Abstract
X-linked sideroblastic anemia (XLSA) is due to deficient activity of erythroid-specific 5-aminolevulinate synthase (ALAS2). We report here a patient who developed sideroblastic anemia at the age of 81 years while undergoing hemodialysis. The diagnosis of sideroblastic anemia was established by the presence of ringed sideroblasts in the bone marrow, and treatment with oral pyridoxine completely eliminated the ringed sideroblasts. We identified a novel point mutation in the fifth exon of this patient's ALAS2 gene, which resulted in an amino acid change at residue 159 from aspartic acid to asparagine (Asp159Asn). In vitro analyses of recombinant Asp159Asn ALAS2 revealed that this mutation accounted for the pyridoxine-responsiveness of this disease. The very late onset in this case of XLSA emphasizes that nutritional deficiencies caused either by dietary irregularities in the elderly or, as in this case, by maintenance hemodialysis therapy, may uncover occult inherited enzymatic deficiencies in the heme biosynthetic pathway.
Introduction
X-linked sideroblastic anemia (XLSA) is an X-linked recessive hypochromic, microcytic anemia with ringed sideroblasts in the bone marrow, resulting from the deficient activity of erythroid-specific 5-aminolevulinate synthase (ALAS2, or ALAS-E; EC 2.3.1.37). XLSA typically presents by the fourth decade of life, however, there are rare cases that may manifest later in life. Late-onset XLSA merits special mention not only because it is extremely rare (only 3 cases have been reported), but also because there has been no direct evidence that the mutations of ALAS2 resulted in the disease.1, 2, 3 In this study, we report a patient with late-onset XLSA who developed the disease following hemodialysis. The diagnosis was confirmed by molecular analysis of the ALAS2 gene.
Study design
Case report
An 81-year-old male patient was admitted to Kyoto Minute-I-Ren Central Hospital in September 2000, with the diagnosis of chronic renal failure and sideroblastic anemia. Prior to admission, he had been treated with hemodialysis for 2 1/2 years. Erythropoietin was administered (3000 U subcutaneously 3 times weekly), yet he remained anemic (red blood cell count [RBC], 2.85 × 1012/L; hemoglobin level [Hb], 81 g/L; hematocrit [Ht], .245; mean corpuscular volume [MCV], 85.9 fL; on June 27, 2000), although blood transfusions were not required. On July 25, 2000, anemia worsened (RBC, 2.32 × 1012/L; Hb, 63 g/L; Ht, .191; and MCV, 82.4 fL). There were no signs of intestinal bleeding, and he received 8 units of packed red blood cells. On admission to the hospital in September 2000, laboratory findings revealed a severe normocytic anemia (RBC, 1.95 × 1012/L; Hb, 56 g/L; Ht, .17; MCV, 87.3 fL; mean corpuscular hemoglobin [MCH], 29.0 pg; mean corpuscular hemoglobin concentration [MCHC], 332 g/L; and white blood cell count [WBC], 4.6 × 109/L) with an increased red cell distribution width of 19.8% (normal, 11.6%-15.0%). The peripheral blood smear revealed both normocytic and microcytic erythrocyte populations. The MCV dropped to 82.4 fL before additional packed red cell transfusions were administered. A bone marrow examination revealed a hypocellular marrow with a normal myeloid-erythroid ratio. Prussian blue staining of bone marrow cells revealed the presence of numerous ringed sideroblasts (49% of the erythroblasts). The serum ferritin concentration was elevated at 797 μg/L. A myelodysplastic syndrome was suspected, namely refractory anemia with ringed sideroblasts (RARS) and additional packed red cell transfusions were administered. A serum pyridoxal level was less than 2.0 ng/mL (normal, 6.0-40.0 ng/mL) and oral pyridoxine (200 mg/d) was started. After 2 weeks of pyridoxine therapy, the anemia stabilized and no further transfusions were required. The bone marrow examination was repeated and ringed sideroblasts were no longer evident. Hemoglobin levels were maintained at or higher than 70 g/L with continued pyridoxine treatment, but the patient died of aspiration pneumonia in November 2000.
Identification of an ALAS2 mutation
An autopsy sample of the patient's liver, which had been stored in a 3.7% formaldehyde solution, was used as a source of DNA. After informed consent was obtained from the patient's family, genomic DNA was extracted from the liver sample. Using the genomic DNA as a template, each exon of the ALAS2 gene was amplified using polymerase chain reaction (PCR); then PCR products were directly sequenced. The primers that were used for PCR and direct sequencing have been previously described.4 The mutation of the ALAS2 gene in the patient was confirmed by 3 independent PCR reactions followed by direct sequencing.
Enzymatic characterization of the mutant ALAS2 protein
Using a QuickChange Site-Directed Mutagenesis kit (Stratagene, La Jolla, CA), the point mutation of ALAS2 gene of the patient was introduced into the wild-type (WT) ALAS2 cDNA, which had been subcloned into a pGEX-3X expression vector for expression of the mature ALAS2 enzyme as a glutathione S-transferase (GST) fusion protein.4 After confirming the sequence of mutant ALAS2 cDNA, BL21(DE3)pLysS Escherichia coli strain was transformed by the expression vector. Induction of the recombinant protein expression, purification, and determination of enzymatic activity were carried out as described previously.4
Results and discussion
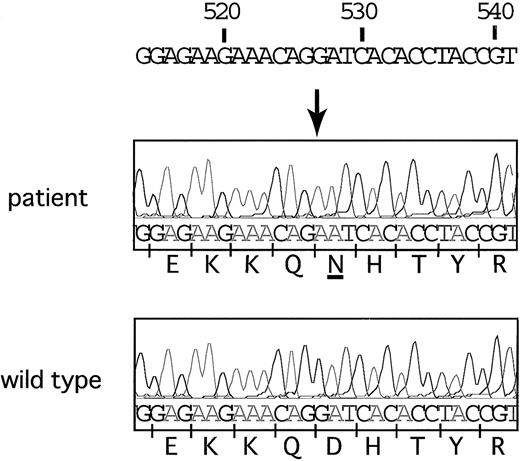
All 11 exons and the 250–base pair (bp) promoter region of the ALAS2 gene were sequenced, and a single nucleotide transition (from guanine to adenine at the 527th nucleotide) was identified in exon 5 (Figure 1). This point mutation resulted in an amino acid change from aspartic acid to asparagine at residue 159 (Asp159Asn). The proband had 5 brothers, 2 sisters, and 3 daughters, but none had a history of anemia. Consent for genetic analysis of living family members could not be obtained. The Asp159Asn mutation does not appear to be a polymorphism, however, as it was not found in 44 control ALAS2 alleles from the Japanese population (data not shown). Recently, an Asp159Tyr ALAS2 mutation was reported in another family with pyridoxine-responsive XLSA.5 In this family, there were 2 males with sideroblastic anemia that was expressed in childhood. Early expression of the Asp159Tyr mutation may be due to the fact that tyrosine, unlike asparagine, is positively charged and would be predicted to have a greater effect on the catalytic activity of ALAS2. These findings suggest that the aspartic acid normally found at residue 159 is important for enzymatic activity and that mutations at this residue are causative for XLSA.
Nucleotide sequence analysis of theALAS2gene. Adenine substituted for guanine at position 527 in this patient. This transition resulted in an amino acid change from aspartic acid to asparagine at the 159th amino acid residue.
Nucleotide sequence analysis of theALAS2gene. Adenine substituted for guanine at position 527 in this patient. This transition resulted in an amino acid change from aspartic acid to asparagine at the 159th amino acid residue.
ALAS requires pyridoxal 5′-phosphate (PLP), the active form of pyridoxine, as a cofactor. Pyridoxine deficiency may cause sideroblastic anemia,6 and pyridoxine deficiency is prevalent in patients undergoing chronic hemodialysis.7 In spite of these points, only one patient has been reported to develop sideroblastic anemia following chronic hemodialysis.8 In our patient, treatment with pyridoxine eliminated the ringed sideroblasts from the marrow and resulted in independence from transfusions. Most patients with XLSA are responsive to pyridoxine (24 of the 29 known ALAS2 mutations).9,10 To confirm that the Asp159Asn mutation was responsible for the sideroblastic anemia in our patient, we examined the effect of PLP on the activity of recombinant Asp159Asn mutant enzyme in vitro. The specific activity of the Asp159Asn mutant was 31.4% of the wild-type enzyme in the absence of PLP. Activity of the mutant enzyme increased to 60.3% of wild type in the presence of PLP (Table 1).
Enzymatic activity of the recombinant ALAS2 protein
Treatment . | GST-WT . | GST-Asp159Asn . |
|---|---|---|
| Without PLP | 24 666 ± 421.6 | 7 755 ± 392.1 |
| With 0.2 mM PLP | 37 743 ± 1 022.2 | 22 767 ± 421.6 |
Treatment . | GST-WT . | GST-Asp159Asn . |
|---|---|---|
| Without PLP | 24 666 ± 421.6 | 7 755 ± 392.1 |
| With 0.2 mM PLP | 37 743 ± 1 022.2 | 22 767 ± 421.6 |
Unit: nmol ALA/h/mg protein, (mean ± SD, n = 3).
Our study indicates that clinically silent mutations of ALAS2 might become evident later in life if cofactor deficiency develops because of dietary peculiarities or, as in this case, if an intervention such as hemodialysis occurs.
Prepublished online as Blood First Edition Paper, January 16, 2003; DOI 10.1182/blood-2002-09-2804.
Supported in part by US Public Health Service grant DK32890 (S. Sassa), American Porphyria Foundation grant (S. Sassa, Grant-in-Aid for Scientific Research (C) from the Ministry of Education, Science, Sports, and Culture of Japan (K.F.), and Research grant from Yamanouchi Foundation for Research on Metabolic Disorders (K.F.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to Ms Luba Garbaczewski and Ms Kiriko Kaneko for their technical assistance in this study, and to Dr A. P. Doke and Ms Taeko Hirabayashi for reviewing the manuscript.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal