The successful induction of a T-cell–mediated tumor-protective immunity against poorly immunogenic malignancies remains a major challenge for cancer immunotherapy. We achieved this by immunization with a tyrosine hydroxylase (mTH)–based DNA vaccine, enhanced with the posttranscriptional regulatory acting RNA element (WPRE), derived from woodchuck hepatitis virus in combination with an antibody-cytokine fusion protein (ch14.18–IL-2) that targets interleukin-2 (IL-2) to the tumor microenvironment. This DNA vaccine mTH-WPRE was carried by attenuated Salmonella typhimurium and applied by oral gavage in a mouse model of neuroblastoma. Mice immunized with the mTH-WPRE vaccine, and which additionally received a boost with suboptimal doses of ch14.18–IL-2, were completely protected against hepatic neuroblastoma metastases. In contrast, all controls presented with disseminated metastases. Both T-cell and natural killer (NK) cell–dependent mechanisms were involved in the induction of a systemic tumor-protective immunity. Thus, up-regulation of interferon-γ (IFN-γ) expression in CD8+ T cells occurred only in those animals that received the mTH-WPRE vaccine plus the ch14.18–IL-2 boost. Up-regulation of this proinflammatory cytokine was not observed in mice immunized with mTH-WPRE vaccine alone. A role for NK cells was indicated by the complete abrogation of systemic tumor-protective immunity in all animals that were depleted of NK cells in vivo. Taken together, these data demonstrate that immunization with a posttranscriptionally enhanced DNA vaccine encoding the WPRE sequence, combined with a boost of the ch14.18–IL-2 fusion protein, completely protects against hepatic metastases in a murine model of neuroblastoma and therefore may lead to a new strategy for immunotherapy and prevention of metastatic neuroblastoma.
Introduction
The induction of an effective systemic protective immunity against malignancies is a major objective for cancer immunotherapy. The goal is to break peripheral tolerance of the immune system toward poorly immunogenic tumors by activating cytotoxic T cells (CTLs) and/or natural killer (NK) cells to effectively eradicate disseminated tumor metastases. In the field of DNA vaccination, it has been shown by others and ourselves1-3 that this can be achieved in melanoma with vaccines encoding TRP1, TRP2, and gp100 antigens4 and in colon carcinoma with the CEA antigen.5-7 In this regard, it has been reported that autoreactive T cells that escape negative selection in the thymus can be activated in the periphery8 to be directed against the tumor, resulting in an efficient tumor-protective immune response. Importantly, there is also evidence that, aside from T cells, the innate immune system plays a role in the host's defense against neuroblastoma via NK cells, in particular if interleukin-2 (IL-2) is included in the therapy protocol. In fact, eradication of murine neuroblastoma metastases by a recombinant human/mouse chimeric antiganglioside GD2–IL-2 fusion protein (ch14.18–IL-2) was entirely NK-cell dependent when administered in therapeutic doses.9Moreover, once neuroblastoma patients were treated with IL-2–transduced autologous neuroblastoma tumor cells, they presented with an increase in activated T cells and NK cells, indicating a role for both effector populations in IL-2 immunotherapy of this malignancy.10 We also recently reported that a mouse tyrosine hydroxylase (mTH)–based DNA vaccine elicits an antitumor effect in a poorly immunogenic murine model of neuroblastoma.11 However, therapy with this DNA vaccine containing solely the tumor-associated antigen mTH was of limited efficacy as indicated by only a partial reduction in subcutaneous tumor growth. Therefore, we tested the hypothesis that the PRE sequence of the woodchuck hepatitis virus (WPRE), in conjunction with mTH as a target antigen, could improve the systemic tumor-protective immune response against neuroblastoma metastases and provide complete tumor protection. The rationale for this approach is based on the observation that human hepatitis B virus (HBV) and woodchuck hepatitis B virus (W-HBV) contain posttranscriptional-acting RNA elements (PRE) that significantly enhance viral gene expression; however, woodchuck PRE sequences enhance gene expression to a greater extent than their equivalents of the human hepatitis B virus.12 In fact, its WPRE sequence acts independently of transcription and splicing and thus may improve gene expression by modification of polyadenylation, RNA export, or translation.13,14 Moreover, recent in vitro studies demonstrated gene expression of human multidrug resistance 1 (MDR1) and enhanced green fluorescent protein (EGFP) genes to be enhanced by posttranscriptional modification with the WPRE sequence.15 In addition, Popa et al suggested that WPRE is directing messages into a CRM1-dependent mRNA export pathway in somatic mammalian cells.16 These observations raised the question as to whether this event could also occur in vivo and be used to optimize the mTH-based DNA vaccine.
Interleukin-2 (IL-2), a well-known, strong promoter of T-cell proliferation, which at low levels induces T-cell expansion,17,18 provides the rationale for boosting an initial immune response provoked by the mTH-WPRE vaccine with the recombinant antibody–IL-2 fusion protein ch14.18–IL-2. This fusion protein uses disialoganglioside GD2 (GD2) as a docking site on neuroblastoma cells, where it is overexpressed and was already successfully used as a target for immunotherapy in neuroblastoma patients19-21 and mouse models of this malignancy as well as other GD2-expressing tumors.22 23 In fact, IL-2 directed to the tumor microenvironment could locally amplify and expand an initial immune response provoked by the mTH-WPRE vaccine.
Here, we demonstrate for the first time that posttranscriptional modification of an antitumor DNA vaccine with the WPRE sequence results in an enhanced immune response that in combination with the ch14.18–IL-2 fusion protein leads to complete T-cell– and NK-cell–mediated protection from neuroblastoma metastases.
Materials and methods
Construction and characterization of DNA vaccines encoding mTH and WPRE
The coding sequence for mTH was generated from the pCMVβ3FUB plasmid encoding for mTH as described earlier11 using primers 5′ HindIIImTH (CCC AAG CTT ATG CCC ACC CCC AGC GCC TC) and 3′mTHXhoI (CCG CTC GAG TTA GCT AAT GGC ACT CAG TG) followed by insertion into the pcDNA3.1+ vector (Invitrogen, Carlsbad, CA). The 650-bp WPRE fragment was amplified from pWHV8 (Genbank accession no. J02442901-1801, nt 900-1550) (kindly provided by Dr H. Will, Heinrich Pette Institut, Hamburg, Germany) using primers 5′XhoIWPRE (GG CTC GAG CTA GCG AGC ATC TTA CCG CCA TTT ATT CCC) and 3′ WPREXbaI (GG GTC GAC CTA GAG AAA GGA CGT CAG CTT CCC CG) followed by ligation into the pcDNA3.1 vector downstream of mTH (Figure 1). Integrity of all vectors was verified by nucleotide sequencing.
Design and characterization of WPRE-enhanced DNA vaccine.
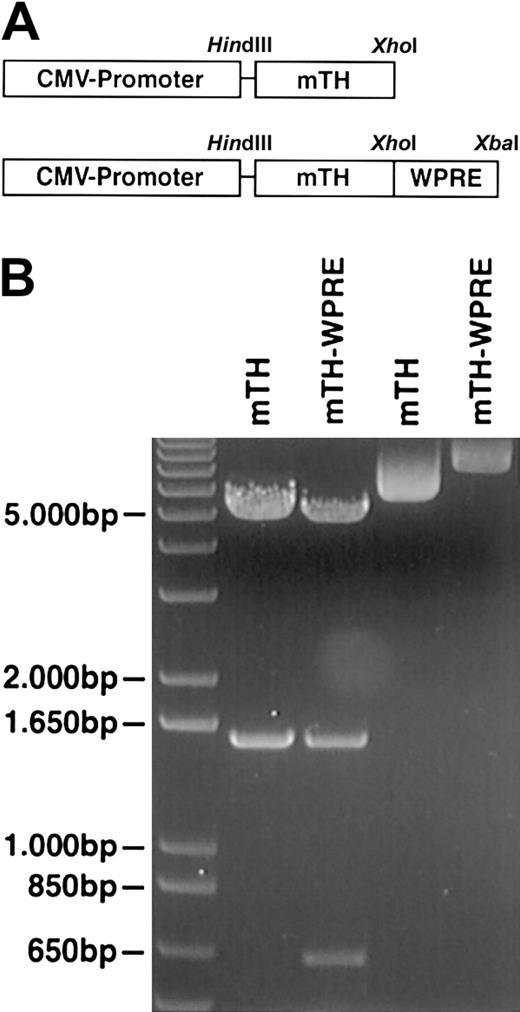
(A) The scheme indicates the composition of the DNA vaccine. The tumor-associated antigen mTH is inserted within the restriction sitesHindIII (5′-end) and XhoI (3′-end) at the multiple cloning site of the pcDNA3.1 vector (Invitrogen, Carlsbad, CA) under the control of the cytomegalovirus (CMV) promoter. The WPRE sequence is inserted after the stop codon within the restriction sites XhoI (5′-end) and XbaI (3′-end). (B) Integrity of genes was demonstrated by restriction enzyme digestion (lanes 1 and 2) with undigested controls shown in lanes 3 and 4 and further verified by nucleotide sequencing. The gel depicts a 1497-bp fragment of mTH and a 641-bp fragment of the WPRE sequence.
Design and characterization of WPRE-enhanced DNA vaccine.
(A) The scheme indicates the composition of the DNA vaccine. The tumor-associated antigen mTH is inserted within the restriction sitesHindIII (5′-end) and XhoI (3′-end) at the multiple cloning site of the pcDNA3.1 vector (Invitrogen, Carlsbad, CA) under the control of the cytomegalovirus (CMV) promoter. The WPRE sequence is inserted after the stop codon within the restriction sites XhoI (5′-end) and XbaI (3′-end). (B) Integrity of genes was demonstrated by restriction enzyme digestion (lanes 1 and 2) with undigested controls shown in lanes 3 and 4 and further verified by nucleotide sequencing. The gel depicts a 1497-bp fragment of mTH and a 641-bp fragment of the WPRE sequence.
Generation and characterization of tumor-specific antibody–IL-2 fusion protein
The ch14.18–IL-2 fusion protein was constructed by fusion of a synthetic sequence coding for human IL-2 to the carboxyl terminal of the human Cγ1 gene of the mouse human chimeric anti-GD2 antibody ch14.18 essentially as described previously.24
Neuroblastoma tumor model and treatment protocol
Syngeneic female A/J mice were obtained at 8 to 12 weeks of age from Harlan Laboratory (Indianapolis, IN). All animal experiments were performed according to the National Institutes of Health's Guide for the Care and Use of Laboratory Animals. Mice were immunized twice at 2-week intervals with 1 × 108attenuated Salmonella typhimurium transformed with a plasmid encoding either mTH-WPRE, mTH, or the empty vector. One week after the last immunization, mice were challenged by intravenous injection of 5 × 105 NXS2 neuroblastoma cells and boosted 2 days thereafter with 10 μg ch14.18–IL-2 fusion protein injected intravenously on 4 consecutive days. The wild-type murine NXS2 neuroblastoma cell line was cultured as described previously.22 The extent of hepatic metastases was determined 28 days after tumor cell challenge by liver weight and by counting the total number of metastatic foci on the liver surface.
Transformation of attenuated S typhimurium (SL 7207) with plasmids encoding a DNA vaccine
Cultured bacteria were washed with deionized water and then admixed with either mTH-WPRE, mTH, or the empty vector DNA (3 × 1010 bacteria, 1 μg plasmid DNA) and electroporated with a Bio-Rad Gene Pulser (Hercules, CA) at 2.5 kV, 25 microfarads (μF), and 200 Ω in a 0.1-cm electroporation cuvette. The transformed bacteria were cultured in Luria-Bertani medium (LB) supplemented with 20 mM glucose (SOC) medium for 30 minutes at 37°C under vigorous shaking and then plated onto LB plates under the selection of 50 μg/mL ampicillin.
Immunofluorescence staining and confocal microscopy
COS-7 cells were maintained in Dulbecco modified Eagle medium supplemented with 10% fetal calf serum (FCS), 1% glutamine, and 1% penicillin/streptomycin. Transfection of COS-7 cells was performed with Superfect (Qiagen, Valencia, CA) according to the manufacturer's guidelines, and 24 hours later cells were analyzed by fluorescence microscopy. Cells grown on coverslips were fixed for 10 minutes with 4% paraformaldehyde; mTH was detected with rabbit–anti-mTH antibody (Chemicon, Temecula, CA) diluted 1:100 in phosphate-buffered saline (PBS) containing 10% FCS and 0.1% saponin and stained with Texas red–labeled donkey antirabbit antibody immunoglobulin G (IgG) (Jackson Immuno Research, West Grove, PA). Cells were analyzed with a MRC1024 confocal microscope (Bio-Rad).
Cytotoxicity assay
Mouse splenocytes were cultured for 2 days with irradiated NXS2 tumor cells at a tumor cell–effector cell ratio of 1:100. Subsequently a 4-hour 51chromium (51Cr) release assay was performed as described previously.25 The percentage of target cell lysis was calculated as follows: experimental release (cpm) − spontaneous release (cpm)/total release (cpm) − spontaneous release (cpm) × 100 = percent cytotoxicity.
Flow cytometry
Staining for expression of cell surface antigens and intracellular antigens was performed as described previously.25 Splenocytes of control mice and immunized mice were isolated as described for the cytotoxicity assay and stimulated for 5 hours with 5 ng/mL phorbol myristate acetate (PMA) (Sigma, St Louis, MO) and 500 ng/mL ionomycin (Sigma). All stimulated cultures contained 1 μg/mL Brefeldin A (Sigma) to block protein transport into post-Golgi compartments and to allow cytokines to accumulate within cells. Cells were analyzed on a FACScan flow cytometer using Cell Quest software (Becton Dickinson, San Diego, CA).
Statistics
The statistical significance of differential findings between experimental groups of animals was determined by the 2-tailed Student t test. Findings were regarded as significant if 2-tailed P values were ≤ .05.
Results
Effect of WPRE-enhanced DNA vaccine on in vitro expression of mTH antigen
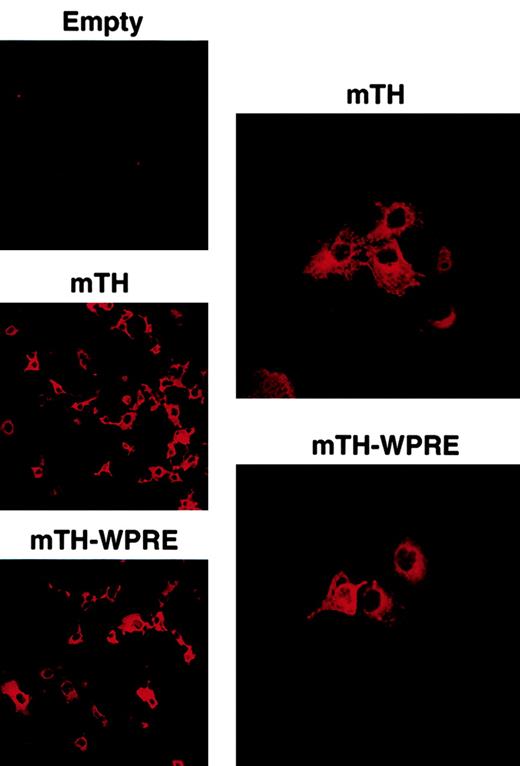
The posttranscriptional regulatory PRE element of the woodchuck hepatitis virus was cloned and inserted as described in “Materials and methods,” directly downstream of the tumor-associated mTH antigen and following the stop codon. The correct sizes of the mTH and the WPRE fragments were analyzed by restriction enzyme digestion with a 1497-bp fragment encoding for mTH and a 641-bp fragment for the WPRE sequence (Figure 1). Integrity of the genes was verified by nucleotide sequencing. Gene expression of the DNA constructs was determined by confocal microscopic analysis. For this purpose, COS-7 cells were transduced with plasmids encoding mTH in the presence or absence of the WPRE sequence. Figure2 illustrates protein expression of the mTH antigen in the cytoplasm (original magnification × 20), with protein accumulation appearing predominantly perinuclear as demonstrated at higher magnification (× 40).
Expression of mTH and mTH-WPRE in COS-7 cells.
Cytoplasmic perinuclear expression of mTH is indicated by immunofluorescence in COS-7 cells in the absence and presence of WPRE following transduction with the plasmid vector DNA or the empty vector (empty). Original magnifications, left column, × 20; right column, × 40.
Expression of mTH and mTH-WPRE in COS-7 cells.
Cytoplasmic perinuclear expression of mTH is indicated by immunofluorescence in COS-7 cells in the absence and presence of WPRE following transduction with the plasmid vector DNA or the empty vector (empty). Original magnifications, left column, × 20; right column, × 40.
Induction of complete systemic tumor protection by posttranscriptionally enhanced WPRE DNA vaccine in combination with a targeted IL-2 boost
To assess whether the WPRE-enhanced DNA vaccine could induce effective systemic tumor-protective immunity, A/J mice were immunized twice by oral gavage at 2-week intervals with an attenuated strain ofS typhimurium (SL7207) as vaccine carrier as described previously.11 This attenuated strain of salmonella is unable to produce aromatic amino acids necessary for its replication in vivo and consequently dies once it reaches the secondary lymphoid tissue after passing through the M cells of intestinal mucosa into the Peyer patches, where it liberates copies of the DNA vaccine. The animals received either mTH, the mTH-WPRE DNA vaccine with or without an additional boost of subcurative doses of ch14.18–IL-2 fusion protein, or the empty vector as a control. One week after immunization, experimental metastases were induced intravenously by a lethal challenge with wild-type NXS2 neuroblastoma cells. Mice immunized with the mTH-WPRE vaccine revealed significantly less metastases as assessed by liver weight and compared with mice immunized with mTH alone or empty vector controls (P ≤ .04), indicating that addition of the WPRE sequence significantly enhanced the antitumor immune response. Moreover, boosts with the ch14.18–IL-2 fusion protein following immunization with the mTH vaccine also significantly enhanced antitumor immune response, with 4 of 6 mice remaining tumor free compared with animals immunized with mTH alone or empty vector controls, where all mice developed fulminant metastases (P ≤ .03). However, only if the mTH-WPRE vaccine was combined with ch14.18–IL-2 boosts, 100% of the mice were completely protected against hepatic neuroblastoma metastases when evaluated after 28 days (Figure 3; Table1). These findings indicate that the WPRE-enhanced DNA vaccine plus the ch14.18–IL-2 fusion protein boost is most effective in inducing complete tumor-protective immunity against neuroblastoma metastases in all experimental animals. Thus, the WPRE sequence can effectively enhance the in vivo antitumor immune response in conjunction with a tumor-associated antigen. However, a combination of both of these components is required to markedly enhance tumor-protective immunity, because neither one of the components alone is capable of protecting 100% of the mice.
Complete protection from hepatic neuroblastoma metastases induced by an mTH-based DNA vaccine with posttranscriptionally enhanced gene expression by WPRE and boosts with ch14.18–IL-2.
(A) Mice were immunized twice by oral gavage at 2-week intervals with 108 attenuated salmonella that contained either the empty vector, pcDNA3.1; pcDNA3.1 plus mTH; or pcDNA3.1 plus mTH-WPRE. All animals were injected intravenously with a lethal challenge of 5 × 105 NXS2 wild-type tumor cells 1 week after the second immunization and boosted 2 days thereafter with 10 μg ch14.18–IL-2 for 4 consecutive days. (B) Mice were killed 4 weeks after tumor cell challenge, and hepatic metastases were assessed by liver weight in milligrams and counting of individual metastatic foci. Ruler below images is marked in centimeters.
Complete protection from hepatic neuroblastoma metastases induced by an mTH-based DNA vaccine with posttranscriptionally enhanced gene expression by WPRE and boosts with ch14.18–IL-2.
(A) Mice were immunized twice by oral gavage at 2-week intervals with 108 attenuated salmonella that contained either the empty vector, pcDNA3.1; pcDNA3.1 plus mTH; or pcDNA3.1 plus mTH-WPRE. All animals were injected intravenously with a lethal challenge of 5 × 105 NXS2 wild-type tumor cells 1 week after the second immunization and boosted 2 days thereafter with 10 μg ch14.18–IL-2 for 4 consecutive days. (B) Mice were killed 4 weeks after tumor cell challenge, and hepatic metastases were assessed by liver weight in milligrams and counting of individual metastatic foci. Ruler below images is marked in centimeters.
Combination therapy with WPRE-enhanced DNA vaccine and boosts with 14.18–IL-2 induces complete protection from metastatic neuroblastoma
| Treatment . | Liver weight . | Metastatic foci . |
|---|---|---|
| mTH-WPRE + ch14.18–IL-2 | 898 ± 109 | 0, 0, 0, 0, 0, 0 |
| Empty vector | 3500 ± 573 | >250, >250, >250, >250, 24, 16 |
| mTH | 3080 ± 953 | >250, >250, >250, 1, 2 |
| mTH + ch14.18–IL-2 | 1166 ± 68 | 7, 5, 0, 0, 0, 0 |
| mTH-WPRE | 1250 ± 113 | 7, 3, 0, 0, 0, 0 |
| ch14.18–IL-2 | 2133 ± 408*,† | >250, >250, >250, 35, 18, 3 |
| Treatment . | Liver weight . | Metastatic foci . |
|---|---|---|
| mTH-WPRE + ch14.18–IL-2 | 898 ± 109 | 0, 0, 0, 0, 0, 0 |
| Empty vector | 3500 ± 573 | >250, >250, >250, >250, 24, 16 |
| mTH | 3080 ± 953 | >250, >250, >250, 1, 2 |
| mTH + ch14.18–IL-2 | 1166 ± 68 | 7, 5, 0, 0, 0, 0 |
| mTH-WPRE | 1250 ± 113 | 7, 3, 0, 0, 0, 0 |
| ch14.18–IL-2 | 2133 ± 408*,† | >250, >250, >250, 35, 18, 3 |
Differences in liver weights of mice that received the combination therapy, mTH-WPRE vaccination, and ch14.18–IL-2 boost were statistically significant compared with the groups of mice that received either mTH plus boosts with the ch14.18–IL-2 or mice that were immunized with the mTH-WPRE vaccine alone.
P = .028.
P = .02
Effector mechanisms involved in systemic tumor-protective immune response induced by the WPRE-enhanced DNA vaccine boosted with the ch14.18–IL-2 fusion protein
To identify immune effector cells responsible for therapy-induced tumor protection, splenocytes were harvested 2 days after the final boost with ch14.18–IL-2 from mice of all experimental groups. These cells were stained for CD8 and intracellular interferon-γ (IFN-γ) and analyzed by flow cytometry. Both treatment groups receiving the antibody–IL-2 fusion protein revealed significant up-regulation of IFN-γ+ CD8+ T cells. This effect was independent of whether previous DNA vaccination included the WPRE-sequence or not, indicating that the boost with targeted IL-2 leads to the activation of tumor-specific T cells. Interestingly, enhancement of the mTH vaccine with the WPRE sequence alone did not affect the CD8+ T-cell compartment, and administration of only the ch14.18–IL-2 fusion protein in subcurative dosages resulted neither in antitumor effects nor in up-regulated IFN-γ+ CD8+ T cells (Figure4). In contrast, only the ch14.18–IL-2 boosts administered after DNA vaccination with or without WPRE produced a doubling of IFN-γ+ CD8+ T cells. This finding points to the importance of combination treatments with DNA vaccines followed by cytokine boosts for the induction of optimal CD8+ T-cell–mediated immune responses.
Effect of posttranscriptional modification of a DNA vaccine with WPRE combined with boosts of ch14.18–IL-2 fusion protein on IFN-γ expression in CD8+ T cells.
Splenocytes from A/J mice, recovered 2 days after final boost with ch14.18–IL-2, were cocultured with irradiated NXS2 tumor cells for antigen-specific proliferation and activated polyclonally as described in “Materials and methods.” IFN-γ expression in CD8+ T cells was determined by intracellular staining. Differences in IFN-γ expression of CD8+ T cells between mice treated with a combination of mTH or mTH-WPRE immunization and ch14.18–IL-2 boosts and all other groups were statistically significant (P ≤ .004). Data are expressed as means ± SE from 2 different experiments.
Effect of posttranscriptional modification of a DNA vaccine with WPRE combined with boosts of ch14.18–IL-2 fusion protein on IFN-γ expression in CD8+ T cells.
Splenocytes from A/J mice, recovered 2 days after final boost with ch14.18–IL-2, were cocultured with irradiated NXS2 tumor cells for antigen-specific proliferation and activated polyclonally as described in “Materials and methods.” IFN-γ expression in CD8+ T cells was determined by intracellular staining. Differences in IFN-γ expression of CD8+ T cells between mice treated with a combination of mTH or mTH-WPRE immunization and ch14.18–IL-2 boosts and all other groups were statistically significant (P ≤ .004). Data are expressed as means ± SE from 2 different experiments.
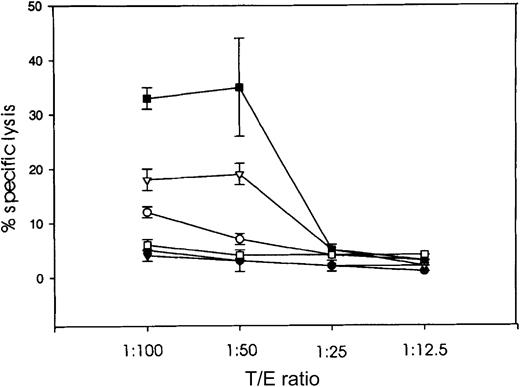
Tumor-specific cytotoxic activity, determined by a standard51Cr release assay with splenocytes isolated from mice of each experimental group, indicated that only animals immunized with the mTH-WPRE DNA vaccine plus ch14.18–IL-2 boosts produced a 3-fold increase in specific cytolysis of NXS2 tumor target cells over controls (Figure 5). This finding indicates that a tumor-specific CTL response requires this combined treatment regimen because neither of these components alone could induce increased tumor-specific cytotoxic activity.
Tumor-specific cytotoxicity induced by combination therapy with WPRE-enhanced DNA vaccine and boosts with ch14.18–IL-2 fusion protein.
Cytotoxicity was induced by mouse splenocytes isolated 2 days after final boost with ch14.18–IL-2 and cocultured for specific expansion with irradiated NXS2 tumor cells for 2 days. Specific lysis was assessed at various T/E ratios in a standard 4-hour 51Cr release assay for splenocytes of mice immunized with either pcDNA3.1mTH (○); boosted with the ch14.18–IL-2 fusion protein in addition to mTH-vaccination (▴); WPRE-enhanced mTH vaccine, pcDNA3.1mTH-WPRE (▿); and a combination therapy of mTH-WPRE vaccination plus ch14.18–IL-2 fusion protein boost (▪). Results were compared with controls either immunized with the empty vector (●) or treated with the ch14.18–IL-2 fusion protein alone (■).
Tumor-specific cytotoxicity induced by combination therapy with WPRE-enhanced DNA vaccine and boosts with ch14.18–IL-2 fusion protein.
Cytotoxicity was induced by mouse splenocytes isolated 2 days after final boost with ch14.18–IL-2 and cocultured for specific expansion with irradiated NXS2 tumor cells for 2 days. Specific lysis was assessed at various T/E ratios in a standard 4-hour 51Cr release assay for splenocytes of mice immunized with either pcDNA3.1mTH (○); boosted with the ch14.18–IL-2 fusion protein in addition to mTH-vaccination (▴); WPRE-enhanced mTH vaccine, pcDNA3.1mTH-WPRE (▿); and a combination therapy of mTH-WPRE vaccination plus ch14.18–IL-2 fusion protein boost (▪). Results were compared with controls either immunized with the empty vector (●) or treated with the ch14.18–IL-2 fusion protein alone (■).
Significantly, previous experiments demonstrated that, once the ch14.18–IL-2 fusion protein was administered in therapeutic dosages, the eradication of neuroblastoma metastases was entirely NK-cell dependent.9 This finding led to the assumption that besides CD8+ T cells the innate immune system may be activated by the mTH-WPRE DNA vaccine, especially following boosts with the ch14.18–IL-2 fusion protein. Therefore, we investigated the role of NK cells in a second set of experiments where all A/J mice in the experimental groups were depleted of NK cells in vivo with anti–asialo-GM1 antiserum (Table 2). In this case, systemic protective immunity against NXS2 wild-type tumor cells was completely abrogated in all treatment groups in contrast to nondepleted controls, indicating that NK cells expressing GM1 play a crucial role in the mediation of systemic tumor-protective immunity in our model system.
Effect of NK cells on systemic tumor-protective immunity induced by the mTH-WPRE vaccine and the ch14.18–IL-2 fusion protein
| Treatment . | Liver weight, mg . | Metastatic foci . |
|---|---|---|
| Empty vector + anti–asialo-GM1 | 5200 ± 500 | >250, >250, >250, >250 |
| mTH + ch14.18–IL-2 + anti–asialo-GM1 | 5450 ± 421 | >250, >250, >250, >250 |
| mTH-WPRE + anti–asialo-GM1 | 3700 ± 200 | >250, >250, >250, >250 |
| mTH-WPRE + ch14.18–IL-2 + anti–asialo-GM1 | 4693 ± 200 | >250, >250, >250, >250 |
| mTH-WPRE | 1250 ± 113 | 7, 3, 0, 0 |
| mTH-WPRE + ch14.18–IL-2 | 1073 ± 103 | 0, 0, 0, 0 |
| Treatment . | Liver weight, mg . | Metastatic foci . |
|---|---|---|
| Empty vector + anti–asialo-GM1 | 5200 ± 500 | >250, >250, >250, >250 |
| mTH + ch14.18–IL-2 + anti–asialo-GM1 | 5450 ± 421 | >250, >250, >250, >250 |
| mTH-WPRE + anti–asialo-GM1 | 3700 ± 200 | >250, >250, >250, >250 |
| mTH-WPRE + ch14.18–IL-2 + anti–asialo-GM1 | 4693 ± 200 | >250, >250, >250, >250 |
| mTH-WPRE | 1250 ± 113 | 7, 3, 0, 0 |
| mTH-WPRE + ch14.18–IL-2 | 1073 ± 103 | 0, 0, 0, 0 |
Mice were treated by oral vaccination with 1 × 108 salmonella containing pcDNA3.1, pcDNA3.1mTH, or pcDNA3.1mTH-WPRE on day 0 (prime) and day 14 (boost); challenged with 5 × 105 NXS2 wild-type tumor cells on day 21; and treated with 10 μg ch14.18–IL-2 2 days thereafter for 4 consecutive days. Mice (n = 4) were humanely killed 28 days after tumor cell challenge, and metastases to the liver were assessed. NK-cell depletion was performed with injection of 100 μL anti–asialo-GM1 weekly for 4 weeks, starting 1 day prior to tumor cell challenge. Differences in liver weights between mice of all experimental groups depleted with anti–asialo-GM1 as compared with nondepleted mice were statistically significant (P ≤ .001). Treatment protocol as described for Figure 3.
Discussion
For immunotherapy to achieve long-term remission in cancer patients with minimal residual disease, it is crucial to break peripheral tolerance against tumor self-antigens. This remains the goal of immunotherapy regimens designed to achieve tumor-protective immunity. Here, we tested the hypothesis that systemic tumor-protective immunity can be induced by a DNA vaccine, which was posttranscriptionally modified to enhance gene expression of the tumor-associated antigen mTH, combined with boosts of an antibody–IL-2 fusion protein. We previously reported that it was possible to induce an antitumor immune response against this mTH self-antigen following DNA vaccination.11 However, this particular DNA vaccine encoding only murine TH induced limited antitumor protection as indicated by a reduction in subcutaneous tumor growth. Therefore, we optimized this mTH-based DNA vaccine by combining its expression unit with the WPRE sequence, leading to a more effective tumor-protective immune response as documented by the absence of tumor growth after immunization. In contrast, the mTH vector alone could not induce full protection, which was in accordance with our previous results.11 Interestingly, although we were unable to detect an enhancement of mTH expression by addition of WPRE in vitro, our in vivo data clearly indicate that immunization with the WPRE-enhanced vaccine leads to significantly improved tumor-protective immunity. However, the mechanisms involved in the enhanced induction of protective immunity by the WPRE element are not entirely understood. Recent reports revealed some mechanistic features of posttranscriptional elements that affect RNA metabolism at the level of RNA stability, polyadenylation, and nucleocytoplasmic transport.13-15,26,27 Remarkably, besides these quantitative aspects, WPRE recruits the nuclear export receptor CRM1 to the transcript and thereby changes the intracellular nucleocytoplasmic transport pathway of the mRNA, which differs from the export pathway of cellular mRNAs.16 Therefore, we propose that when the mTH vaccine is applied alone the transcript expressed is exported via the cellular mRNA export pathway; however, in the presence of WPRE the mTH transcript leaves the nucleus via a pathway involving CRM1. This difference in nucleocytoplasmic transport may result in a qualitatively different association of the resulting mTH transcript with cellular compartments involved in antigen processing and presentation, which, in turn, may lead to a more effective antitumor immune response in vivo. We indeed demonstrated this to be the case, because the WPRE sequence markedly enhanced the efficacy of our DNA vaccine. Specifically, systemic tumor protection occurred in 4 of 6 animals remaining tumor free after vaccination with mTH-WPRE. In contrast, all mice immunized with mTH alone developed extensive tumor metastases. The extent of tumor metastases was assessed by liver weight and number of metastatic foci. While metastatic foci indicate visible macroscopic changes, a change in liver weight indicates even microscopic changes and therefore appears to be a more sensitive parameter. Consequently, the tumor-protective effect achieved in our experiments clearly indicates that the WPRE sequence is an important tool to enhance the efficacy of DNA-based tumor vaccines.
Boosts with subcurative dosages of ch14.18–IL-2 fusion protein targeted to the tumor microenvironment lead to an efficient amplification of the initial antitumor immunity induced by our DNA vaccine. Two lines of evidence support this conclusion. First, boosts with the ch14.18–IL-2 fusion protein produced a 3-fold increase in intracellular expression of IFN-γ in the CD8+ T-cell compartment, indicating a major role for this T-cell subset. The assessment of a potential minor role of the CD4+ T-cell subset in the priming and effector phase was not within the scope of this study and therefore remains to be determined in the context of this tumor model. However, a role for CD4+ T-cell help, particularly in the priming phase, was established for a murine model of melanoma following immunization with a gp100- and TRP1-based DNA vaccine in our previous work.1 In analogy to the melanoma tumor model, CD4+ T cells may thus also be required in the priming phase to generate an antitumor immune response against neuroblastoma as well. Second, specific CTL activity was increased in animals that received the mTH-WPRE DNA vaccine in combination with ch14.18–IL-2 boosts. In this regard, IL-2 was reported to phosphorylate Jak1 and Jak3 and lead to the recruitment and activation of signal transducers and activators of transcription-1 (STAT1, STAT3, and STAT5), which, in turn, translocate to the nucleus and activate target genes.28-30 Because these signaling events are identical for both T and NK cells, it is likely that these pathways mediate effects of IL-2 in both T and NK cells and hence lead to the proliferation of both cell types. These observations may explain why combination treatment with the DNA vaccine and boosts of ch14.18–IL-2 in our neuroblastoma model induce systemic tumor-protective immunity mediated by both T and NK effector cells. Moreover, an alternative pathway was also reported for selective activation of NK cells by IL-2 through Jak2 and STAT4, which enhances their cytolytic activity.31 This pathway could be responsible for tumor cell killing following boosts with ch14.18–IL-2. In addition, consistent with our findings and in support of a “threshold” model for IL-2, von Parijs et al32demonstrated that lower levels of this cytokine tend to promote T-cell survival, whereas higher levels are required for apoptosis-induced cell death. Because we used low amounts of IL-2 targeted to the tumor microenvironment via an antibody, this observation may explain the significant up-regulation of IFN-γ+ CD8 T cells following boosts with ch14.18–IL-2 in our tumor model.
In summary, we demonstrated that a DNA vaccine based on the mTH tumor self-antigen and enhanced with the WPRE sequence can induce tumor-protective immunity and that this immune response can be amplified by subcurative doses of IL-2 targeted to the tumor microenvironment by the ch14.18–IL-2 fusion protein. Each component of the combined treatment regimen played a role, and 100% protection was obtained only when all components were included to induce effective immune responses. The mechanisms of action involved included at least 2 effector cell-types: CD8+ T cells and NK cells. It is anticipated that this strategy may lead to an improvement in the efficacy of DNA vaccines for cancer therapy.
We express our appreciation to Collette Beaton for the preparation of this manuscript.
Prepublished online as Blood First Edition Paper, September 12, 2002; DOI 10.1182/blood-2002-02-0391.
Supported by National Institutes of Health grant 1R21CA83140-01 (H.N.L., R.A.R.) and Emmy Noether Programm of the Deutsche Forschungsgemeinschaft (H.N.L.) (Lo 635/2-1). J.M.R. is a fellow of the Deutsche Forschungsgemeinschaft.
U.P. and H.W. contributed equally to this study, and H.N.L. and R.A.R. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Ralph A. Reisfeld, The Scripps Research Institute, Department of Immunology, R218, IMM13, 10550 N Torrey Pines Rd, La Jolla, CA 92037; e-mail:reisfeld@scripps.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal