Reduced-intensity or nonmyeloablative stem cell transplantation (NST) is designed to induce host-versus-graft tolerance by engraftment of donor stem cells. The rationale behind NST is to induce optimal graft-versus-leukemia (GVL) effects for elimination of all malignant cells by donor alloreactive immunocompetent cells as an alternative to standard high-dose myeloablative chemoradiotherapy. NST based on the use of fludarabine, low-dose busulfan, and anti–T-lymphocyte globulin (ATG) was employed in 24 patients aged 3 to 63 years with chronic myeloid leukemia (CML) in first chronic phase (CP). Graft-versus-host disease (GVHD) prophylaxis consisted of low-dose cyclosporine (CSP), in some cases with low-dose methotrexate. Early discontinuation of CSP was attempted in cases of mixed chimerism in an attempt to amplify GVL effects. All 24 patients showed rapid 3-lineage engraftment, mostly without complete aplasia; 6 patients did not require transfusion of any blood products. NST was associated with minimal procedure-related toxicity. The incidence of acute GVHD (grade I or higher) was 54%; however, this incidence increased following CSP withdrawal. After a follow-up of up to 70 months (median, 42 months), 21 of 24 patients remained alive and disease free. The GVL effects induced by donor immunocompetent lymphocytes eradicated all host hematopoietic cells, as evidenced by molecular testing. The Kaplan-Meier probability of survival and disease-free survival at 5 years is 85% ± 8% (95% confidence interval, 70%-100%). NST may successfully replace myeloablative stem cell transplantation, providing a safer, well-tolerated therapeutic option for all patients with CML in first CP with a matched donor. However, this conclusion must be tested in a prospective randomized clinical trial.
Introduction
The use of bone marrow transplantation (BMT) from a fully matched donor for chronic myeloid leukemia (CML) is considered a most effective curative modality.1,2 Until recently, myeloablative conditioning was considered mandatory for the elimination of malignant hematopoietic cells and for prevention of allograft rejection. However, animal experiments in the 1960s3,4 and clinical observations in the 1970s confirmed that immune-mediated graft-versus-leukemia (GVL) effects play the most important role in the course of BMT.5-7 Furthermore, since 1987 we have documented that donor lymphocyte infusion (DLI) after grafting, with no additional chemotherapy, can eliminate leukemia cells even in patients fully resistant to maximally tolerated doses of chemoradiotherapy.8,9 Since then, DLI has been confirmed as the most effective therapeutic modality, especially for patients with CML with residual or recurrent disease following BMT.10-12 The rate of complete remission in response to DLI has been impressive, with 70% to 80% of relapsed CML patients accomplishing durable remissions following DLI8-12; success rates have been lower in other diseases treated by BMT.13-21 Given the unequivocal therapeutic role of donor lymphocytes in patients with CML22 and considering the documented therapeutic potential of alloreactive donor lymphocytes administered after BMT in CML patients relapsing following maximally tolerated doses of chemoradiotherapy,8-12 it seemed reasonable to exploit the therapeutic use of alloreactive donor lymphocytes following establishment of host-to-graft transplantation tolerance induced by engraftment of donor stem cells after reduced-intensity, lymphoablative yet nonmyeloablative conditioning.23
The present report summarizes our cumulative experience in a cohort of 24 consecutive patients with CML in first chronic phase and confirms that durable remission, with eradication of molecular evidence of disease, can be accomplished without myeloablative conditioning, thus avoiding or minimizing procedure-related toxicity and mortality.
Patients and methods
Twenty-four consecutive patients with Philadelphia-positive CML in first chronic phase were enrolled in this study, according to previously published criteria.24 Patients were included if they were proved to be in stable first chronic phase and consented to participate in the clinical trial, which was approved by the institutional review boards of the Hadassah Hospital in Jerusalem and the Souraski Hospital in Tel Aviv. The 15 male and 9 female patients ranged in age from 3 to 63 (median 35) years (Table1). All the patients were referred to BMT between May 1996 and January 2001. Each adult participant in the study signed an approved informed consent form. Parental approval endorsed by the court was obtained for 3 minors. Nineteen patients received transplants from family members (16 siblings and one father) fully matched for human leukocyte antigen (HLA) class I and II; 5 patients received marrow grafts from fully matched unrelated donors (MUDs) mildly reactive or nonreactive in mixed lymphocyte culture.
Characteristics and outcomes of patients with CML in first chronic phase undergoing NST from fully matched donors
| Unique patient no. . | Age, y . | Sex . | Donor's relationship to patient . | Interval from diagnosis to NST, mo . | Acute GVHD* . | Chronic GVHD4* . | ||
|---|---|---|---|---|---|---|---|---|
| Recipient . | Donor . | +CSP . | −CSP . | |||||
| 1031 | 50 | M | M | Sibling | 19 | 0 | II | Mild |
| 1053† | 57 | M | M | Sibling | 7 | 0 | II | 0 |
| 1119†,‡ | 3 | M | M | Father | 15 | 0 | IV | Severe→mild |
| 1135† | 34 | F | F | Sibling | 32 | 0 | IV | 0 |
| 1137† | 39 | M | M | Sibling | 3 | 0 | 0 | Mild |
| 1143† | 37 | M | F | Sibling | 5 | 0 | I | Mild |
| 1159 | 46 | M | M | Sibling | 9 | 0 | II | Moderate→resolved |
| 1176 | 48 | M | F | Sibling | 8 | II | NA | 0 |
| 1179 | 48 | F | M | MUD | 40 | II | NA | Mild |
| 1239 | 51 | M | F | Sibling | 6 | II | NA | Mild |
| 1286 | 26 | F | F | Sibling | 8 | II | NA | 0 |
| 1357 | 11 | M | M | MUD | 13 | III | NA | 0 |
| 1372 | 17 | M | F | MUD | 54 | IV | NA | Severe |
| 1379 | 45 | F | F | Sibling | 11 | IV | NA | Severe |
| 1384 | 20 | F | M | Sibling | 5 | III | NA | Severe |
| 1393 | 18 | M | F | MUD | 49 | II | NA | 0 |
| 1411 | 44 | F | F | Sibling | 3 | IV | NA | Moderate→mild |
| 1486 | 21 | F | F | Sibling | 13 | 0 | 0 | 0 |
| 1502 | 24 | F | M | Sibling | 7 | 0 | 0 | 0 |
| 1521 | 20 | F | F | MUD | 9 | II | NA | 0 |
| 1524 | 35 | M | M | Sibling | 4 | II | NA | Mild |
| 1575 | 25 | M | M | Sibling | 1 | I | 0 | Mild |
| 15761-153 | 25 | M | M | Sibling | 9 | 0 | 0 | 0 |
| 15771-153 | 63 | F | M | Sibling | 10 | 0 | 0 | 0 |
| Unique patient no. . | Age, y . | Sex . | Donor's relationship to patient . | Interval from diagnosis to NST, mo . | Acute GVHD* . | Chronic GVHD4* . | ||
|---|---|---|---|---|---|---|---|---|
| Recipient . | Donor . | +CSP . | −CSP . | |||||
| 1031 | 50 | M | M | Sibling | 19 | 0 | II | Mild |
| 1053† | 57 | M | M | Sibling | 7 | 0 | II | 0 |
| 1119†,‡ | 3 | M | M | Father | 15 | 0 | IV | Severe→mild |
| 1135† | 34 | F | F | Sibling | 32 | 0 | IV | 0 |
| 1137† | 39 | M | M | Sibling | 3 | 0 | 0 | Mild |
| 1143† | 37 | M | F | Sibling | 5 | 0 | I | Mild |
| 1159 | 46 | M | M | Sibling | 9 | 0 | II | Moderate→resolved |
| 1176 | 48 | M | F | Sibling | 8 | II | NA | 0 |
| 1179 | 48 | F | M | MUD | 40 | II | NA | Mild |
| 1239 | 51 | M | F | Sibling | 6 | II | NA | Mild |
| 1286 | 26 | F | F | Sibling | 8 | II | NA | 0 |
| 1357 | 11 | M | M | MUD | 13 | III | NA | 0 |
| 1372 | 17 | M | F | MUD | 54 | IV | NA | Severe |
| 1379 | 45 | F | F | Sibling | 11 | IV | NA | Severe |
| 1384 | 20 | F | M | Sibling | 5 | III | NA | Severe |
| 1393 | 18 | M | F | MUD | 49 | II | NA | 0 |
| 1411 | 44 | F | F | Sibling | 3 | IV | NA | Moderate→mild |
| 1486 | 21 | F | F | Sibling | 13 | 0 | 0 | 0 |
| 1502 | 24 | F | M | Sibling | 7 | 0 | 0 | 0 |
| 1521 | 20 | F | F | MUD | 9 | II | NA | 0 |
| 1524 | 35 | M | M | Sibling | 4 | II | NA | Mild |
| 1575 | 25 | M | M | Sibling | 1 | I | 0 | Mild |
| 15761-153 | 25 | M | M | Sibling | 9 | 0 | 0 | 0 |
| 15771-153 | 63 | F | M | Sibling | 10 | 0 | 0 | 0 |
Patient 1135 died of acute GVHD on day 116 after transplantation; patients 1372 and 1379 died of severe chronic GVHD on days 499 and 726 after transplantation, respectively.
CSP indicates cyclosporine A; and MUD, matched unrelated donor.
Graded according to International Bone Marrow Transplant Registry (IBMTR) severity indices.
Abrupt withdrawal of CSP.
Juvenile chronic myeloid leukemia.
Patients treated at the Souraski Medical Center.
NST consisted of intensive immunosuppression with intravenously administered fludarabine (30 mg/m2/d on days −10 to −5) and oral busulfan (4 mg/kg/d administered in 4 daily doses of 1 mg/kg on days −6 and −5) as previously described.18 All recipients of transplants from MUDs also received rabbit antihuman T-lymphocyte globulin (ATG; Fresenius, Gräfelfing, Germany) at a dose of 10 mg/kg/d on days −4 to −1). The dosage of ATG was lowered to 5 mg/kg/d in the last 10 patients receiving transplants from first-degree relatives. Donors were injected subcutaneously with granulocyte-colony stimulating factor (Neupogen, 5 μg/kg twice daily for 5 days) and mobilized peripheral blood stem cells were collected on days 5 and 6.
The details of the mobilized inoculum are presented in Table2. Prior to NST, all patients received trimethoprim/sulfamethoxazole (10 mg/kg/d trimethoprin) on days −10 to −2, acyclovir (500 mg/m2 × 3/d) from days −6 until day 100, and allopurinol (300 mg/d) on days −10 to −1. Administration of trimethoprim/sulfamethoxazole (twice weekly) was reinstituted after recovery from neutropenia as a preventive measure againstPneumocystis carinii infection. Cytomegalovirus (CMV) infections (diagnosed by 2 successive positive polymerase chain reactions [PCRs]) were treated with ganciclovir (10 mg/kg/d).
Mobilized blood stem cells used for transplantation on day 0 and 1 in patients undergoing NST for CML in first chronic phase
| . | Median . | Range . |
|---|---|---|
| Nucleated cells × 108/kg | 7.85 | 1.8-84.4 |
| CD34+cells × 106/kg | 0.94 | 0.11-4.50 |
| CD34+T cells × 107/kg | 46.2 | 31.50-72.60 |
| . | Median . | Range . |
|---|---|---|
| Nucleated cells × 108/kg | 7.85 | 1.8-84.4 |
| CD34+cells × 106/kg | 0.94 | 0.11-4.50 |
| CD34+T cells × 107/kg | 46.2 | 31.50-72.60 |
Graft-versus-host disease prophylaxis consisted of single-drug, low-dose, short-term cyclosporine (CSP) (3 mg/kg daily, administered intravenously in 2 divided doses) starting on day −1 in the first 19 patients. The remaining 5 patients received methotrexate in addition (15 mg/m2 on day 1 and 10 mg/m2 on days 3 and 6). Once the patients were dehospitalized, CSP was administered orally. CSP dosage was tapered during the second or third month after transplantation, according to chimeric status and evidence of GVHD.
Neutropenic patients with culture-negative fever received a combination of gentamicin, cefazolin, and mezlocillin as a first-line antibiotic protocol. Persisting fever was treated with amikacin and tazocin as a second-line protocol, while imipenem was used as the third-line protocol. In cases of persistent fever that did not respond to antibiotic therapy within 5 days, amphotericin B (1 mg/kg every other day) was added until the neutropenia resolved.
Starting on day −10, a DNA PCR test was done weekly to detect CMV. Two consecutive positive PCR results served as an indication for replacing acyclovir with ganciclovir until a minimum of 2 negative tests was obtained. Patients were treated in reverse isolation rooms equipped with high-efficiency particulate air (HEPA) filters and received a regular diet. Additional supportive measures, such as parenteral nutrition and blood component transfusion, were administered as necessary.
Acute and chronic GVHD were graded according to the Glucksberg et al criteria.25 Immediately upon the appearance of signs and symptoms of GVHD, methylprednisolone (2 mg/kg) and CSP were administered intravenously.
In order to assess engraftment, degree of chimerism, minimal residual disease, and early relapse, patients were monitored at regular intervals by cytogenetic analysis; by donor- and host-specific DNA markers, including male and female amelogenine gene PCR bands26; and by variable number tandem repeat (VNTR)–PCR assay.27 Cytogenetic analysis for the Philadelphia chromosome and the bcr/abl reverse transcriptase (RT)–PCR test were applied at the time of diagnosis and for detection of relapse during follow-up.28
Results
The protocol used for conditioning was well tolerated by all 24 patients. Patients were free to leave the hospital between treatment schedules, and 9 were treated partially on an outpatient basis. The large majority of patients did not experience oral mucositis and thus were maintained on normal oral intake; 34% of the entire group required supplemental parenteral nutrition. Moderate to severe hepatic veno-occlusive disease (VOD) occurred in 3 patients, but resolved completely within 3 months. Fever (temperature higher than 38°C) was noted in 16 patients and positive blood cultures were documented in 4 patients.
All patients displayed evidence of engraftment shortly after NST and none exhibited immune-mediated rejection. Mixed chimerism was detected in 6 patients and lasted from 4 to 57 (median 11) weeks; all 6 converted to full donor chimerism. Degree of chimerism was evaluated weekly in patients with documented mixed chimerism and monthly in patients with documented full donor engraftment. In the other 18 patients, rapid full engraftment of donor cells was confirmed, without evidence of transient mixed chimerism. In 20 of the 24 patients the white blood cell (WBC) count remained above 0.1 × 109/L, and neutrophils were observed in the blood smear throughout the posttransplantation course, whereas in 4 patients the WBC count never dropped below 0.5 × 109/L. In one patient the WBC count dropped to a level of 0.1 × 109/L for 3 days. The period of leukopenia (WBC count < 0.5 × 109/L) ranged between 0 and 12 (median 4) days. Time to recovery of absolute neutrophil count (ANC) above 0.5 × 109/L ranged between 0 and 26 (median 16) days. In 4 patients, ANC never dropped below 0.5 × 109/L. The period of neutropenia (ANC < 0.5 × 109/L) ranged from 0 to 12 (median 4) days. The time interval to platelet recovery (≥ 20 × 109/L) ranged from 0 to 26 (median 10) days. In 6 patients, platelet support was not required. The period of thrombocytopenia, with platelet transfusion requirements (platelet count < 20 × 109/L) ranged between 0 and 16 (median 3) days.
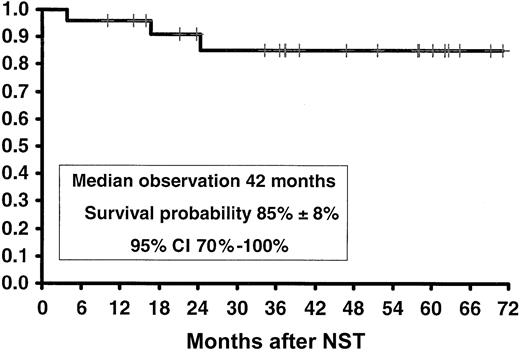
Mortality at day 100 was zero, indicating that NST was well tolerated by all patients in all age groups. Within an observation period of 7 to 63 months (median, 37 months) following NST, both the overall survival and disease-free survival (Figure 1) were 85% ± 8% (95% confidence interval [CI], 70%-100%), with no patients relapsing during this period.
Kaplan-Meier actuarial survival and disease-free survival of patients with CML in first chronic phase treated with nonmyeloablative stem cell transplantation (NST).
Kaplan-Meier actuarial survival and disease-free survival of patients with CML in first chronic phase treated with nonmyeloablative stem cell transplantation (NST).
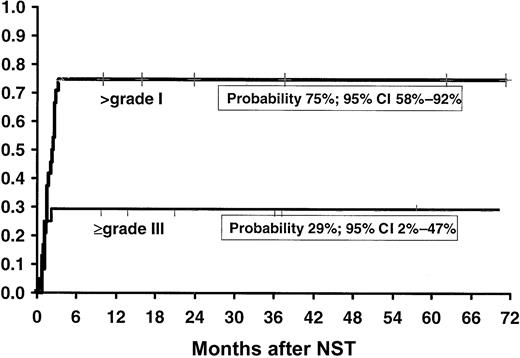
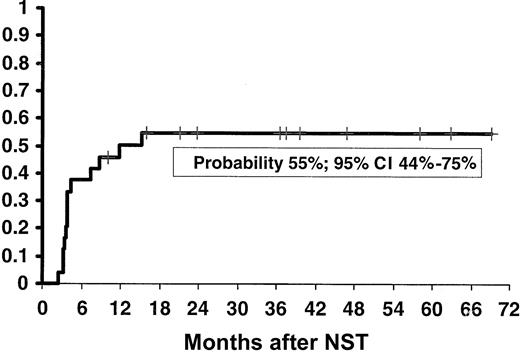
Acute GVHD (≥ grade I) occurred in 13 of 24 patients (probability, 54%; 95% CI, 34%-74%) while on CSP (1 grade I, 7 grade II, 2 grade III and 3 grade IV). The early withdrawal of CSP in 7 patients, in order to facilitate engraftment and eliminate residual host cells, resulted in 6 additional cases of acute GVHD (1 grade I, 3 grade II, and 2 grade IV), thus accounting for an overall cumulative probability of acute GVHD of 75% (95% CI, 58%-92%; Figure2). The overall incidence of severe acute GVHD (grades III and IV), including 2 patients who developed grade IV GVHD only when CSP was discontinued, was 29% (95% CI, 2%-47%; Figure 2). In 11 patients acute GVHD progressed to chronic GVHD. Four of these patients developed acute GVHD while on CSP, and 1 developed acute GVHD following early discontinuation of CSP. Only one patient developed de novo mild chronic GVHD (Table 1). The overall probability of chronic GVHD was 55% (95% CI, 44%-75%; Figure3). Three patients died as a consequence of GVHD: one on day 116 owing to acute GVHD, another on day 499 owing to chronic GVHD and invasive fungal infection, and the third on day 726 owing to chronic GVHD and recurrent infections. These were the only fatalities recorded in this series.
Kaplan-Meier actuarial probability of overall incidence of acute (higher than grade I) and severe (grade III or higher) GVHD in patients with CML in first chronic phase treated with nonmyeloablative stem cell transplantation (NST).
Kaplan-Meier actuarial probability of overall incidence of acute (higher than grade I) and severe (grade III or higher) GVHD in patients with CML in first chronic phase treated with nonmyeloablative stem cell transplantation (NST).
Kaplan-Meier actuarial probability of chronic GVHD in patients with CML in first chronic phase treated with nonmyeloablative stem cell transplantation (NST).
Kaplan-Meier actuarial probability of chronic GVHD in patients with CML in first chronic phase treated with nonmyeloablative stem cell transplantation (NST).
Mixed chimerism was observed in 6 patients who were receiving CSP therapy, hence posttransplantation immunosuppression was withdrawn abruptly. Three of these patients responded with total elimination of molecular evidence of disease (negative bcr/abl RT-PCR) and host-type DNA by VNTR-PCR or amelogenine gene PCR. Of the remaining 3 patients, whose chimeric studies showed a majority of host cells, 3 received DLI. One patient (no. 1119) received DLI in the form of blood stem cells obtained from the original donor on day 38, after which he converted to 100% donor cells with no residual circulating host DNA in the blood or in the marrow. The second patient (no. 1137) underwent a course of incremental doses of DLI, starting on day 111, with T-cell doses of 105/kg initially, 106 and 107 T cells/kg during the first 2 months, and 3 × 108 T cells when last given on day 308. The patient responded with a shift to 100% donor-type cells by day 399, with no residual circulating host DNA in the blood or in the marrow. The third patient (no. 1486) received DLI (105 T cells/kg) on day 210, again resulting in complete elimination of all detectable host cells. All the patients remain with 100% donor cells, with negative RT-PCT for bcr/abl. At an observation period ranging from 14 to 70 (median 42) months, none of the patients featured any evidence of molecular relapse. Consequently, the Kaplan-Meier probability of disease-free survival in this cohort remains 85% ± 8% (95% CI, 70%-100%) (Figure 1).
Discussion
Our data based on a cohort of 24 patients with CML in first chronic phase suggest that durable engraftment of fully matched HLA allografts from related or unrelated donors and durable elimination of molecular evidence of disease may be accomplished with minimal procedure-related toxicity and no early mortality following nonmyeloablative conditioning. The absence of graft rejection in this (admittedly small) group of patients is in keeping with our recent publications summarizing larger cohorts of patients conditioned with NST for other indications,17,20 as well as with the cumulative international experience comprising larger numbers of patients treated with different NST regimens based on the same principles.18,19,29-31 The common denominator of most recent NST protocols is the use of fludarabine for prevention of graft rejection. This drug induces effective apoptosis of malignant as well as normal lymphocytes. Furthermore, fludarabine has synergistic effects in combination with other alkylating agents, such as busulfan,17,20 cytoxan,30,32 or melphalan,18,29,33 or total body irradiation (TBI),19,31,34,35 thus explaining proven efficacy for consistent engraftment of matched related and unrelated stem cell allografts. In fact, fully matched related or unrelated donor stem cell engraftment can be accomplished while avoiding or minimizing early marrow aplasia and pancytopenia. Using the same NST regimen, engraftment was observed in all 16 consecutive recipients of allografts from unrelated donors, with 15 of 16 achieving 100% donor type chimerism,36 while the incidence of procedure-related toxicity (eg, severe mucositis, VOD, and multiorgan failure) and mortality were reduced. Clinical application of NST is based on the concept that the transplantation procedure is an improved immunotherapy protocol rather than an attempt to eliminate all host tumor cells by aggressive chemoradiotherapy up front, prior to rescue with donor stem cells. This explains the markedly reduced early mortality rate among our patients compared with the figures generally encountered in relation to the conventional myeloablative approach.37-39These observations, if confirmed, may justify the use of NST for elderly patients in need and patients with poor performance status who would not normally qualify for standard myeloablative BMT.
Allogeneic stem cell transplantation is the only proven cure for CML; however, the standard myeloablative procedure is associated with early toxicity and mortality and late complications.1,2,37-39 Therefore, there is a dilemma in using BMT for patients with asymptomatic CML that can be well controlled, though not necessarily cured, with conventional cytoreductive agents such as hydroxyurea, interferon, or the tyrosine kinase inhibitor STI571.40 Delaying transplantation in CML involves a complex decision, as best results are achieved if patients receive transplants while still in chronic phase, and preferably within the first year of diagnosis. Whereas postponement of BMT may be justified in patients responding to α-interferon or Glivec, other patients showing resistance to these agents, or patients with advanced stages of disease, should be offered BMT immediately. Because of anticipated procedure-related toxicity, BMT is seldom offered to elderly patients, even when the indication is unequivocal, owing to increased procedure-related mortality.24 Results from younger patients who underwent BMT following a conventional myeloablative preparatory regimen with high-dose cytoxan and busulfan or TBI, which were considered mandatory until recently, are unsatisfactory in many centers owing to acceptable procedure-related toxicity and mortality in young patients.37-39 However, using more intensive cytoreductive reigmens, including higher-dose TBI or additional splenic irradiation as part of the conditioning, confers no significant benefit over conventional protocols.38,39,41,42 The efficacy of well-tolerated NST in CML as suggested by our data could be predicted by the efficacy of elimination of molecular evidence of disease in the large majority of CML patients who relapse following myeloablative BMT after treatment with DLI alone.8-12 Occasionally, as shown here and as reported earlier in recipients of conventional BM transplants, discontinuation of CSP alone can result in regression or relapse.43 Likewise, there seems to be an increased risk of leukemia relapse in patients receiving high-dose CSP after allogeneic BMT,44 thus again indicating the important role of alloreactive T cells in controlling CML.
Our data may also suggest that the use of NST may offer an advantage to recipients with CML in first chronic phase receiving an allograft from a phenotypically matched unrelated donor. However, the number of patients in this series is small and a prospective randomized clinical trial with a larger number of patients is required to confirm these clinical impressions.
In spite of the possible advantage of lymphoablative over myeloablative conditioning prior to BMT in the treatment of CML, the problem of severe acute and chronic GVHD remains. Considering the therapeutic role of donor T cells, there is uncertainty as to whether CML patients should receive stem cell allografts derived from the blood, which contain higher proportions of immunocompetent T cells as well as committed stem cells, or bone marrow cells. In general, engraftment is more rapid with blood-derived stem cells, probably with the likelihood of an increased incidence of chronic GVHD.45 The feasibility of controlling CML by immunotherapy mediated by in vitro–activated alloreactive T cells46 and/or natural killer (NK) cells or NK T cells that may mediate more potent antitumor effects, occasionally with no GVHD,47 or using hematopoietic-specific48,49 or disease-specific cytotoxic donor lymphocytes,50 may provide an option to amplify antitumor effects while controlling or eliminating GVHD.
The elimination of all measurable evidence of disease and documentation of durable remission in our small cohort of patients, despite the use of reduced-intensity conditioning, is most encouraging. Based on the data presented and considering the cumulative international experience, it appears that reduced-intensity conditioning may be safely applied for all patients with CML in need while still in the chronic phase of the disease, especially those with recognized risk factors prohibitive for standard BMT. Confirmation of the benefits of NST as a possible replacement of conventional BMT will require a large prospective multicenter clinical trial, currently in progress. However, considering the documented efficacy of NST in a small cohort of patients with CML in first chronic phase, it is not impossible that this approach may ultimately develop into an optimal treatment of choice for patients in need of BMT, especially elderly patients and patients with poor performance status who fail to respond to STI571 or interferon, or patients not eligible for a conventional myeloablative regimen, and have a matched related or unrelated donor available.
This work was carried out in the Danny Cunniff Leukemia Research Laboratory.
Prepublished online as Blood First Edition Paper, September 19, 2002; DOI 10.1182/blood-2002-02-0535.
Supported by the Gabrielle Rich Leukemia Research Foundation; the Novotny Trust; the Fig Tree Foundation; and the Cancer Treatment Research Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Shimon Slavin, Department of Bone Marrow Transplantation and Cancer Immunotherapy, PO Box 12000, Jerusalem 91120, Israel; e-mail: slavin@huji.ac.il.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal