The inv(16)(p13q22) rearrangement is present in approximately 10% of cases with de novo acute myeloid leukemia (AML) and results in a CBFB-MYH11 gene fusion.1 Patients with this fusion gene define a specific subgroup with a relatively good prognosis, and the accurate identification of CBFB-MYH11/inv(16)–positive cases is therefore essential. Recent studies have shown that CBFB-MYH11 reverse transcriptase–polymerase chain reaction (RT-PCR)–positive cases can be missed by cytogenetic analysis.2,3 RT-PCR may be an efficient method for identifying CBFB-MYH11– positive cases. To date, at least 12 different CBFB-MYH11 fusion transcripts have been described that are caused by alternative splicing and variable breakpoints in both CBFB and MYH11.1,4-8 This diversity complicates routine CBFB-MYH11 RT-PCR diagnosis. Real-time quantitative PCR (qPCR) is currently being used for routine identification and quantification of many fusion genes and transcripts associated with hematologic malignancies. The amplicon size used in qPCR should be 300 bp or less. Because the distance between the smallest and longest CBFB-MYH11 fusion transcript is more than 1200 bp the efficient detection of all fusion transcripts requires at least 4 different qPCRs.8
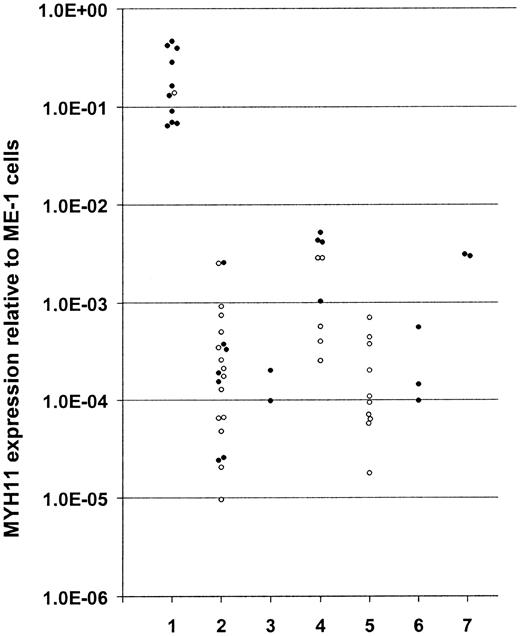
Because of the fusion to CBFB, the expression of the involved MYH11 RNA sequences might be significantly altered compared with normal levels from the unrearranged alleles. This would allow for rapid identification of CBFB-MYH11–positive cases by quantifying MYH11 mRNA expression. To test this hypothesis, we designed a MYH11 qPCR downstream of all known MYH11 fusion points. We determined the MYH11 expression in 32 bone marrow and blood samples taken from cases with newly diagnosed AML. Of these samples, 11 were CBFB-MYH11 positive as determined by cytogenetics and conventional RT-PCR.8 Of the CBFB-MYH11– positive cases, 6 were positive for the most frequently occurring fusion transcript (type A), 2 were positive for transcript type D, 2 were positive for the longest transcript (type E), and one was positive for the smallest transcript (type S).1,8 Within the group of CBFB-MYH11–positive cases the MYH11 expression varied 7-fold. This is in line with an earlier observation that the CBFB-MYH11 expression levels in a different group of 6 cases varied less than 5-fold at diagnosis.9 A significantly higher MYH11 expression was measured in all CBFB-MYH11–positive cases compared with negative cases (P = .000 0046, Mann-Whitney test, Figure 1). The median MYH11 expression detected in CBFB-MYH11–positive cases was 298-fold higher compared with the negative cases. The smallest difference between the CBFB-MYH11–positive patient with the lowest MYH11 expression and CBFB-MYH11–negative patient with the highest MYH11 expression was 25-fold. In 2 CBFB-MYH11–positive cases where follow-up material was available, remission samples showed MYH11 expression levels comparable to those observed in inv(16)-negative AML patients. Finally, we measured the MYH11 expression in bone marrow and blood samples taken from cases with other hematologic malignancies (n = 22) and from healthy volunteers (n = 2) and observed, as in CBFB-MYH11–negative AML cases, a significant lower expression compared with CBFB-MYH11–positive AML cases (Figure 1). We conclude that up-regulation of MYH11 expression because of the fusion to CBFB can be used to rapidly identify CBFB-MYH11– positive cases in newly diagnosed AML.
MYH11 overexpression in CBFB-MYH11– positive cells. The expression in CBFB-MYH11–positive ME-1 cells was set at 1.0. From all cases informed consent was obtained in accordance with the Declaration of Helsinki. The numbers indicate the following: (1) newly diagnosed CBFB-MYH11–positive AML (n = 11); (2) newly diagnosed CBFB-MYH11–negative AML (n = 21); (3) CBFB-MYH11–positive cases in complete hematologic remission (n = 2); (4) de novo chronic myeloid leukemia (n = 9); (5) history of chronic myeloid leukemia (n = 10); (6) de novo acute lymphocytic leukemia (n = 3); and (7) healthy volunteers (n = 2). ○ and • indicate blood and bone marrow samples, respectively. Primers and probes were developed downstream of all known MYH11 fusion points using Primer Express version 1.5 (Applied Biosystems, Foster City, CA). Sequences (5′-3′) of MYH11 forward and reverse primers and probe are respectively, AGTAGCCTGTCGGGAAGGAAC, GCCTGCTGTGTGGCTTTG, CACTCCAGGACGAGAAGCGCCG. The cDNA synthesis and input, cycling conditions, and PBGD expression measured for normalization were as described.9 For quantification, serial log dilutions of cDNA in H2O derived from the CBFB-MYH11–positive cell line ME-1 were used. Linear amplification extended down to a 4 log dilution.
MYH11 overexpression in CBFB-MYH11– positive cells. The expression in CBFB-MYH11–positive ME-1 cells was set at 1.0. From all cases informed consent was obtained in accordance with the Declaration of Helsinki. The numbers indicate the following: (1) newly diagnosed CBFB-MYH11–positive AML (n = 11); (2) newly diagnosed CBFB-MYH11–negative AML (n = 21); (3) CBFB-MYH11–positive cases in complete hematologic remission (n = 2); (4) de novo chronic myeloid leukemia (n = 9); (5) history of chronic myeloid leukemia (n = 10); (6) de novo acute lymphocytic leukemia (n = 3); and (7) healthy volunteers (n = 2). ○ and • indicate blood and bone marrow samples, respectively. Primers and probes were developed downstream of all known MYH11 fusion points using Primer Express version 1.5 (Applied Biosystems, Foster City, CA). Sequences (5′-3′) of MYH11 forward and reverse primers and probe are respectively, AGTAGCCTGTCGGGAAGGAAC, GCCTGCTGTGTGGCTTTG, CACTCCAGGACGAGAAGCGCCG. The cDNA synthesis and input, cycling conditions, and PBGD expression measured for normalization were as described.9 For quantification, serial log dilutions of cDNA in H2O derived from the CBFB-MYH11–positive cell line ME-1 were used. Linear amplification extended down to a 4 log dilution.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal