Constitutive E-selectin expression on dermal microvascular endothelial cells plays a critical role in mediating rolling adhesive interactions of human skin–homing T cells and in pathologic accumulation of lymphocytes in skin. The major E-selectin ligand on human skin–homing T cells is cutaneous lymphocyte–associated antigen (CLA), a specialized glycoform of P-selectin glycoprotein ligand-1 (PSGL-1) defined by monoclonal antibody HECA-452. Since HECA-452 reactivity, and not PSGL-1 polypeptide itself, confers the specificity of human T cells to enter dermal tissue, inhibition of HECA-452 expression is a potential strategy for modulating lymphocyte migration to skin. In this study, we examined the efficacy of several well-characterized metabolic inhibitors of glycosylation and of a novel fluorinated analog of N-acetylglucosamine (2-acetamido-1,3,6-tri-O-acetyl-4-deoxy-4-fluoro-D-glucopyranose [4-F-GlcNAc]) to alter HECA-452 expression on human CLA+ T cells and prevent cell tethering and rolling on selectins under shear stress. At concentrations that did not affect PSGL-1 expression, we found that swainsonine (inhibitor of complex-typeN-glycan synthesis) had no effect on HECA-452 expression or selectin ligand activity, whereas benzyl-O-N-acetylgalactosamide (BAG; inhibitor of O-glycan biosynthesis) ablated HECA-452 expression on PSGL-1 and significantly lowered selectin ligand activity. We found that 4-F-GlcNAc (putative inhibitor of poly-N-acetyllactosamine biosynthesis) was more potent than BAG at lowering HECA-452 expression and selectin binding. In addition, we show that 4-F-GlcNAc was directly incorporated into native CLA expressed on T cells, indicating direct inhibition on poly-N-acetyllactosamine elongation and selectin-binding determinants on PSGL-1 O-glycans. These observations establish a potential treatment approach for targeting pathologic lymphocyte trafficking to skin and indicate that 4-F-GlcNAc may be a promising agent for treatment of dermal tropism associated with malignancies and inflammatory disorders.
Introduction
Tissue-specific migration of lymphocytes in neoplastic and inflammatory processes is critically dependent on the expression of cell membrane adhesion molecules that regulate adhesive interactions with target tissue endothelium. Skin disorders, including cutaneous T-cell lymphomas, cutaneous graft-versus-host disease (GVHD), and other inflammatory diseases (eg, psoriasis), are mediated by infiltrations of skin-homing T cells that express cutaneous lymphocyte–associated antigen (CLA).1-10 CLA is a sialyl Lewis X–like carbohydrate epitope displayed on the mucinlike molecule P-selectin glycoprotein ligand-1 (PSGL-1) recognized by the rat monoclonal antibody HECA-452.2,11,12 CLA functions as the P-selectin ligand and the principal E- and L-selectin ligand on human skin–homing T cells; specifically, the presence of HECA-452 epitopes on PSGL-1 directly correlates with the capacity of skin-homing memory T cells (including skin-disease–related lymphocytes) to bind E-selectin, which is constitutively expressed on dermal microvasculature.2,11 13 CLA–E-selectin binding interactions mediate lymphocyte trafficking to skin, and therefore, posttranslational glycosylations on skin-homing leukocytes represent a potential therapeutic target for controlling cutaneous tropism.
The α2,3 sialyltransferase, ST3Gal IV, and α1,3 fucosyltransferases, FucTIV and FucTVII, expressed in human skin–homing lymphocytes are responsible for synthesizing terminal sialofucosylations, recognized by HECA-452, that serve as E-selectin–binding determinants on PSGL-1.14-23 These terminal sialyl Lewis X structures are expressed on poly-N-acetyllactosamines [(Galβ1,4GlcNAcβ1,3)n] found on core 2 serine/threonine (O)–linked glycans and asparagine (N)–linked glycans displayed by PSGL-1.24-30 Although the majority of sialyl Lewis X epitopes appear to reside on PSGL-1 O-glycans, there are 3 potential sites for N-glycosylation and sialyl Lewis X decoration.30 Consequently, interfering with the synthesis of O-glycans or N-glycans or of poly-N-acetyllactosamine structures on either O- or N-glycans (or both) would prevent the synthesis of HECA-452 epitopes and could dampen the E-selectin–binding function of PSGL-1 (CLA) on skin-homing leukocytes.
With the aim of modulating CLA expression, we investigated the relative potency of metabolic inhibitors of glycosylation withO-glycan, N-glycan, or poly-N-acetyllactosaminyl glycan inhibitory specificities to diminish CLA expression and function as natively expressed on human skin–homing T lymphocytes. The well-characterized O-glycan inhibitor benzyl-O-N-acetylgalactosamide (BAG), theN-glycan inhibitors tunicamycin and swainsonine, and the newly described putative inhibitor of poly-N-acetyllactosamine synthesis, 2-acetamido-1,3,6-tri-O-acetyl-4-deoxy-4-fluoro-D-glucopyranose (4-F-GlcNAc),31-34 were used to metabolically modify oligosaccharide structures. Since there is little direct evidence on the mechanism of the anticarbohydrate action of 4-F-GlcNAc, we performed lectin blotting experiments and metabolic studies with radiolabeled 4-F-GlcNAc to analyze the relevant glycan modifications. Results from these studies suggest that metabolic modulation of CLA structure and function with 4-F-GlcNAc (or with modifiers of core 2 structures) could prevent the capacity of skin-homing lymphocytes to interact with dermal microvascular endothelial selectins. Standard anti-inflammatory immunosuppressant and antineoplastic treatment modalities alter homeostatic immunologic processes and cause toxicity to uninvolved normal tissues. Modulation of CLA expression represents a new and relatively nontoxic treatment paradigm that could specifically interfere with the migration of lymphocytes to cutaneous inflammatory sites and with the progression and dissemination of cutaneous lymphomas.
Materials and methods
Antibodies, enzymes, metabolic inhibitors, and radiochemicals
Rat immunoglobulin M (IgM) antihuman CLA HECA-452 monoclonal antibody and fluorescein isothiocyanate (FITC)–rat IgM HECA-452 were purchased from BD PharMingen (San Diego, CA). Peridinin chlorophyll A protein (PerCP)–mouse IgG1, PerCP–mouse IgG antihuman CD4 (clone SK3), and PerCP–mouse IgG antihuman CD8 (SK1) were purchased from BD Biosciences (San Jose, CA). Goat antimouse IgG, alkaline phosphatase (AP)–goat antirat IgM, and AP–goat antimouse IgG were purchased from Southern Biotechnology Associates (Birmingham, AL). Mouse IgG antihuman CD3 and mouse IgG antihuman PSGL-1 monoclonal antibody (PL-1 or PL-2) were purchased from Beckman Coulter (Brea, CA). Mouse IgG antihuman PSGL-1 monoclonal antibodies (moAbs) (clones 2G3, 4F9, and 4D8) were generously provided by Wyeth/Genetics Institute (Cambridge, MA).Vibrio cholerae neuraminidase was purchased from Roche Molecular Biochemicals (Indianapolis, IN). Human IgG, bromelain, tunicamycin, swainsonine, N-acetylglucosamine (GlcNAc), and benzyl-O-N-acetylgalactosamide (BAG) were from Sigma Chemical (St Louis, MO). Easy Tag [35S]-protein labeling mix was purchased from NEN Life Science products (Boston, MA). The 2-acetamido-1,3,6-tri-O-acetyl-4-deoxy-4-fluoro-D-glucopyranose (4-F-GlcNAc), which possesses a fluorine substitution at carbon-4 of the pyranose ring (Figure 1A) and putatively blocks poly-N-acetyllactosamine elongation31-34 (Figure 1B-C), and tritiated 4-F-Glc[3H]NAc (16 μCi/μmol [0.592 MBq/μmol]) were synthesized and provided by the Chemical Resource Laboratory at Roswell Park Cancer Institute (Buffalo, NY).
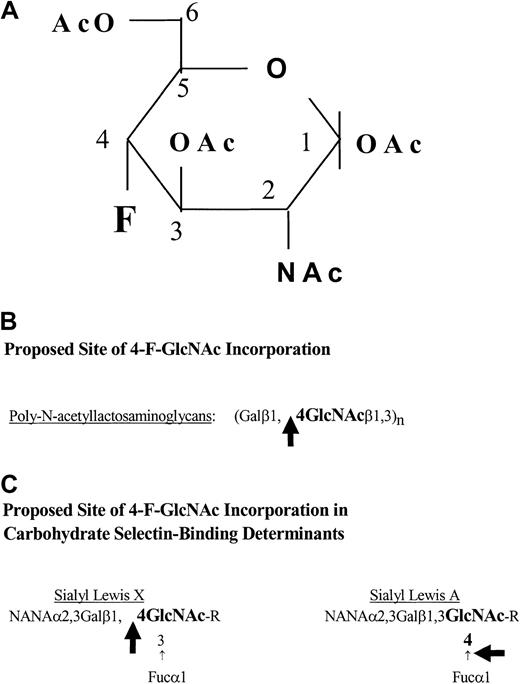
Structure of 4-F-GlcNAc and potential sites of its incorporation.
(A) Structure of 2-acetamido-1,3,6-tri-O-acetyl-4-fluoro-D-glucopyranose (4-F-GlcNAc). The carbons on the pyranose ring are indicated numerically, starting with the anomeric carbon 1, which forms a glycosidic bond with the acceptor sugar in a β (up) or α (down) position. (B-C) Potential sites of 4-F-GlcNAc incorporation into poly-N-acetyllactosamine chains and into carbohydrate selectin-binding determinants. The large black arrows indicate the potential points of blockage or chain termination. Symbols: fucose (Fuc), galactose (Gal), N-acetylneuraminic acid (NANA), andN-acetylglucosamine (GlcNAc).
Structure of 4-F-GlcNAc and potential sites of its incorporation.
(A) Structure of 2-acetamido-1,3,6-tri-O-acetyl-4-fluoro-D-glucopyranose (4-F-GlcNAc). The carbons on the pyranose ring are indicated numerically, starting with the anomeric carbon 1, which forms a glycosidic bond with the acceptor sugar in a β (up) or α (down) position. (B-C) Potential sites of 4-F-GlcNAc incorporation into poly-N-acetyllactosamine chains and into carbohydrate selectin-binding determinants. The large black arrows indicate the potential points of blockage or chain termination. Symbols: fucose (Fuc), galactose (Gal), N-acetylneuraminic acid (NANA), andN-acetylglucosamine (GlcNAc).
Generation of human cutaneous lymphocyte–associated antigen (CLA)–expressing T cells treated with glycosylation modifiers or protease
Human CLA+ T cells were prepared as previously described.3 Human peripheral blood mononuclear cells (PBMCs) were isolated from citrated whole blood by Histopaque-1077 (Sigma Diagnostics, St Louis, MO) density-gradient centrifugation under pathogen-free conditions. PBMCs were suspended in X-VIVO 15 serum-free medium (BioWhittaker, Walkersville, MD), plated on antihuman CD3-coated 24-well plates (2 × 106 cells per well), and activated for 48 hours at 37°C. Alternatively, as negative control for CLA expression, PBMCs were suspended in RPMI 1640/10% fetal bovine serum (FBS; Invitrogen, Carlsbad, CA) and activated on anti-CD3–coated plastic to produce T cells expressing low levels of CLA (CLAlow T cells). For preparation of antihuman CD3-coated 24-well plates, goat antimouse IgG (5 μg per well in 500 μL 0.1 M NaCO3, pH 9.6) was first added and incubated for 2 hours at 37°C. After washing 3 × with PBS, mouse anti-CD3 (0.1 μg per well in 500 μL PBS/2% FBS) was added to each well and incubated for 2 hours at 37°C, and plates were stored at 4°C until ready for use. After cell activation on anti-CD3–coated plates (48 hours), T cells were harvested, suspended in X-VIVO 15 serum-free medium containing 5 ng/mL recombinant human interleukin 2 (IL-2), and replated in non–antibody-coated 24-well plates at 2 × 106 cells per well for 48 hours at 37°C. This step was repeated 3 more times (48-hour intervals), and cells were examined for CLA, CD4, and CD8 expression by flow cytometry to confirm elevated CLA expression (99% CLA+ versus < 20% on CLAlow T cells or on freshly isolated peripheral blood lymphocytes [PBLs]) and frequency of CD4 and CD8 subsets before being used in functional adherence assays or in preparation of cell lysates.
For analysis of CLA expression on T cells treated with glycoconjugate modifiers, CLA+ T cells were first pretreated with neuraminidase (0.1 U/mL at 37°C for 1 hour) to eliminate functional CLA and cleave all HECA-452–reactive epitopes from the cell surface.35-38 Since we wanted to investigate whether these compounds directly affected CLA expression and function, neuraminidase pretreatment followed by incubation with glycosylation modifiers allowed for examination of de novo synthesized CLA/HECA-452 epitopes re-expressed on the cell surface. After neuraminidase digestion, cells were then incubated with 0.02 mM tunicamycin (an inhibitor of GlcNAc phosphotransferase, the initial step of N-glycosylation), 0.23 mM swainsonine (an inhibitor of α-mannosidase II and complex-typeN-glycosylation), 0.05 mM 4-F-GlcNAc (putative inhibitor of poly-N-acetyllactosamine biosynthesis), less than 2 mM BAG (an inhibitor of O-glycan biosynthesis), or 5 mM GlcNAc (negative control, naturally occurring metabolic counterpart of 4-F-GlcNAc) for 30 hours in the presence of human recombinant IL-2. Concentrations of tunicamycin and swainsonine were based on theirN-glycan–inhibitory effects on human hematopoietic cell cultures, which possess growth rates identical to those of activated T cells generated for these studies.35-38Concentrations for BAG were selected on the basis of maximalO-glycosylation–inhibitory effects at non–growth-inhibitory concentrations as previously reported.39 To determine a suitable concentration of 4-F-GlcNAc for examining glycoconjugate-modifying effects without influencing cell growth or protein synthesis, we performed preliminary growth-inhibitory experiments of cells incubated with 4-F-GlcNAc for 2 cell doublings (approximately 36 hours) at 37°C (cell viability assessed by trypan-blue exclusion). We found that 4-F-GlcNAc had an inhibitory concentration–10% (IC10) exceeding 0.2 mM; thus, data generated from 4-F-GlcNAc treatments below 0.2 mM would not reflect inhibition of cell growth or protein synthesis.33 34
To assess the level of selectin ligand activity conferred by cell surface glycoprotein compared with the effects of glycosylation inhibitor treatments, cells were treated with bromelain (0.1% for 1 hour at 37°C), a protease known to remove membrane proteins, including all P- and L-selectin ligand activity expressed on human hematopoietic cell membrane glycoproteins (ie, PSGL-1 and hematopoietic cell E- and L-selectin ligand [HCELL]).35-38Residual E-selectin ligand activity after bromelain treatment would, therefore, be indicative of activity contributed by a non–PSGL-1 glycolipid component.40-44 Bromelain-treated cells were then analyzed for both HECA-452 and PSGL-1 expression by flow cytometry. In addition, to further verify the complete disappearance of HECA-452 and PSGL-1 expression from the cell surface after bromelain treatment, membrane proteins were prepared as previously described by our laboratory35-37 and analyzed for HECA-452 antigen and PSGL-1 polypeptide by Western blotting.
Flow cytometric analysis
Flow cytometric analysis was performed on human leukocytes with the use of both direct and indirect immunofluorescence staining. All cells used for these experiments were washed twice with cold PBS/2% FBS and suspended in 107/mL PBS/1% FBS. Primary antibodies anti-CLA (HECA-452), anti-CD4, anti-CD8, anti-CD43 (L60), and anti–PSGL-1 (PL-2), along with the appropriate isotype-matched control antibodies, were incubated with the cells for 30 minutes on ice. Following 2 washes with PBS/2% FBS, cells were resuspended in PBS/1% FBS and fluorochrome-conjugated secondary antibodies (2 μL) and incubated for 30 minutes on ice. Cells were washed twice with PBS/2% FBS and resuspended in PBS, and flow cytometry was performed on a FACScan apparatus equipped with an argon laser tuned at 488 nm (Becton Dickinson, San Jose, CA).
Cell lysate preparation and immunoprecipitations
For lysate preparation, cells (including radiolabeled cells) were washed 3 × in ice-cold PBS and lysed in buffer containing 150 mM NaCl; 50 mM Tris-HCl (tris(hydroxymethyl)aminomethane–HCl), pH 7.4; 1 mM EDTA (ethylenediaminetetraacetic acid); 0.02% NaAzide; 20 μg/mL phenylmethyl sulfonyl fluoride (PMSF); Complete protease inhibitor cocktail tablets (Roche Molecular Biochemicals); and 2% Nonidet P-40 (NP-40) (250 μL/108 cells). Following 2-hour incubation on ice, insoluble cellular debris was pelleted by centrifugation for 30 minutes at 10 000g at 4°C, and solubilized protein lysate was collected and quantified by Bradford protein assay (Sigma Chemical). Sodium dodecyl sulfate (SDS) was added to a final concentration of 1% in lysate preparations used for immunoprecipitation experiments.
For immunoprecipitation of PSGL-1, anti–PSGL-1 moAbs PL-2 2G3, 4F9, and 4D8 (2 μg each) were added to nonradiolabeled or radiolabeled cell lysates (containing 1% SDS) precleared in recombinant protein G–agarose (Invitrogen) for 18 hours at 4°C on a rotator. Immunoprecipitations with mouse IgG isotype control at a similar Ab-lysate ratio were also performed to serve as negative controls. The antibody-lysate mixture was added to protein G–agarose, preincubated with lysis buffer/2% NP-40/1% SDS/1% BSA, and incubated for longer than 4 hours at 4°C under constant rotation. Immunoprecipitates were washed 5 × with lysis buffer/2% NP-40/1% SDS/1% BSA; washed 3 × with lysis buffer/2% NP-40/1% SDS without BSA; and then boiled in reducing sample buffer for analysis.
SDS-PAGE/Western blotting, lectin blotting, and autoradiography
For SDS–polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting, cell lysates, membrane proteins, or immunoprecipitates were diluted and boiled in reducing sample buffer, and separated on 7% or 9% SDS-PAGE gels. Resolved protein was transferred to Sequi-blot poly(vinylidene difluoride) (PVDF) membrane (Bio-Rad, Hercules, CA) and blocked with FBS for 1 hour at room temperature (RT). Blots were incubated with rat IgM antihuman CLA HECA-452 (1 μg/mL); mouse IgG antihuman PSGL-1 moAbs PL-2, 2G3, 4F9, and 4D8 (1-2 μg each per milliliter); or mouse IgG antihuman CD43 (L60) (1 μg/mL) for 1 hour at RT. Isotype control immunoblots using either rat IgM or mouse IgG were performed in parallel to evaluate nonspecific reactive proteins. After 3 washes with PBS/0.1%Tween-20 (10 minutes per wash), blots were incubated with the respective secondary Ab, AP-conjugated goat antirat IgM (1:400), or goat antimouse IgG (1:8000). AP substrate, Western Blue (Promega, Madison, WI), was then added to develop blots.
For lectin blotting, cell lysates (25 μg per spot) were spotted onto methanol-permeabilized PVDF membrane and blocked in FBS for 1 hour at RT. Blots were then probed with AP–Canavalia ensiformisagglutinin (ConA) (2.0 μg/mL PBS; specificity, α-mannose); AP-Triticum vulgaris (wheat germ) agglutinin (WGA) (0.5 μg/mL PBS; specificity, terminal GlcNAc or GlcNAcβ1,4GlcNAc); or AP-Lycopersicon esculentum (tomato lectin) agglutinin (LEA) (1.0 μg/mL PBS; specificity, (Galβ1,4GlcNAc)n) for 1 hour at RT (all lectins from EY Labs, San Mateo, CA). After washing 3 × in PBS/0.1% Tween-20 and 1 × in PBS (10 minutes each), blots were developed with Western Blue.
For autoradiography, lysates were prepared from cells metabolically radiolabeled with Easy Tag [35S]-protein labeling mix (100 μCi/mL [3.7 MBq/mL]) in complete X-VIVO 15 medium for 30 hours or with 4-F-Glc[3H]NAc (0.1 mM [16 μCi/μmol (0.592 MBq/μmol)]) for 36 hours in complete X-VIVO 15 growth medium. Radiolabeled lysates or immunoprecipitates were resolved on reducing SDS-PAGE gels, and gels were dried and exposed to Kodak Biomax MR film (Rochester, NY). Densitometric scans of both anti–PSGL-1 and isotype control immunoprecipitates resolved by SDS-PAGE were performed by means of NIH ImageJ software (National Institutes of Health, Bethesda, MD), and 8-bit grayscale values were plotted versus the length of the lane (from high molecular weight range to the dye front) by means of Microsoft Excel (Bothell, WA).
TCA precipitation of radiolabeled human CLA+ T-cell macromolecules
Human CLA+ T cells (5 × 106/mL complete X-VIVO 15 medium) were grown in the presence of 4-F-Glc[3H]NAc (0.025 to 1.0 mM; 16 μCi/μmol [0.592 MBq/μmol]) for 12 to 36 hours. Cells were then harvested, washed 2 × in ice-cold PBS and incubated with 10% trichloroacetic acid (TCA) (250 μL/5 × 106 cells) on ice for 30 minutes. Cellular precipitates were passed over Whatman GF/C microfiber glass paper (Fisher Scientific, Springfield, NJ) under vacuum pressure and washed 5 × with ice-cold 5% TCA and 3 × with ice-cold ddH2O. Filter paper was placed into scintillation fluid and counted by a Beckman LS6000IC Scintillation Counter (Beckman Coulter, Fullerton, CA). After determining the counting efficiency of a known amount of tritiated 4-F-Glc[3H]NAc, counts per minute were corrected to nanomoles (16 μCi/μmol [0.592 MBq/μmol] 4-F-Glc[3H]NAc), and fluorosugar analog content was expressed on per cell basis.
Parallel-plate flow chamber analysis
Tethering and rolling of human T cells on recombinant human E- and P-selectin–immunoglobulin chimera (provided by Dr Robert Fuhlbrigge, Harvard Medical School, Boston, MA) and human L-selectin–immunoglobulin (gift from Dr Ray Camphausen, Wyeth/Genetics Institute) were analyzed in the parallel-plate flow chamber under physiologic shear stress conditions.3 To prepare E- and P-selectin–immunoglobulin chimera spots, protein A (300 μg/15 μL 0.1 M NaHCO3) was adsorbed to Ten-twenty-nine Petri dishes (Becton Dickinson) for 2 hours at 37°C. Human serum albumin (2 μg/mL PBS) was then added, and the mixture was incubated for 2 hours at 37°C to block nonspecific binding sites. E-selectin–immunoglobulin (50 ng/50 μL PBS) or P-selectin–immunoglobulin (50 ng/50 μL PBS) solution was pipetted directly over the pre-existing protein A spots for 18 hours at 4°C. To prepare L-selectin–immunoglobulin spots, L-selectin–immunoglobulin (300 ng/15 μL PBS) or isotype control human IgG (300 ng/15 μL) was adsorbed directly to plastic (non–protein A–coated) for 18 hours at 4°C and blocked in 100% FBS for 2 hours at 4°C.
CLA+ T cells or freshly isolated PBLs were treated with neuraminidase and metabolic glycosylation inhibitors or with protease (bromelain) as described above, washed twice in Hanks balanced salt solution (HBSS), suspended at 2 × 106/mL in HBSS/10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid)/2 mM CaCl2 (H/H/Ca++), and infused into the chamber over selectin chimeras. Protease treatment was performed to control for residual E-selectin ligand activity not attributable to the expression of CLA on a protein scaffold. Cell tethering was permitted at 0.6 dyne/cm2 for 1 minute; then stepwise increments in shear stress every 15 seconds were employed to a final shear stress level of 60 dyne/cm2. The number of cells rolling per viewing field (magnification × 100 at the midpoint of the chamber) was quantified at each level of shear stress in 4 fields for a minimum of 3 experiments. All experiments were observed in real time and videotaped for off-line analysis. Negative control experiments were performed in parallel; in these, (1) cell binding was examined in H/H adhesion assay medium containing 5 mM EDTA, and (2) binding of cells to human IgG isotype control was assayed.
Results
In this report, we compared and contrasted the ability of glycosylation inhibitors 4-F-GlcNAc, BAG, tunicamycin, and swainsonine to modulate the structure and selectin-binding function of CLA as natively expressed by human CLA+ T cells.3 In addition, we further investigated the mechanism of 4-F-GlcNAc action, confirming its capacity to convert to a nucleotide sugar, uridine diphosphate–4-F-GlcNAc (UDP–4-F-GlcNAc); to incorporate into a growing poly-N-acetyllactosamine chain; and to block the addition of UDP-galactose. Human CLA+ T cells were derived from human peripheral blood mononuclear cells (PBMCs) activated by anti-CD3 antibody stimulation and cultured in serum-free X-VIVO 15 medium as previously described.3 These T cells (approximately 40% CD4+ and approximately 60% CD8+) were 99% HECA-452+, compared with only 18% of the cells when grown in RPMI 1640/10% FBS (CLAlow T cells) or on freshly isolated PBLs. Before culturing these T cells in 4-F-GlcNAc to perform structural and functional expression analysis on CLA, we assessed 4-F-GlcNAc treatment on CLA+ T-cell cultures in log-phase growth to determine pertinent concentrations for evaluating anticarbohydrate effects and selectin-binding capabilities. Indeed, growth-inhibitory concentrations could alter protein synthesis or reduce the turnover rate of cell surface glycoproteins, lowering cell surface expression levels of relevant selectin ligands and leading to erroneous conclusions on the proposed mechanism of anticarbohydrate action. We found that 4-F-GlcNAc concentrations below 0.2 mM (less than IC10) did not affect T-cell growth and did not affect the expression of T-cell markers CD4 and CD8 (data not shown). Similarly, tunicamycin, swainsonine, and BAG were used for cell treatments at concentrations previously shown to inhibit sialyl Lewis X,N-glycan, or O-glycan expression, but not cell growth.35-39
4-F-GlcNAc and BAG prevent the expression of HECA-452 epitopes on PSGL-1
To examine the effects of glycosylation inhibitors on de novo synthesis of HECA-452 expression, we first treated T-cell cultures withVibrio cholerae neuraminidase to cleave terminal sialic acid residues critical for HECA-452 recognition and cellular selectin ligand activities.37,38 This approach allowed for direct assessment of the effects of the glycosylation inhibitor on de novo CLA biosynthesis and obviates the contribution of preformed CLA on the cell surface. Cells were then recultured in the presence of glycosylation inhibitor, diluent control (PBS), or molecular control (GlcNAc) for 30 hours and then harvested for CLA expression and functional analysis. By Western blot analysis, we found that tunicamycin, BAG, and 4-F-GlcNAc treatments following neuraminidase digestion resulted in a marked reduction in de novo synthesized HECA-452 epitopes on PSGL-1, which resolves as a dimer (220 kDa) and monomer (140 kDa) form (Figure2A).3,36 37 Recovery of HECA-452 expression on cells grown in PBS, swainsonine, or GlcNAc (drug control) was not affected. Of note, data from preliminary experiments showed that BAG treatments at concentrations below 1.0 mM and tunicamycin treatment below 0.015 mM had minimal effects on HECA-452 expression (data not shown). On a molar basis, although tunicamycin was 2.5-fold more potent than 4-F-GlcNAc at lowering HECA-452 expression, 4-F-GlcNAc was more potent than BAG. Notably, whereas PSGL-1 expression in tunicamycin-treated cells was ablated, 4-F-GlcNAc had no effect on PSGL-1 expression itself (Figure 2B), showing the ability of 4-F-GlcNAc to selectively inhibit glycosylation without interfering with homeostatic pathways of protein synthesis and cell growth. To assess the duration of anticarbohydrate effects on HECA-452 expression on PSGL-1, cells were treated with 4-F-GlcNAc for 30 hours and were then recultured in non–4-F-GlcNAc–containing medium for 72 hours. By HECA-452 immunoblotting, we found that HECA-452 expression on PSGL-1 was suppressed for the first 48 hours with re-expression thereafter, suggesting that the lipophilicity of 4-F-GlcNAc results in maximal uptake during exposure to cells and, once converted to a nucleotide sugar (which is not exported from a cell), results in metabolic inhibition of selectin ligand synthesis for less than 48 hours.
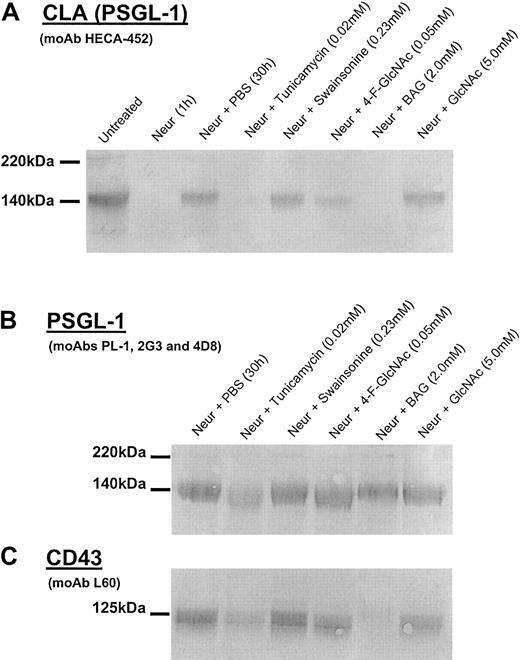
Western blot analysis of CLA, PSGL-1, and CD43 isolated from human CLA+ T cells treated with glycosylation inhibitors.
Cell lysates were prepared from human CLA+ T-cell cultures treated with neuraminidase (Neur.) and then grown in the presence of diluent control (PBS), tunicamycin (0.02 mM), swainsonine (0.23 mM), 4-F-GlcNAc (0.05 mM), BAG (2.0 mM), or GlcNAc (5.0 mM, negative control) for 30 hours. Lysates (25 μg per lane) were resolved on 6% reducing SDS-PAGE gels and blotted onto PVDF membrane. Blots were then probed with either antihuman CLA moAb HECA-452 (1 μg/mL; panel A); antihuman PSGL-1 moAbs PL-1, 2G3, and 4D8 (1 μg/mL each; panel B); or antihuman CD43 moAb L60 (1 μg/mL; panel C) and were developed in Western Blue stain. In panel A, note the disappearance of HECA-452–reactive PSGL-1 (CLA) after neuraminidase treatment and after recovery in tunicamycin, 4-F-GlcNAc, and BAG. In panel B, note the marked reduction of PSGL-1 polypeptide in cells recovered in tunicamycin. Also, in panel C, note the reduction inO-glycan–dependent L60 epitopes on CD43 from cells recovered in tunicamycin and BAG with no effect by complexN-glycan inhibitor swainsonine.
Western blot analysis of CLA, PSGL-1, and CD43 isolated from human CLA+ T cells treated with glycosylation inhibitors.
Cell lysates were prepared from human CLA+ T-cell cultures treated with neuraminidase (Neur.) and then grown in the presence of diluent control (PBS), tunicamycin (0.02 mM), swainsonine (0.23 mM), 4-F-GlcNAc (0.05 mM), BAG (2.0 mM), or GlcNAc (5.0 mM, negative control) for 30 hours. Lysates (25 μg per lane) were resolved on 6% reducing SDS-PAGE gels and blotted onto PVDF membrane. Blots were then probed with either antihuman CLA moAb HECA-452 (1 μg/mL; panel A); antihuman PSGL-1 moAbs PL-1, 2G3, and 4D8 (1 μg/mL each; panel B); or antihuman CD43 moAb L60 (1 μg/mL; panel C) and were developed in Western Blue stain. In panel A, note the disappearance of HECA-452–reactive PSGL-1 (CLA) after neuraminidase treatment and after recovery in tunicamycin, 4-F-GlcNAc, and BAG. In panel B, note the marked reduction of PSGL-1 polypeptide in cells recovered in tunicamycin. Also, in panel C, note the reduction inO-glycan–dependent L60 epitopes on CD43 from cells recovered in tunicamycin and BAG with no effect by complexN-glycan inhibitor swainsonine.
To further analyze O-glycan–inhibitory effects and help assess the specificity of decreases in HECA-452 epitopes displayed by core 2 O-glycans on PSGL-1, we blotted lysate from BAG-treated cells with moAb L60, which recognizes a sialylated epitope expressed on O-glycans of CD43. BAG treatment resulted in complete abrogation of L60 reactivity (Figure 2C). Interestingly, tunicamycin also reduced L60 recognition (Figure 2C), suggesting a general inhibitory effect on cellular protein synthesis. Furthermore, 4-F-GlcNAc did not affect L60 reactivity, indicating that 4-F-GlcNAc did not affect O-glycosylation per se. Because 4-F-GlcNAc resulted in decrements in HECA-452 epitopes that reside on poly-N-acetyllactosamine backbones displayed by core 2O-glycosylations on PSGL-1 (Figure 2A), unchanged L-60 reactivity of CD43 from lysates of 4-F-GlcNAc–treated cells suggests that L-60 epitopes do not reside on poly-N-acetyllactosamine backbones.45 46
To address the possibility of tunicamycin affecting the blotting capacity of PSGL-1 with anti–PSGL-1 moAbs, which could account for the lack of detection of PSGL-1 polypeptide by Western blotting (Figure 2B), we performed autoradiography of PSGL-1 immunoprecipitated from cells metabolically radiolabeled with [35S]-protein labeling mix concurrently with glycosylation-inhibitor treatment. PSGL-1 expression on cells grown in tunicamycin was completely eliminated, indicating that ablation of HECA-452 epitopes was due to inhibition of PSGL-1 biosynthesis (Figure3). There was also a slight reduction in the incorporation of [35S]-protein labeling mix into PSGL-1 from cells treated with BAG, whereas the level of radioactive PSGL-1 isolated from cells grown in swainsonine or 4-F-GlcNAc was not changed compared with PSGL-1 from cells grown with PBS (diluent control) or GlcNAc (drug control) (Figure 3).
Autoradiography of PSGL-1 isolated from metabolically radiolabeled CLA+T cells treated with glycosylation inhibitors.
Radiolabeled lysates were prepared from human CLA+ T-cell cultures treated with neuraminidase (Neur.) and then regrown in [35S]-protein labeling mix (100 μCi/mL [3.7 MBq/mL]) in the presence of PBS (diluent control), tunicamycin (0.02 mM), swainsonine (0.23 mM), 4-F-GlcNAc (0.05 mM), BAG (2.0 mM), or GlcNAc (5.0 mM, negative control) for 30 hours. PSGL-1 was then immunoprecipitated from lysates (200 μg) with mouse IgG isotype control or antihuman PSGL-1 moAbs PL-1, 2G3, 4F9, and 4D8 (2 μg/mL each) from lysates (200 μg) and resolved on a 6% reducing SDS-PAGE gel. Autoradiography reveals that tunicamycin markedly inhibited PSGL-1 expression, while BAG treatment slightly reduced PSGL-1 expression and mobility of the 140-kDa monomer form. Also, 4-F-GlcNAc did not affect PSGL-1 expression, while it did reduce the molecular weight of the monomer (to 130 kDa).
Autoradiography of PSGL-1 isolated from metabolically radiolabeled CLA+T cells treated with glycosylation inhibitors.
Radiolabeled lysates were prepared from human CLA+ T-cell cultures treated with neuraminidase (Neur.) and then regrown in [35S]-protein labeling mix (100 μCi/mL [3.7 MBq/mL]) in the presence of PBS (diluent control), tunicamycin (0.02 mM), swainsonine (0.23 mM), 4-F-GlcNAc (0.05 mM), BAG (2.0 mM), or GlcNAc (5.0 mM, negative control) for 30 hours. PSGL-1 was then immunoprecipitated from lysates (200 μg) with mouse IgG isotype control or antihuman PSGL-1 moAbs PL-1, 2G3, 4F9, and 4D8 (2 μg/mL each) from lysates (200 μg) and resolved on a 6% reducing SDS-PAGE gel. Autoradiography reveals that tunicamycin markedly inhibited PSGL-1 expression, while BAG treatment slightly reduced PSGL-1 expression and mobility of the 140-kDa monomer form. Also, 4-F-GlcNAc did not affect PSGL-1 expression, while it did reduce the molecular weight of the monomer (to 130 kDa).
Suppression of selectin ligand activities on human CLA+ T cells
Using the parallel-plate flow chamber under physiologic shear stress conditions, we analyzed E-, P-, and L-selectin ligand activities of human CLA+ T cells treated in a manner identical to that of cells prepared for Western blotting and autoradiography studies. Human CLA+ T cells grown in PBS or GlcNAc (drug control) for 30 hours following neuraminidase digestion resulted in a greater than 80% recovery of selectin ligand activities, which were all inhibitable by adding 5 mM EDTA to the adhesion assay medium (Figure 4). As expected, E-selectin and L-selectin ligand activities of negative-control CLAlow T cells grown in RPMI 1640/10% FBS or freshly isolated PBLs were considerably less than CLA+ T-cell activities (25% of CLA+ T-cell activity), whereas P-selectin ligand activities were not different from those of CLA+ T cells (data not shown). At 2.0 dyne/cm2 we observed significant reductions in rolling adhesions on E-, P-, and L-selectin chimeras of cells treated with tunicamycin, 4-F-GlcNAc, and BAG (P < .01; Student t test). However, inhibition of PSGL-1 expression in tunicamycin-treated cells (as shown in Figures 2B and 3) may explain for the marked decrement in selectin binding. Swainsonine had no effect on cell rolling on selectins, suggesting that complex-typeN-glycans, particularly on PSGL-1, do not contribute to selectin ligand activities. Together, these observations indicate that functional modulation of selectin ligand activities with 4-F-GlcNAc and BAG directly correlate with inhibitory effects on HECA-452 expression on PSGL-1 and that selectin-binding determinants are displayed by core 2 O-glycans.
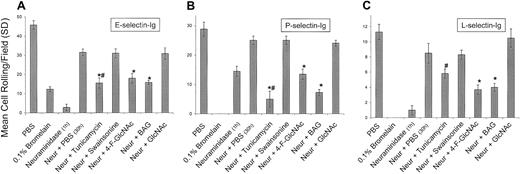
Effects of glycosylation inhibitors on human CLA+ T-cell rolling on E-, P-, and L-selectins.
To assess the effects of glycosylation inhibitors on the de novo synthesis of selectin ligands expressed by CLA+ T cells, cells were treated with neuraminidase to cleave all preformed selectin ligand on the cell surface. Cells were then recultured for 30 hours in PBS (diluent control), tunicamycin (0.02 mM), swainsonine (0.23 mM), 4-F-GlcNAc (0.05 mM), BAG (2.0 mM), and GlcNAc (5.0 mM; negative drug control). Cell rolling assessments were made at 2.0 dyne/cm2 from the midpoint of the chamber viewing field (4 fields per selectin spot; 3 different experiments). When compared with recovery in PBS, tunicamycin, 4-F-GlcNAc, and BAG significantly inhibited the re-expression of E- and P-selectin ligand activity (panels A and B), whereas 4-F-GlcNAc and BAG also inhibited L-selectin ligand activity (panel C) (*P < .01; Student ttest). Inhibition of P- and L-selectin–mediated T-cell rolling after bromelain treatment confirmed the role of PSGL-1 and revealed that a possible glycolipid component might be contributing to residual E-selectin ligand activity. # indicates that 0.02 mM tunicamycin inhibited protein synthesis and PSGL-1 expression as determined by flow cytometry and autoradiography.
Effects of glycosylation inhibitors on human CLA+ T-cell rolling on E-, P-, and L-selectins.
To assess the effects of glycosylation inhibitors on the de novo synthesis of selectin ligands expressed by CLA+ T cells, cells were treated with neuraminidase to cleave all preformed selectin ligand on the cell surface. Cells were then recultured for 30 hours in PBS (diluent control), tunicamycin (0.02 mM), swainsonine (0.23 mM), 4-F-GlcNAc (0.05 mM), BAG (2.0 mM), and GlcNAc (5.0 mM; negative drug control). Cell rolling assessments were made at 2.0 dyne/cm2 from the midpoint of the chamber viewing field (4 fields per selectin spot; 3 different experiments). When compared with recovery in PBS, tunicamycin, 4-F-GlcNAc, and BAG significantly inhibited the re-expression of E- and P-selectin ligand activity (panels A and B), whereas 4-F-GlcNAc and BAG also inhibited L-selectin ligand activity (panel C) (*P < .01; Student ttest). Inhibition of P- and L-selectin–mediated T-cell rolling after bromelain treatment confirmed the role of PSGL-1 and revealed that a possible glycolipid component might be contributing to residual E-selectin ligand activity. # indicates that 0.02 mM tunicamycin inhibited protein synthesis and PSGL-1 expression as determined by flow cytometry and autoradiography.
Importantly, treatment of cells with the protease bromelain completely eliminated PSGL-1 expression, as determined by flow cytometry (Table 1; Figure5A), as well as P- and L-selectin ligand activities (Figure 4), confirming that PSGL-1 is the primary glycoprotein P-selectin/L-selectin ligand on CLA+ T cells. However, although PSGL-1 was completely removed by bromelain digestion (Table 1; Figure 5A), E-selectin ligand activity (lower than 25% of control) was observed (Figure 4). This remaining E-selectin ligand activity of CLA+ T cells parallels that of the baseline E-selectin ligand activity of CLAlow T cells or of fresh PBLs (lower than 25% of CLA+ T-cell activity) and probably reflects glycolipid E-selectin ligand activity. Notably, flow cytometry analysis of PSGL-1 and HECA-452 expression on CLAlow T cells or PBLs after bromelain digestion indicated that HECA-452 expression was relatively unchanged, whereas PSGL-1 levels were completely abolished (Table 1), indicating that HECA-452 expression on glycolipids is robust, though these structures contribute only a minor component of functional E-selectin binding that does not correlate with lymphocyte skin-homing and CLA/PSGL-1 expression. Although we expected that tunicamycin and BAG would not lower E-selectin ligand activity below that of protease treatment alone (as these inhibitors are glycoprotein specific), the finding that 4-F-GlcNAc did not reduce E-selectin ligand activity below that of protease treatment may reflect a selectivity for incorporation of 4-F-GlcNAc into poly-N-acetyllactosamines displayed by proteins. The presence of protease-resistant HECA-452 epitopes and E-selectin ligand activity, distinct from reductions due to 4-F-GlcNAc decrement, suggests that N-acetyllactosamine structures on glycolipids, such as neolacto-glycosphingolipids, may not be affected by 4-F-GlcNAc treatment. Further studies are ongoing to investigate the effects of 4-F-GlcNAc treatment on glycolipid structures.
FACS assessment of HECA-452 and PSGL-1 expression on human T cells after bromelain treatment
| . | Cell staining (% positive [MCF]) . | |||
|---|---|---|---|---|
| CLA+ T cells . | CLAlow T cells . | |||
| Untreated . | Bromelain . | Untreated . | Bromelain . | |
| Anti-CLA (moAb HECA-452) | 99% (2304) | 68% (1869) | 18% (51) | 16% (48) |
| Anti–PSGL-1 (moAb PL-2) | 99% (97) | 1% (10) | 96% (105) | 0% (25) |
| . | Cell staining (% positive [MCF]) . | |||
|---|---|---|---|---|
| CLA+ T cells . | CLAlow T cells . | |||
| Untreated . | Bromelain . | Untreated . | Bromelain . | |
| Anti-CLA (moAb HECA-452) | 99% (2304) | 68% (1869) | 18% (51) | 16% (48) |
| Anti–PSGL-1 (moAb PL-2) | 99% (97) | 1% (10) | 96% (105) | 0% (25) |
Flow cytometric analysis and Western blot results (Figure5) showed the complete digestion of PSGL-1 polypeptide on both CLA+ and CLAlow T cells following bromelain treatment, and although HECA-452 expression on CLA+ T cells was markedly reduced, HECA-452 levels on CLAlow T cells were relatively unaffected by bromelain.
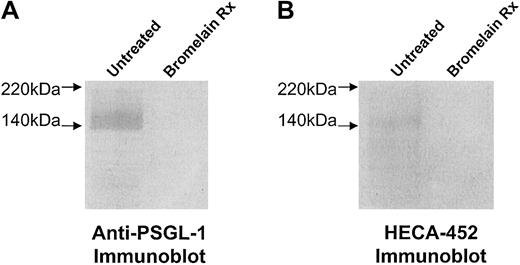
Expression of HECA-452 epitopes and PSGL-1 after bromelain treatment.
To remove HECA-452 epitopes displayed by PSGL-1 and disrupt the selectin-binding function of CLA, human CLA+ and CLAlow T cells were treated with 0.1% bromelain and then examined for HECA-452 and PSGL-1 polypeptide expression. In panels A and B, membrane proteins were prepared from human CLA+T-cell cultures treated with bromelain in parallel with flow cytometry experiments. Membrane proteins (10 μg per lane) were resolved on 6% reducing SDS-PAGE gels and blotted onto PVDF membrane. Western blotting with anti–PSGL-1 moAbs PL-1, 2G3, 4F9, and 4D8 (2 μg/mL each) or moAb HECA-452 (1.0 μg/mL) revealed that HECA-452 epitopes (panel A, lane 2) and PSGL-1 polypeptide (panel B, lane 2) were absent after bromelain treatment.
Expression of HECA-452 epitopes and PSGL-1 after bromelain treatment.
To remove HECA-452 epitopes displayed by PSGL-1 and disrupt the selectin-binding function of CLA, human CLA+ and CLAlow T cells were treated with 0.1% bromelain and then examined for HECA-452 and PSGL-1 polypeptide expression. In panels A and B, membrane proteins were prepared from human CLA+T-cell cultures treated with bromelain in parallel with flow cytometry experiments. Membrane proteins (10 μg per lane) were resolved on 6% reducing SDS-PAGE gels and blotted onto PVDF membrane. Western blotting with anti–PSGL-1 moAbs PL-1, 2G3, 4F9, and 4D8 (2 μg/mL each) or moAb HECA-452 (1.0 μg/mL) revealed that HECA-452 epitopes (panel A, lane 2) and PSGL-1 polypeptide (panel B, lane 2) were absent after bromelain treatment.
4-F-GlcNAc functions as a metabolic modulator of poly-N-acetyllactosamine synthesis and selectin-binding determinants by direct incorporation into CLA
Prior studies evaluating the inhibitory efficacy of 4-F-GlcNAc on human tumor cell binding to lectins indicate that 4-F-GlcNAc inhibits poly-N-acetyllactosamine synthesis and resultant terminalN-acetylneuraminic acid or galactose-bearing structures.33 34 Even though protein synthesis and cell growth are unaffected by 4-F-GlcNAc at concentrations that inhibit glycosylation, there is little direct evidence on the mechanism of anticarbohydrate action. To help determine whether 4-F-GlcNAc is directly incorporated into glycoconjugates and why reductions were observed in terminal glycan decorations or HECA-452 expression on human CLA+ T cells, we performed metabolic radiolabeling of CLA+ T cells with tritiated 4-F-GlcNAc (4-F-Glc[3H]NAc) and quantified the amount of 4-F-Glc[3H]NAc incorporated into TCA-precipitated macromolecules on a per cell basis. In a concentration-dependent manner, we found that 4-F-Glc[3H]NAc was incorporated into TCA-precipitated macromolecules (Figure6). In addition, inhibition of cell growth and metabolic activity after 36-hour incubation with 1.0 mM 4-F-Glc[3H]NAc was noted, resulting in a significant reduction in cell frequency (lower than 25% cell number at time 0) and a lower level of radioactivity in TCA-precipitated macromolecules (Figure 6).
Incorporation of 4-F-Glc[3H]NAc into TCA-precipitated macromolecules isolated from human CLA+ T cells.
Cellular macromolecules were precipitated from 4-F-Glc[3H]NAc–treated CLA+ T cells (0-1.0 mM or 0-16.7 μCi/mL [0-0.6179 MBq/mL] for 36 hours or less) with 10% TCA and collected on GF/C microfiber glass filters for liquid scintillation counting. Background counts per minute from non–4-F-Glc[3H]NAc–treated cells were subtracted from counts per minute measured from TCA precipitates of 4-F-Glc[3H]NAc–treated cells, and data in triplicate were expressed as nmol 4-F-Glc[3H]NAc incorporation per cell (SEM) at 12, 24, and 36 hours. Please note that 4-F-Glc[3H]NAc was detectable in TCA precipitates in a concentration-dependent manner over a 24-hour period.
Incorporation of 4-F-Glc[3H]NAc into TCA-precipitated macromolecules isolated from human CLA+ T cells.
Cellular macromolecules were precipitated from 4-F-Glc[3H]NAc–treated CLA+ T cells (0-1.0 mM or 0-16.7 μCi/mL [0-0.6179 MBq/mL] for 36 hours or less) with 10% TCA and collected on GF/C microfiber glass filters for liquid scintillation counting. Background counts per minute from non–4-F-Glc[3H]NAc–treated cells were subtracted from counts per minute measured from TCA precipitates of 4-F-Glc[3H]NAc–treated cells, and data in triplicate were expressed as nmol 4-F-Glc[3H]NAc incorporation per cell (SEM) at 12, 24, and 36 hours. Please note that 4-F-Glc[3H]NAc was detectable in TCA precipitates in a concentration-dependent manner over a 24-hour period.
To demonstrate that modulation of CLA by 4-F-GlcNAc treatment was due specifically to the direct incorporation into PSGL-1, radiodetection of PSGL-1 immunoprecipitated from 4-F-Glc[3H]NAc–labeled CLA+ T-cell lysates was performed. Although the low specific activity of 4-F-Glc[3H]NAc prevented a strong signal after a 28-day exposure time, autoradiography and scanning densitometry revealed that both dimer (220 kDa) and monomer (140 kDa) forms of PSGL-1 were still detectable, whereas isotype control immunoprecipitates did not contain any radiolabeled PSGL-1 (Figure7A, lane 2; 7B, lane 2). These data indicate that termination of poly-N-acetyllactosamine extension and sialofucosylations (ie, HECA-452 epitopes) on PSGL-1 by 4-F-GlcNAc treatment is due to inhibition of UDP-galactose (donor) linkage to the carbon 4-position of 4-F-GlcNAcβ1-3 Gal-R (acceptor).
Autoradiography of PSGL-1 immunoprecipitated from human CLA+ T cells metabolically radiolabeled with 4-F-Glc[3H]NAc.
Radiolabeled lysate was prepared from human CLA+ T-cell cultures grown in 4-F-Glc[3H]NAc (0.2 mM or 3.4 μCi/mL [0.1258 MBq/mL]; 16 μCi/μmol [0.592 MBq/μmol]) for 36 hours. PSGL-1 was then immunoprecipitated from radiolabeled lysate (2 mg) with mouse IgG isotype control or antihuman PSGL-1 moAbs (PL-1, 2G3, 4F9, and 4D8, 2 μg for each 100 μg lysate), resolved on a 6% reducing SDS-PAGE gel, and exposed to film for 28 days. Autoradiography revealed that both dimer (220 kDa) and monomer (140 kDa) forms of PSGL-1 were detectable (panel A, lane 1) and that isotype control antibody did not immunoprecipitate any detectable material (panel A, lane 2). Comparative densitometric scans of lanes 1 and 2 in panel A by means of NIH ImageJ software confirmed the appearance of 220- and 140-kDa forms of PSGL-1 immunoprecipitated from radiolabeled lysate (panel B, lane 1).
Autoradiography of PSGL-1 immunoprecipitated from human CLA+ T cells metabolically radiolabeled with 4-F-Glc[3H]NAc.
Radiolabeled lysate was prepared from human CLA+ T-cell cultures grown in 4-F-Glc[3H]NAc (0.2 mM or 3.4 μCi/mL [0.1258 MBq/mL]; 16 μCi/μmol [0.592 MBq/μmol]) for 36 hours. PSGL-1 was then immunoprecipitated from radiolabeled lysate (2 mg) with mouse IgG isotype control or antihuman PSGL-1 moAbs (PL-1, 2G3, 4F9, and 4D8, 2 μg for each 100 μg lysate), resolved on a 6% reducing SDS-PAGE gel, and exposed to film for 28 days. Autoradiography revealed that both dimer (220 kDa) and monomer (140 kDa) forms of PSGL-1 were detectable (panel A, lane 1) and that isotype control antibody did not immunoprecipitate any detectable material (panel A, lane 2). Comparative densitometric scans of lanes 1 and 2 in panel A by means of NIH ImageJ software confirmed the appearance of 220- and 140-kDa forms of PSGL-1 immunoprecipitated from radiolabeled lysate (panel B, lane 1).
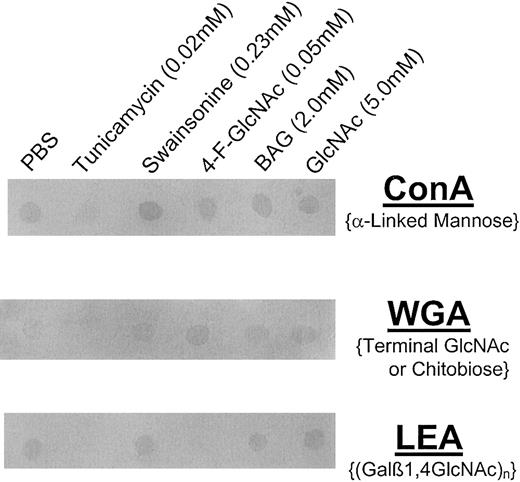
To analyze and help identify the glycan modifications on CLA+ T cells treated with 4-F-GlcNAc, we performed lectin blotting experiments with ConA (specificity, α-mannose), WGA (specificity, terminal GlcNAc or GlcNAcβ1,4GlcNAc), and LEA (specificity, (Galβ1,4GlcNAc)n). These experiments were also performed to help confirm the expected inhibitory effects on glycosylation by swainsonine and tunicamycin. Compared with cells grown in PBS or GlcNAc (negative controls), we found that ConA, WGA, and LEA staining of lysates prepared from tunicamycin-treated cells was eliminated (Figure 8). ConA staining of lysates from swainsonine-treated cells was enhanced, whereas there was no effect on ConA staining by 4-F-GlcNAc or BAG treatment (Figure 8) compared with PBS or GlcNAc treatments. Furthermore, LEA staining of lysates from 4-F-GlcNAc–treated cells was notably diminished, whereas WGA staining was elevated (Figure 8) compared with PBS, swainsonine, BAG, or GlcNAc treatments. Interestingly, BAG treatment did not affect LEA staining, suggesting that residual poly-N-acetyllactosamine units onN-glycans are sufficient for observable LEA staining and that 4-F-GlcNAc was inhibiting poly-N-acetyllactosamines onO-glycans and N-glycans. These data helped confirm the expected changes in glycosylation due to swainsonine treatment in which α-mannosidase is inhibited and thus ConA staining (ie, level of α-mannose residues) was increased. More importantly, reduction in poly-N-acetyllactosamine structures (LEA staining) due to 4-F-GlcNAc treatment corroborated with previous findings on 4-F-GlcNAc glycan modulation,34 and an elevation in WGA staining in conjunction with data on 4-F-Glc[3H]NAc incorporation into CLA strongly suggests that 4-F-GlcNAc acts as a terminator of poly-N-acetyllactosamine elongation. Although the presence of fluorine at the carbon-4 position of 4-GlcNAc may block glycosidic linkage to UDP-Gal, recognition of terminal 4-F-GlcNAc residues by WGA was apparently not affected.
Lectin blot analysis of glycoconjugates isolated from human CLA+ T cells treated with glycosylation inhibitors.
Cell lysates were prepared from human CLA+ T-cell cultures treated with diluent control (PBS), tunicamycin (0.02 mM), swainsonine (0.23 mM), 4-F-GlcNAc (0.05 mM), BAG (2.0 mM), or GlcNAc (5.0 mM, negative control) for 30 hours. Lysates (25 μg per spot) were spotted onto permeabilized PVDF membrane, blocked in FBS for 1 hour at RT, and blotted with AP-conjugated ConA (2.0 μg/mL PBS), WGA (0.5 μg/mL PBS), or LEA (1.0 μg/mL PBS). Blots were then washed 3 × with PBS/0.1% Tween-20 and developed with Western Blue stain. Please note the reduction in ConA, WGA, and LEA staining intensity of lysates from tunicamycin-treated cells; LEA staining was also diminished, yet WGA staining was increased in lysates from 4-F-GlcNAc–treated cells. ConA staining of lysates from swainsonine-treated cells was slightly increased.
Lectin blot analysis of glycoconjugates isolated from human CLA+ T cells treated with glycosylation inhibitors.
Cell lysates were prepared from human CLA+ T-cell cultures treated with diluent control (PBS), tunicamycin (0.02 mM), swainsonine (0.23 mM), 4-F-GlcNAc (0.05 mM), BAG (2.0 mM), or GlcNAc (5.0 mM, negative control) for 30 hours. Lysates (25 μg per spot) were spotted onto permeabilized PVDF membrane, blocked in FBS for 1 hour at RT, and blotted with AP-conjugated ConA (2.0 μg/mL PBS), WGA (0.5 μg/mL PBS), or LEA (1.0 μg/mL PBS). Blots were then washed 3 × with PBS/0.1% Tween-20 and developed with Western Blue stain. Please note the reduction in ConA, WGA, and LEA staining intensity of lysates from tunicamycin-treated cells; LEA staining was also diminished, yet WGA staining was increased in lysates from 4-F-GlcNAc–treated cells. ConA staining of lysates from swainsonine-treated cells was slightly increased.
Discussion
Tissue-specific migration of lymphocytes to skin is critical to the pathobiology of cutaneous GVHD, of neoplastic conditions (eg, lymphoma cutis), and of inflammatory skin diseases (eg, psoriasis). The expression of CLA on normal human lymphocytes (and malignant leukocytes) directly correlates with the functional capacity of these cells to enter skin.1-10 Although CLA is expressed on a subset of primitive human hematopoietic progenitor cells,35-38 as well as on dendritic cells, monocytes, and neutrophils,12 CLA expression is conspicuously up-regulated on effector lymphocytes and on malignant cells in patients with cutaneous inflammatory disease or leukemia/lymphoma, respectively.1,5-10,14 47-50 Therefore, targeting the expression of CLA offers an attractive therapeutic approach for controlling leukocyte migration to skin. Moreover, since HECA-452–reactive epitopes on PSGL-1 define CLA expression and selectin-binding function, posttranslational glycosylations, particularly the addition of poly-N-acetyllactosamines, within pathologic skin-homing lymphocytes reveal a selective feature for potential therapies targeting the synthesis of HECA-452 epitopes.
In this study, we investigated the capacity of metabolic agents to modify HECA-452 expression and selectin-binding activity as a potential strategy for preventing lymphocyte migration to skin. We analyzed the effects of well-characterized glycosylation inhibitors, tunicamycin, swainsonine, and BAG, and of a novel fluorinated analog ofN-acetylglucosamine, 4-F-GlcNAc, in lowering HECA-452 expression on PSGL-1 and reducing selectin ligand activity as natively expressed on human CLA+ T cells. To further elucidate the mechanism of 4-F-GlcNAc anticarbohydrate action, we also performed metabolic radiolabeling experiments with 4-F-Glc[3H]NAc and lectin blotting studies to analyze glycan structural changes related to 4-F-GlcNAc treatment.
Our studies show that 4-F-GlcNAc lowers the expression of HECA-452 epitopes on CLA+ T cells at concentrations that affect neither PSGL-1 expression nor cell proliferation. Although tunicamycin and BAG treatments also markedly reduce CLA expression, tunicamycin conspicuously inhibits PSGL-1 expression and protein synthesis in general, whereas a significantly higher concentration of BAG compared with 4-F-GlcNAc is required for the observed reduction in HECA-452 expression. Furthermore, the inhibitory effects on O-glycans expressed on CD43 by BAG, but not by 4-F-GlcNAc, and the reduction of HECA-452 expression by both BAG and 4-F-GlcNAc strongly suggest that HECA-452 epitopes on PSGL-1 and selectin ligand activity related to CLA expression are displayed byO-glycans and that 4-F-GlcNAc is acting as a terminator of poly-N-acetyllactosamines on core 2 O-glycans present on PSGL-1, but not on O-glycans expressed by CD43. Interestingly, although core 2 O-glycan structures are up-regulated on CD43 expressed by activated human lymphocytes, structural analysis of CD43 O-glycans from these cells indicates that elongated poly-N-acetyllactosamines are expressed at a low level (fewer than 2% of all CD43O-glycans).45,46,51 We speculate, therefore, that synthesis of poly-N-acetyllactosamines on PSGL-1, which exist on approximately 50% of its O-glycans, has a higher chance for incorporation and termination with 4-F-GlcNAc than a singleN-acetyllactosamine structure as expressed on most core 2O-glycans displayed by CD43.30 Elevated WGA (terminal GlcNAc) staining of lysates from 4-F-GlcNAc–treated cells and reductions in LEA ((Galβ1,4GlcNAc)n) staining, in addition to data verifying 4-F-Glc[3H]NAc incorporation into CLA, provide further evidence of the proposed blockade of glycosidic linkage by fluorine following incorporation of 4-F-GlcNAc into the growing poly-N-acetyllactosamine chain (Figure 1B-C).
Interestingly, though there is greater than 3-fold more E-selectin ligand activity on CLA+ T cells compared with CLAlow T cells or PBLs, a minor E-selectin ligand activity, which parallels baseline E-selectin ligand activity of CLAlow T cells or PBLs, persisted after protease treatment. The residual protease-resistant E-selectin ligand activity (below 25%) suggests that glycolipids contribute a minor portion of total E-selectin ligand activity. These data further support that CLA is the major E-selectin ligand on CLA+ T cells and that CLA/PSGL-1 is the most relevant therapeutic target for preventing pathologic lymphocyte migration to skin. Because 4-F-GlcNAc did not reduce E-selectin ligand activity beyond that observed with protease treatment, the formation of poly-N-acetyllactosamine structures, specifically those found on neolacto series glycosphingolipids, appears unaffected. Nonetheless, the high activity of 4-F-GlcNAc in blunting CLA expression supports its utility in modifying the migration of lymphocytes to skin.
In conclusion, these data show that 4-F-GlcNAc can directly incorporate into a CLA molecule and selectively function as a poly-N-acetyllactosamine inhibitor, most likely by conversion to UDP–4-F-GlcNAc and, after addition to a poly-N-acetyllactosamine backbone, by blocking the addition of subsequent nucleotide sugar (ie, UDP-Gal) to the carbon-4 position of 4-F-GlcNAc. At low glycosylation-inhibitory concentrations, this putative mechanism of action does not impinge on other cellular activities relating to cell growth. We also demonstrate for the first time that glycolipids have a major contribution as scaffolds for expression of HECA-452 on circulating lymphocytes, but a minor functional contribution on human CLA+(skin-homing) T cells in mediating E-selectin ligand activity. Disruption of CLA expression or HECA-452 epitopes on CLA+ T cells represents a new treatment strategy and supports the use of glycosylation inhibitors, namely 4-F-GlcNAc, as nontoxic therapeutic agents for preventing the trafficking of lymphocytes (or lymphoblasts) associated with skin pathologies. Future studies evaluating the in vivo efficacy of 4-F-GlcNAc on cutaneous models of inflammation and cancer, which critically involve the functional collaboration of CLA and selectins for elaboration of model disease, are currently ongoing and will further test our hypothesis that CLA expression is a plausible target for therapeutic exploitation.
We would like to thank Drs Brajeswar Paul, Khushi L. Matta, and Moheswar Sharma at Roswell Park Cancer Institute, Buffalo, NY, for synthesizing and providing 4-F-GlcNAc and 4-F-Glc[3H]NAc. We also would like to dedicate this manuscript to the memory of Dr Walter Korytnyk, who was instrumental in initiating this work more than 30 years ago.
Prepublished online as Blood First Edition Paper, September 5, 2002; DOI 10.1182/blood-2002-06-1736.
Supported by National Institutes of Health (NIH) grants National Cancer Institute (NCI) RO1 CA84156 (R.S.), National Heart, Lung, and Blood Institute (NHLBI) RO1 HL60528 (R.S.), and NCI RO1 CA73872 (R.J.B.); Roswell Park Cancer Institute Core Grant CA16056 (R.J.B.); NIH National Research Service Award, CA91780-01 (C.J.D.); and Harvard Skin Disease Research Center Core Grant P30AR42689.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Robert Sackstein, Harvard Institutes of Medicine, Room 671, 77 Ave Louis Pasteur, Boston, MA 02115; e-mail:rsackstein@rics.bwh.harvard.edu.

![Fig. 3. Autoradiography of PSGL-1 isolated from metabolically radiolabeled CLA+T cells treated with glycosylation inhibitors. / Radiolabeled lysates were prepared from human CLA+ T-cell cultures treated with neuraminidase (Neur.) and then regrown in [35S]-protein labeling mix (100 μCi/mL [3.7 MBq/mL]) in the presence of PBS (diluent control), tunicamycin (0.02 mM), swainsonine (0.23 mM), 4-F-GlcNAc (0.05 mM), BAG (2.0 mM), or GlcNAc (5.0 mM, negative control) for 30 hours. PSGL-1 was then immunoprecipitated from lysates (200 μg) with mouse IgG isotype control or antihuman PSGL-1 moAbs PL-1, 2G3, 4F9, and 4D8 (2 μg/mL each) from lysates (200 μg) and resolved on a 6% reducing SDS-PAGE gel. Autoradiography reveals that tunicamycin markedly inhibited PSGL-1 expression, while BAG treatment slightly reduced PSGL-1 expression and mobility of the 140-kDa monomer form. Also, 4-F-GlcNAc did not affect PSGL-1 expression, while it did reduce the molecular weight of the monomer (to 130 kDa).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/2/10.1182_blood-2002-06-1736/4/m_h80233687003.jpeg?Expires=1769124177&Signature=dR7GQtgOADAnTQJ5r4B1GxrYWoMJ9H0IvzCN9sxPtJqdHXpPt-AFIG8X6eOHSaIGID-W9GrueAfn6D0Zimf4FJiPQESsNQnPPQR8KtBVgoRLYYb-gOHULH~RBuHwbgRbnq6Ls7Q6om4UbLUWMAGc3Gb1QXf12j3m9aXK1R220MgVFlh5Ev9zTFHz21j-y-ubmwvebJBaRZeTmE6kSENFbyMJhrDfxWT4tVIMvcsGgfZuQeB84f~dr08Jc~bdEcItrqN2VM-cVVLqEohK4VVum9lCMqtUWGrgBWUcZ~3Aodb3l6lQfKUHElVnSCWc6m3JKdzkL78UOWRJOocpiTqNzw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Incorporation of 4-F-Glc[3H]NAc into TCA-precipitated macromolecules isolated from human CLA+ T cells. / Cellular macromolecules were precipitated from 4-F-Glc[3H]NAc–treated CLA+ T cells (0-1.0 mM or 0-16.7 μCi/mL [0-0.6179 MBq/mL] for 36 hours or less) with 10% TCA and collected on GF/C microfiber glass filters for liquid scintillation counting. Background counts per minute from non–4-F-Glc[3H]NAc–treated cells were subtracted from counts per minute measured from TCA precipitates of 4-F-Glc[3H]NAc–treated cells, and data in triplicate were expressed as nmol 4-F-Glc[3H]NAc incorporation per cell (SEM) at 12, 24, and 36 hours. Please note that 4-F-Glc[3H]NAc was detectable in TCA precipitates in a concentration-dependent manner over a 24-hour period.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/2/10.1182_blood-2002-06-1736/4/m_h80233687006.jpeg?Expires=1769124177&Signature=DAH8lHovtomi1iddYZEuO20~DuXaC8qTzoREv0stPpNv61UAZWJ3v32ABFXbp1oX93vaXdRITBHMmeiIU3Z2DlzDQf8g7E0S~h3s5h1TuhP~scVUTqu3yr~snDyOEdM0NVfwgk~brP89A1epU5lki2F-e3kq8CqmGHDEIGiiIoPI~tJt6EtF8VAbOSZQcLgqWjdGB4F76z3ISnx~INQlgeEyqtwGkhoW0Db9T-zitTJJMj94ovq5cj7dBU-wy~sQHOd8FwG28611EWfDYyJcLVEF4~KC3okGszpr4FCuybJZjNXN79jsGgeARD5JGdaKzMbNpNe-hdrKurSXvOOZYQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 7. Autoradiography of PSGL-1 immunoprecipitated from human CLA+ T cells metabolically radiolabeled with 4-F-Glc[3H]NAc. / Radiolabeled lysate was prepared from human CLA+ T-cell cultures grown in 4-F-Glc[3H]NAc (0.2 mM or 3.4 μCi/mL [0.1258 MBq/mL]; 16 μCi/μmol [0.592 MBq/μmol]) for 36 hours. PSGL-1 was then immunoprecipitated from radiolabeled lysate (2 mg) with mouse IgG isotype control or antihuman PSGL-1 moAbs (PL-1, 2G3, 4F9, and 4D8, 2 μg for each 100 μg lysate), resolved on a 6% reducing SDS-PAGE gel, and exposed to film for 28 days. Autoradiography revealed that both dimer (220 kDa) and monomer (140 kDa) forms of PSGL-1 were detectable (panel A, lane 1) and that isotype control antibody did not immunoprecipitate any detectable material (panel A, lane 2). Comparative densitometric scans of lanes 1 and 2 in panel A by means of NIH ImageJ software confirmed the appearance of 220- and 140-kDa forms of PSGL-1 immunoprecipitated from radiolabeled lysate (panel B, lane 1).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/2/10.1182_blood-2002-06-1736/4/m_h80233687007.jpeg?Expires=1769124177&Signature=WIF2aZd38FA1Jx5lQNqYZwSLX~Dajduzs-Zj88zdJePW01xU03cQSR7V2BRuk-tepNApZWdI10ijHRcMj3bZpnUgYKP-jEu70Bbtp-RxwFt9yFzjkZFxFp46EersAsLzfRsgaXrJ4QfEw0TwpF3E3RY98i5~-kK0Zl1s7mXzEXPOO8QxX5AIRnkWrvlHZJDfD0iSN4-lgChXydJ9RNvqM3ZePhrkLadt0EMYpUt2u28FdHmdaFRr9UtjJF7DlnZDFzSS4RWEDwgAYWS4h3tMhCb5bA-gdle8lgozRM77Jei~XX8biEfpy52Cj2IinOl0J9-fo~0~1FAidFDiiRIvuw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal