Abstract
We have investigated the significance of telomerase activity (TA) and telomere length (TL) in multiple myeloma (MM). The analyses were undertaken on CD138+ MM cells isolated from the marrow of 183 patients either at diagnosis or in relapse. There was heterogeneity in telomerase expression; 36% of the patients had TA levels comparable to those detected in normal plasma cells, and 13% of patients had levels 1- to 4-fold greater than in a neuroblastoma cell line control. The TL of MM cells was significantly shorter than that of the patients' own leukocytes; in 25% of patients, the TL measured less than 4.0 kbp. Analysis of TL distribution indicated selective TA-mediated stabilization of shorter telomeres when mean TL fell below 5.5 kbp. Unusually long (10.8-15.0 kbp) telomeres were observed in 7 patients, and low TA was observed in 5 of 7 patients, suggesting the operation of a TA-independent pathway of telomere stabilization. A strong negative correlation existed between TA and TL or platelet count. TL negatively correlated with age and with interleukin-6 (IL-6) and β2-microglobulin levels. Various cytogenetic abnormalities, including those associated with poor prognosis, strongly correlated with TA and, to a lesser extent, with short TL. High TA and short TL defined a subgroup of patients with poor prognosis. At 1 year the survival rate in patients with TA levels lower than 25% of neuroblastoma control and TL greater than 5.5 kbp was 82%, whereas in patients with higher TA and shorter TL the survival rate was 63% (P = .004). The 2-year survival rate for patients with TA levels lower than 25% was 81%, and it was 52% in those with higher TA levels (P <.0001).
Introduction
As normal cells undergo repeated rounds of DNA replication, their telomeres shorten because of the inability of traditional DNA polymerases to completely replicate the end of the chromosomal DNA.1 This shortening continues until the cells reach a proliferative block referred to as crisis, which is characterized by chromosomal instability, end-to-end fusions, and cell death. Stabilization of the telomeric DNA through telomerase activation or activation of the alternative mechanism of telomere maintenance (ALT) is essential if the cells are to survive and proliferate indefinitely.2-4 In approximately 85% of all human cancer, immortalization is associated with the up-regulation of telomerase. Direct evidence for the role of telomerase in cancer has been obtained through studies in which normal human lung or breast epithelium5,6 or marrow endothelium7 is transfected with the catalytic subunit of telomerase, hTERT, together with the simian virus 40 large T antigen and ras oncogenes. This is sufficient to confer immortality and malignant behavior in vivo. Conversely, loss of telomere stabilization by an already immortalized cell, such as after telomerase inhibition, results in loss of immortality and cell death.1,8-10 Together, these studies indicate that telomere maintenance is a critical component of cancer cell immortality.
The significance of telomerase expression in human malignancy has been evaluated extensively in solid tumors,11-18 hematopoietic malignancies,19-21 and, on a smaller scale, in lymphoid malignancies.22-28 The number of studies in which tumor telomere length (TL) was evaluated in parallel with telomerase has been more limited, and, though in some solid tumors the concept that high telomerase levels lead to tumor telomere elongation has been supported, it is by no means clear whether this simple relationship is generally applicable, particularly in view of the increasing understanding of the complexity of molecular interactions at the chromosome ends. In B-cell malignancies such as non-Hodgkin lymphoma (NHL), elevated telomerase levels of chronic lymphocytic leukemia (CLL) and hairy cell leukemia were reported relative to normal B lymphocytes; however, benign germinal center B cells also showed high levels of telomerase activity (TA).22,24 This has led to the suggestion that telomerase expression in malignant B cells can be explained by an induction-and-retention model of reactivation of telomerase in malignant clones.22 In B-CLL a high inverse correlation was noted between TL and TA, and patients in early-stage disease had significantly longer telomeres than those at late stages.22 Short telomeres and high TA levels were significantly associated with shorter mean survival. In multiple myeloma (MM), 2 small studies on TA, but not on TL, have been reported. In one, telomerase level was not elevated in plasma cells (PCs) from 5 patients with monoclonal gammopathy of undetermined significance (MGUS), but it was elevated in 21 of 27 patients with MM and in all 4 patients with plasma cell leukemia.27 In another study of 25 patients with MM, elevated TA level correlated with high β2-microglobulin (β2M) levels, clinical stage III disease, and shorter survival.28 The need for more extensive analysis of MM is attributed to its heterogeneity and to what has been described as its genetic chaos, as reflected in its marked aneuploidy and the difficulty in establishing correlations between genetic abnormalities and clinical outcome.29 Gene expression profiling by microarray analysis has recently been applied to MM and MGUS. On hierarchical clustering analysis, normal and MM plasma cells could be differentiated, and 4 distinct subgroups of MM were identified.29 One group (MM1) was similar to normal plasma cells and MGUS, whereas at the other extreme (MM4) the profile was similar to that of MM cell lines and was associated with clinical parameters linked to poor prognosis, such as abnormal karyotype and high serum β2M levels. Microarray analysis can determine whether certain genes involved in TA (hTR, hTERT) or telomere organization or stabilization (telomeric repeat-binding factor-1 [TRF-1], TRF-2, tankyrase)1,11,30,31 are differentially expressed in normal versus MM cells or MM subsets. It is well established that hTR mRNA expression does not correlate with TA level; therefore, it is hard to evaluate TA based on microarray hTR mRNA levels. Our Affymetrix microarray analysis did not detect significant changes in hTERT mRNA expression in enriched myeloma cells relative to normal plasma cells (J.S., unpublished data, 2002). This may be because of the low mRNA transcription level or our observation that normal plasma cells expressed hTERT and hTR. However, increased telomerase activity can occur without altering hTERT mRNA and protein expression in a process involving phosphorylation of the protein and its nuclear localization.32,33 This is seen after antigen stimulation of normal T cells,32 or in MM cells after exposure to interleukin-6 (IL-6) or insulin-like growth factor 1 (IGF-1).33
We have demonstrated in a large group of MM patients that there is considerable heterogeneity in tumor TA and TL. Increased TA correlated positively with age and negatively with TL. Strong correlations were determined between various prognostic features (β2M and IL-6 levels, platelet counts at diagnosis) and TA, TL, or both. Various cytogenetic abnormalities, including poor prognosis abnormalities involving chromosome 13, strongly correlated with TA and, in many instances, with short TL. Detailed comparison of TA and TL has shed light on the molecular mechanisms involved in telomere stabilization and tumor immortalization. High TA significantly correlated with 2-year survival among patients, pointing to the prognostic value of TA evaluation in MM survival.
Patients, materials, and methods
Patients
All samples were collected from the Myeloma Institute for Research and Therapy (University of Arkansas for Medical Sciences, Little Rock). Table 1 shows the patients' characteristics. Written informed consent was obtained from each patient and donor.
Patient characteristics
. | Median . | Range . | Cutoff (patients, %) . |
|---|---|---|---|
| Age, y | 59 | 24-87 | ≥ 65 (32) |
| Age, healthy donors, y | 32 | 21-41 | — |
| β2-microglobulin, mg/L | 3.3 | 1.0-153.4 | ≥ 4.0 (41) |
| C-reactive protein, mg/L | 2.8 | 0.3-101.0 | ≥ 4.0 (60) |
| Hemoglobin level, g/dL | 11.0 | 6.5-15.7 | < 10 (32) |
| Albumin, mg/L | 3.7 | 1.0-5.4 | < 3.5 (33) |
| Lactic acid dehydrogenase, IU/L | 167 | 55-1035 | ≥ 190 (34) |
| PCs in bone marrow, % | 50 | 4-99 | ≥ 50 (52) |
| Platelet count, × 109/L* | 200 | 17-572 | < 150 (25) |
| IL-6, pg/mL, n = 83 patients | 5.7 | < 0.7-227 | ≥ 3.5 (70) |
| Labeling index, %, n = 91 patients | 0.4 | 0-4.6 | ≥ 0.5 (47) |
. | Median . | Range . | Cutoff (patients, %) . |
|---|---|---|---|
| Age, y | 59 | 24-87 | ≥ 65 (32) |
| Age, healthy donors, y | 32 | 21-41 | — |
| β2-microglobulin, mg/L | 3.3 | 1.0-153.4 | ≥ 4.0 (41) |
| C-reactive protein, mg/L | 2.8 | 0.3-101.0 | ≥ 4.0 (60) |
| Hemoglobin level, g/dL | 11.0 | 6.5-15.7 | < 10 (32) |
| Albumin, mg/L | 3.7 | 1.0-5.4 | < 3.5 (33) |
| Lactic acid dehydrogenase, IU/L | 167 | 55-1035 | ≥ 190 (34) |
| PCs in bone marrow, % | 50 | 4-99 | ≥ 50 (52) |
| Platelet count, × 109/L* | 200 | 17-572 | < 150 (25) |
| IL-6, pg/mL, n = 83 patients | 5.7 | < 0.7-227 | ≥ 3.5 (70) |
| Labeling index, %, n = 91 patients | 0.4 | 0-4.6 | ≥ 0.5 (47) |
Total sample, 183 patients. Male patients constituted 66% of sample; and white patients, 85%.
Platelet count was taken at diagnosis.
Cell preparations
Marrow PCs were collected from 183 patients with newly diagnosed or relapsed MM and 7 healthy donors (normal PCs) after receiving informed consent according to the University's Institutional Review Board (IRB) guidelines. Patient in relapse was defined as the recurrence of monoclonal protein or bone marrow plasmacytosis or evidence of extramedullary disease in patients in complete remission (CR) or near CR or as any new disease manifestation, including hypercalcemia. Disease progression for patients not in CR implied at least a 25% increase in tumor mass or any new disease manifestation. PC isolation from the marrow mononuclear cell fraction was performed using immunomagnetic bead selection with monoclonal mouse antihuman CD138 antibody and the AutoMACS separation system (Miltenyi Biotec, Auburn, CA). PC purity of at least 95% homogeneity was confirmed through 2-color flow cytometry using CD138+/CD45- and CD38+/CD45- criteria (Becton Dickinson, San Jose, CA), immunocytochemistry for cytoplasmic light-chain immunoglobulin, and morphology by Wright-Giemsa staining.29 Blood granulocytes and lymphocytes were separated by continuous Percoll (Amersham Pharmacia Biotech AB, Uppsala, Sweden) gradient centrifugation with 62% and 78% Percoll, respectively. Harvested cells were washed in phosphate-buffered saline, and cytospin slides were made to evaluate the purity of the separated cells. The purity of granulocytes and lymphocytes was generally greater than 90%. MM cell lines (U266, ARP-1, RPMI-8226, SKO-007, and CAG) were cultured as recommended (ATCC, Chantilly, VA), and cells were harvested for TA and TL assay.
Determination of telomerase activity
A modified version of the telomeric repeat amplification protocol (TRAP), the TRAP-eze telomerase detection kit (Intergen, Purchase, NY), was used to measure TA. Briefly, cellular protein was extracted, protein concentration was determined by the Bradford assay (Bio-Rad Laboratories, Richmond, CA), and 1 μg protein extract was used for each reaction. A standard batch of protein extracted from the SK-N-SH neuroblastoma cell line was used as an internal positive control for each run. After 30 minutes of telomerase extension at 30°C, samples were subjected to polymerase chain reaction (PCR) for 28 cycles in the presence of a 32γP[ATP] end-labeled TS primer, followed by electrophoresis on 12.5% polyacrylamide gel. Gels were exposed to a Fuji Imaging plate, scanned on a Fujifilm BAS-2500 Bio-imaging analyzer, and quantitated using MacBas V-2.5 (Fuji, Stamford, CT) as described previously.10 Background activity was subtracted, and the ratio of sample intensity to that of a 36-bp internal control was calculated. All values were then expressed as a percentage of telomerase activity in a control SK-N-SH neuroblastoma cell line.
Southern blot analysis of terminal restriction fragment
Approximately 0.5 to 1 million cells were used for the preparation of high-molecular–weight DNA by the Nucleon BACC2 DNA extraction kit (RPN 8502; Amersham Life Science, Buckinghamshire, United Kingdom). Mean length of terminal restriction fragment (TRF) was measured using the TeloTAGGG telomere length assay kit (Roche Molecular Biochemical, Indianapolis, IN). Briefly, 2 μg purified DNA was digested with HinfI/RsaI mixture (10 U/1 μg DNA) for 2 hours, separated on 0.8% agarose gel electrophoresis in 1 × Tris-acetate-EDTA (-ethylenediaminetetraacetic acid), and transferred onto a nylon membrane (Hybond N+; Amersham Life Science) according to the protocol described by the supplier. After rinsing in 2 × SSC and neutralizing solution, the filter was hybridized to digoxigenin-labeled probe specific for telomeric repeats for 3 hours at 42°C. The filter was washed twice in 2 × SSC/0.1% sodium dodecyl sulfate (SDS) for 5 minutes each time at room temperature and then was placed in 0.2 × SSC/0.1% SDS solution at 50°C for 15 minutes with one repeat. To determine TRF length, the hybridized probe was incubated with a digoxigenin-specific antibody covalently coupled to alkaline phosphatase. Finally, the immobilized telomere probe was visualized by a highly sensitive chemiluminescent substrate for alkaline phosphatase, CDP-Star, and was used to detect TRF DNA on Hyperfilm (Amersham Life Science). Overall mean TRF length was determined as described previously.34 In addition, mean TRF of the longer telomeres (longer than the overall mean TRF distribution) and mean TRF of the shorter telomeres (shorter than the overall mean TRF distribution) were calculated using MacBasV-2.5 software (Fuji).35
Serum IL-6 level assay
IL-6 is measured by a quantitative sandwich enzyme immunoassay technique (R & D Systems, Minneapolis, MN). Briefly, a monoclonal antibody specific for IL-6 was precoated onto a microplate. Standards and samples were pipetted into the wells, and the immobilized antibody bound any IL-6 that was present. After washing away unbound substances, an enzyme-linked polyclonal antibody specific for IL-6 was added to the wells. After a wash to remove the unbound antibody-enzyme reagent, a substrate solution was added to the wells with color developing in proportion to the amount of IL-6 bound in the initial step. The color development was stopped, and the intensity of the color was measured.
Plasma cell labeling index assay
A specimen of aspirate of bone marrow or of a tumor was processed to isolate the mononuclear cells. These cells were then stained for cytoplasmic light-chain proteins (κ or λ) and with a 5-bromo-2′-deoxyuridine (BUdR) antibody to detect all plasma cells in the S phase. Five hundred plasma cells were counted, and a percentage of double-positive cells was obtained. This percentage is the plasma cell labeling index.
Statistical analysis
Because of the highly skewed distributions of TA and TL in this patient population, nonparametric tests were used for most comparisons. Distributions of TA and TL within subgroups of clinical characteristics were compared using the Wilcoxon rank sum test for dichotomous clinical parameters and the Kruskal-Wallis test for clinical parameters with 3 or more groups. Correlations between TA, TL, and continuous clinical or laboratory measures were computed using Spearman rank-order correlation. Associations between categorical variables were compared using the χ2 test. Survival distributions were presented using the Kaplan-Meier method36 and were compared using the log-rank test.37 Analyses were performed using SAS statistical software (Chicago, IL).
Results
Telomerase activity detected in CD138+ PCs from patients with MM and healthy donors
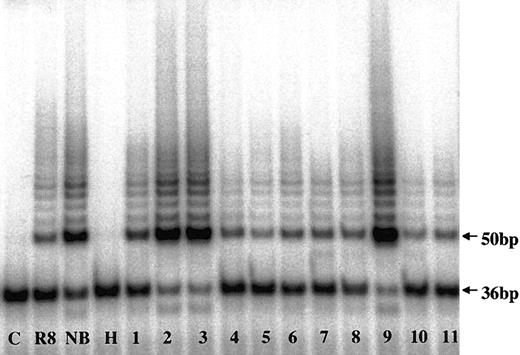
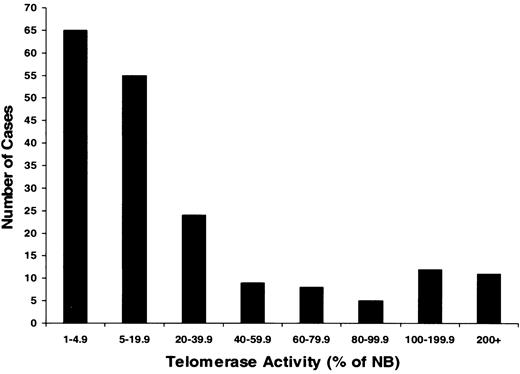
To evaluate telomerase expression in CD138+ cells, TA in enriched MM cells from 183 MM patients and PCs from 7 healthy donors were measured (Figures 1, 2; Table 2). There was a heterogeneous distribution of TA in the MM samples, with median activity 8.2% of the neuroblastoma (NB) cell line control (Figures 1, 2). Thirty-six percent of samples had no significant TA level, and 64% had TA levels greater than 5% of the NB control. Thirteen percent (n = 24) of the total had very high TA levels—100% to 427% of the NB levels. The median TA of MM cells from newly diagnosed patients (n = 86) was significantly (P = .001) lower (6.3%) than found with specimens from the 97 patients in relapse who had prior therapy (16.2%). In healthy donors, the TA of marrow PCs ranged from 0.3% to 22.5%, with a median of 3.7% (Table 2). All 5 myeloma cell lines expressing high TA levels ranged from 87% to 266%, with a median of 180% (Table 2).
Telomerase activity in CD138+ myeloma and normal plasma cells by TRAP assay. Telomerase activity in marrow myeloma cells from MM patients (lanes 1-9) and in marrow plasma cells from healthy donors (lanes 10-11) are shown. C indicates PCR-negative control; R8, TA-positive control template; NB, neuroblastoma cell line; H, heat-inactivated control; 36bp, internal standard.
Telomerase activity in CD138+ myeloma and normal plasma cells by TRAP assay. Telomerase activity in marrow myeloma cells from MM patients (lanes 1-9) and in marrow plasma cells from healthy donors (lanes 10-11) are shown. C indicates PCR-negative control; R8, TA-positive control template; NB, neuroblastoma cell line; H, heat-inactivated control; 36bp, internal standard.
Heterogeneous telomerase activity in CD138+-enriched cells from patients with multiple myeloma. Telomerase activity (TA) was determined in myeloma cells from 183 patients. Monoclonal CD138 antibody was used to isolate myeloma cells with a purity of more than 95% from bone marrow of patients with active disease. Results were expressed as a percentage of activity in a neuroblastoma (NB) cell line standard. Results showed that TA ranged from 0% to 427% of the activity in an NB cell line standard and that approximately 36% of myeloma patients had TA levels less than 5%; 51% had TA levels between 5% and 100%; and 13% had greater activity than this highly telomerase-positive tumor (higher than 100%).
Heterogeneous telomerase activity in CD138+-enriched cells from patients with multiple myeloma. Telomerase activity (TA) was determined in myeloma cells from 183 patients. Monoclonal CD138 antibody was used to isolate myeloma cells with a purity of more than 95% from bone marrow of patients with active disease. Results were expressed as a percentage of activity in a neuroblastoma (NB) cell line standard. Results showed that TA ranged from 0% to 427% of the activity in an NB cell line standard and that approximately 36% of myeloma patients had TA levels less than 5%; 51% had TA levels between 5% and 100%; and 13% had greater activity than this highly telomerase-positive tumor (higher than 100%).
TA and TL in myeloma cells and normal plasma cells
. | TA, % of neuroblastoma cell line control . | . | . | . | TL, kbp . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | N . | Median . | Min . | Max . | N . | Median . | Min . | Max . | ||||||
| Myeloma patients | 183 | 8.0 | 0.02 | 426.8 | 115 | 5.6 | 3.0 | 15.0 | ||||||
| Diagnosed | 86 | 6.3 | 0.02 | 325.3 | 55 | 6.6 | 3.2 | 15.0 | ||||||
| Relapsed | 97 | 16.2 | 0.07 | 426.8 | 60 | 5.3 | 3.0 | 13.4 | ||||||
| MM cell lines | 5 | 180.1 | 86.6 | 265.8 | 5 | 3.7 | 1.7 | 8.6 | ||||||
| Healthy donors | 7 | 3.7 | 0.3 | 22.5 | 7 | 10.6 | 9.6 | 11.3 | ||||||
. | TA, % of neuroblastoma cell line control . | . | . | . | TL, kbp . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | N . | Median . | Min . | Max . | N . | Median . | Min . | Max . | ||||||
| Myeloma patients | 183 | 8.0 | 0.02 | 426.8 | 115 | 5.6 | 3.0 | 15.0 | ||||||
| Diagnosed | 86 | 6.3 | 0.02 | 325.3 | 55 | 6.6 | 3.2 | 15.0 | ||||||
| Relapsed | 97 | 16.2 | 0.07 | 426.8 | 60 | 5.3 | 3.0 | 13.4 | ||||||
| MM cell lines | 5 | 180.1 | 86.6 | 265.8 | 5 | 3.7 | 1.7 | 8.6 | ||||||
| Healthy donors | 7 | 3.7 | 0.3 | 22.5 | 7 | 10.6 | 9.6 | 11.3 | ||||||
Min indicates minimum; and max, maximum.
Telomere length in CD138+ MM cells and normal PCs relative to patients' peripheral blood granulocytes and lymphocytes
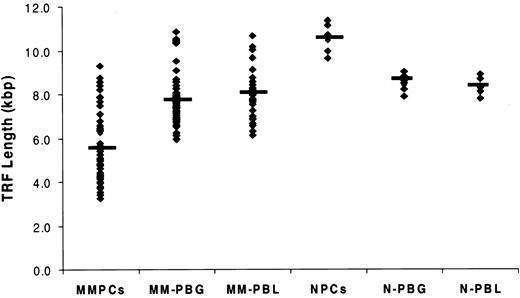
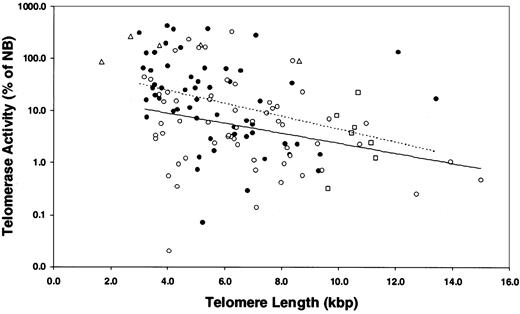
TL in marrow PCs from 115 MM patients and 7 healthy donors was determined by TRF Southern blot analysis (Figure 3; Table 2). The results exhibited significantly reduced TL in MM patients compared with the TL in PCs of healthy donors. The median TRF was 5.3 kbp, and the range was 3.0 to 15.0 kbp; 25% of patients had TL shorter than 4.0 kbp. In 7 patients with MM in whom unusually long (10.8-15.0 kbp) telomeres were observed, 5 patients had TA levels equal to or less than 5.8% of the NB control, and 2 patients had TA levels comparable to those of other patients with MM (Figure 4). In marked contrast to the MM cells, the TL in PCs from healthy donors ranged from 9.6 to 11.3 kbp, with a median TL of 10.6 kbp (Figure 3; Table 2). Although the mean age of the healthy donors was significantly younger than that of the MM population, when matched with MM patients of comparable ages, the TL in PCs from MM was still significantly shorter than it was in normal PCs (P < .01) (Figure 3; Table 1). In addition, TL was measured in purified peripheral blood granulocytes and lymphocytes from 48 of 115 patients with MM, allowing us to evaluate TL alterations in different cell types from the same patient. Results showed that TL was significantly shorter in marrow MM cells than in granulocytes and lymphocytes from the same person (P < .001). No significant difference in TL was found between granulocytes and lymphocytes of the same patient or between granulocytes and lymphocytes of patients and those of donors (Figure 3).
Telomere length was significantly shorter in marrow myeloma plasma cells (MMPCs) than in peripheral blood granulocytes and lymphocytes from patients with MM. Telomere length (TL) was measured in 48 patients who had myeloma cells and peripheral blood granulocytes (PBGs) and peripheral blood lymphocytes (PBLs) available. TL from normal plasma cells (NPCs) from 7 healthy donors and normal PBG (N-PBG) and normal PBL (N-PBL) are also included. The value indicated in each group is the TL median. Results showed significantly reduced TL in MM cells compared with TL in healthy donor PCs (P < .01), and they showed that TL was significantly shorter in marrow MM cells than in granulocytes and lymphocytes from the same person (P < .001). There was no significant difference in TL between the granulocytes and lymphocytes in the same patient or between granulocytes and lymphocytes of patients and those of donors.
Telomere length was significantly shorter in marrow myeloma plasma cells (MMPCs) than in peripheral blood granulocytes and lymphocytes from patients with MM. Telomere length (TL) was measured in 48 patients who had myeloma cells and peripheral blood granulocytes (PBGs) and peripheral blood lymphocytes (PBLs) available. TL from normal plasma cells (NPCs) from 7 healthy donors and normal PBG (N-PBG) and normal PBL (N-PBL) are also included. The value indicated in each group is the TL median. Results showed significantly reduced TL in MM cells compared with TL in healthy donor PCs (P < .01), and they showed that TL was significantly shorter in marrow MM cells than in granulocytes and lymphocytes from the same person (P < .001). There was no significant difference in TL between the granulocytes and lymphocytes in the same patient or between granulocytes and lymphocytes of patients and those of donors.
Correlations between telomerase activity and telomere length in myeloma and normal plasma cells. Results showed that there was a strong negative correlation between TA and TL in the myeloma samples from treated (•) and untreated (○) patients, but there was no difference between them (P > .05). Dotted line indicates treated MM patients; linear trend, y = -0.285 × +4.336. Solid line indicates untreated MM patients; linear trend, y = -0.221 × +3.079. MM cell lines (Δ) had high TA and short TL, and 7 healthy donors (□) had low TA and relatively long TL. Seven MM patients exhibited unusually long TL (longer than 10.8 kbp) with TA less than 5.8% of NB control in 5 patients.
Correlations between telomerase activity and telomere length in myeloma and normal plasma cells. Results showed that there was a strong negative correlation between TA and TL in the myeloma samples from treated (•) and untreated (○) patients, but there was no difference between them (P > .05). Dotted line indicates treated MM patients; linear trend, y = -0.285 × +4.336. Solid line indicates untreated MM patients; linear trend, y = -0.221 × +3.079. MM cell lines (Δ) had high TA and short TL, and 7 healthy donors (□) had low TA and relatively long TL. Seven MM patients exhibited unusually long TL (longer than 10.8 kbp) with TA less than 5.8% of NB control in 5 patients.
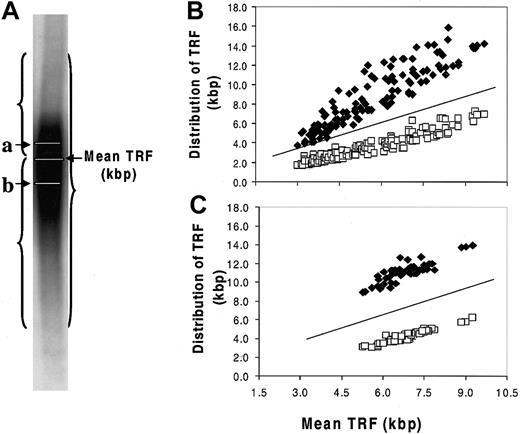
A strong negative correlation was found between TA and TL in the MM samples (Figure 4; Table 3). Two opposing concepts have been evoked to explain the existence of short telomeres in TA-positive cell populations without associated senescence or apoptosis. In one concept, telomerase caps the short telomeres, blocking their recognition as damaged DNA.31,38 The second concept was based on studies in which hTERT transduction was used to transiently express telomerase in human fibroblasts.35,39 In the latter studies, preferential shortening of the longest telomeres was observed, accompanied by narrowing of the size distribution of all the telomeres, so that the average length was closer to the shortest length of that in the parental cells. Thus, when the cells eventually reached senescence, the mean TL was 1.5 to 2.0 kbp shorter than in the nonsenescent control, whereas the length of the shortest telomere at senescence was unchanged. To test the validity of either of these concepts in the MM model, we evaluated the telomere range in the TRF Southern blot smear, determining the means of the telomeric DNA either greater than or less than the overall mean TRF (Figure 5A). To establish a nonmalignant, TA-negative control, we determined these parameters in the patients' granulocyte population. As shown in Figure 5B-C, the shorter and longer mean TRFs were constant relative to the overall mean TRF over the 5.5- to 9.5-kbp range in MM cells and granulocytes. However, in myeloma cells with mean TRF in the 5.5- to 3.0-kbp range, though the shorter telomeres were stable, the contribution of the longer telomeres was significantly reduced as the mean TRF progressively shortened (Figure 5B). This was not seen in the granulocytes, though the shortest mean TRF was 5.0 kbp or longer (Figure 5C).
TA and TL correlate with continuous laboratory variables
. | TA, % of neuroblastoma cell line control . | . | . | TL, kbp . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
. | N . | Corr . | P . | N . | Corr . | P . | ||||
| TA | — | — | — | 112 | -0.412 | < .001 | ||||
| TL | 112 | -0.412 | < .001 | — | — | — | ||||
| Age, y | 183 | 0.234 | .002 | 115 | -0.197 | .034 | ||||
| β2-microglobulin | 175 | 0.154 | .041 | 115 | -0.332 | < .001 | ||||
| C-reactive protein | 176 | 0.025 | .767 | 115 | -0.084 | .366 | ||||
| Hemoglobin level | 178 | -0.126 | .095 | 115 | 0.148 | .114 | ||||
| Albumin level | 174 | -0.158 | .037 | 113 | 0.148 | .616 | ||||
| Lactic acid dehydrogenase | 177 | 0.086 | .255 | 115 | -0.115 | .222 | ||||
| PCs in bone marrow, % | 145 | 0.097 | .245 | 97 | -0.172 | .092 | ||||
| Platelet count | 177 | -0.204 | .007 | 115 | 0.194 | .037 | ||||
| IL-6 | 81 | 0.124 | .272 | 41 | -0.408 | .008 | ||||
| Labeling index | 89 | -0.092 | .389 | 59 | -0.231 | .087 | ||||
. | TA, % of neuroblastoma cell line control . | . | . | TL, kbp . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
. | N . | Corr . | P . | N . | Corr . | P . | ||||
| TA | — | — | — | 112 | -0.412 | < .001 | ||||
| TL | 112 | -0.412 | < .001 | — | — | — | ||||
| Age, y | 183 | 0.234 | .002 | 115 | -0.197 | .034 | ||||
| β2-microglobulin | 175 | 0.154 | .041 | 115 | -0.332 | < .001 | ||||
| C-reactive protein | 176 | 0.025 | .767 | 115 | -0.084 | .366 | ||||
| Hemoglobin level | 178 | -0.126 | .095 | 115 | 0.148 | .114 | ||||
| Albumin level | 174 | -0.158 | .037 | 113 | 0.148 | .616 | ||||
| Lactic acid dehydrogenase | 177 | 0.086 | .255 | 115 | -0.115 | .222 | ||||
| PCs in bone marrow, % | 145 | 0.097 | .245 | 97 | -0.172 | .092 | ||||
| Platelet count | 177 | -0.204 | .007 | 115 | 0.194 | .037 | ||||
| IL-6 | 81 | 0.124 | .272 | 41 | -0.408 | .008 | ||||
| Labeling index | 89 | -0.092 | .389 | 59 | -0.231 | .087 | ||||
Corr indicates correlation coefficient.
Stabilization of the shortest telomeres in myeloma cells. (A) Telomere distribution in a Southern blot smear. a indicates mean TRF of longer telomeres (⋄, kbp); b, mean TRF of shorter telomeres (□, kbp). Results showed that the distribution of longer telomeres and shorter telomeres was constant relative to the mean TRF in the 5.5- to 9.0-kbp range (B), and the patients' own granulocytes showed a similar relationship (C). Progressive shortening of the longest telomeres was seen in MM cells when mean TRF fell from 5.5 to 3.0 kbp, with stabilization of the shortest telomeres (B). This was not seen in the granulocytes, though the shortest mean TRF was 5.0 kbp or more (C). The lines shown in panels B and C were y = x reference lines.
Stabilization of the shortest telomeres in myeloma cells. (A) Telomere distribution in a Southern blot smear. a indicates mean TRF of longer telomeres (⋄, kbp); b, mean TRF of shorter telomeres (□, kbp). Results showed that the distribution of longer telomeres and shorter telomeres was constant relative to the mean TRF in the 5.5- to 9.0-kbp range (B), and the patients' own granulocytes showed a similar relationship (C). Progressive shortening of the longest telomeres was seen in MM cells when mean TRF fell from 5.5 to 3.0 kbp, with stabilization of the shortest telomeres (B). This was not seen in the granulocytes, though the shortest mean TRF was 5.0 kbp or more (C). The lines shown in panels B and C were y = x reference lines.
Correlation of telomerase activity and telomere length with MM clinical and cytogenetic parameters
We explored the correlation between TA and TL in MM cells and clinical parameters (Table 3). In addition to the strong negative correlation between TA and TL (P ≤ .001), a strong positive correlation existed between age and TA (P = .002) and a weaker negative correlation with TL (P = .034). Ethnicity exhibited no significant influence on TA or TL. There was a strong negative correlation between platelet count and TA (P = .007) and a weak positive correlation with TL (P = .037). This relationship could not simply be explained by marrow replacement with MM cells because we did not observe a correlation between the percentage of plasma cells in the marrow and either TA or TL. In staging MM, β2-M levels have proved of value40 —we observed a weak positive correlation (P = .041) between levels of β2-M and TA but a strong negative correlation with TL (P ≤ .001). IL-6 has an important role in the pathogenesis of MM and has been implicated in the up-regulation of TA.33 However, serum levels of IL-6 did not correlate with TA but did strongly negatively correlate with TL (P = .008). The presence of any cytogenetic abnormality correlated positively with TA (P ≤ .001) and negatively with TL (P ≤ .001) (Table 4), as did any whole chromosome addition or deletion. TA correlated with abnormalities involving chromosome 13 (P ≤ .001), a marker of poor prognosis, and with the duplication of chromosome 3 (P ≤ .001), which is the chromosome location of the gene for human telomerase RNA component (hTR). In a multivariate analysis model, TA also correlated with chromosome 1 translocations and jumping 1q (P ≤ .001), additions of chromosomes 8, 9, 11, 19, and 20, deletions of 13, 19, and 16q arm, translocation 8q and 14q, and MM expressing a myelodysplastic syndrome (MDS) cytogenetic profile (one or more of the following abnormalities: 5q-, -5, 7q-, -7, t(1;7), +8, 20q-, and additional abnormalities typical of MM). TL negatively correlated with 8 of the 14 chromosome subgroups.
Median TA and TL by presence of selected cytogenetic abnormalities
. | . | Median TA, % of neuroblastoma cell line control . | . | . | Median TL, kbp . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CA . | % . | CA+ . | CA- . | P . | CA+ . | CA- . | P . | ||||
| Translocation 1q-arm | 25 | 26.7 | 6.4 | < .001 | 5.3 | 6.2 | .051 | ||||
| Jumping 1q-arm | 10 | 57.0 | 6.9 | < .001 | 5.0 | 5.8 | .200 | ||||
| Addition 3 | 22 | 44.4 | 6.4 | < .001 | 5.1 | 6.3 | .006 | ||||
| Addition 8 | 5 | 59.1 | 7.5 | .033 | 3.4 | 5.8 | .009 | ||||
| Translocation 8q-arm | 10 | 73.5 | 6.9 | < .001 | 5.1 | 5.7 | .200 | ||||
| Addition 9 | 23 | 19.6 | 6.7 | .003 | 5.4 | 6.1 | .105 | ||||
| Addition 11 | 25 | 15.3 | 6.8 | .037 | 5.1 | 6.2 | .009 | ||||
| Deletion 13 | 25 | 32.1 | 6.9 | .001 | 5.2 | 6.1 | .029 | ||||
| Translocation 14q-arm | 7 | 31.4 | 6.9 | .003 | 4.3 | 5.7 | .115 | ||||
| Deletion 16q-arm | 7 | 60.4 | 7.0 | .005 | 5.1 | 5.7 | .076 | ||||
| Addition 19 | 25 | 48.2 | 6.2 | < .001 | 5.1 | 6.2 | .011 | ||||
| Deletion 19 | 2 | 134.7 | 7.5 | < .001 | 5.4 | 5.6 | .466 | ||||
| Addition 20 | 6 | 70.3 | 7.1 | .006 | 4.0 | 5.7 | .029 | ||||
| Any whole addition | 39 | 23.3 | 5.9 | < .001 | 5.1 | 6.4 | .005 | ||||
| Any whole deletion | 38 | 28.5 | 6.1 | .003 | 5.2 | 6.2 | .027 | ||||
| Any abnormality | 53 | 23.3 | 4.1 | < .001 | 5.1 | 6.6 | < .001 | ||||
| MM-MDS | 20 | 34.9 | 6.8 | < .001 | 4.3 | 6.2 | .001 | ||||
. | . | Median TA, % of neuroblastoma cell line control . | . | . | Median TL, kbp . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CA . | % . | CA+ . | CA- . | P . | CA+ . | CA- . | P . | ||||
| Translocation 1q-arm | 25 | 26.7 | 6.4 | < .001 | 5.3 | 6.2 | .051 | ||||
| Jumping 1q-arm | 10 | 57.0 | 6.9 | < .001 | 5.0 | 5.8 | .200 | ||||
| Addition 3 | 22 | 44.4 | 6.4 | < .001 | 5.1 | 6.3 | .006 | ||||
| Addition 8 | 5 | 59.1 | 7.5 | .033 | 3.4 | 5.8 | .009 | ||||
| Translocation 8q-arm | 10 | 73.5 | 6.9 | < .001 | 5.1 | 5.7 | .200 | ||||
| Addition 9 | 23 | 19.6 | 6.7 | .003 | 5.4 | 6.1 | .105 | ||||
| Addition 11 | 25 | 15.3 | 6.8 | .037 | 5.1 | 6.2 | .009 | ||||
| Deletion 13 | 25 | 32.1 | 6.9 | .001 | 5.2 | 6.1 | .029 | ||||
| Translocation 14q-arm | 7 | 31.4 | 6.9 | .003 | 4.3 | 5.7 | .115 | ||||
| Deletion 16q-arm | 7 | 60.4 | 7.0 | .005 | 5.1 | 5.7 | .076 | ||||
| Addition 19 | 25 | 48.2 | 6.2 | < .001 | 5.1 | 6.2 | .011 | ||||
| Deletion 19 | 2 | 134.7 | 7.5 | < .001 | 5.4 | 5.6 | .466 | ||||
| Addition 20 | 6 | 70.3 | 7.1 | .006 | 4.0 | 5.7 | .029 | ||||
| Any whole addition | 39 | 23.3 | 5.9 | < .001 | 5.1 | 6.4 | .005 | ||||
| Any whole deletion | 38 | 28.5 | 6.1 | .003 | 5.2 | 6.2 | .027 | ||||
| Any abnormality | 53 | 23.3 | 4.1 | < .001 | 5.1 | 6.6 | < .001 | ||||
| MM-MDS | 20 | 34.9 | 6.8 | < .001 | 4.3 | 6.2 | .001 | ||||
CA values listed had P < .01 in test of association between TA or TL and cytogenetic abnormality. Percentage in the second column is incidence of CA in patient population. CA indicates cytogenetic abnormalities; CA+, with cytogenetic abnormalities; CA-, without cytogenetic abnormalities; MM-MDS, myeloma cells expressing cytogenetic features seen also in myelodysplastic syndrome.
Telomerase activity and telomere length associated with overall survival in subset of MM
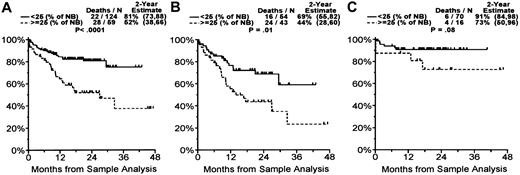
Elevated TA was associated with significantly shorter survival. At 1 year the survival rate was 82% in patients with TA level less than 25% of NB control and TL greater than 5.5 kbp versus a 63% survival rate and shorter TL in patients with higher TA level (P = .004). Patients with MM whose TA level was 25% or greater than that of NB control (59 of 183 patients) had significantly shorter survival (28 of 59 died; 2-year survival estimate, 52%) than patients with lower levels of TA (22 of 124 died; 2-year survival estimate, 81%; P < .0001) (Figure 6A). When divided into groups with newly diagnosed or relapsed disease, the patients in relapse whose MM cells had TA levels 25% or greater than the levels in the NB controls (43 of 97 patients) had significantly shorter survival (24 of 43 died; 2-year survival estimate, 44%) than patients with lower levels of TA (16 of 54 died; 2-year survival estimate, 69%; P = .01) (Figure 6B). In the group with newly diagnosed disease, we still observed a trend to poorer survival in patients with higher TA than in those with lower TA, but this did not reach statistical significance (P = .08; Figure 6C), probably because of the small number of patients in this group. Dividing into newly diagnosed or relapsed groups with TL levels less than versus more than or equal to the median of 5.5 kbp did not reveal any significant difference in survival rate (data not shown).
Overall survival by telomerase activity in patients with multiple myeloma. When TA was less than 25% of NB cell control, the estimated patient 2-year overall survival rate was 81%, and when TA was 25% or more of NB cell control, the estimated patient 2-year overall survival rate was 52% (P < .0001) (A). When divided into groups with newly diagnosed and relapsed disease, the patients in relapse whose MM cells had a TA of 25% or more the activity of NB control had a significantly shorter survival than patients with lower levels of TA (P = .01) (B). In the newly diagnosed group there was a trend to poorer survival in patients with higher TA than lower TA, but this did not reach statistical significance (P = .08) (C). Numbers in parentheses represent 95% confidence intervals.
Overall survival by telomerase activity in patients with multiple myeloma. When TA was less than 25% of NB cell control, the estimated patient 2-year overall survival rate was 81%, and when TA was 25% or more of NB cell control, the estimated patient 2-year overall survival rate was 52% (P < .0001) (A). When divided into groups with newly diagnosed and relapsed disease, the patients in relapse whose MM cells had a TA of 25% or more the activity of NB control had a significantly shorter survival than patients with lower levels of TA (P = .01) (B). In the newly diagnosed group there was a trend to poorer survival in patients with higher TA than lower TA, but this did not reach statistical significance (P = .08) (C). Numbers in parentheses represent 95% confidence intervals.
Discussion
The current study demonstrated that TA negatively correlated with TL and strongly correlated with MM patients' 2-year survival, though there was a heterogeneous distribution of TA in the myeloma samples with the median activity 8.2% that of the NB cell line control. This low median TA, relative to what has been reported in solid tumors, reflects the fact that one third of samples had either no significant TA level or TA level less than 5% that of the NB control. In contrast, 13% of patients had very high TA levels. In many tumors and in normal lymphoid or hematopoietic precursors, TA is down-modulated in quiescent cells and is rapidly up-regulated when cells are activated into cycle.16,41,42 Although there was a trend, the relationship between myeloma proliferative activity as determined by labeling index did not significantly correlate with TA (or TL). Thus, low telomerase levels cannot simply be explained by cycle-arrested cells. It has recently been reported that IGF-1 and IL-6 up-regulate TA in human myeloma cell lines and was mediated by phosphatidylinositol 3 kinase (PI3K)/Akt/NFκB signaling.33 However, we found no correlation between TA and serum IL-6 level. There was a strong negative correlation between IL-6 level and telomere length (P = .008). We found that TA was strongly correlated with age (P = .002), in contrast to results in large studies of primary lung cancer16 and breast cancer18 in which a negative correlation with age was observed.
There was a significant positive correlation of TA and a negative correlation of TL with poor prognosis features such as β2-M levels40 and chromosome 13 deletions.43 However, our analysis showed that TA, but not TL, was strongly predictive of 1- and 2-year survival. The observation that low platelet count at diagnosis correlated with high TA and low TL could not be explained by replacement of marrow hematopoiesis by infiltrating MM cells because there was no significant correlation with hemoglobin levels, neutrophil or white blood cell counts, or percentage of PCs in the marrow.
The data showing a strong correlation between short telomeres and high TA in MM cells can be used to support the concept of protection of critically shortened telomeric DNA by telomerase capping.31,37 Our analysis of telomeric DNA distribution in myeloma cells showed preferential stabilization of shorter telomeres with progressive erosion of longer telomeres, particularly when mean TRF fell below 5.5 kbp. Nevertheless, the data support the concept of preferential telomerase stabilization of short telomeres because length becomes a critical trigger for the initiation of cell death/senescence induced by either the p53 or the p16-RB pathway.35,39 A further mechanism that has been evoked to explain short telomeres in the absence of senescence and chromosome end-fusion is overexpression of the sequence-specific telomere binding protein TRF-2.44 It is of interest that microarray analysis of CD19-enriched B cells and CD138-enriched plasma cells from tonsil, bone marrow, and myeloma showed that TRF-2 is expressed in B cells but is extinguished in all PC samples.45
In our analysis of a group of CD138+-enriched plasma cells derived from healthy donors, we observed 2 unusual features. The first was the presence of significant levels of TA in what are considered growth-arrested cells. Weng et al46 previously reported moderate levels of TA in plasma cells isolated from human tonsil. The second was the surprising length of telomeres (median length, 10.6 kbp) relative to peripheral blood lymphocytes (median length, 7.9 kbp) and myeloma CD138+ cells (median length, 5.3 kbp). In contrast to what occurs in most cell systems in which telomere shortening with concomitant loss of replicative potential occurs in the process of somatic cell differentiation and cell division, the B cells in germinal centers are characterized by extensive clonal expansion and selection and have telomeres significantly longer than in precursor naive B cells.46,47 Furthermore, TA was at least 128-fold higher in germinal center B cells than in naive or memory B cells, accounting for this telomeric elongation phenomenon.47 By comparing the median TL of the normal PC (10.6 kbp) with that of the myeloma population (5.3 kbp) and accepting 100 bp as an upper limit of base pair loss per cell division, a single transformed PC could undergo approximately 50 population doublings before reaching a point at which telomere shortening precipitated a crisis and telomere stabilization/elongation would be required to achieve tumor immortality. With this proliferative potential, 1014 to 1015 MM cells could be generated without the need for telomerase. Theoretically, therefore, a clinical tumor burden of MM at diagnosis (approximately 1011 cells) could be achieved without the need for the selection of immortalized clones.
Our identification of a small group of patients (7 of 115) whose tumor cells have excessively long telomeres (10.8-15.0 kbp) is particularly significant in view of the particularly short mean TL characteristic in most patients (Table 2). This group may use a nontelomerase mechanism for telomere maintenance (ALT) involving intertelomeric homologous recombination.48 ALT is reported in approximately 5% of tumors, mostly carcinoma-derived cell lines.48 Because 2 of 7 of our candidate ALT patients had elevated telomerase levels, we propose that ALT may sometimes coexist with telomerase-induced telomere stabilization, as previously shown in immortalized W138 cells.49 The existence of an ALT pathway in normal B-cell development has been documented in mice that have lost TA because of a knockout mutation (mTR-/-).50 ALT-associated promyelocytic leukemia (PML) bodies have been reported as large donut-shaped nuclear structures containing PML protein, telomeric DNA, and TRF-1 and TRF-2.51 We are evaluating our myeloma population, both prospectively and retrospectively, for such PML bodies and their association with particularly long telomeres.
Myeloma is associated with considerable cytogenetic instability involving translocations and additions or deletions of whole chromosomes. We observed a very strong correlation between any cytogenetic abnormality, as well as any whole chromosome addition or deletion, and the coexistence of high TA and short TL (Table 4). Of 13 specific chromosomal abnormalities that correlated strongly with high TA, 7 also correlated with short TL. Deletion of chromosome 13 was associated with both high TA and short TL. Cytogenetic analysis has shown partial or complete deletion of chromosome 13 in 15% to 20% of MM patients at diagnosis, and this imparts a poor prognosis suggestive of a tumor-suppressor gene(s) on this chromosome.43 We observed a very strong correlation between high telomerase, short telomeres, and a cytogenetic profile in the myeloma cells that has been associated with MDS. In a cytogenetic study of 868 patients with myeloma, abnormalities typical of MDS (for example, 5q-, -5, 7q-, -7, t(1;7), +8, 20q-) were found as sole abnormalities in individual metaphases in 5% of patients or as associated with abnormalities characteristic of myeloma within the same metaphases in 10% of patients.52 In 6 patients whose myeloma cells showed MDS cytogenetic abnormalities, MDS independently developed. The evolution of these specific cytogenetic abnormalities may be attributed to a common mechanism operating in malignant myeloid and lymphoid cells that could be directly linked to telomere shortening. Genetic instability associated with gross cytogenetic change is observed in many cancers and points to the role of shortened telomeres, leading to chromosomal fusion and to the formation of dicentric structures that are resolved by fragmentation or unequal distribution to daughter cells, with repeated cycles of breakage-fusion-bridge formation probably coupled with the capacity to survive such changes—for example, by loss of p53.53 Such cytogenetic instability is seen in cells with short telomeres, as in late-generation telomerase null mice (mTR-/-).54
We observed a highly significant correlation between chromosome 3 duplication and high TA with short telomeres (Table 4). This may be linked to a gene-dosage effect because the hTR gene is located on this chromosome. However, this raises a number of issues because hTR expression is not normally the limiting component of TA, and the transfer of chromosome 3 to immortal human tumor cell lines initiates the suppression of telomerase and telomere shortening and the induction of senescence.55-58 The latter studies indicate the existence of a telomerase suppressor gene(s) mapping to 3p14.2-p21.1,56 or to 3p21.3-p22 and 3p12-21.1,57 that induced complete silencing of endogenous hTERT mRNA.58 The absence of a telomerase suppressor effect associated with chromosome 3 duplication in myeloma may be related to a tissue-specific suppressor activity because suppression was reported in tumors of the kidney and breast in which TA is strongly suppressed in the normal tissues of these organs, versus myeloma as a malignancy in the B-cell compartment in which telomerase is strongly expressed in the course of normal development.
We observed a strong correlation between TA and frequency of jumping 1q arm abnormality in myeloma cells. This abnormality has been reported in up to 23% of myeloma patients with abnormal karyotype, and the speculation is that it involves the decondensation of pericentric heterochromatin.59 In MDS with jumping 1q21, the region distal to 1q21 jumps onto numerous different telomeres, leading to the suggestion that critical telomere shortening can trigger the jump.60 We did not observe a significant correlation between telomere shortening and this abnormality, favoring the decondensation model.
In conclusion, these studies have provided insight into the role of telomerase in cancer. This investigation has indicated 2 mechanisms for achieving myeloma cell immortality (TA stabilization of short telomeres and ALT) and has supported a link between cytogenetic instability and TL. In addition, TA has proven to be predictive of MM patient prognosis as determined by 2-year survival. The identification of more than 25% of patients with TL of less than 4.0 kbp indicates that MM should be highly amenable to anti-telomerase therapy because the rapid onset of senescence or apoptosis would be expected given the limited amount of telomeric DNA on certain chromosome ends.
Prepublished online as Blood First Edition Paper, February 27, 2003; DOI 10.1182/blood-2002-11-3451.
Supported by National Cancer Institute grants CA55819 (B.B., J.S., M.A.S.M.) and U19-CA67842 (M.A.S.M.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Shiek Baksh and Olga Whitman for excellent technical assistance, Kate deBeer for preparing the manuscript, and Clyde Bailey for providing clinical data management.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal