C-reactive protein (CRP) is a major acute-phase protein in humans. Elevated plasma CRP levels are a risk factor for cardiovascular disease. CRP is predominantly expressed in hepatocytes and is induced by interleukin-1 (IL-1) and IL-6 under inflammatory situations, such as the acute phase. Fibrates are hypolipidemic drugs that act through the nuclear receptor peroxisome proliferator-activated receptor-α (PPAR-α). Fibrates have been shown to reduce elevated CRP levels in humans, but the molecular mechanism is unknown. In this study, we demonstrate that different PPAR-α activators suppress IL-1–induced, but not IL-6–induced, expression of CRP in primary human hepatocytes and HuH7 hepatoma cells. Induction of CRP expression by IL-1 occurs at the transcriptional level. Site-directed mutagenesis experiments show that IL-1 induces CRP expression through 2 overlapping response elements, the binding sites for CCAAT-box/enhancer–binding protein-β (C/EBP-β) and p50-nuclear factor-κB (p50-NFκB). Cotransfection of C/EBP-β and p50-NFκB enhances CRP promoter activity, and coimmunoprecipitation experiments indicate that the increase in CRP promoter activity by IL-1 is related to the generation and nuclear accumulation of C/EBP-β–p50-NFκB complexes. Interestingly, PPAR-α activators reduce the formation of nuclear C/EBP-β–p50-NFκB complexes, and thereby CRP promoter activity, by 2 mechanisms. First, PPAR-α increases IκB-α expression and thus prevents p50-NFκB translocation to the nucleus. Second, fibrates decrease hepatic C/EBP-β and p50-NFκB protein levels in mice in a PPAR-α–dependent way. Our findings identify C/EBP-β and p50-NFκB as novel targets for PPAR-α and provide a molecular explanation for the reduction of plasma CRP levels by fibrates.
Introduction
Among the liver-specific or liver-enriched genes whose expression is strongly modulated during the acute phase of inflammation is C-reactive protein (CRP). CRP is a major acute-phase protein in humans, its plasma concentration increasing more than 1000-fold in severe inflammatory states.1,2 Several studies have reported a predictive association between elevated plasma CRP and coronary artery disease.3,4 There is increasing evidence that CRP is not merely an important and unique risk marker but that it also has a role in the pathogenesis of inflammation and atherosclerosis.5-8 CRP is synthesized predominantly in human liver, and the stimulation of CRP biosynthesis in response to trauma and inflammation is mainly mediated by interleukin-1 (IL-1) and IL-6.
Treatment of hyperlipidemia with fibrates reduces plasma CRP concentrations.9,10 Fibrates are clinically used hypolipidemic drugs that lower plasma levels of triglycerides and cholesterol, both of which are established risk factors for cardiovascular disease. Fibrates exert these beneficial activities on lipid and lipoprotein metabolism through the activation of the nuclear receptor, peroxisome proliferator-activated receptor-α (PPAR-α).11 It is unclear whether PPAR-α is also involved in the down-regulation of CRP by fibrates.
PPAR-α belongs to the superfamily of nuclear receptors that activate gene expression on ligand binding and dimerization with the retinoid X receptor (RXR). PPAR-α RXR heterodimers bind to specific sequences localized in the promoter region of target genes, termed peroxisome proliferator response elements. In addition to its role in mediating the hypolipidemic effects of fibrates, PPAR-α has been shown to act as a negative regulator of inflammatory processes by antagonizing the activity of the transcription factor pathways, such as NF-κB and AP-1.9 12
Recently, we reported another anti-inflammatory mode of action of fibrate-activated PPAR-α, namely through the binding of GRIP1/TIF2 (glucocorticoid receptor–interacting protein 1/transcription intermediary factor 2), a coactivator of the CCAAT box/enhancer-binding protein-β (C/EBP-β).13 This mechanism appeared relevant for quenching the expression of several C/EBP-β/GRIP1–regulated acute-phase response genes by fibrates, including the down-regulation of the expression of fibrinogen-α, fibrinogen-β, and serum amyloid A (SAA) genes. In accordance with these findings, the effect of fibrates on the fibrinogen gene expression was absent in PPAR-α knock-out mice.14 Because CRP is not an acute-phase protein in mice,2 knowledge of the role of PPAR-α in the regulation of CRP remained elusive.
Several recent studies indicate that the induction of CRP by IL-1 and IL-6 is at the transcriptional level, and it has been narrowed down to a 300-bp promoter fragment that harbors binding sites for the transcription factors signal transducer and activator of transcription 3 (STAT3),15 C/EBP-β, and p50-nuclear factor-κB (p50-NFκB).16,17 Because the classical binding partner of p50-NFκB—p65-NFκB—is not involved in the transcriptional activation of CRP and p50/p65 heterodimers are not capable of binding to the promoter, p50-NFκB is thought to enhance CRP transcription by facilitating the binding of transcriptionally active C/EBP.16 It is possible that transcription factors that transduce the effects of IL-1 and IL-6 on CRP expression are targets for fibrate-activated PPAR-α.
In the present paper we have investigated in detail the induction of CRP by IL-1 and the mechanism of CRP promoter inhibition by fibrates. It is shown that IL-1 and IL-6 strongly induce CRP expression in primary cultures of human hepatocytes, but only the IL-1 effect could be suppressed by fibrates and by a specific PPAR-α activator, Wy 14643. Evidence is provided that the induction of CRP gene expression by IL-1 requires the integrity of the overlapping response elements (REs) for p50-NFκB and C/EBP-β and correlates with the accumulation of IL-1–inducible p50-NFκB–C/EBP-β complexes in the nucleus. We demonstrated that fibrates and Wy 14643 reduce the amount of p50-NFκB–C/EBP-β complexes in the nucleus, and thereby CRP gene expression, by preventing the translocation of p50-NFκB from the cytosol into the nucleus. It is shown that fibrates and Wy 14643 induce a cytoplasmic inhibitor of NF-κB—IκB-α— thus trapping p50-NFκB in the cytosol. A second effect of fibrates on p50-NFκB–C/EBP-β was observed in vivo when fenofibrate treatment almost completely blocked the basal generation of p50-NFκB and C/EBP-β in the livers of wild-type, but not PPAR-α knock-out, mice. Our findings provide a specific molecular mechanism for the fibrate-induced down-regulation of CRP not shared with other acute-phase response genes repressed by these drugs, and they explain the uniqueness of CRP as a marker for inflammatory processes.
Materials and methods
Reagents
Ciprofibrate and bezafibrate were from Sanofi-Synthelabo (Aramon, France) and Roche Molecular Biochemicals (Almere, The Netherlands), respectively. Fenofibric acid was a kind gift of Dr A. Edgar (Laboratoires Fournier, Daix, France). Wy 14643 was from Chemsyn (Lenexa, KS), and simvastatin was from Merck (Amsterdam, The Netherlands). Human recombinant IL-1 and IL-6 were purchased from Sanvertech (Heerhugowaard, The Netherlands). All antibodies used in this study were from Santa Cruz Biotechnology (Heerhugowaard, The Netherlands). Molecular biology reagents were obtained from Life Technologies (Breda, The Netherlands). All other chemicals were specified in the references cited or were purchased from Sigma-Aldrich Chemicals (Zwijndrecht, The Netherlands).
Cell culture
Primary hepatocytes were isolated from human donor livers as described.18 Experiments with primary hepatocytes were approved by the institutional committees of the Leiden University Medical Center and TNO Prevention and Health. Cellular viability was greater than 85%, as determined by trypan blue exclusion. Cell culture conditions and experimental conditions for primary hepatocytes were as reported.19
The human hepatoma cell line HuH7, a cell line with endogenous CRP expression and responsiveness to IL-1, was a kind gift of Dr J. Rijntjes (Organon Teknika, Boxtel, The Netherlands). Hepatoma cells were cultured in Dulbecco modified Eagle medium (DMEM) (Life Technologies, Breda, The Netherlands) supplemented with 10% (vol/vol) fetal calf serum, 100 IU penicillin, and 100 μg/mL streptomycin.
Animal studies
Animal studies were performed in compliance with European Community specifications regarding the use of laboratory animals. Experimental conditions have been described previously.14Briefly, male Sv/129 homozygous wild-type (+/+) and PPAR-α knock-out (−/−) mice (10 to 12 weeks of age) were fed for 17 days with either a standard mouse chow or one containing 0.2% (wt/wt) fenofibrate. Animals were killed by exsanguination under ether anesthesia. Livers were removed immediately, weighed, rinsed with 0.9% (wt/vol) NaCl, frozen in liquid nitrogen, and stored at −80°C until use. For immunoblotting, livers were homogenized in phosphate-buffered saline (PBS) containing proteinase inhibitor (PI) (Roche Diagnostics) and centrifuged at 10 000g and 4°C for 5 minutes, and soluble proteins were immediately boiled in Laemmli electrophoresis buffer for immunoblot analysis.
Enzyme-linked immunosorbent assay measurements
Plasmids and luciferase assay
A genomic fragment corresponding to nucleotides −300 to −1 of the human CRP promoter21 was amplified using the primers 5′ CCT AGA TCT AGA GCT ACC TCC TCC TGC CTG G 3′ and 5′ CCG ACG CGT ACC CAG ATG GCC ACT CGT TTA ATA TGT TAC C 3′. The primers were designed to contain the BglII and MluI restriction sites, respectively. PCR products were then cloned into the luciferase reporter vector pGL3 (Promega, Leiden, The Netherlands). DNA sequences were confirmed by bidirectional sequencing of the clones. Constructs containing mutated binding sites for C/EBP-β and p50-NFκB were generated following a published procedure17 using the Quick Change Mutagenesis kit (Stratagene, Amsterdam, The Netherlands) and the primers 5′ GGA AAA TTA TTT ACA TAG TGT AGC TTA CTC CCT TAC TGC TTT GG 3′ and 5′ CCA AAG CAG TAA GGG AGT AAG CTA CAC TAT GTA AAT AAT TTT CC 3′ for mutation of the C/EBP-β binding site and 5′ CAT AGT GGC GCA AAC GAT ATT ACT GCT TTG GAT A 3′ and 5′ TAT CCA AAG CAG TAA TAT CGT TTG CGC CAC TAT G 3′ for mutation of the p50-NFκB–binding site. Mutant plasmids were bidirectionally sequenced to confirm sequence identity. Generation of the human fibrinogen-β promoter construct was described previously.13
The human hepatoma cell line HuH7 was used for all reporter gene assays. Applied fibrate concentrations did not affect cell viability, as determined by the MTT (3-(4,5-cimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) viability assay.22 Reporter gene assays were performed as described13 with the following modifications: the FUGENE6 (Roche Diagnostics) reagent was used, and 100 ng luciferase reporter plasmid was transiently transfected in 1.2 × 105 cells. After 20 hours, luciferase activity was quantified with the dual-luciferase reporter assay system (Promega) according to the manufacturer's protocol.
Cell extracts and coimmunoprecipitations
Total and nuclear cell extracts were prepared in the presence of PI (Roche Diagnostics), and all steps were performed at 4°C.23 24 Briefly, cells were washed twice with cold PBS, scraped off in 1.3 mL cold PBS containing PI, and collected by centrifugation at 800g for 5 minutes. Cell pellets were resuspended in cold PBS–protease inibitor, and equal amounts were used for the preparation of total and nuclear extracts. For the preparation of total cell extracts, resuspended cells were boiled in Laemmli electrophoresis buffer and stored at −80°C until use. For preparation of nuclear extracts, cells were pelleted again and resuspended in 1 mL cold hypotonic buffer containing 10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), pH 7.9, 10 mM KCl, 0.1 mM EDTA (ethylenediaminetetraacetic acid), 0.1 mM EGTA (ethyleneglycoltetraacetic acid), 1 mM dithiothreitol (DTT), and PI. Cells were allowed to swell for 15 minutes on ice. Then 62.5μL 10% (vol/vol) Nonidet P-40 was added, and cells were lysed within 2 minutes under shaking. After centrifugation at 1000g for 10 minutes, the supernatant was removed and the pellet containing the nuclei was washed with cold PBS/PI. Nuclei were incubated for 30 minutes in 20 mM HEPES, pH 7.9, 0.4 M KCl, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, and PI and were centrifuged for 5 minutes at 10 000g, and the supernatant corresponding to the nuclear extract was collected, boiled in Laemmli electrophoresis buffer, and stored at −80°C until use. Protein concentration was determined by the method of Bradford using a kit from Bio-Rad Laboratories (Veenendaal, The Netherlands).
Coimmunoprecipitation was performed as described previously.13 Briefly, lysed nuclei were centrifuged, and soluble nuclear proteins were incubated in 1.3 mL PBS/PI with anti–p50-NFκB antibody (1.5 μg/mL) or anti–MMP-8 (anti–matrix metalloproteinase 8) control antibody (1.5 μg/mL) for 16 hours at 4°C. Complexes were immunoprecipitated with protein A–Sepharose (Amersham Pharmacia Biotech, Roosendaal, The Netherlands) and washed 3 times in PBS/PI and 3 times in 50 mM Tris-HCl, pH 8.0, 170 mM NaCl, 0.5% (vol/vol) Nonidet P-40, and 50 mM NaF in the presence of PI. Washed complexes were immediately boiled in Laemmli electrophoresis buffer and analyzed by immunoblotting.
Western blotting
For sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), samples were electrophoresed as described.13Proteins were blotted onto Immobilon-P polyvinylidene fluoride transfer membranes (Millipore, Bedford, MA). Blots were blocked with 5% (wt/vol) skim milk powder (Merck, Amsterdam, The Netherlands) diluted in 20 mM Tris (pH 7.4), 55 mM NaCl, and 0.1% (vol/vol) Tween-20. Blots were developed with a goat anti–p50-NFκB primary antibody, a rabbit anti–C/EBP-β primary antibody, or a rabbit anti–IκB-α primary antibody and horseradish peroxidase–conjugated secondary immunoglobulin, respectively. Antihistone H1 and anti–β-actin antibodies were used for control. All antibodies were diluted in 20 mM Tris (pH 7.4), 55 mM NaCl, 0.1% (vol/vol) Tween-20, and 5% (wt/wt) bovine serum and were used at a final concentration of 0.2 μg/mL. The Super Signal West Dura Extended Duration Substrate (Pierce, St Augustin, Germany) and the luminescent image workstation (Roche Diagnostics) were used for visualization.
Statistical analysis
All data are presented as mean ± SD. Statistical analysis was performed with the Student t test, andP < .05 was considered statistically significant.
Results
Effects of ciprofibrate and Wy 14643 on CRP and fibrinogen expression in primary human hepatocytes
Figure 1 shows the effects of ciprofibrate and the PPAR-α activator Wy 14643 on the synthesis of CRP and, as a control, fibrinogen in primary human hepatocytes under basal and IL-1– or IL-6–induced conditions during a 24-hour incubation period. CRP concentrations were increased 23 times and 68 times by IL-1 and IL-6, respectively (Figure 1A). Ciprofibrate and Wy 14643 had no significant effect on basal CRP expression but strongly reduced the IL-1 induction of CRP. In contrast, IL-6–induced CRP expression was not or was only slightly reduced in the presence of ciprofibrate and Wy 14643, respectively. Fibrinogen concentrations were moderately but significantly increased 1.3 times with IL-1 and 2.1 times with IL-6 (P < .05). Ciprofibrate and Wy 14643 strongly decreased IL-6–induced fibrinogen production but showed no (ciprofibrate) or only moderate (Wy 14643) suppressive effect on basal and IL-1–increased fibrinogen synthesis (Figure 1B). These differential effects of ciprofibrate and Wy 14643 on IL-1– and IL-6–induced CRP and fibrinogen expression point to differences in CRP and fibrinogen gene regulation mechanisms.
Effect of ciprofibrate and Wy 14643 on CRP and fibrinogen expression in human hepatocytes stimulated by IL-1 and IL-6.
CRP (A) and fibrinogen (B) concentrations were measured in culture medium by ELISA and were expressed as means ± SDs. Human hepatocytes were isolated and treated for 16 hours with 250 μM ciprofibrate (CF), 250 μM Wy 14643 (WY), or vehicle (dimethyl sulfoxide [DMSO]; C) and subsequently were stimulated with 25 ng/mL IL-1 or IL-6 for an additional 24 hours. Results of 1 representative experiment of 3 experiments with different donors are shown. *P < .05 compared with control.
Effect of ciprofibrate and Wy 14643 on CRP and fibrinogen expression in human hepatocytes stimulated by IL-1 and IL-6.
CRP (A) and fibrinogen (B) concentrations were measured in culture medium by ELISA and were expressed as means ± SDs. Human hepatocytes were isolated and treated for 16 hours with 250 μM ciprofibrate (CF), 250 μM Wy 14643 (WY), or vehicle (dimethyl sulfoxide [DMSO]; C) and subsequently were stimulated with 25 ng/mL IL-1 or IL-6 for an additional 24 hours. Results of 1 representative experiment of 3 experiments with different donors are shown. *P < .05 compared with control.
CRP repression by fibrates is at the transcriptional level and is specific for activators of PPAR-α
We next investigated whether the negative interference of fibrates with the IL-1–induced expression of CRP is at the transcriptional level. A 300-bp promoter fragment of the human CRP gene21,25 containing the essential regulatory elements for IL-1–mediated promoter activity26,27 was cloned in front of a luciferase reporter gene, giving rise to pCRP-luc. The human hepatoma cell line HuH7 was transiently transfected with pCRP-luc, and cells were incubated with IL-1. Induction was optimal at 10 ng/mL IL-1 and resulted in 5- to 6-fold increased luciferase activity, as shown in Figure 2. Treatment of HuH7 cells with increasing concentrations (10-250 μM) of the structurally different fibrates, gemfibrozil, ciprofibrate, or bezafibrate or the PPAR-α activator Wy 14643 resulted in a concentration-dependent inhibition of IL-1–stimulated CRP promoter activity (Figure 2), with the strongest inhibitory effect observed with the specific PPAR-α activator Wy 14643. By contrast, BRL 49653 (10 μM), a specific activator of PPAR-γ,28 did not display an effect (Figure 2), supporting the notion that specific activation of PPAR-α is necessary to inhibit CRP transcription.
Inhibition of IL-1–induced human CRP promoter activity by PPAR-α activators in HuH7 cells.
HuH7 cells were transiently transfected with the human CRP promoter (pCRP-luc) linked to a luciferase reporter and incubated with increasing concentrations (10, 30, 50, 125, 250 μM) of gemfibrozil (GF), ciprofibrate (CF), bezafibrate (BF), and Wy 14643 (WY) or BRL49653 (BRL) (10 μM) for 5 hours and subsequently stimulated with IL-1 (10 ng/mL) for 18 hours. Luciferase activities are expressed as means ± SDs of several (3 or more) transfection experiments performed in triplicate.
Inhibition of IL-1–induced human CRP promoter activity by PPAR-α activators in HuH7 cells.
HuH7 cells were transiently transfected with the human CRP promoter (pCRP-luc) linked to a luciferase reporter and incubated with increasing concentrations (10, 30, 50, 125, 250 μM) of gemfibrozil (GF), ciprofibrate (CF), bezafibrate (BF), and Wy 14643 (WY) or BRL49653 (BRL) (10 μM) for 5 hours and subsequently stimulated with IL-1 (10 ng/mL) for 18 hours. Luciferase activities are expressed as means ± SDs of several (3 or more) transfection experiments performed in triplicate.
IL-1–induced CRP expression is mediated by p50-NFκB and C/EBP-β
Transcription factors that transduce the IL-1 effect on CRP expression have not been investigated. To that end we analyzed the CRP promoter fragment cloned in front of the luciferase reporter gene. This fragment contains a 17-bp stretch with 2 distinct response elements. One was a binding site for C/EBP-β and the other a binding site for p50-NFκB (Figure 3A).16 To delineate whether either of these sites was involved in IL-1–stimulated CRP promoter activity, binding sites for C/EBP-β and p50-NFκB within the pCRP-luc construct were mutated (Figure 3B). Mutation of the p50-NFκB binding site did not affect basal pCRP-luc activity, but it completely repressed induction by IL-1 (Figure 3B). Mutation of the C/EBP-β response element core site in pCRP-luc did not affect basal transcriptional activity either, but it significantly reduced the induction of CRP promoter activity by IL-1. These results point to a crucial role of p50-NFκB and C/EBP-β binding sites in IL-1–induced CRP promoter activity.
C/EBP-β and p50-NFκB mediate the induction of the CRP promoter by IL-1.
(A) The fragment of the human CRP core promoter containing the overlapping REs for C/EBP-β and p50-NFκB. (B) HuH7 cells were transfected with the wild-type human CRP promoter construct pCRP-luc and the mutated constructs pCRPΔp50NF-κB-luc and pCRPΔC/EBP-β-luc, as indicated, and subsequently were stimulated with IL-1. (C) Dose-dependent 18-hour stimulation of HuH7 cells with IL-1, as indicated, and Western blot analysis of p50-NFκB and C/EBP-β in nuclear extracts. Levels of histone H1 are shown for confirmation of equal loading. (D) HuH7 cells were transfected with pCRP-luc in the presence of p50-NFκB and C/EBP-β expression vectors or control vector (Con) and were stimulated with 10 ng/mL IL-1 for 18 hours. Luciferase activities represent means ± SDs of several (3 or more) transfection experiments.
C/EBP-β and p50-NFκB mediate the induction of the CRP promoter by IL-1.
(A) The fragment of the human CRP core promoter containing the overlapping REs for C/EBP-β and p50-NFκB. (B) HuH7 cells were transfected with the wild-type human CRP promoter construct pCRP-luc and the mutated constructs pCRPΔp50NF-κB-luc and pCRPΔC/EBP-β-luc, as indicated, and subsequently were stimulated with IL-1. (C) Dose-dependent 18-hour stimulation of HuH7 cells with IL-1, as indicated, and Western blot analysis of p50-NFκB and C/EBP-β in nuclear extracts. Levels of histone H1 are shown for confirmation of equal loading. (D) HuH7 cells were transfected with pCRP-luc in the presence of p50-NFκB and C/EBP-β expression vectors or control vector (Con) and were stimulated with 10 ng/mL IL-1 for 18 hours. Luciferase activities represent means ± SDs of several (3 or more) transfection experiments.
In accordance with this, stimulation with IL-1 increased the nuclear amount of p50-NFκB and C/EBP-β (Figure 3C), and overexpression of p50-NFκB or C/EBP-β by cotransfection resulted in a 2-fold and a 10-fold increase in basal CRP promoter activity, respectively (Figure3D). These increases in luciferase activity were further enhanced in the presence of IL-1, which can be explained by the increased nuclear translocation of p50-NFκB (data not shown).
The absence of an IL-1 effect on mutation of the p50-NFκB binding site and the proximity of the binding sites for C/EBP-β and p50-NFκB on the CRP promoter suggested to us that the 2 transcription factors act as a complex. To determine whether C/EBP-β and p50-NFκB are associated in regular human hepatocytes and HuH7 cells, coimmunoprecipitation experiments were performed with nuclear protein extracts. Specific coimmunoprecipitation of C/EBP-β–p50-NFκB was detected by anti–C/EBP-β Western blot when anti–p50-NFκB, but not control antibody, was used for precipitation (Figure4). Treatment of HuH7 cells and primary human hepatocytes with IL-1 strongly increased nuclear C/EBP–p50-NFκB levels, thus providing a molecular mechanism for the IL-1–stimulated CRP transcription rate.
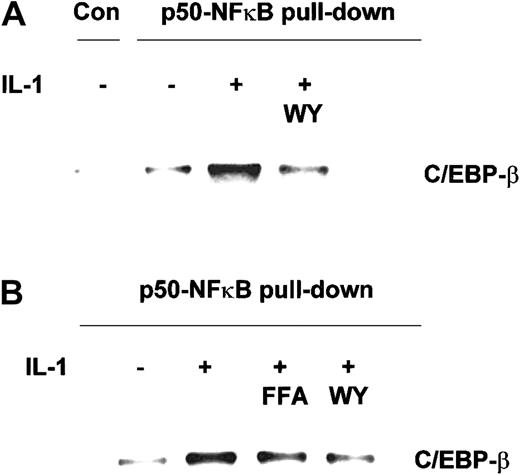
Effect of PPAR-α activators on the IL-1–induced formation of C/EBP-β–p50-NFκB complexes.
Coimmunoprecipitation was performed on nuclear extracts prepared from HuH7 cells (A) and freshly isolated primary human hepatocytes (B), preincubated for 6 hours with Wy 14643 (WY), fenofibric acid (FFA), or vehicle, and subsequently stimulated with 10 ng/mL IL-1 (HuH7 cells) or 25 ng/mL IL-1 (primary hepatocytes) for 17 hours. PPAR-α activators were used at a final concentration of 50 μM and 250 μM in HuH7 cells and primary hepatocytes, respectively. Anti–p50-NFκB or anti–MMP-8 control antibody (Con) were used for immunoprecipitation. C/EBP-β bound to precipitated p50-NFκB was detected by Western blot analysis.
Effect of PPAR-α activators on the IL-1–induced formation of C/EBP-β–p50-NFκB complexes.
Coimmunoprecipitation was performed on nuclear extracts prepared from HuH7 cells (A) and freshly isolated primary human hepatocytes (B), preincubated for 6 hours with Wy 14643 (WY), fenofibric acid (FFA), or vehicle, and subsequently stimulated with 10 ng/mL IL-1 (HuH7 cells) or 25 ng/mL IL-1 (primary hepatocytes) for 17 hours. PPAR-α activators were used at a final concentration of 50 μM and 250 μM in HuH7 cells and primary hepatocytes, respectively. Anti–p50-NFκB or anti–MMP-8 control antibody (Con) were used for immunoprecipitation. C/EBP-β bound to precipitated p50-NFκB was detected by Western blot analysis.
Fibrates and Wy 14643 prevent nuclear C/EBP-β–p50-NFκB complex formation
To determine whether the suppressors of IL-1–induced CRP promoter activity—that is, fibrates and Wy 14643—interfere with nuclear C/EBP-β–p50-NFκB complex formation, we performed coimmunoprecipitation on nuclear extracts from IL-1–treated HuH7 cells and primary human hepatocytes preincubated with activators of PPAR-α. As shown in Figure 4A-B, Wy 14643 and fenofibric acid markedly reduced nuclear C/EBP–p50-NFκB complex accumulation in IL-1–treated hepatocytes, thus explaining their suppressive effects on IL-1–stimulated CRP expression.
To evaluate at which level the various compounds interfere with IL-1–induced nuclear C/EBP-β–p50-NFκB accumulation, we analyzed total cellular and nuclear levels of C/EBP-β and p50-NFκB in HuH7 cells stimulated with IL-1 in the presence or absence of Wy 14643 or ciprofibrate by Western blotting (Figure5A). None of the compounds affected IL-1–induced total and nuclear C/EBP-β levels. However, though Wy 14643 and ciprofibrate were without effect on IL-1–induced total p50-NFκB levels, they strongly reduced IL-1–induced nuclear p50-NFκB accumulation. These findings indicate that the inhibition of p50-NFκB translocation is the prime cause for the suppression of the IL-1–induced C/EBPβ–p50-NFκB accumulation by PPAR-α activators in cultured human hepatocytes.
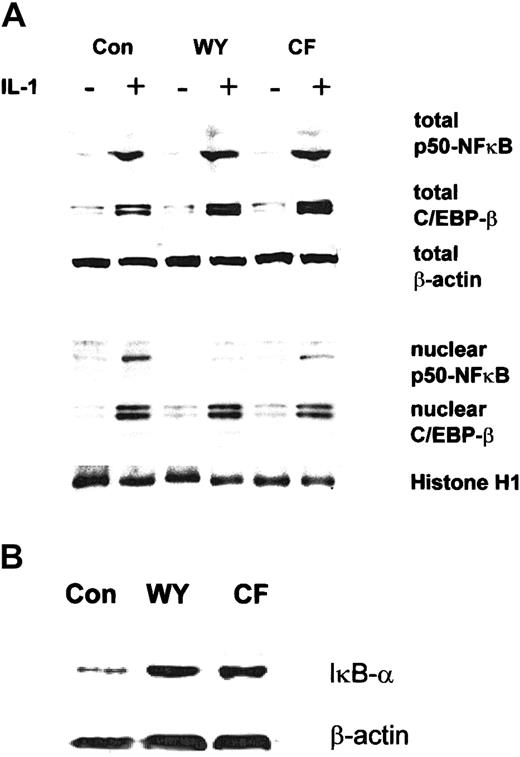
Effect of PPAR-α activators on the expression of C/EBP-β, p50-NFκB, and IκB-α in HuH7 cells.
(A) HuH7 cells were preincubated with Wy 14643 (WY; 50 μM), ciprofibrate (CF; 125 μM), or DMSO (Con) for 5 hours and subsequently were stimulated with 10 ng/mL IL-1 for 17 hours. Total cellular (upper panel) and nuclear (lower panel) extracts were analyzed for p50-NFκB and C/EBP-β expression. (B) HuH7 cells were incubated with PPAR-α activators for 24 hours, and total cellular extracts were analyzed for IκB-α by Western blotting. Equal loading was ensured by demonstration of uniform β-actin and histone H1 expression for total and nuclear extracts, respectively.
Effect of PPAR-α activators on the expression of C/EBP-β, p50-NFκB, and IκB-α in HuH7 cells.
(A) HuH7 cells were preincubated with Wy 14643 (WY; 50 μM), ciprofibrate (CF; 125 μM), or DMSO (Con) for 5 hours and subsequently were stimulated with 10 ng/mL IL-1 for 17 hours. Total cellular (upper panel) and nuclear (lower panel) extracts were analyzed for p50-NFκB and C/EBP-β expression. (B) HuH7 cells were incubated with PPAR-α activators for 24 hours, and total cellular extracts were analyzed for IκB-α by Western blotting. Equal loading was ensured by demonstration of uniform β-actin and histone H1 expression for total and nuclear extracts, respectively.
A possible explanation for impaired p50-NFκB translocation from the cytosol to the nucleus would be trapping of p50-NFκB by a cytoplasmic inhibitor of NFκB, termed IκB-α. Indeed, Wy 14643 and ciprofibrate all up-regulated IκB-α protein concentrations (Figure5B). Because IκB-α effectively binds p50-NFκB in the cytosol, it prevents nuclear C/EBP-β–p50-NFκB complex formation.
PPAR-α–dependent down-regulation of basal C/EBP-β and p50-NFκB expression in vivo
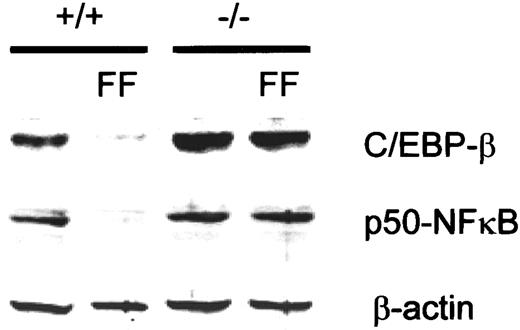
Previously, it had been shown that PPAR-α activation also induces IκB-α expression in mice.29 Evaluation of the hepatic expression of C/EBP-β and p50-NFκB in fenofibrate-treated mice revealed novel targets for the activity of PPAR-α. Treatment of mice with fenofibrate for 17 days resulted in strongly reduced C/EBP-β and p50-NFκB protein levels as analyzed by Western blotting (Figure 6). This reduction of C/EBP-β and p50-NFκB was dependent on PPAR-α because the effect of fenofibrate was absent in PPAR-α (−/−) mice. These effects may further contribute to the observed reduction of plasma CRP levels in patients treated with fibrates. The results also suggest that under chronic conditions of PPAR-α activation, a second mechanism—suppression of C/EBP-β and p50-NFκB levels—is operative.
Down-regulation of basal p50-NFκB and C/EBP-β expression on activation of PPAR-α in vivo.
Wild-type (+/+) and PPAR-α (−/−) mice were fed normal chow or 0.2% (w/w) fenofibrate (FF)–containing chow for 17 days, and total liver extracts were analyzed for p50-NFκB and C/EBP-β by Western blotting. Levels of β-actin are shown for confirmation of equal loading.
Down-regulation of basal p50-NFκB and C/EBP-β expression on activation of PPAR-α in vivo.
Wild-type (+/+) and PPAR-α (−/−) mice were fed normal chow or 0.2% (w/w) fenofibrate (FF)–containing chow for 17 days, and total liver extracts were analyzed for p50-NFκB and C/EBP-β by Western blotting. Levels of β-actin are shown for confirmation of equal loading.
Discussion
Fibrates reportedly lower plasma CRP levels in humans,9 but the regulatory mechanism of this effect remains to be clarified. In this report, we demonstrate that fibrates strongly inhibit IL-1–induced, but not IL-6–induced, CRP expression in human hepatocytes. We show that the induction of CRP expression by IL-1 is at the transcriptional level, requires the integrity of the overlapping REs for p50-NFκB and C/EBP-β, and correlates with increasing nuclear concentrations of C/EBPβ–p50-NFκB complexes. We found that fibrates and the PPAR-α activator Wy 14643 inhibit CRP transcription by reducing the formation of nuclear C/EBP-β–p50-NFκB complexes in 2 ways. First, we demonstrated that PPAR-α activators up-regulate IκB-α expression in vitro (current study) and in vivo,29 thereby preventing p50-NFκB translocation into the nucleus. Second, we showed a PPAR-α–dependent strong reduction of basal C/EBP-β and p50-NFκB expression levels in the livers of mice treated with fibrate for several days. More important, the results presented here give detailed insight into how fibrates inhibit CRP expression in humans.
Our findings show that IL-1 and IL-6 strongly induce the expression of CRP in human hepatocytes. This is in line with previous reports in which dual control of CRP gene expression by IL-1 and IL-6 was demonstrated in hepatoma cells,27 primary human hepatocytes,30 and mice carrying the human CRP transgene (hCRP).31 Interestingly, we found that fibrates and the PPAR-α activator Wy 14643 strongly suppressed the induction of CRP by IL-1 but did not affect or only slightly affected the induction by IL-6 in primary human hepatocytes, suggesting different regulatory mechanisms. Induction of CRP by IL-6 has been reported to involve the IL-6–inducible transcription factors STAT3,15 C/EBP-β, and C/EBP-δ,21,32 but the factors that participate in transducing the effects of IL-1 on CRP expression, such as those observed in IL-6(−/−) hCRP transgenic mice,31 remained unclear. Our study provides the first molecular explanation for the induction of CRP by IL-1. This IL-1 induction pathway in HuH7 cells is direct and independent of IL-6, because an IL-6–inducible, IL-6–RE–containing promoter fragment was not activated by IL-1 (R.K., unpublished data, December 2001). Functional analysis of the promoter of the human CRP gene in HuH7 human hepatoma cells revealed the existence of 2 overlapping response elements that are crucial for full induction by IL-1—the binding sites for C/EBP-β and p50-NFκB. Because IL-1 induces the expression and nuclear translocation of C/EBP-β and p50-NFκB and the cotransfection of C/EBP-β and p50-NFκB was found to enhance CRP promoter activity, we conclude that the induction of CRP by IL-1 involves the binding of the transcription factors C/EBP-β and p50-NFκB to their respective CRP promoter-binding sites. These findings also explain the unique position of CRP among the acute-phase proteins. Although C/EBP-β and STAT3 also mediate the expression of other IL-6–inducible acute-phase proteins, such as haptoglobin and fibrinogen,13,32,33 the stimulation of promoter activity by p50-NFκB and C/EBP-β is specific for CRP. Indeed, a computer search for sequences similar to the CRP promoter region harboring the (overlapping) REs for C/EBP-β and p50-NFκB was without results. These differences in gene regulation also provide a rationale for the use of CRP and fibrinogen as independent risk markers34 in clinical and epidemiologic studies to predict future cardiovascular events and to underscore their additive value.
Our observation that deletion of the p50-NFκB binding site completely abolishes CRP transcription by IL-1 supports the idea of concerted action of p50-NFκB and C/EBP-β and suggests interaction between the 2 transcription factors.17 We demonstrate for the first time that C/EBP-β and p50-NFκB physically interact in the nucleus under physiologically relevant expression levels, thereby providing a physiologic basis and a functional role for earlier in vitro overexpression studies.35,36 Because p50-NFκB lacks a transactivation domain and p65-NFκB abolishes CRP transcription,17 the mechanism by which p50-NFκB transactivates CRP is uncertain. Interaction with C/EBP-β under physiologic conditions gives strength to the hypothesis put forward by Cha-Molstad et al17 that p50-NFκB binds to the promoter region followed by C/EBP-β, which carries the required transactivation domain.
The finding that ciprofibrate and Wy 14643 strongly suppress IL-1– but not IL-6–induced CRP expression, together with the finding that these 2 cytokines differ in the induction of p50-NFκB, suggested to us that PPAR-α activation might interfere with the induction or the nuclear translocation of p50-NFκB. Indeed, activators of PPAR-α reduced the formation of IL-1–inducible nuclear p50-NFκB–C/EBP-β complexes by inhibition of the p50-NFκB translocation. Cytosolic retention of p50-NFκB extends the anti-inflammatory properties of PPAR-α that have been mainly characterized by direct binding and inactivation of transcription factors, such as p65-NFκB and c-jun.9,37Induction of IκB-α may also explain the broad inhibitory effect of PPAR-α activators on NF-κB–regulated genes, including IL-6, VCAM-1, and SAA.38 39
Among the nonlipid blood markers, CRP and fibrinogen are widely used in clinical and epidemiologic studies as independent risk markers34 to predict future cardiovascular events, but it has remained unclear why they provide additive predictive value. We showed that fibrates and activators of PPAR-α markedly inhibited the expression of fibrinogen, which is in accordance with previous observations.13 14 In contrast to fibrinogen, IL-6–induced expression of CRP was not, or was only slightly, inhibited by fibrates and Wy 14643, pointing to differences in CRP and fibrinogen gene regulation mechanisms and their additive predictive value as inflammation markers. This observation is of clinical relevance because CRP levels provide additional information on the activities of a given drug caused by its unique molecular mechanism of regulation.
Elevated plasma CRP levels are associated with an increased inflammatory state and a higher risk for atherosclerosis and coronary heart disease.40 It is becoming increasingly clear that CRP is not only a risk marker but also a risk factor, playing an active role in inducing adhesion molecule and monocyte chemoattractant protein-1 (MCP-1) expression.5 6 Given that direct CRP effects are considered to worsen the patient's situation, the down-regulation of CRP expression, as demonstrated here by the activation of PPAR-α, may contribute to the prevention and treatment of cardiovascular diseases beyond merely risk factor correction.
The results of the present study demonstrate that IL-1 stimulates hepatic CRP gene expression through C/EBP-β and p50-NFκB. Fibrates and PPAR-α activators reduce the formation of nuclear p50-NFκB–C/EBP-β complexes, and thereby CRP promoter activity, by 2 novel mechanisms, the inhibition of p50-NFκB translocation and the down-regulation of basal p50-NFκB and C/EBP-β expression in a PPAR-α dependent fashion, providing a first molecular explanation for the reduced plasma CRP levels of humans treated with fibrates9 and possibly contributing to a general PPAR-α–dependent reduction of other acute-phase genes. These observations extend our knowledge of the mechanisms of anti-inflammatory activities of PPAR-α effects, which are undoubtedly complimentary to the beneficial effects of fibrates on lipid metabolism and provide an additional rationale for the use of these drugs in atherosclerosis therapy.
We thank Dr Alt Zantema for helpful discussions and Annette de Jong for assistance with the experiments.
Prepublished online as Blood First Edition Paper, August 29, 2002; DOI 10.1182/blood-2002-06-1762.
Supported by Netherlands Heart Foundation grants 99.104 (L.V.) and 99.110 (R.K.) and by European Community Marie Curie Fellowship QLK-1-CT-1999-51206 (P.P.G.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
R. Kleemann, Gaubius Laboratory, TNO Prevention and Health, PO Box 2215, 2301 CE Leiden, The Netherlands; e-mail: r.kleemann@pg.tno.nl.

![Fig. 1. Effect of ciprofibrate and Wy 14643 on CRP and fibrinogen expression in human hepatocytes stimulated by IL-1 and IL-6. / CRP (A) and fibrinogen (B) concentrations were measured in culture medium by ELISA and were expressed as means ± SDs. Human hepatocytes were isolated and treated for 16 hours with 250 μM ciprofibrate (CF), 250 μM Wy 14643 (WY), or vehicle (dimethyl sulfoxide [DMSO]; C) and subsequently were stimulated with 25 ng/mL IL-1 or IL-6 for an additional 24 hours. Results of 1 representative experiment of 3 experiments with different donors are shown. *P < .05 compared with control.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/2/10.1182_blood-2002-06-1762/4/m_h80233682001.jpeg?Expires=1765883874&Signature=FIxWFry8ziVDzDWUEgFI5sc68~6HR-T1oiOXcapB-V42DR7YaLTVanxcfS~tLCxKQPXbUuYN4hNSXL52a-xEgAnJJuskl05oOpH2J3Svy38uPV4n2JXI4Ojmn8nDN~wuq9ykMkMGRuVciJ0kd2Znugb7DLO-w72cIfe3cP54bsNWmC-Py0wW-oeOpAq0-ZDBDYFZdizSMC06wMj4elPgtYPrdZbRDpTWXwVmobYqslCBcJP3DSm6eSggecCnThHtB~JDYSWv7BQZFL5rh7a2qwF3jUGd3xlvYZuQ4bjzSuKPgH0y6qSlS5A2OalRi4QsfF~-3plN3Hoh~Rv3Hzmj1Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal