The introduction of an inducible suicide gene has been proposed as a strategy to exploit the antitumor reactivity of donor T cells after allogeneic hematopoietic stem cell transplantation but permit control of graft-versus-host disease. However, there are several obstacles to this approach that may impair the ability of T cells to function and survive in vivo. These include the requirement for in vitro activation or long-term culture to introduce the transgene and obtain therapeutic cell numbers, the toxicity of drug selection to enrich transduced cells, and the immunogenicity of the transgene-encoded products. Here we have developed a transduction and selection strategy for generating large numbers of polyclonal T cells transduced with a retroviral vector encoding the human low-affinity nerve growth factor receptor (LNGFR) for selection and a Fas-based suicide construct (LV'VFas). Ligation of CD28 in conjunction with a T-cell receptor signal permitted efficient transduction, substantially promoted T-cell growth, and contributed to the generation of gene-modified T cells that retained clonal diversity, functional properties, and a homing receptor profile similar to untransduced peripheral blood lymphocytes. Microbeads conjugated directly to antibody specific to LNGFR significantly improved the immunomagnetic selection of LV'VFas-modified T cells and assisted in scaling of the selection procedure to therapeutic cell numbers. Thus, these studies identified a strategy that requires only a brief ex vivo culture and does not use drug selection to obtain large numbers of functional gene-modified polyclonal T cells that can be used for adoptive immunotherapy.
Introduction
Advances in the genetic modification of T lymphocytes have led to the development of novel therapeutic approaches including the introduction of an inducible suicide gene into allogeneic donor lymphocytes as a strategy to modulate the graft-versus-leukemia effect after allogeneic hematopoietic stem cell transplantation (HSCT).1-4 However, published clinical trials and animal studies with transduced T cells have not consistently shown in vivo function or survival of the ex vivo manipulated cells.2,4-9 Limitations that were identified include the use of immunogenic bacterial genes to permit drug selection of transduced cells and extensive activation or prolonged ex vivo propagation to obtain therapeutic numbers of purified gene-modified T cells, which may account for the decreased in vivo function and survival.4-9 Thus, more effective immunotherapy will require the identification of rapid, nontoxic, and nonimmunogenic strategies to obtain therapeutic numbers of genetically engineered T cells with retained functional properties.
A variety of attempts have been made to generate gene-modified polyclonal T cells for antileukemia/lymphoma therapy. Donor T cells transduced to express the herpes simplex virus thymidine kinase (HSV-tk) suicide gene and selected in a single stimulation cycle in vitro have been adoptively transferred to allogeneic transplant recipients, but the transferred cells failed to mediate sufficient antitumor and anti-infectious immunity.5-7 The problem may have in part been caused by the use of anti-CD3 monoclonal antibodies (mAbs), supraphysiologic doses of interleukin 2 (IL-2) of up to 1000 U/mL, and drug selection to propagate and enrich transduced T cells in vitro. This approach can result in a substantial reduction of T-cell receptor (TCR) diversity,10,11 a dramatic loss of antigen-reactive T cells,5,12 and an increase in susceptibility to apoptosis.12-14 Moreover, bacterial and viral transgene products are immunogenic in humans and may result in premature elimination of transduced cells, thereby abrogating the desired therapeutic effect.4 15
An alternative to drug selection has been to express a cell-surface marker gene such as the intracytoplasmic truncated human low-affinity nerve growth factor receptor (ΔLNGFR) for specific and nontoxic immunomagnetic enrichment of transduced T cells.2,16 Donor lymphocytes transduced with both the ΔLNGFR and HSV-tkgenes were used to treat patients with Epstein-Barr virus lymphoproliferative disease or leukemic relapse after T-cell– depleted HSCT.2,15 In these reports, peripheral blood lymphocytes (PBLs) were activated in vitro using phytohemagglutinin (PHA), transduced, and enriched for ΔLNGFR expression using immunomagnetic selection with anti-LNGFR mAb and anti–immunoglobulin G (IgG)–coated magnetic microbeads. No immunity to the human ΔLNGFR selectable marker was detected. However, the HSV-tk suicide gene was a target for immune-mediated rejection of modified cells and this negated the benefit of using ΔLNGFR as a selectable marker.2 15
The goal of our studies was to develop an approach to obtain polyclonal T cells modified to express ΔLNGFR linked to an inducible suicide gene construct that is not immunogenic, for a clinical trial to control graft-versus-host disease (GVHD) after allogeneic HSCT. Several of the factors that may have contributed to the impaired function and survival of transduced T cells in prior studies were addressed in the preclinical development of this approach. First, since costimulatory signals delivered through CD28 improve the sensitivity of TCR signaling,17 augment IL-2 production and T-cell proliferation,18-20 and promote survival by increasing expression of Bcl-XL,21 we examined if CD28 costimulation can overcome the inherent problems of TCR and cytokine stimulation. Second, we assessed whether the efficiency of immunomagnetic selection could be improved by conjugating the anti-LNGFR mAb directly to magnetic beads and developed a methodology to scale selection for a large starting PBL population. Finally, to circumvent the immunogenicity of the HSV-tk suicide gene, we used a humanized suicide construct that is based on the induction of apoptosis through oligomerization of the human Fas protein by a synthetic drug (AP1903).22 Our studies demonstrate that large numbers of gene-modified T cells, which retain TCR-repertoire diversity, functional properties, and homing receptor expression, can be generated with a single cycle of in vitro stimulation. This strategy could be applied to both human and macaque (Macaca nemestrina) T cells and will facilitate in vivo evaluation of the adoptive transfer of gene-modified T cells in nonhuman primates and in humans.
Materials and methods
Retroviral vectors
The retroviral vector LV'VFas encodes for 3 human proteins expressed as a chimeric molecule and was provided by ARIAD Pharmaceuticals (Cambridge, MA).22 The fusion protein consists of the ΔLNGFR, 2 copies of the FK506-binding protein containing a single amino acid substitution at position 36 to serve as drug-binding domains, and the cytoplasmic domain of Fas. The construct was packaged in PG13 cells at the National Gene Vector Laboratory (Indiana University, Indianapolis, IN). The LV'V retroviral vector22 lacks the cytoplasmic domain of Fas and was used as a control in experiments to determine whether crosslinking of ΔLNGFR by anti-LNGFR mAb might cluster the Fas domains and activate signaling.
Retroviral transduction of primary human and macaque T lymphocytes
PBLs were isolated from heparinized blood obtained from healthy human donors or macaques by Ficoll-Hypaque gradient separation and cultured in RPMI 1640 medium (GIBCO-BRL, Gaithersburg, MD) supplemented with 10% human serum. Cells were activated in 6-well plates coated with anti-CD3 (OKT3, 30 ng/mL-100 ng/mL; Ortho Biotech, Raritan, NJ, or SP34; 20 ng/mL; PharMingen, San Diego, CA) and anti-CD28 mAbs (9.3; 1 μg/mL, P. Martin, Fred Hutchinson Cancer Research Center, Seattle, WA) or with soluble anti-CD3 mAb at identical concentrations. On day 1 after activation, recombinant IL-2 (50 U/mL) (Chiron, Emeryville, CA) was added and cells were placed in phosphate-deficient T-cell medium (GIBCO-BRL). On days 2 and 3, cells were exposed to LV'VFas retroviral supernatant containing 8 μg/mL protamine sulfate and IL-2 (50 U/mL), spun at 1000g for 1 hour at 32°C, and incubated overnight. T cells were then cultured in medium containing IL-2 (25 U/mL-50 U/mL) for at least 48 hours prior to analysis for transgene expression and function. For certain experiments, T cells were restimulated with anti-CD3 and anti-CD28 mAbs as described.23 24
Flow cytometry
PBLs and T cells from the same donors were analyzed by cell-surface staining with fluorescein isothiocyanate (FITC)– or phycoerythrin (PE)–conjugated mAbs to CD3 (SP34, PharMingen), CD4, CD8, CD16, CD28, and isotype-matched irrelevant control mAbs (Becton Dickinson, Mountain View, CA). Expression of ΔLNGFR at the cell surface was determined by staining with PE-conjugated murine anti–LNGFR IgG1 mAb (clone ME20.4; Chromaprobe, Mountain View, CA). In some experiments cells were stained for surface expression of α4β7-integrin (CD49d) (Immunotech Coulter, Marseille, France), L-selectin (CD62L), and CCR7 (PharMingen). All analyses were performed on a FACSCalibur flow cytometer and data were analyzed using CellQuest Software (Becton Dickinson).
Immunomagnetic selection of LV'VFas-modified T cells
For immunomagnetic selection, ΔLNGFR-modified T cells were either first incubated with a primary anti-LNGFR mAb (clone ME20.4, Chromaprobe), washed, and labeled with secondary rat anti–mouse IgG1-coupled magnetic beads (Miltenyi Biotec, Auburn, CA), or incubated with directly anti-LNGFR–conjugated microbeads alone. The directly conjugated microbeads were provided by Miltenyi Biotec. ΔLNGFR-expressing cells were enriched using MidiMACS techniques or the CliniMACS device (Miltenyi Biotec) and stained with an FITC-coupled anti–mouse IgG1 mAb (Tago Immunologicals, Camarillo, CA) or a PE-labeled anti-LNGFR mAb (Chromaprobe). The percentage of ΔLNGFR-expressing cells was determined by gating on CD3+cells, and using cell scatter gate or 7-amino-actinomycin D (0.75 μg/mL; Sigma Chemical, St Louis, MO) to exclude dead cells. Transduced, immunoselected, and cultured LV'V or LV'VFas-modified cells will from now on be referred to as LV'V+ or LV'VFas+ T cells.
Statistical analysis to compare selection using either anti-LNGFR mAb and IgG1+ microbeads or directly anti-LNGFR–conjugated beads was performed using the Wilcoxon rank-sum test.
AP1903-sensitivity and cell death assays
Sensitivity of unmodified, LV'V+, or LV'VFas+ T cells to drug-induced apoptosis was determined by exposing 0.5 × 106 cells/mL to various concentrations of AP1903 for 2 hours.22 Induction of cell death was determined after 24 hours by trypan blue exclusion. In some experiments the pan-specific caspase inhibitor Z-Val-Ala-Asp(OMe)-FMK was added at a final concentration of 20 μM (Enzyme Systems Products, Livermore, CA).
Apoptosis assays
In experiments, in which the viability of cultured PBLs and gene-modified T cells was assessed, cells were stained with FITC-labeled annexin V (PharMingen) and propidium iodide (PI) according to the manufacturer's instruction.
HLA-A* 0201 tetramer and intracellular cytokine staining of CMV-specific T cells
An HLA-A* 0201 tetramer folded with the peptide NLVPMVATV (pp65, aa 495-503) was constructed as described and used for detection of cytomegalovirus (CMV)pp65–specific CD8+ T cells.25 PBLs and the cultured T cells from HLA-A* 0201–positive donors were evaluated for CMVpp65-specific CD8+ T cells by staining with PE-conjugated HLA-A* 0201-peptide tetramer and with FITC-conjugated anti-CD3 and PE-Cy5–conjugated anti-CD8 mAbs (Becton Dickinson).
To evaluate antigen-specific intracellular cytokine secretion of CD8+ T cells, PBLs or cultured T cells were stimulated for 6 hours at 37°C with equal numbers of the HLA-A* 0201 Tap-deficient T2 cell line either unpulsed or pulsed with the pp65495-503peptide in the presence of anti-CD28 and anti-CD49d mAbs and 10 μg/mL Brefeldin A (Sigma). As a positive control, 1 μg/mL Staphylococcal enterotoxin B (Sigma) was used. CMV-specific CD4+ T-cell responses were evaluated by incubating PBLs or T cells with plastic-adherent monocytes that had been pulsed for 2 hours with CMV antigen (1:100). Cells were permeabilized using FACS Permeabilizing Solution and stained with an FITC-labeled interferon-γ (IFN-γ) mAb (Becton Dickinson). Cells were also stained for cell-surface expression of CD3 and CD8, or CD4 respectively, and analyzed by flow cytometry.
Lymphoproliferative assay
PBLs or T cells (1 × 105/well) were plated in triplicate in the presence of γ-irradiated autologous PBLs (3500 rad) with medium alone, PHA (5 μg/mL; Sigma), CMV, varizella-zoster virus (1:400; M. Boeckh, Fred Hutchinson Cancer Research Center, Seattle, WA), or candida antigen (40 μg/mL; Greer Laboratories, Lenoir, NC). For mixed lymphocyte reactions, an equal number of γ-irradiated allogeneic PBLs (3500 rad) was added. In some experiments, cyclosporine A (100 ng/mL) was added to selected wells. Cultures were pulsed with 2.5 μCi/well (.093 MBq/well) of [3H]thymidine (NEN Products, Boston, MA) for the final 16 hours of a 4- or 6-day assay.
Evaluation of TCR Vβ gene usage
TCR Vβ gene usage was assessed using a multiplex polymerase chain reaction (PCR) method based on measurement of the complementary determining region 3 (CDR3) length.26 Briefly, total RNA was extracted from human PBLs or cultured T cells (RNeasy Mini Kit; Qiagen, Valencia, CA). Complementary DNA was synthesized from RNA using M-MLV reverse transcriptase and primer p(dT)12-18(GIBCO-BRL) and used for multiplex PCR. Each reaction contained an optimal concentration of TCR Vβ primers specific for 4 or 5 different families, a single TCR Cβ primer tagged with the fluorescent FAM (6-carboxyfluorescein) dye, 500 μM dNTPs, 2 mM MgCl2, 1 U AmpliTaq Gold DNA polymerase (Applied Biosystems, Branchbury, NJ) in 1X buffer. The PCR conditions were incubation at 95°C for 10 minutes, 35 cycles of 94°C for 30 seconds, 58°C for 20 seconds, 72°C for 30 seconds, and extension at 78°C for 10 minutes. The PCR products were size-fractionated on 2.2% agarose gels or analyzed on a GeneScan-3100 sequencer with the assistance of GeneScan software (Applied Biosystems).
To evaluate the TCR Vβ repertoire by flow cytometry, the following FITC- or PE-labeled anti–human TCR Vβ mAbs were used: anti–TCR Vβ1, Vβ2, Vβ3, Vβ3.1, Vβ5, Vβ5.1, Vβ5.2, Vβ5a, Vβ5c, Vβ6.7, Vβ7, Vβ8, Vβ11, Vβ12, Vβ12.1, Vβ13.1, Vβ13.6, Vβ14, Vβ16, Vβ17, Vβ18, Vβ20, Vβ21.3, Vβ22, and Vβ24 (Immunotech Coulter or Endogen, Woburn, MA). For multiparameter analysis, staining was paired with anti-CD3 and anti-CD8 mAbs.
Results
CD28 costimulation permits efficient transduction and expansion of primary T cells
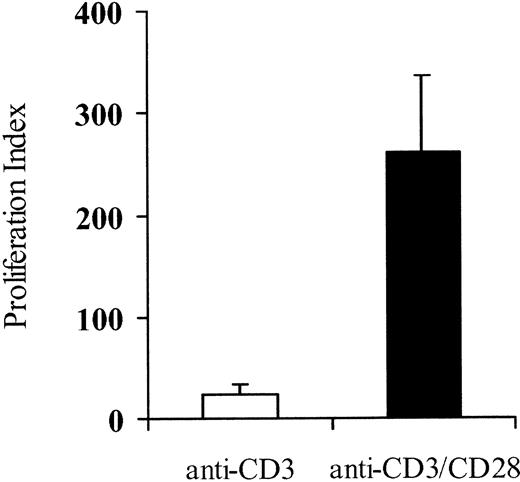
Activation of PBLs is required to permit retroviral-mediated gene transfer27 and to expand functional T cells to therapeutic numbers. Current methods to stimulate polyclonal T cells using anti-CD3 alone may initially result in an efficient recruitment of T cells into the dividing pool,28,29 but mitotic progression is poor and cells generally fail to maintain this proliferative response unless supraphysiologic doses of IL-2 are provided.28,30 By contrast, costimulatory signals delivered through CD28 promote sustained clonal expansion.19 20 Therefore, to determine whether the addition of anti-CD28 would facilitate the propagation of gene-modified polyclonal T cells, human PBLs from 4 different donors were stimulated with anti-CD3 alone or with both anti-CD3 and anti-CD28, transduced with LV'VFas retroviral supernatant, and evaluated for ΔLNGFR expression, proliferation, and phenotype. Optimal transduction as measured by expression of ΔLNGFR was achieved by combining phosphate-free media, low temperature, and spin-infection. In these experiments, the addition of anti-CD28 to cultures stimulated with anti-CD3 only slightly increased gene transfer compared with anti-CD3 alone (35.0%; range, 27.6%-46.2% vs 32.2%; range, 24.6%-42.2%). Similar gene-transfer was achieved in the context of polystyrene beads coated with anti-CD3 and anti-CD28 mAbs (data not shown). Proliferation was measured by numerical expansion. There was a high degree of proliferation early after stimulation with both activation methods (data not shown), but T cells cultured with anti-CD3 and anti-CD28 in the presence of low doses of IL-2 (25 U/mL-50 U/mL) demonstrated a consistent growth advantage with a mean 261-fold expansion (range, 137-fold-336-fold) during the 12 to 14 days of culture as compared with 22-fold (range, 11-fold-33-fold) with anti-CD3 alone (Figure 1).
CD28 costimulation enhances growth of primary T lymphocytes.
PBLs from 4 different donors were stimulated with anti-CD3 alone (■) or both anti-CD3 and anti-CD28 (▪), transduced with the LV'VFas retroviral vector, and cultured in the presence of low doses of IL-2 (25-50 U/mL). Proliferation of viable cells was measured by numerical expansion and trypan blue exclusion. The proliferation index was calculated by the ratio of total cell number at days 13 to 14 of the culture to the starting cell number at day 0. Means and standard deviations are displayed.
CD28 costimulation enhances growth of primary T lymphocytes.
PBLs from 4 different donors were stimulated with anti-CD3 alone (■) or both anti-CD3 and anti-CD28 (▪), transduced with the LV'VFas retroviral vector, and cultured in the presence of low doses of IL-2 (25-50 U/mL). Proliferation of viable cells was measured by numerical expansion and trypan blue exclusion. The proliferation index was calculated by the ratio of total cell number at days 13 to 14 of the culture to the starting cell number at day 0. Means and standard deviations are displayed.
Finally, we examined the phenotype of gene-modified T cells and compared the results to the starting PBL population (n = 3). As compared with CD28 costimulation, stimulation with anti-CD3 alone was found to result in a lower frequency of CD3+ cells with decreased CD28 expression. The CD4+/CD8+ ratio was reversed in the anti-CD3 cultures as compared with the starting PBLs, and a mean of 10.4% of the cells displayed lineage markers for natural killer cells (CD16+) (Table1). Collectively, these results indicated that CD28 costimulation had the potential to permit for an efficient gene transfer and to markedly augment anti-CD3–induced proliferation without the need for extensive stimulation during cell growth.
Phenotypic characteristics of human LV'VFas-modified T cells stimulated with anti-CD3 alone or with both anti-CD3 and anti-CD28
| Phenotype . | PBLs, % mean (range) no stimulation . | LV'VFas-modified T cells, % mean (range) . | |
|---|---|---|---|
| Anti-CD3 . | Anti-CD3 and anti-CD28 . | ||
| CD3+ T cells | 66.2 (53.5-74.0) | 88.3 (81.9-93.5) | 97.3 (95.1-99.3) |
| CD3+CD4+ T cells | 58.0 (43.1-71.5) | 26.4 (10.8-36.2) | 47.6 (30.4-65.9) |
| CD3+CD8+ T cells | 39.3 (25.1-57.4) | 69.9 (63.6-81.4) | 48.7 (34.8-58.3) |
| CD3+CD28+ T cells | 74.2 (62.2-86.1) | 43.9 (35.6-52.3) | 88.6 (73.5-97.6) |
| CD16+ T cells | 12.8 (9.3-16.3) | 10.4 (6.5-12.7) | 1.2 (0.1-3.3) |
| Phenotype . | PBLs, % mean (range) no stimulation . | LV'VFas-modified T cells, % mean (range) . | |
|---|---|---|---|
| Anti-CD3 . | Anti-CD3 and anti-CD28 . | ||
| CD3+ T cells | 66.2 (53.5-74.0) | 88.3 (81.9-93.5) | 97.3 (95.1-99.3) |
| CD3+CD4+ T cells | 58.0 (43.1-71.5) | 26.4 (10.8-36.2) | 47.6 (30.4-65.9) |
| CD3+CD8+ T cells | 39.3 (25.1-57.4) | 69.9 (63.6-81.4) | 48.7 (34.8-58.3) |
| CD3+CD28+ T cells | 74.2 (62.2-86.1) | 43.9 (35.6-52.3) | 88.6 (73.5-97.6) |
| CD16+ T cells | 12.8 (9.3-16.3) | 10.4 (6.5-12.7) | 1.2 (0.1-3.3) |
Enrichment of ΔLNGFR+ T cells is scalable to clinically relevant numbers
Current cell culture techniques often require prolonged ex vivo propagation or extensive T-cell activation to achieve therapeutic numbers of gene-modified T cells. This may diminish clonal diversity because of the selective overgrowth of a small number of clones,10,11 reduce the ability of the cells to function and survive,5,9 12-14 and increase the probability of contamination. Since optimized methods for T-cell activation alone may not always circumvent all of these problems, we investigated methods for early immunomagnetic selection of ΔLNGFR-modified T cells derived from a large starting PBL population to determine if therapeutic numbers of T cells could be obtained within a single cycle of in vitro stimulation.
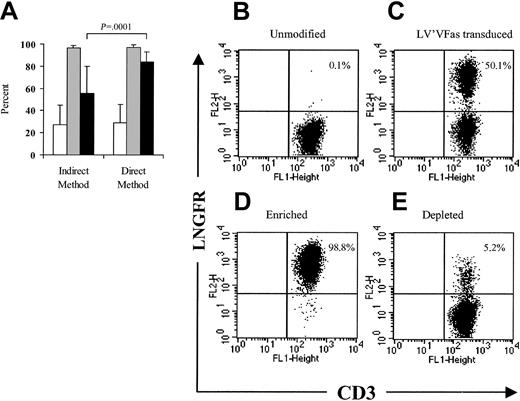
A 2-step immunomagnetic procedure in which the T cells were first incubated with anti-LNGFR mAb followed by the addition of anti-IgG1+ magnetic beads has been used for the enrichment of ΔLNGFR-expressing cells,2 but this approach may result in a relatively low yield of 23.5% ± 6.4%.31Conjugating the anti-LNGFR mAb directly to magnetic beads would allow for a shorter selection procedure and could provide more specific binding and improve selection yield. A series of small-scale experiments in which human or macaque PBLs (1 × 108-4 × 108) were transduced to express ΔLNGFR and enriched with anti-LNGFR+ microbeads alone was performed and compared with selections using the 2-step method. Both the indirect and direct methods enriched the ΔLNGFR-expressing cells to almost 100% purity (96.7% ± 2.0%; n = 27 vs 97.1% ± 2.3%; n = 26). However, the direct method provided a significantly improved yield (84.6% ± 8.4% vs 55.4% ± 24.4%; P = .0001) when compared with the indirect method (Figure 2A). A representative direct selection experiment is shown in Figure 2B-E.
Efficient immunomagnetic selection of LV'VFas-modified T cells using anti-LNGFR+ microbeads.
(A) LV'VFas-modified T cells were isolated by immunomagnetic selection using either anti-LNGFR mAb and anti-IgG1–conjugated microbeads (indirect method, n = 27) or anti-LNGFR–coated microbeads (direct method, n = 26). The number of viable cells was assessed by trypan blue exclusion. The frequency of the ΔLNGFR-expressing cells in cultures prior to selection (■) or after selection (░) was examined by flow cytometry using anti-LNGFR and anti-CD3 mAbs. ▪ denotes the yield of the selection procedure. Means and standard deviations are displayed. (B-C) Representative flow cytometry analysis of ΔLNGFR expression in macaque T cells either unmodified (B) or transduced with the LV'VFas retroviral vector (C). Macaque PBLs were stimulated with anti-CD3 and anti-CD28 and exposed to LV'VFas retroviral supernatant. T cells were then stained with anti–LNGFR-PE and anti-CD3 FITC mAbs and analyzed by flow cytometry. The percentage of cells positive for both ΔLNGFR and CD3 are as indicated. These data are representative of 16 experiments with macaque (n = 7) or human (n = 9) T cells. (D-E) LV'VFas+ T cells were then enriched with anti-LNGFR+ microbeads, as described in “Materials and methods.” The enriched fraction (D) and the depleted fraction (E) of T cells were evaluated for expression of ΔLNGFR and CD3.
Efficient immunomagnetic selection of LV'VFas-modified T cells using anti-LNGFR+ microbeads.
(A) LV'VFas-modified T cells were isolated by immunomagnetic selection using either anti-LNGFR mAb and anti-IgG1–conjugated microbeads (indirect method, n = 27) or anti-LNGFR–coated microbeads (direct method, n = 26). The number of viable cells was assessed by trypan blue exclusion. The frequency of the ΔLNGFR-expressing cells in cultures prior to selection (■) or after selection (░) was examined by flow cytometry using anti-LNGFR and anti-CD3 mAbs. ▪ denotes the yield of the selection procedure. Means and standard deviations are displayed. (B-C) Representative flow cytometry analysis of ΔLNGFR expression in macaque T cells either unmodified (B) or transduced with the LV'VFas retroviral vector (C). Macaque PBLs were stimulated with anti-CD3 and anti-CD28 and exposed to LV'VFas retroviral supernatant. T cells were then stained with anti–LNGFR-PE and anti-CD3 FITC mAbs and analyzed by flow cytometry. The percentage of cells positive for both ΔLNGFR and CD3 are as indicated. These data are representative of 16 experiments with macaque (n = 7) or human (n = 9) T cells. (D-E) LV'VFas+ T cells were then enriched with anti-LNGFR+ microbeads, as described in “Materials and methods.” The enriched fraction (D) and the depleted fraction (E) of T cells were evaluated for expression of ΔLNGFR and CD3.
The results for purity and yield were independent of the proportion of ΔLNGFR-expressing cells prior to enrichment and the day of selection following transduction (data not shown). In fact, direct selection of LV'VFas-modified T cells as early as day 6 after activation, 2 days after the transduction procedure, resulted in a purity of 97.1% (range, 93.2%-99.6%, n = 12) and a recovery of 85.8% (range, 71%-93.5%).
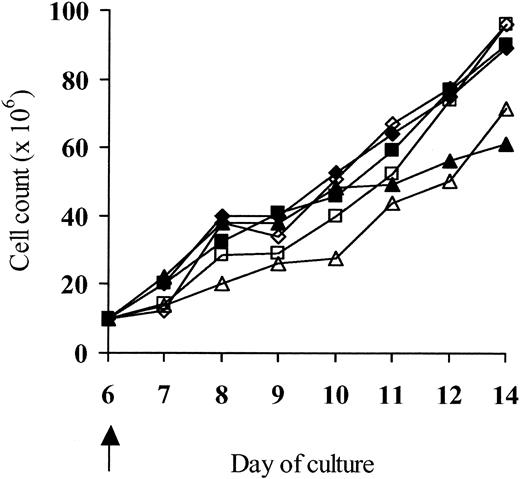
Crosslinking of ΔLNGFR on the surface of LV'VFas-modified T cells by the mAb-coated beads could theoretically induce clustering of the intracellular Fas domains, activate Fas signaling, and result in cell death. To test this possibility, T cells were transduced to express the LV'VFas or the control LV'V transgene, enriched on day 6 by immunomagnetic selection, and then assessed for viability by trypan blue exclusion and growth. There was no specific loss of viability after selection (viability > 93%) and the LV'VFas+and LV'V+ T cells proliferated similarly to unselected cells or the ΔLNGFR-depleted fractions (Figure3).
Binding of ΔLNGFR on the surface of LV'VFas+ cells by the LNGFR+ microbeads does not induce signaling of the intracellular Fas domains and cell death.
Macaque T cells were transduced with either the LV'VFas or the LV'V retroviral vector and on day 6 enriched for ΔLNGFR expression. Aliquots of cells were then evaluated for growth. LV'V-modified: unselected (▵), positive fraction (■), depleted fraction (⋄); LV'VFas-modified: unselected (▴), positive fraction (▪), depleted fraction (♦). The arrow indicates the day of the ΔLNGFR selection. Data shown are from 1 of 2 separate experiments.
Binding of ΔLNGFR on the surface of LV'VFas+ cells by the LNGFR+ microbeads does not induce signaling of the intracellular Fas domains and cell death.
Macaque T cells were transduced with either the LV'VFas or the LV'V retroviral vector and on day 6 enriched for ΔLNGFR expression. Aliquots of cells were then evaluated for growth. LV'V-modified: unselected (▵), positive fraction (■), depleted fraction (⋄); LV'VFas-modified: unselected (▴), positive fraction (▪), depleted fraction (♦). The arrow indicates the day of the ΔLNGFR selection. Data shown are from 1 of 2 separate experiments.
We next examined whether this early selection could be accomplished with a large starting number of ΔLNGFR-modified cells. Human PBLs (0.5 × 109-1 × 109) were obtained via leukapheresis, transduced with LV'VFas, and enriched using anti-LNGFR+ microbeads and the CliniMACS device originally developed for clinical-scale CD34+ cell enrichment.32 A high degree of purity of 98.5% (range, 96.6%-99.6%) was achieved from a starting population of up to 5.4 × 109 cells containing a mean of 20.1% (range, 10.1%-40.2%) ΔLNGFR-expressing T cells (n = 3). The mean yield of LV'VFas+ cells was 63.6% (range, 39.7%-79.4%). The large-scale selection procedure did not alter the phenotypic composition or growth of the enriched fraction (data not shown) and up to 3.7 × 1010 LV'VFas+ T cells were obtained at day 14 of the culture. Thus, this activation, transduction, and selection procedure is capable of generating large numbers of gene-modified T cells for immunotherapy in humans in a single stimulation cycle.
Function of the LV'VFas suicide construct
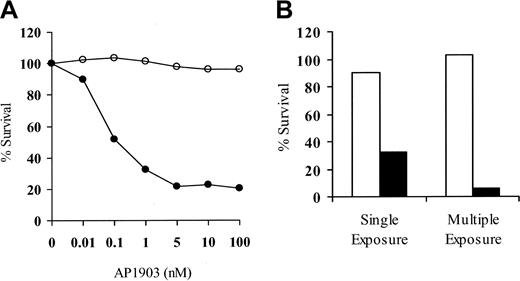
A goal of our studies was to develop a strategy to obtain donor T cells modified to express the LV'VFas suicide construct to allow the control of GVHD after allogeneic HSCT. To assess transgene function after direct immunomagnetic selection, LV'VFas+ T cells were cultured in the presence of AP1903 (0.01 nM-100 nM) to induce apoptosis.22 The viability of unmodified or LV'V+ T cells was not affected (data not shown), but 70% to 80% of both human and macaque LV'VFas+ T cells were killed after a single 2-hour exposure to more than 5 nM of AP1903 (Figure 4A). Cell killing increased to more than 90% after 4 drug exposures (Figure 4B).
LV'VFas+ T cells are sensitive to AP1903-induced cell death in vitro.
(A) Representative dose-response curve of AP1903-induced killing of primary LV'VFas+ T cells after purification for ΔLNGFR expression. Aliquots of macaque LV'VFas+ T cells were exposed for 2 hours with the indicated concentrations of AP1903 (●) or medium alone (○) and evaluated 24 hours later by trypan blue exclusion. The result shown is representative of 10 independent experiments with human or macaque T cells, indicating maximal killing at 5 nM. (B) Repeated exposure of LV'VFas+ T cells to AP1903 will eliminate the majority of LV'VFas-modified T cells. Aliquots of LV'VFas+ T cells were exposed once or 4 times (48 hours apart) for 2 hours to medium alone (■) or to medium containing 10 nM AP1903 (▪). Cells were washed, and survival was evaluated 24 hours later by trypan blue exclusion.
LV'VFas+ T cells are sensitive to AP1903-induced cell death in vitro.
(A) Representative dose-response curve of AP1903-induced killing of primary LV'VFas+ T cells after purification for ΔLNGFR expression. Aliquots of macaque LV'VFas+ T cells were exposed for 2 hours with the indicated concentrations of AP1903 (●) or medium alone (○) and evaluated 24 hours later by trypan blue exclusion. The result shown is representative of 10 independent experiments with human or macaque T cells, indicating maximal killing at 5 nM. (B) Repeated exposure of LV'VFas+ T cells to AP1903 will eliminate the majority of LV'VFas-modified T cells. Aliquots of LV'VFas+ T cells were exposed once or 4 times (48 hours apart) for 2 hours to medium alone (■) or to medium containing 10 nM AP1903 (▪). Cells were washed, and survival was evaluated 24 hours later by trypan blue exclusion.
Induction of apoptosis was abrogated by addition of the caspase-inhibitor Z-Val-Ala-Asp(OMe)-FMK, confirming the apoptotic mechanism of cell death (data not shown). Over a more than 2-month period, in which LV'VFas+ T cells were restimulated every 14 days, more than 90% of T cells continued to express ΔLNGFR and remained sensitive to AP1903 (data not shown). The steady-state plasma level of AP1903 that is achieved in vivo in humans33and macaques (C.B., S.R.R., and D. C. Dalgarno, unpublished data, May to August 2001) with a dose of 0.1 mg/kg of AP1903 administered over 2 hours is more than 80 nM. Thus, LV'VFas+ T cells are sensitive to concentrations of AP1903 that can be easily achieved in vivo.
LV'VFas+ T cells retain a broad TCR repertoire, antigen-specific function, and homing receptor expression after the transduction and selection
TCR Vβ repertoire.
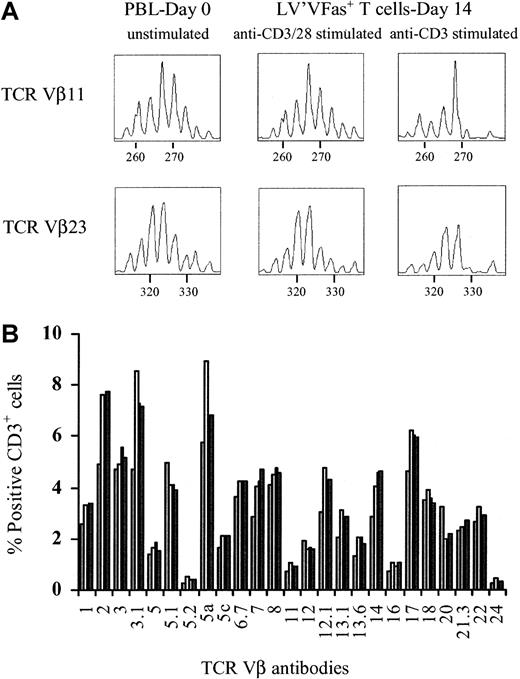
The maintenance of a diverse TCR Vβ repertoire in polyclonal donor lymphocytes may be critical for the induction of antitumor immunity or for restoring immunity to pathogens by adoptive immunotherapy after allogeneic HSCT. However, significant perturbations of the TCR Vβ repertoire have been observed even after brief anti-CD3– and IL-2–driven T-cell proliferation10,11 and could potentially result in the loss of T cells required for therapeutic efficacy. CD28 signaling promotes polyclonal proliferation of T cells20 and enhances the expression of intrinsic survival molecules21 which may assist in maintaining a diverse TCR Vβ repertoire. We first examined whether untransduced PBLs, cultured with anti-CD3 or combined anti-CD3 and anti-CD28 for 14 days, exhibited any changes in the TCR Vβ repertoire. PBLs from 2 donors were stimulated with anti-CD3 or both anti-CD3 and anti-CD28 and cultured for 12 to 14 days. The TCR Vβ gene usage and CDR3 size length of cultured T cells were then compared with the starting PBL population using a multiplex PCR technique that detects transcripts encoded by 46 of the known TCR Vβ genes comprising 23 TCR Vβ families.26 We found significant alterations of the TCR Vβ repertoire when anti-CD3 was used alone. In contrast, the addition of anti-CD28 allowed the diverse repertoire of the T cells to be maintained during the 14-day culture (data not shown). Based on these findings, we transduced PBLs from 7 donors with LV'VFas following anti-CD3 and anti-CD28 stimulation, enriched ΔLNGFR-expressing cells by immunomagnetic selection, and compared the TCR Vβ gene usage and CDR3 size length with the starting PBL population. In 2 of these donors, T cells were transduced after stimulation with anti-CD3 alone, selected, and subjected to the same TCR Vβ analysis. The CDR3 length distribution in the LV'VFas+ cells generated after anti-CD3 and anti-CD28 stimulation was almost identical to that in the starting PBL population in all 7 donors, whereas clonal diversity in cultures generated with anti-CD3 alone was impaired (Figure5A).
The TCR repertoire is maintained in cultures of transduced and immunomagnetic-selected LV'VFas-modified T cells.
(A) Spectratype profile for TCR Vβ11 and TCR Vβ23 from a single individual in response to stimulation to different activation methods. Multiplex PCR analysis of TCR Vβ gene usage in freshly isolated human PBLs and LV'VFas+ T cells cultured for 14 days was performed as described in “Materials and methods.” The profiles are of PBLs before and of LV'VFas+ T cells after stimulation with anti-CD3 and anti-CD28, or anti-CD3 alone. The horizontal axis denotes the length in base pairs. The data are representative of 2 separate experiments performed for comparison. (B) Representative flow cytometric analysis of the TCR Vβ gene usage in PBLs (▧) and unmodified T cells (■), or LV'VFas-transduced T cells either unselected (■) or ΔLNGFR-enriched (▪). Cells were stained with a panel of 24 human anti–TCR Vβ and anti-CD3 mAbs and evaluated by flow cytometry. The percentage of CD3+ cells positive for the individual TCR Vβ mAbs are as indicated.
The TCR repertoire is maintained in cultures of transduced and immunomagnetic-selected LV'VFas-modified T cells.
(A) Spectratype profile for TCR Vβ11 and TCR Vβ23 from a single individual in response to stimulation to different activation methods. Multiplex PCR analysis of TCR Vβ gene usage in freshly isolated human PBLs and LV'VFas+ T cells cultured for 14 days was performed as described in “Materials and methods.” The profiles are of PBLs before and of LV'VFas+ T cells after stimulation with anti-CD3 and anti-CD28, or anti-CD3 alone. The horizontal axis denotes the length in base pairs. The data are representative of 2 separate experiments performed for comparison. (B) Representative flow cytometric analysis of the TCR Vβ gene usage in PBLs (▧) and unmodified T cells (■), or LV'VFas-transduced T cells either unselected (■) or ΔLNGFR-enriched (▪). Cells were stained with a panel of 24 human anti–TCR Vβ and anti-CD3 mAbs and evaluated by flow cytometry. The percentage of CD3+ cells positive for the individual TCR Vβ mAbs are as indicated.
The TCR Vβ repertoire in the anti-CD3 and anti-CD28 stimulated population was also examined by specific mAbs, allowing for precise quantification of T cells expressing individual TCR Vβ genes by flow cytometry.34 We used 24 different anti–human TCR Vβ mAbs detecting 18 TCR Vβ families and covering the majority (> 70%) of the TCR Vβ repertoire to stain uncultured PBLs and T cells from 2 donors on days 12 to 14 of the culture. The LV'VFas+ T cells demonstrated substantial diversity comparable with uncultured PBLs or unmodified T cells (Figure 5B). Multiparameter analysis indicated that the contribution of CD4+ and CD8+ T cells to this diverse T-cell repertoire was equivalent (data not shown).
Antigen-reactive T cells.
The ability of CD28 costimulation and immunomagnetic selection to maintain clonal diversity suggested that functional antigen-specific T cells in the cultures might be similarly retained. We initially evaluated anti-CMV immunity since CMV causes significant morbidity and mortality following allogeneic HSCT and CMV-specific CD4+ and CD8+ T cells are present at easily detectable levels in PBLs from CMV-seropositive donors.
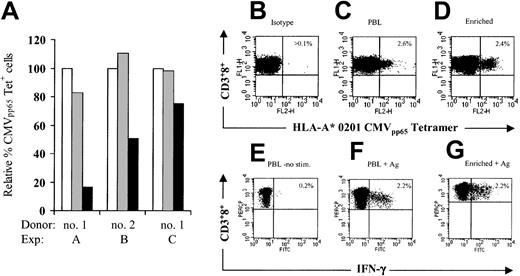
PBLs from CMV-seropositive donors were stimulated with anti-CD3 alone or with both anti-CD3 and anti-CD28, and then stained with the HLA-A* 0201/CMVpp65/495-503 tetramer. Consistent with the finding that engagement of the TCR results in internalization of the TCR-CD3 complex from the cell surface,23 28 there was a decrease of detectable tetramer+ CD8+ T cells early after activation in both cultures. However, 12 to 14 days after anti-CD3 and anti-CD28 stimulation, tetramer+CD8+ cells were detectable at similar levels as in the starting PBL population (Figure 6A). By contrast, the frequency of CMVpp65 tetramer+ T cells was reduced in cultures stimulated with anti-CD3 alone and only a mean of 49% (range, 14%-77%) could be visualized as compared with uncultured PBLs (Figure 6A). PBLs from 6 additional donors were then stimulated with anti-CD3 and anti-CD28, and after transduction with LV'VFas and immunomagnetic selection, the frequency of tetramer+ T cells was assessed. The results showed almost identical levels of tetramer+ CD8+ T cells as compared with uncultured PBLs, indicating that the ex vivo manipulation did not impair the frequency of the CMVpp65tetramer+ T cells (Table 2). A representative experiment is shown in Figure 6B-D.
Antigen-specific CD8+ T lymphocytes are maintained in cultures of anti-CD3– and anti-CD28–stimulated PBLs.
(A) Prevention of CMV-specific CD8+ T-cell loss by CD28 costimulation after 12 to 14 days of culture. PBLs (■) from CMV-seropositive donors were stimulated with anti-CD3 (▪) or both anti-CD3 and anti-CD28 (░), and frequency of HLA A* 0201 CMVpp65 tetramer+CD3+CD8+ cells was assessed as described in “Materials and methods.” Data are expressed as percentage of tetramer+ cells in CD3+CD8+ cells from fresh PBLs. Panels C-E indicate staining with HLA A* 0201 CMVpp65 tetramer-PE. Human PBLs (panel C) and LV'VFas+ T cells (panel D) grown with anti-CD3 and anti-CD28 were stained with PE-coupled HLA A* 0201 CMVpp65 tetramer, anti-CD3 and anti-CD8 mAbs, or isotype-matched control mAbs (panel B) and evaluated by flow cytometry. Values indicate the percentage of HLA-peptide tetramer+cells in CD3+CD8+ T cells. The results are representative of assays from 6 donors. Panels E-G indicate staining of cell-associated IFN-γ production after antigen stimulation. PBLs (panel F) and LV'VFas+ T cells (panel G) from the same donor were incubated for 6 hours with CMVpp65peptide-pulsed T2 cells or T2 cells alone (panel E) and stained with FITC-coupled anti–IFN-γ mAb, anti-CD3 mAb, and anti-CD8 mAb. Cells were gated to identify CD3+CD8+ T cells, and assessed for cytokine production. Values indicated the percentage of cells producing the relevant cytokine.
Antigen-specific CD8+ T lymphocytes are maintained in cultures of anti-CD3– and anti-CD28–stimulated PBLs.
(A) Prevention of CMV-specific CD8+ T-cell loss by CD28 costimulation after 12 to 14 days of culture. PBLs (■) from CMV-seropositive donors were stimulated with anti-CD3 (▪) or both anti-CD3 and anti-CD28 (░), and frequency of HLA A* 0201 CMVpp65 tetramer+CD3+CD8+ cells was assessed as described in “Materials and methods.” Data are expressed as percentage of tetramer+ cells in CD3+CD8+ cells from fresh PBLs. Panels C-E indicate staining with HLA A* 0201 CMVpp65 tetramer-PE. Human PBLs (panel C) and LV'VFas+ T cells (panel D) grown with anti-CD3 and anti-CD28 were stained with PE-coupled HLA A* 0201 CMVpp65 tetramer, anti-CD3 and anti-CD8 mAbs, or isotype-matched control mAbs (panel B) and evaluated by flow cytometry. Values indicate the percentage of HLA-peptide tetramer+cells in CD3+CD8+ T cells. The results are representative of assays from 6 donors. Panels E-G indicate staining of cell-associated IFN-γ production after antigen stimulation. PBLs (panel F) and LV'VFas+ T cells (panel G) from the same donor were incubated for 6 hours with CMVpp65peptide-pulsed T2 cells or T2 cells alone (panel E) and stained with FITC-coupled anti–IFN-γ mAb, anti-CD3 mAb, and anti-CD8 mAb. Cells were gated to identify CD3+CD8+ T cells, and assessed for cytokine production. Values indicated the percentage of cells producing the relevant cytokine.
Frequency of CMVpp65 tetramer+cells within CD3+CD8+ T cells before and after anti-CD3/CD28 activation, transduction, and immunomagnetic selection
| Donor no. . | PBLs, % . | T cells, % . | ||
|---|---|---|---|---|
| Untransduced . | LV'VFas+unselected . | LV'VFas+ enriched . | ||
| 1 | 2.1 | 2.5 | 2.1 | 2.6 |
| 2 | 0.4 | 0.4 | 0.3 | 0.4 |
| 3 | 1.1 | 0.8 | 0.9 | 1.1 |
| 4 | 0.8 | 0.6 | 0.6 | 0.7 |
| 5 | 4.1 | 3.0 | 2.9 | 3.2 |
| 6 | 3.1 | 3.2 | 2.4 | 3.0 |
| Donor no. . | PBLs, % . | T cells, % . | ||
|---|---|---|---|---|
| Untransduced . | LV'VFas+unselected . | LV'VFas+ enriched . | ||
| 1 | 2.1 | 2.5 | 2.1 | 2.6 |
| 2 | 0.4 | 0.4 | 0.3 | 0.4 |
| 3 | 1.1 | 0.8 | 0.9 | 1.1 |
| 4 | 0.8 | 0.6 | 0.6 | 0.7 |
| 5 | 4.1 | 3.0 | 2.9 | 3.2 |
| 6 | 3.1 | 3.2 | 2.4 | 3.0 |
To assess functional activity, we performed intracellular staining of IFN-γ after antigen stimulation. Aliquots of fresh PBLs and LV'VFas+ T cells that were generated by anti-CD3 and anti-CD28 stimulation were prepared and cultured with the CMVpp65/495-503 cognate peptide. The frequency of CD3+CD8+ T cells staining positive for IFN-γ remained nearly unchanged in LV'VFas+ T cells as compared with the starting PBLs (Figure 6E-G).
To ascertain that CMV-specific CD4+ T cells were similarly maintained in cultures generated with anti-CD3 and anti-CD28, we examined the antigen-stimulated cytokine production by CD4+T cells in freshly isolated PBLs and unmodified or LV'VFas+ T cells obtained from 4 CMV-seropositive donors. The PBLs and cultured T cells were stimulated for 6 hours with CMV antigen and examined by flow cytometry after staining with anti–IFN-γ and anti-CD4 mAbs. Similar to our findings with CD8+ T cells, the CMV-specific CD4+ T cells were detectable at nearly unchanged levels in cultures of LV'VFas+ T cells as compared with freshly isolated PBLs or unmodified T cells (Table 3).
Frequency of IFN-γ secreting CMV-specific CD4+ T cells before and after anti-CD3/CD28 activation, transduction, and immunomagnetic selection
| Donor no. . | PBLs, % . | T cells, % . | |
|---|---|---|---|
| Untransduced . | LV'VFas+ . | ||
| 1 | 2.0 | 2.0 | 1.9 |
| 2 | 5.0 | 5.8 | 7.5 |
| 3 | 10.3 | 7.3 | 11.7 |
| 4 | 6.0 | — | 6.2 |
| Donor no. . | PBLs, % . | T cells, % . | |
|---|---|---|---|
| Untransduced . | LV'VFas+ . | ||
| 1 | 2.0 | 2.0 | 1.9 |
| 2 | 5.0 | 5.8 | 7.5 |
| 3 | 10.3 | 7.3 | 11.7 |
| 4 | 6.0 | — | 6.2 |
— indicates data are missing.
We also examined whether T cells reactive to antigens other than CMV persisted in the cultures. PBLs from 3 donors were stimulated with anti-CD3 and anti-CD28 mAbs and, after transduction and selection for ΔLNGFR expression, stimulated with antigen preparations derived from different pathogens or with allogeneic γ-irradiated HLA-disparate PBLs and evaluated for [3H]thymidine incorporation. ΔLNGFR+ T cells proliferated in response to these antigens similarly to freshly isolated PBLs and to cultures of T cells either unmodified or transduced but unselected (Figure 7A). Similarly, the response to alloantigen was maintained in LV'VFas+ T cells and this alloreactivity was abrogated by addition of cyclosporin A to block TCR-induced IL-2 synthesis (Figure 7B). Collectively, these observations demonstrate that a diverse repertoire of functional CD4+ and CD8+ gene-modified T cells is obtained with the activation, transduction, and selection approach we used.
LV'VFas+ T cells retain responses to antigen derived from pathogens and to allogeneic cells.
(A) Freshly isolated PBLs (▪) and anti-CD3– and anti-CD28–stimulated T cells either unmodified (■), LV'VFas-transduced but unselected (▧), or LV'VFas+(░) were stimulated with PHA, or antigen derived from CMV, varizella-zoster virus (VZV), or candida, respectively, or with media alone. Cultures were pulsed with [3H]thymidine 16 hours prior to harvest. The [3H]thymidine incorporation was determined in counts per minute (cpm). The stimulation index was calculated by the ratio of cpm incorporated in wells stimulated with antigen to cpm in wells stimulated with medium alone. (B) Freshly isolated PBLs (▪) and T cells either unmodified (■) or LV'VFas+ (░) were plated with γ-irradiated HLA-disparate PBLs from 2 donors either in medium alone or in medium containing 100 ng/mL cyclosporin A (CSP) in a conventional mixed lymphocyte culture as described in “Materials and methods.” The stimulation index was calculated as described above. The data shown are from 1 of 3 separate experiments with different donors.
LV'VFas+ T cells retain responses to antigen derived from pathogens and to allogeneic cells.
(A) Freshly isolated PBLs (▪) and anti-CD3– and anti-CD28–stimulated T cells either unmodified (■), LV'VFas-transduced but unselected (▧), or LV'VFas+(░) were stimulated with PHA, or antigen derived from CMV, varizella-zoster virus (VZV), or candida, respectively, or with media alone. Cultures were pulsed with [3H]thymidine 16 hours prior to harvest. The [3H]thymidine incorporation was determined in counts per minute (cpm). The stimulation index was calculated by the ratio of cpm incorporated in wells stimulated with antigen to cpm in wells stimulated with medium alone. (B) Freshly isolated PBLs (▪) and T cells either unmodified (■) or LV'VFas+ (░) were plated with γ-irradiated HLA-disparate PBLs from 2 donors either in medium alone or in medium containing 100 ng/mL cyclosporin A (CSP) in a conventional mixed lymphocyte culture as described in “Materials and methods.” The stimulation index was calculated as described above. The data shown are from 1 of 3 separate experiments with different donors.
Homing receptor expression.
The ability of the transferred T cells to traffic to secondary lymphoid organs may be required for optimal antitumor response. Since antigen or mitogen stimulation may substantially modify the cell-surface expression of molecules that mediate homing or recirculating properties of T cells,35-38 we examined the impact of the activation, transduction, and selection procedure on the expression of several homing receptors by LV'VFas+ T cells. PBLs from 9 different donors were stimulated with anti-CD3 and anti-CD28 mAbs and, after transduction and selection, assessed for cell-surface expression of CD62L and CCR7,36,37 which are prerequisites for localization of T cells to secondary lymphoid organs, and for expression of CD49d, which mediates migration across endothelial barriers.36 PBLs from 3 donors were also stimulated with anti-CD3 alone for comparison. After 12 to 14 days of culture with anti-CD3 and anti-CD28, LV'VFas+ T cells displayed levels of both CD62L and CD49d that were comparable with uncultured PBLs, while levels of CCR7 were moderately reduced (Table4). In contrast, homing-receptor expression by T cells stimulated with anti-CD3 alone, transduced with LV'VFas, and enriched for ΔLNGFR expression, was markedly altered and only a few T cells were CD62L+ or CCR7+(Table 4). Expression of CD49d on anti-CD3–stimulated T cells was comparable with PBLs.
Surface expression of homing receptors by human LV'VFas+ T cells stimulated with anti-CD3 and anti-CD28 or with anti-CD3 alone
| T-cell subset . | PBLs, % mean (range) . | Stimulated T cells, % mean (range) . | |||
|---|---|---|---|---|---|
| Anti-CD3 and anti-CD28 . | Anti-CD3 . | ||||
| Untransduced . | LV'VFas+unselected . | LV'VFas+ enriched . | LV'VFas+enriched . | ||
| CD62L+ | 50.5 (21.6-66.7) | 50.9 (29.3-79.5) | 53.9 (31.5-81.0) | 51.0 (30.9-83.0) | 7.4 (6.2-8.7) |
| CCR7+ | 51.8 (30.7-74.8) | 34.5 (18.8-52.6) | 31.5 (17.9-48.8) | 31.0 (18.4-51.0) | 3.8 (2.8-4.5) |
| CD49d+ | 94.3 (83.4-99.5) | 99.2 (98.9-99.5) | 99.3 (98.9-99.8) | 99.5 (99.1-99.9) | 99.4 (99.0-99.8) |
| T-cell subset . | PBLs, % mean (range) . | Stimulated T cells, % mean (range) . | |||
|---|---|---|---|---|---|
| Anti-CD3 and anti-CD28 . | Anti-CD3 . | ||||
| Untransduced . | LV'VFas+unselected . | LV'VFas+ enriched . | LV'VFas+enriched . | ||
| CD62L+ | 50.5 (21.6-66.7) | 50.9 (29.3-79.5) | 53.9 (31.5-81.0) | 51.0 (30.9-83.0) | 7.4 (6.2-8.7) |
| CCR7+ | 51.8 (30.7-74.8) | 34.5 (18.8-52.6) | 31.5 (17.9-48.8) | 31.0 (18.4-51.0) | 3.8 (2.8-4.5) |
| CD49d+ | 94.3 (83.4-99.5) | 99.2 (98.9-99.5) | 99.3 (98.9-99.8) | 99.5 (99.1-99.9) | 99.4 (99.0-99.8) |
Data are representative of independent experiments from 9 different donors. In 3 experiments, PBLs were stimulated with anti-CD3 alone for comparison.
Susceptibility to cell death.
Successful T-cell therapy will require the ability of the ex vivo–manipulated cells to survive following transfer. However, there is evidence that T cells exposed to anti-CD3 mAb or continuously cultured in IL-2 become increasingly susceptible to cell death upon IL-2 withdrawal.13,14 39 To determine whether the ex vivo–manipulated T cells exhibit an increased rate of apoptosis, LV'VFas+ T cells cultured with anti-CD3 or combined anti-CD3 and anti-CD28 for 12 to 13 days were washed and plated on autologous γ-irradiated PBLs as feeder cells in the absence of IL-2 or mitogen stimulation. Fresh PBLs were plated in a similar fashion. After 7 days, cell viability was assessed by PI and annexin V staining. T cells stimulated with anti-CD3 alone demonstrated a marked increase in PI and annexin V staining as compared with freshly isolated PBLs (Figure 8A-B). In contrast, T cells cultured with anti-CD3 and anti-CD28 exhibited nearly identical staining to PBLs (n = 2) (Figure 8C), indicating that a single cycle of activation, transduction, and culture in low-dose IL-2 did not program the T cells to die or express a marker of apoptosis after cytokine withdrawal.
Survival of cultured polyclonal T cells stimulated with anti-CD3 and anti-CD28 after cytokine withdrawal.
Flow cytometric analysis of cultured T cells stained with FITC-coupled annexin V and PI. LV'VFas+ T cells cultured with anti-CD3 (B) or combined anti-CD3 and anti-CD28 (C) for 12 to 13 days were washed and plated on autologous γ-irradiated peripheral blood mononuclear cells as feeder cells in the absence of IL-2 or mitogen stimulation. Fresh PBLs (A) were plated in a similar fashion, and after 7 days cell viability was assessed by staining with annexin V and PI. T cells stimulated with anti-CD3 alone demonstrated a marked increase in apoptosis as compared with freshly isolated PBLs.
Survival of cultured polyclonal T cells stimulated with anti-CD3 and anti-CD28 after cytokine withdrawal.
Flow cytometric analysis of cultured T cells stained with FITC-coupled annexin V and PI. LV'VFas+ T cells cultured with anti-CD3 (B) or combined anti-CD3 and anti-CD28 (C) for 12 to 13 days were washed and plated on autologous γ-irradiated peripheral blood mononuclear cells as feeder cells in the absence of IL-2 or mitogen stimulation. Fresh PBLs (A) were plated in a similar fashion, and after 7 days cell viability was assessed by staining with annexin V and PI. T cells stimulated with anti-CD3 alone demonstrated a marked increase in apoptosis as compared with freshly isolated PBLs.
Discussion
The use of ex vivo–expanded gene-modified T cells is being explored as a therapeutic strategy to augment host T-cell immunity to chronic viral diseases and cancer.2-4 Several obstacles have been identified, including the immunogenicity of bacterial genes introduced to permit drug selection of transduced cells and impairments in repertoire, function, or in vivo survival related to procedures used for in vitro activation and culture. These problems have been especially apparent in studies in which donor lymphocytes were engineered to express a suicide gene to permit control of GVHD after allogeneic HSCT.5-7 Here we have developed a strategy for preparing large numbers of gene-modified T cells that requires only brief ex vivo culture and does not use drug selection.
Signals other than TCR triggering through CD3 have been shown to participate in T-cell activation. We found that CD28 costimulation substantially improved the generation of functional gene-modified T cells. First, CD28 costimulation enhanced cell growth compared with stimulation using anti-CD3 and thus may alleviate the requirements for extensive cytokine-driven stimulation or prolonged culture to achieve therapeutic cell numbers.20 This is important since there is abundant evidence for dual effects of IL-2 on T-cell survival.30,39-41 IL-2 has been shown to promote T-cell growth early after activation, but may induce cell death if stimulation is persistent or IL-2 concentrations increase above a threshold, by increasing expression of proapoptotic proteins or suppression of Fas-inhibitory molecules.40
Second, the addition of anti-CD28 to T cells activated by anti-CD3 led to the retention of clonal diversity and antigen-specific CD4+ and CD8+ T cells in the cultures. Previous work has shown that skewing of the TCR repertoire can be demonstrated following stimulation with anti-CD3 and IL-2 alone,10,11and that costimulation with anti-CD28 can prevent these alterations.10,20 Our results confirm and extend these studies, showing that costimulation can yield T cells with unmodified polyclonality and functional properties of both CD4+ and CD8+ T cells. These improvements may in part reflect the ability of CD28 signaling to enhance mitotic progression,28 maintain T-cell proliferation,20,28 and increase expression of intrinsic survival molecules such as Bcl-XL.21 Previous reports have indicated that coimmobilization of anti-CD3 and anti-CD28 mAbs on polystyrene beads,20 or on artificial antigen-presenting cells (APCs),42,43 may permit better formation of the immunologic synapse compared with plate-bound anti-CD3 and anti-CD28 mAbs and these approaches might provide further benefit. Moreover, other costimulatory molecules such as NKG2D44and 4-1BB45 have been identified and stimulation of these molecules may be beneficial. Expression of 4-1BB ligand by APCs in conjunction with anti-CD3 and anti-CD28 mAbs has been examined for T-cell stimulation and improved ex vivo expansion and function of antigen-specific CD8+ T cells.43 However, 4-1BB stimulation may preferentially activate CD8+ T cells46 and whether this approach will promote polyclonal expansion of both CD4+ and CD8+ PBL has not been reported.
Third, CD28 costimulation substantially improved the retention of homing receptor expression by cultured T cells and their survival properties after cytokine withdrawal. Effective immunotherapy will largely rely on the ability of the T cells to survive and traffic to appropriate target sites in vivo. Thus the loss of homing receptor expression and the increased susceptibility to apoptosis of T cells cultured with anti-CD3 alone may have contributed to the impaired in vivo function and survival of transferred T cells in previous studies.5-7 The ability to apply our methodology to macaque T cells will enable us to examine these questions in a large animal model.
The immunomagnetic selection strategy facilitated the generation of large numbers of functional LV'VFas+ T cells in a single stimulation cycle and in the absence of extensive cytokine stimulation. This was accomplished by numerically increasing the starting PBL population and utilizing directly conjugated LNGFR+microbeads, which improved yield. Orchard et al31 have selected large numbers of ΔLNGFR+ T cells derived from PBLs obtained by peripheral venipuncture using a 2-step selection procedure. However, the mean yields achieved with this methodology and a Miltenyi-selection device were 23.5% ± 6.4%, making it necessary to expand the cells with high doses (1000 U/mL) of IL-2.31The improved results observed in our studies might be in part attributed to the directly conjugated beads that allow for a shorter one-step selection procedure and appear to decrease cell loss. Moreover, the direct immunomagnetic selection did not compromise viability, phenotype, or functional properties of ΔLNGFR-modified T cells.
The human origin of ΔLNGFR may reduce the potential for immune recognition of gene-modified T cells and makes this molecule an attractive cell-surface marker for selection. However, recent studies in mice receiving transplants of ΔLNGFR-transduced bone marrow have suggested that ΔLNGFR expressed in stem cells may have cooperated with insertional activation of the Evi1 gene to cause leukemia in mice.47 The intracellular truncated version of LNGFR has been shown to functionally collaborate with some members of the Trk family of receptor tyrosine kinases and subsequently induce cell growth in certain cell types.48 49 Transformation of primary T cells by expression of ΔLNGFR has not been reported, but studies of the selectable marker gene will need to address this possibility. In this regard, the macaque model may provide an important large animal model for such studies.
In conclusion, we have shown that therapeutic numbers of purified LV'VFas+ T cells can be obtained following a single cycle of in vitro stimulation in the absence of extensive cytokine stimulation. The transduction and selection procedure described here did not compromise the functional capabilities of the cells. This, and the use of a nonimmunogenic suicide gene may allow the transferred cells to survive, traffic, and function after transfer. Future studies of T-cell transfer in nonhuman primates and in humans will provide insights into the efficacy and potential limitations of this approach in vivo.
We thank Ted Gooley for the statistical analysis and Michele Brown, Bryce Baril, and Dave Yadock for their technical assistance. We thank Carole Elliott and the staff of the University of Washington Regional Primate Research Center for technical assistance. We also thank Dr Kenneth G. Cornetta and Lilith A. Reeves at the National Gene Vector Laboratory (Indiana University, Indianapolis, IN) for vector production, and Ulf Bethke and Juergen Schmitz at Miltenyi Biotec (Bergisch Gladbach, Germany) for providing the directly conjugated reagent.
Prepublished online as Blood First Edition Paper, August 22, 2002; DOI 10.1182/blood-2002-07-2142.
Supported by National Institutes of Health grants HL66947 (S.H., S.R.R.), CA15704 (S.H.), CA18029 (S.H., S.R.R.), DK56465 (S.H.), and HL54881 (S.H.).
T.C. has declared a financial interest in Ariad Gene Therapeutics whose potential product was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Shelly Heimfeld, Clinical Research, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, Seattle, WA 98109; e-mail: sheimfel@fhcrc.org.

![Fig. 7. LV'VFas+ T cells retain responses to antigen derived from pathogens and to allogeneic cells. / (A) Freshly isolated PBLs (▪) and anti-CD3– and anti-CD28–stimulated T cells either unmodified (■), LV'VFas-transduced but unselected (▧), or LV'VFas+(░) were stimulated with PHA, or antigen derived from CMV, varizella-zoster virus (VZV), or candida, respectively, or with media alone. Cultures were pulsed with [3H]thymidine 16 hours prior to harvest. The [3H]thymidine incorporation was determined in counts per minute (cpm). The stimulation index was calculated by the ratio of cpm incorporated in wells stimulated with antigen to cpm in wells stimulated with medium alone. (B) Freshly isolated PBLs (▪) and T cells either unmodified (■) or LV'VFas+ (░) were plated with γ-irradiated HLA-disparate PBLs from 2 donors either in medium alone or in medium containing 100 ng/mL cyclosporin A (CSP) in a conventional mixed lymphocyte culture as described in “Materials and methods.” The stimulation index was calculated as described above. The data shown are from 1 of 3 separate experiments with different donors.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/2/10.1182_blood-2002-07-2142/4/m_h80233672007.jpeg?Expires=1765891498&Signature=XbBo3RhCE4fr08C77YEk1f9yRyb4~OX-KJH0yIfXjachVFD6sidSjAj3f1UAMiwX-f2Kci-NC2pYHuZnZ4KK0dYrwQQlq2PZHKdb8ux-W83ORFEa1j-3qSGkb9UkkqVp5al0oyXGxo3YYwS2hc8lQ1j8pDhZySmD0vKOpDAYpcnCBrV-ouHSSVUlEGF3IViRbjcYQmN4RCQDyiaupvtO-GLizrWzz2XE1LUrLZnXp2ZtOCWfha0psZf-2ptcD7NvWXAf2l4Q5Qr4YUQ2MgM7wSrD4YEhdjdh2khykdD1EPROBnA8BTCMchUF3jXESoYgE5pu5W27GrltwxYhDaRNpw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal