, UT), 2 mM L-glutamine (Invitrogen, Carlsbad, CA), and penicillin (100 U/mL) and streptomycin (100 μg/mL) (Sigma-Aldrich, St Louis, MO). As noted in “Results,” normal B cells and T cells from healthy volunteers were isolated using CD19 or CD3 microbeads and magnetic-activated cell separation (MACS) columns according to the manufacturer's instructions (Miltenyi Biotec, Auburn, CA). The broad caspase inhibitor z-VAD-fmk was obtained from Calbiochem, San Diego, CA. KRN5500 was obtained from the Developmental Therapeutics Program, Division of Cancer Treatment, National Cancer Institute.
Blood viability and apoptosis assays
Viability assays of isolated mononuclear cells from CLL patients and normal B cells and T cells were performed utilizing the MTT assay as previously described by our group.19 Cell death was measured relative to cells incubated in media. The annexin V–propidium iodide (PI) assay was also performed as previously described by our group.19 Assessment of caspase-3 activation using a phycoerythrin (PE)–labeled antiactive caspase-3 polyclonal antibody (BD Pharmingen, San Diego, CA) was performed using the manufacturer's instructions. Rhodamine-123 was used to monitor the integrity of mitochondria following treatment with KRN5500. Media and KRN5500-treated cells were washed once in RPMI 1640 media and then incubated in RPMI 1640 media containing 50 ng/mL rhodamine-123 (Molecular Probes, Eugene, OR) for 30 minutes at 37°C. Stained cells were washed once in RPMI 1640 media, placed on ice, and then quickly analyzed by flow cytometry (Becton Dickinson, San Jose, CA).
Protein extraction and Western blot analysis
Caspase-8, capase-9, poly(ADP-ribose) polymerase (PARP), and Bcl-2 protein expression was analyzed by immunoblot after incubation either in medium or in 2 concentrations of KRN5500 (0.134 μM and 1.34 μM) for 24 hours. Western blot analysis of whole cell lysates was done as previously described.19 Primary antibodies included caspase-8 (monoclonal antibody 3-1-9; BD Pharmingen), caspase-9 (rabbit polyclonal antibody Ab-1; Oncogene Research Products, San Diego, CA), Bcl-2 (Dako, San Diego, CA), and PARP (monoclonal antibody C-2-10; Oncogene Research Products). Following antibody incubation, the blots were detected with chemilu-minescent substrate (SuperSignal; Pierce, Rockford, IL). Gel loading equivalence was confirmed by reprobing with monoclonal antihuman glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Chemicon, Temecula, CA). Protein bands were quantified by digital analysis of the chemiluminescence signals by ChemiDoc (BioRad, Hercules, CA).
Results
KRN5500 produces cytotoxicity in human CLL cells
Peripheral mononuclear cells from 11 patients with CLL were exposed to varying (0.01, 0.033, 0.1, 0.33, 1, 3.3, 10, 33, and 100 μM) concentrations of KRN5500. The clinical features of these patients are summarized in Table 1. Cells were incubated as follows: 4 hours and then MTT reduction immediately assessed; 4 hours and then incubated in fresh medium without drug for an additional 92 hours; 24 hours and then MTT reduction immediately assessed; 24 hours and then incubated in fresh medium without drug for an additional 72 hours; and, finally, MTT reduction assessed after 96 hours of continuous incubation with drug. All of the patients with CLL demonstrated in vitro response to KRN5500, although there was significant variability in response from patient to patient as summarized in Table 1. The mean concentration of KRN5500 required to produce 50% cytotoxicity (LC50) after 4 hours of agent exposure followed by incubation in fresh medium until 96 hours was 2.5 μM (median, 0.209; range, 0.106-22.47; 95% confidence interval (CI), ± 3.93). In contrast, the 24 hours of drug incubation followed by incubation in fresh medium until 96 hours and the 96 hours of continuous exposure to KRN5500 had an LC50 of 0.287 μM (median, 0.091; range, 0.023-1.88; 95% CI, ± 0.329) and 0.139 μM (median, 0.09; range, 0.024-0.443; 95% CI, ± 0.0793), respectively. Examination of the sensitivity of the human CLL cells relative to the time of exposure to KRN5500 demonstrated no significant advantage to exposure beyond 4 hours for most patients. However, the LC50 concentration did decrease substantially in the 3 patients (1, 4, and 11) with initial LC50 values above 1.0 μM. These data suggest that the ideal time of KRN5500 administration in CLL is 4 to 24 hours.
KRN5500 demonstrates less cytotoxicity to normal T cells and B cells
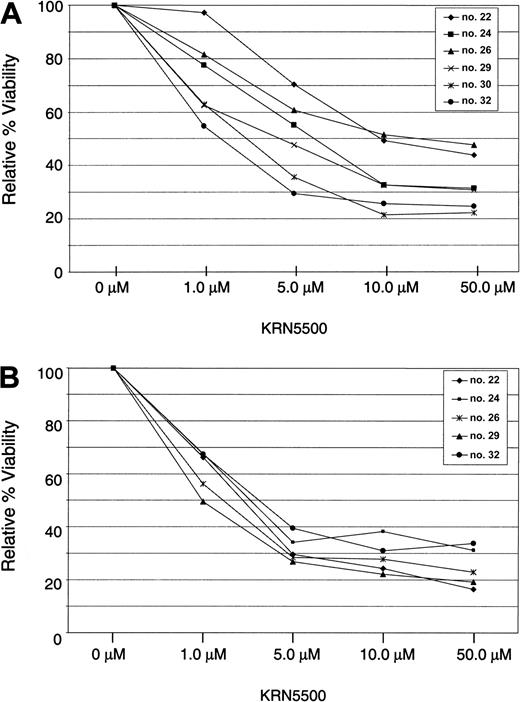
Fludarabine and Campath-1H are both approved for the treatment of CLL but produce significant cellular immune suppression. To assess the impact of KRN5500 on normal T cells and B cells, these were isolated from healthy volunteers and incubated in KRN5500 for 4 hours followed by incubation in fresh medium until 96 hours. As shown in Figure 1, the viability of both normal T cells and B cells exceeded 50% of the media control in all patients at the 1 μM or less concentration. In contrast, the median LC50 for the CLL patients studied was 0.209 μM, suggesting KRN5500 demonstrates some selectivity for the malignant B cell.
KRN5500 is less cytotoxic toward normal T cells and B cells. (A) T cells were selected from normal peripheral blood mononuclear cells using CD3 MACS beads. (B) B cells were selected from normal peripheral blood mononuclear cells using CD19 MACS beads. In both panels, 1 × 106 cells were incubated in each of 4 wells for each drug concentration. Plates were incubated for 4 hours, and cells were then washed and replated in fresh media without drug for an additional 92 hours. MTT reagent was then added, and after a further 24-hour incubation plates were processed and analyzed. Viability is expressed as percent of media control.
KRN5500 is less cytotoxic toward normal T cells and B cells. (A) T cells were selected from normal peripheral blood mononuclear cells using CD3 MACS beads. (B) B cells were selected from normal peripheral blood mononuclear cells using CD19 MACS beads. In both panels, 1 × 106 cells were incubated in each of 4 wells for each drug concentration. Plates were incubated for 4 hours, and cells were then washed and replated in fresh media without drug for an additional 92 hours. MTT reagent was then added, and after a further 24-hour incubation plates were processed and analyzed. Viability is expressed as percent of media control.
In an attempt to determine if the cytotoxicity induced by KRN5500 was due to an increase in apoptosis, mononuclear cells from 5 CLL patients were incubated in medium alone or 0.134 μM or 1.34 μM KRN5500 for 24 hours. At this point, cleavage of caspase-3 and PARP, which serves as a substrate for this activated effector caspase, was assessed. Figure 2A demonstrates one such representative patient demonstrating a dose-dependent increase in active 29 kDa heterodimer of caspase-3 as assessed by flow cytometry at 0.134 μM and 1.34 μM concentration of KRN5500. Utilizing annexin V–PI staining, we demonstrated similar findings at the KRN5500 concentrations of 0.134 μM or 1.34 μM (data not shown), but apoptosis was completely abrogated by addition of 100 μM of the pan-caspase inhibitor z-VAD-fmk (data not shown). Figure 2B demonstrates the appearance of the 85 kDa cleaved product of PARP that is typically observed in the setting of caspase-mediated apoptosis. These data support the conclusion that KRN5500 is inducing cytotoxicity at least in part through the pathway of caspase-dependent apoptosis.
KRN5500 induces caspase-3 activation and cleavage of PARP. (A) KRN5500 induces caspase-3 activation in human CLL cells in vitro. CLL cells were exposed to 24 hours of media (i) or KRN5500 (0.134 [ii] and 1.34 μM [iii]) and then examined for the presence of the active 29 kDa heterodimer of effector caspase-3. An increase in fluorescence is observed with KRN5500 exposure at 24 hours that is indicative of caspase-3 activation. (B) KRN5500 induces cleavage of PARP in human CLL cells in vitro. CLL cells were exposed to 24 hours of media or KRN5500 (0.134 and 1.34 μM) followed by immunoblotting for PARP. A dose-dependent increase in the cleaved p85 fragment of PARP is observed following 24 hours of exposure of KRN5500.
KRN5500 induces caspase-3 activation and cleavage of PARP. (A) KRN5500 induces caspase-3 activation in human CLL cells in vitro. CLL cells were exposed to 24 hours of media (i) or KRN5500 (0.134 [ii] and 1.34 μM [iii]) and then examined for the presence of the active 29 kDa heterodimer of effector caspase-3. An increase in fluorescence is observed with KRN5500 exposure at 24 hours that is indicative of caspase-3 activation. (B) KRN5500 induces cleavage of PARP in human CLL cells in vitro. CLL cells were exposed to 24 hours of media or KRN5500 (0.134 and 1.34 μM) followed by immunoblotting for PARP. A dose-dependent increase in the cleaved p85 fragment of PARP is observed following 24 hours of exposure of KRN5500.
KRN5500 induces activation of the intrinsic pathway of apoptosis
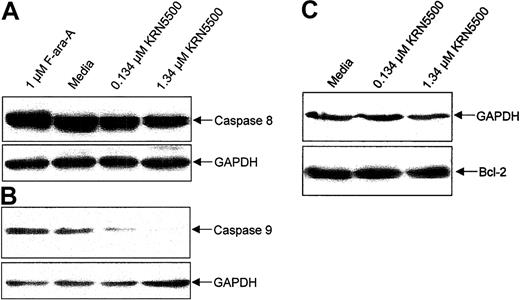
Caspase-3–mediated apoptosis can occur both through activation of the tumor necrosis receptor family members via caspase-8 cleavage (extrinsic pathway) or through the mitochondria (intrinsic pathway) of apoptosis that involves activation of caspase-9. We sought to determine which pathway of apoptosis was activated by KRN5500. CLL cells from 5 patients were incubated with 0.134 μM or 1.34 μM KRN5500 or medium for 24 hours and examined for processing of caspase-8 and caspase-9. Figure 3A demonstrates that KRN5500 induces processing of caspase-9, as seen by the reduction in the 46 kDa proform of the enzyme relative to the housekeeping protein GAPDH. We noted no significant reduction in the 58 kDa proform of caspase-8 in these same cells. Because loss of mitochondria membrane potential heralds this occurrence, we examined this and demonstrate appropriate loss as demonstrated in Figure 4 at the time processing of the caspase-9 proform is noted. These data suggest KRN5500 utilizes the intrinsic pathway of apoptosis to promote cell death of CLL cells.
KRN5500 exposure to CLL cells in vitro causes caspase-dependent apoptosis via the intrinsic but not the extrinsic pathway of apoptosis and no change in Bcl-2 expression. (A) Expression of caspase-9 zymogen protein (48 kDa) in human CLL cells at 24 hours following incubation with medium or KRN5500 (0.134 or 1.34 μM) demonstrating processing of caspase-9 (and, thus, decrease in 45 kDa band), a finding supportive of activation of the intrinsic pathway of apoptosis. (B) Expression of caspase-8 zymogen protein (55 kDa) in human CLL cells at 24 hours following incubation with medium or KRN5500 (0.134 or 1.34 μM) demonstrating no processing of procaspase-8 (and, thus, no decrease in 55 kDa band), a finding supportive of KRN5500 apoptosis occurring independent of the extrinsic pathway of apoptosis. (C) Expression of the Bcl-2 protein in human CLL cells at 24 hours following incubation with KRN5500 (0.134 or 1.34 μM) demonstrating no change in Bcl-2 expression following treatment with this agent.
KRN5500 exposure to CLL cells in vitro causes caspase-dependent apoptosis via the intrinsic but not the extrinsic pathway of apoptosis and no change in Bcl-2 expression. (A) Expression of caspase-9 zymogen protein (48 kDa) in human CLL cells at 24 hours following incubation with medium or KRN5500 (0.134 or 1.34 μM) demonstrating processing of caspase-9 (and, thus, decrease in 45 kDa band), a finding supportive of activation of the intrinsic pathway of apoptosis. (B) Expression of caspase-8 zymogen protein (55 kDa) in human CLL cells at 24 hours following incubation with medium or KRN5500 (0.134 or 1.34 μM) demonstrating no processing of procaspase-8 (and, thus, no decrease in 55 kDa band), a finding supportive of KRN5500 apoptosis occurring independent of the extrinsic pathway of apoptosis. (C) Expression of the Bcl-2 protein in human CLL cells at 24 hours following incubation with KRN5500 (0.134 or 1.34 μM) demonstrating no change in Bcl-2 expression following treatment with this agent.
Loss of mitochondria integrity with KRN5500 treatment of B-CLL cells. After 24 hours of incubation without (A) or with (B) KRN5500, cells were incubated with 50 nM rhodamine-123 and then analyzed by flow cytometry. The rise in the lower intensity peak, with KRN5500 treatment, indicates loss of the fluorescent dye rhodamine-123 from mitochondria.
Loss of mitochondria integrity with KRN5500 treatment of B-CLL cells. After 24 hours of incubation without (A) or with (B) KRN5500, cells were incubated with 50 nM rhodamine-123 and then analyzed by flow cytometry. The rise in the lower intensity peak, with KRN5500 treatment, indicates loss of the fluorescent dye rhodamine-123 from mitochondria.
Bcl-2 is an antiapoptotic protein that functions through stabilization of the mitochondrial membrane, and its increased expression has been shown to confer resistance to cytotoxic agents. Because of this, we next assessed whether Bcl-2 protein expression was affected by incubation with KRN5500. Cellular lysates from the previous experiments were subjected to immunoblotting with antihuman Bcl-2 antibodies, and protein expression was measured relative to GAPDH. As shown in Figure 3, we did not detect changes in expression of Bcl-2 protein in KRN5500-treated versus untreated cells.
Discussion
This report represents the first preclinical evaluation of the novel semisynthetic antibiotic KRN5500 in human chronic lymphocytic leukemia cells. Data derived from these studies demonstrate that KRN5500 has marked preclinical activity against CLL cells, requiring only a 4- to 24-hour exposure time to induce apoptosis in most patients tested. A dose- and time-dependent increase in loss of viability was observed in the 3 most resistant patients from 4 to 96 hours of exposure to KRN5500, but the activity of this agent in the whole group was not significantly accentuated by more extended incubation. We also documented that at concentrations of approximately 1 μM we can detect processing of caspase-9 but not caspase-8 proforms in CLL cells as well as cleavage of the activated caspase substrate PARP. Taken together, these findings strongly support that KRN5500 exerts its cytotoxic effects via the intrinsic pathway of apoptosis.
The mechanism by which KRN5500 promotes mitochondria damage in CLL cells with subsequent activation of caspase-9 and -3 is currently unknown. Given the variable sensitivity of both normal mononuclear cell isolates and CLL, it is possible that the conversion of KRN5500 to SAN-gly is variable and possibly due to a polymorphism in the yet unidentified metabolizing enzyme. Resistance to KRN5500 does not appear to correlate with drug resistance to other types of chemotherapy, because the most resistant patients studied in this series had lower in vitro sensitivity to KRN5500. Similar findings have been noted by Lee et al,12 who showed that cell lines resistant to cisplatin were still quite sensitive to the effects of KRN5500. Identifying the enzyme that metabolizes the prodrug to the active form is of great interest. Once activated, KRN5500 has a variety of potential mechanisms, including both inhibition of protein synthesis and glycoprotein processing.11,13 Initial studies demonstrated that KRN5500 decreased protein synthesis in a variety of human tumor cell lines11 but not in a rabbit reticulocyte system. Subsequent studies performed by Burger and colleagues13 demonstrated that KRN5500 inhibited protein synthesis at high concentrations of drug but also increased mannose concentration on glycoproteins at lower concentrations where growth inhibition was noted. This alteration in mannose concentration, combined with the observation that lectin binding was diminished, implied that KRN5500 might be acting through alteration of cell glycoprotein processing.13 Electron microscopy of KRN5500-treated cells supported this possible mechanism of action, because cells treated with this agent had altered Golgi apparatus with dilated cisternae as compared with control cells.13 Our data extend the cell line cytotoxicity data with KRN5500, demonstrating that it can effectively induce apoptosis in a predominately G0 arrested population of human tumor cells. This suggests that KRN5500 may have efficacy in tumors with low proliferation rates. Based on these data, inclusion of CLL patients on these phase 1 studies appears warranted.
Prepublished online as Blood First Edition Paper, February 20, 2003; DOI 10.1182/blood-2002-08-2623.
Supported by the National Cancer Institute (P01 CA81534-02 and CA98099), The Sidney Kimmel Cancer Research Foundation, The Leukemia and Lymphoma Society of America, and The D. Warren Brown Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Figure 2. KRN5500 induces caspase-3 activation and cleavage of PARP. (A) KRN5500 induces caspase-3 activation in human CLL cells in vitro. CLL cells were exposed to 24 hours of media (i) or KRN5500 (0.134 [ii] and 1.34 μM [iii]) and then examined for the presence of the active 29 kDa heterodimer of effector caspase-3. An increase in fluorescence is observed with KRN5500 exposure at 24 hours that is indicative of caspase-3 activation. (B) KRN5500 induces cleavage of PARP in human CLL cells in vitro. CLL cells were exposed to 24 hours of media or KRN5500 (0.134 and 1.34 μM) followed by immunoblotting for PARP. A dose-dependent increase in the cleaved p85 fragment of PARP is observed following 24 hours of exposure of KRN5500.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/11/10.1182_blood-2002-08-2623/5/m_h81134410002.jpeg?Expires=1765021243&Signature=EqaPz0rJbjC6Eq3S05VMMd31AynivWF3FGxxa0zkH9arGuwt7OPwHzI1Fr7XZrrIF9AL7SCSqUfPaJSZzPE8d~b9OjnXspGhC1F~~jov6vXoNq04YMtpBlWubYIz6ADsi~y848I2SpQ1CY9DTreRqPhllDIzokLaMF3CHG3amS~y7LYN-01T3CRqXn63x7ebNRNEKglu98VtebGxtVdSxRywfIuuXJRIGlRyGjLwcOVGYNSwlTHD7b8z79zWwwBcP8dzvmfMWo3LGxGwNT8OXv0fyayZxbMB~S~jqEJ-KyQrjkVxfSoXeTXn255tLnQUS7CNkdXEm2Yp1UEiNDx3og__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal