Glycosphingolipids (GSLs) are complex macromolecules on cell membranes that have been shown to play a role in neutrophil differentiation, activation, phagocytosis, and adhesion to both microorganisms and vascular endothelium. Because GSLs are often cryptic antigens on cell membranes, little is known regarding GSL expression in early myelopoiesis. To study the latter, myeloblasts were collected from patients with acute nonlymphocytic leukemia (ANLL) who required therapeutic leukocytopheresis for hyperleukocytosis. The neutral GSLs were isolated and identified by high-performance thin-layer chromatography (HPTLC), HPTLC immunostaining, gas chromatography, nuclear magnetic resonance, and fast atom bombardment–mass spectrometry. Like mature peripheral blood neutrophils, myeloblasts expressed glucosylceramide, lactosylceramide, and the neolacto-family GSLs, lactotriaosylceramide and neolactotetraosylceramide. Unlike neutrophils and chronic myeloid leukemia, most ANLL samples also expressed the globo-series GSLs, globotriaosylceramide and globotetraosylceramide. Globo GSL expression was strongly associated with a myeloblastic (ANLL M0-M2) and monoblastic phenotype (M5). A weak association was also noted with expression of either lymphoid (P < .10) or early hematopoietic markers (terminal deoxynucleotidyl transferase [TdT], CD34; P < .10). Globo-positive ANLL samples bound both shiga toxin and parvovirus B19 on HPTLC immunostaining. Based on these findings, we propose that neolacto and globo GSLs are expressed during early myeloid differentiation. Globotriaosylceramide expression on myeloblasts, and possibly myeloid stem cells, may have important implications for the use of shiga toxin as an ex vivo purging agent in autologous stem cell transplantation. Expression of globotetraosylceramide, the parvovirus B19 receptor, on myeloblasts may also explain the association between B19 infection, aplastic anemia, and chronic neutropenia of childhood.
Introduction
Glycosphingolipids (GSLs) are information-rich biomolecules present in the cell membrane of all animal cells. Structurally, GSLs are composed of an antigenically active carbohydrate head group covalently linked to a ceramide (N-acyl sphingosine) lipid tail, which anchors the molecule within the cell membrane.1 More than 300 GSL species have been identified to date, reflecting differences in either the carbohydrate or lipid moiety.1,2 GSLs can be classified by their charge (neutral, acidic) and the presence of chemical modifications, such as sialylation (gangliosides) or sulfation (sulfatides). GSLs are also classified by family, based on the sequence and anomeric linkage of the first 3 to 4 carbohydrates. Of 12 GSL families identified, only 5 are typically expressed in human tissues (gala, globo, lacto, neolacto, and ganglio; Table 1; Figure1).1 3
Glycosphingolipid (GSL) structures
| Name . | Symbol . | Family . | Structure . |
|---|---|---|---|
| Glucosylceramide | GlcCer | — | Glcβ1→1′Cer |
| Lactosylceramide | LacCer | — | Galβ1→4Glcβ1→1′Cer |
| Galactosylceramide | GalCer | Gala | Galβ1→1′Cer |
| Galabiosylceramide | Gal2Cer | Gala | Galα1→4Galβ1→1′Cer |
| Globotriosylceramide | Gb3(Pk) | Globo | Galα1→4Galβ1→4Glcβ1→1′Cer |
| Globotetraosylceramide | Gb4(P) | Globo | GalNAcβ1→3Galα1→4Galβ1→4Glcβ1→1′Cer |
| Gangliotriaosylceramide | Gg3 | Ganglio | GalNAcβ1→4Galβ1→4Glcβ1→1′Cer |
| Gangliotetraosylceramide | Gg4 | Ganglio | Galβ1→3GalNAcβ1→4Galβ1→4Glcβ1→1′Cer |
| Lactotriaosylceramide | Lc3 | Lacto | GlcNAcβ1→3Galβ1→4Glcβ1→1′Cer |
| Lactotetraosylceramide | Lc4 | Lacto | Galβ1→3GlcNAcβ1→3Galβ1→4Glcβ1→1′Cer |
| Neolactotetraosylceramide | nLc4 | Neolacto | Galβ1→4GlcNAcβ1→3Galβ1→4Glcβ1→1′Cer |
| Neolactopentaosylceramide | nLc5 | Neolacto | GlcNAcβ1→3Galβ1→4GlcNAcβ1→3Galβ1→4Glcβ1→1′Cer |
| Neolactohexaosylceramide | nLc6 | Neolacto | Galβ1→4GlcNAcβ1→3Galβ1→4GlcNAcβ1→3Galβ1→4Glcβ1→1′Cer |
| Neolactooctaosylceramide | nLc8 | Neolacto | Galβ1→4GlcNAcβ1→3Galβ1→4GlcNAcβ1→3Galβ1→4GlcNAcβ1→3Galβ1→4Glcβ1→1′Cer |
| Name . | Symbol . | Family . | Structure . |
|---|---|---|---|
| Glucosylceramide | GlcCer | — | Glcβ1→1′Cer |
| Lactosylceramide | LacCer | — | Galβ1→4Glcβ1→1′Cer |
| Galactosylceramide | GalCer | Gala | Galβ1→1′Cer |
| Galabiosylceramide | Gal2Cer | Gala | Galα1→4Galβ1→1′Cer |
| Globotriosylceramide | Gb3(Pk) | Globo | Galα1→4Galβ1→4Glcβ1→1′Cer |
| Globotetraosylceramide | Gb4(P) | Globo | GalNAcβ1→3Galα1→4Galβ1→4Glcβ1→1′Cer |
| Gangliotriaosylceramide | Gg3 | Ganglio | GalNAcβ1→4Galβ1→4Glcβ1→1′Cer |
| Gangliotetraosylceramide | Gg4 | Ganglio | Galβ1→3GalNAcβ1→4Galβ1→4Glcβ1→1′Cer |
| Lactotriaosylceramide | Lc3 | Lacto | GlcNAcβ1→3Galβ1→4Glcβ1→1′Cer |
| Lactotetraosylceramide | Lc4 | Lacto | Galβ1→3GlcNAcβ1→3Galβ1→4Glcβ1→1′Cer |
| Neolactotetraosylceramide | nLc4 | Neolacto | Galβ1→4GlcNAcβ1→3Galβ1→4Glcβ1→1′Cer |
| Neolactopentaosylceramide | nLc5 | Neolacto | GlcNAcβ1→3Galβ1→4GlcNAcβ1→3Galβ1→4Glcβ1→1′Cer |
| Neolactohexaosylceramide | nLc6 | Neolacto | Galβ1→4GlcNAcβ1→3Galβ1→4GlcNAcβ1→3Galβ1→4Glcβ1→1′Cer |
| Neolactooctaosylceramide | nLc8 | Neolacto | Galβ1→4GlcNAcβ1→3Galβ1→4GlcNAcβ1→3Galβ1→4GlcNAcβ1→3Galβ1→4Glcβ1→1′Cer |
Glycosphingolipid (GSL) families.
Structural and biosynthetic relationship of the 5 major GSL families in humans.
Glycosphingolipid (GSL) families.
Structural and biosynthetic relationship of the 5 major GSL families in humans.
Biologically, GSLs and their derivatives play critical roles in receptor modulation, cell signaling, apoptosis, adhesion, growth, and differentiation.4-7 Cellular differentiation and malignant transformation are often accompanied by dramatic changes in GSL expression, with many GSLs capable of inducing differentiation, bone marrow suppression, platelet activation, and metastasis.8-11 GSLs also play a role in the pathophysiology of many infectious diseases through modulation of cytokine receptors, phagocytosis, and T-cell and neutrophil activation.3,12-16 GSLs also serve as membrane receptors for several viruses, toxins, and bacterial adhesins including P-fimbria, HIV, parvovirus B19, and shiga toxin (Stx).3 17-19
Recently, LaCasse et al proposed using Stx1 to purge residual tumor cells present in autologous stem cell collections.20Stx's are a family of ricinlike bacterial cytotoxins that recognize specific GSLs of the gala, globo, and neolacto families.19Once bound, Stx is endocytosed, followed by enzymatic cleavage of rRNA, inhibition of protein synthesis, and cell death.19 GSL receptors for Stx and Shiga-like toxins (Stx1 and Stx2) are galabiosylceramide (Gal2Cer; Table 1; Figure 1), globotriaosylceramide (Gb3, Pk antigen), and P1 antigen, which all terminate in a Galα1-4Gal or galabiosyl epitope.19,21 Stx2e, the causative agent of pig edema disease, preferentially binds globoside (Gb4) or P blood group antigen.19 In mice, Stx1 successfully purged Burkitt lymphoma, which is rich in Gb3, from murine bone marrow grafts prior to transplantation.22 Preliminary studies in humans indicate Stx1 may be a useful purging agent in breast cancer, non-Hodgkin lymphoma, and multiple myeloma.20 Stx1 does not appear to be toxic to human hematopoietic progenitor cells (HPCs) based on flow cytometry and in vitro proliferation assays.22
Although evidence suggests early HPCs do not bind Stx1, Stx1 receptors might be expressed on committed myeloid progenitors. Gb3and other globo-family GSLs are richly expressed on early myeloid cells of mice.23 They are also major neutral GSLs on human peripheral blood monocytes, erythrocytes, and platelets,21,24,25 which ultimately arise from the same pleuripotent progenitor cell—the granulocyte-erythroid-monocyte-megakaryocyte colony-forming unit (CFU-GEMM).26 Gb3 and/or Gb4 has also been identified in 3 immortalized human myeloid leukemia cell lines, KG1, K526, and THP-1, as well as a small number of clinical acute nonlymphocytic leukemia (ANLL) samples.27-32 In addition, there is evidence that parvovirus B19, which recognizes Gb4, can infect early myeloid cells, decrease myeloid progenitors, and inhibit granulocyte-monocyte colony (CFU-GM) formation in vivo.18,33-36 In contrast, globo GSLs are not expressed by either mature neutrophils or chronic myeloid leukemia (CML) cells.24,25,37,38 The presence of globo GSLs on mature monocytes and possibly myeloblasts, but not neutrophils, suggests that globo GSLs might be developmentally regulated antigens during myelomonocytic differentiation. The latter has been demonstrated during human lymphopoiesis and murine myelopoiesis.23 39-41
At present, very little is known regarding neutral GSL expression and differentiation during human myelopoiesis. Such studies have been hampered by the cryptic nature of neutral GSLs on cell membranes,42-45 coupled with the difficulty in obtaining sufficient numbers of well-characterized myeloid precursors from human marrow for biochemical analysis. To combat these difficulties, we examined myeloid cells collected from 13 patients with ANLL and CML who required therapeutic leukocytopheresis for severe leukocytosis. This permitted collection of large numbers of well-defined, immature myeloid cells for GSL isolation and characterization. GSL expression was examined by high-performance thin-layer chromatography (HPTLC), HPTLC immunostaining, compositional analysis, nuclear magnetic resonance spectroscopy (NMR), and fast atom bombardment–mass spectroscopy (FAB-MS). GSL samples were also tested for their ability to bind Stx and parvovirus B19. Unlike earlier studies,27,29-31 we were able to examine GSL expression relative to myeloid differentiation, as defined by histologic and immunophenotypic markers. Based on our data and those of earlier investigators,27 29we present a modified model of neutral GSL differentiation during human myelopoiesis and discuss the implications for mature granulocyte function, parvovirus B19 infection, and transplantation.
Patients, materials, and methods
Leukemic and normal peripheral blood cells
Therapeutic leukocytopheresis was performed on 13 patients for treatment of hyperleukocytosis and clinical evidence of leukostasis. Eleven patients were diagnosed with ANLL and 2 patients with CML and CML in blast crisis with transformation to ANLL M2 (CML-B/M2). Except for patient 6, all patients were newly diagnosed with leukemia at the time of leukocytopheresis and had not received prior chemotherapy. Patient 6 was previously diagnosed with chronic myelomonocytic leukemia 3 years earlier and was subsequently diagnosed with ANLL M2, 10 months prior to leukopheresis. The patient's chemotherapy in the 3 years prior to leukopheresis included daunarubicin, cytosine arabinoside, and hydroxyurea. All patients had bone marrow biopsies at the time of admission and were diagnosed according to French-American-British (FAB) criteria.46 In most patients, cytogenetic and immunophenotyping were performed as part of their diagnostic evaluation (Table 2).
Cytogenetic, cytochemical, and immunophenotypic characteristics of myeloid leukemias
| Sample . | FAB subtype . | Immunophenotype* . | Cytogenetic phenotype . | Cytochemistry . | ||
|---|---|---|---|---|---|---|
| Per . | NSE . | PAS . | ||||
| 1 | M0 | CD13, CD34, CD45, HLA-DR | NL | <3% | − | − |
| 2 | M1 | CD33, CD45, HLA-DR, TdT | NL | <10% | − | − |
| 3 | M1 | CD5, CD7, CD11b, CD13, HLA-DR, CD45 | NL | 20% | − | − |
| 4 | M1 | CD13, CD33, CD45 | NL | + | − | − |
| 5 | M1 | ND | ND | <10% | − | − |
| 6 | M2 | CD13, CD15, CD34, CD45, TdT | NL | + | − | − |
| 7 | M3 | CD2, CD13, CD33, CD45 | t(15:17) | + | + | − |
| 8 | M4 | CD33, CD45 | inv(16)(p32q32) | + | − | − |
| 9 | M5 | ND | ND | ND | ND | ND |
| 10 | M5a | ND | ND | + | + | − |
| 11 | M5b | CD5, CD11b, CD13, CD14, CD15, CD24, CD45 | ND | + | + | + |
| 12 | CML-B/M2 | CD2, CD13, CD33 CD34, CD38, CD45, HLA-DR | 47XY t(9:22) | + | − | − |
| 13 | CML | ND | NL | + | + | + |
| Sample . | FAB subtype . | Immunophenotype* . | Cytogenetic phenotype . | Cytochemistry . | ||
|---|---|---|---|---|---|---|
| Per . | NSE . | PAS . | ||||
| 1 | M0 | CD13, CD34, CD45, HLA-DR | NL | <3% | − | − |
| 2 | M1 | CD33, CD45, HLA-DR, TdT | NL | <10% | − | − |
| 3 | M1 | CD5, CD7, CD11b, CD13, HLA-DR, CD45 | NL | 20% | − | − |
| 4 | M1 | CD13, CD33, CD45 | NL | + | − | − |
| 5 | M1 | ND | ND | <10% | − | − |
| 6 | M2 | CD13, CD15, CD34, CD45, TdT | NL | + | − | − |
| 7 | M3 | CD2, CD13, CD33, CD45 | t(15:17) | + | + | − |
| 8 | M4 | CD33, CD45 | inv(16)(p32q32) | + | − | − |
| 9 | M5 | ND | ND | ND | ND | ND |
| 10 | M5a | ND | ND | + | + | − |
| 11 | M5b | CD5, CD11b, CD13, CD14, CD15, CD24, CD45 | ND | + | + | + |
| 12 | CML-B/M2 | CD2, CD13, CD33 CD34, CD38, CD45, HLA-DR | 47XY t(9:22) | + | − | − |
| 13 | CML | ND | NL | + | + | + |
Per indicates peroxidase; NSE, nonspecific esterase; PAS, periodic acid-Schiff; NL, normal (45 XX or XY); +, positive; −, negative; and ND, not done.
Positive markers listed only. All patients were negative for cytoplasmic μ, CD3, CD19, glycophorin A, and CD41.
Leukemic cells were collected on a Cobe Spectra 2997 (Cobe Laboratories, Lakewood, CO).47 A total of 1011to 1012 leukocytes were collected from each patient with minimal lymphocyte and platelet contamination (average platelet to white blood cells [PLT/WBC] less than 0.23) when compared with a normal granulocyte control (PLT/WBC = 22.6, Table3). A white blood cell differential of the collected leukocytes showed predominantly blasts (more than 80%) in 8 of 11 ANLLs: Exceptions were samples 7 (M3; blasts + promyelocytes = 82%) and 10 and 11 (M5; monocytes + monoblasts ≈ 85%). Normal peripheral blood lymphocytes, granulocytes, and red blood cells (RBCs) (AB+, P1+) were obtained from the DeGowin Blood Center (Department of Pathology, University of Iowa, Iowa City). Outdated peripheral blood lymphocytes and granulocyte concentrates were collected on a Fenwal CS3000 (Fenwal Laboratories, Deerfield, IL) as described.47 48 Outdated platelet concentrates (5 to 7 days) were purchased from Siouxland Red Cross (Sioux City, IA). Human kidney was obtained from the hospital autopsy service. All tissue procurement was carried out with the supervision and approval of the institutional human investigation committee.
Quantitative cytometric analysis of leukemic and normal granulocyte collections by cytopheresis
| Sample . | Total WBCs,3-150 × 1011 . | WBC differential, % total WBCs3-151 . | Ratio3-152 PLT/WBC . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Blast . | Pro . | Myelo . | Meta . | Gran . | Mono3-153 . | Lymph . | |||
| 1 | 2.6 | 96 | — | — | — | — | 1 | 3 | 0.61 |
| 2 | 3.5 | 95 | — | — | — | — | 2 | 2 | 0.07 |
| 3 | 6.0 | 91 | 1 | — | — | 4 | 1 | 3 | 0.34 |
| 4 | 2.6 | 94 | — | — | — | 2 | — | 4 | 0.45 |
| 5 | 14.0 | >99 | — | — | — | — | — | — | 0.05 |
| 6 | 9.7 | 85 | — | — | 2 | 10 | — | 3 | 0.34 |
| 7 | 2.9 | 45 | 37 | — | 12 | 3 | — | 3 | 0.38 |
| 8 | 3.8 | 97 | 1 | 1 | — | 1 | — | — | 0.01 |
| 9 | 3.1 | 80 | — | — | — | 8 | 6 | 6 | 0.09 |
| 10 | 1.9 | 73 | — | — | — | 3 | 11 | 13 | 0.12 |
| 11 | 4.2 | 96 | — | — | — | — | — | 4 | 0.39 |
| 12 | 10.0 | 47 | 6 | 4 | 3 | 35 | 2 | 3 | 0.06 |
| 13 | 4.1 | 0 | 1 | 18 | 18 | 54 | 8 | 1 | 0.09 |
| Granulocyte control | 0.27 | 0 | — | — | 1 | 92 | 2 | 5 | 22.6 |
| Sample . | Total WBCs,3-150 × 1011 . | WBC differential, % total WBCs3-151 . | Ratio3-152 PLT/WBC . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Blast . | Pro . | Myelo . | Meta . | Gran . | Mono3-153 . | Lymph . | |||
| 1 | 2.6 | 96 | — | — | — | — | 1 | 3 | 0.61 |
| 2 | 3.5 | 95 | — | — | — | — | 2 | 2 | 0.07 |
| 3 | 6.0 | 91 | 1 | — | — | 4 | 1 | 3 | 0.34 |
| 4 | 2.6 | 94 | — | — | — | 2 | — | 4 | 0.45 |
| 5 | 14.0 | >99 | — | — | — | — | — | — | 0.05 |
| 6 | 9.7 | 85 | — | — | 2 | 10 | — | 3 | 0.34 |
| 7 | 2.9 | 45 | 37 | — | 12 | 3 | — | 3 | 0.38 |
| 8 | 3.8 | 97 | 1 | 1 | — | 1 | — | — | 0.01 |
| 9 | 3.1 | 80 | — | — | — | 8 | 6 | 6 | 0.09 |
| 10 | 1.9 | 73 | — | — | — | 3 | 11 | 13 | 0.12 |
| 11 | 4.2 | 96 | — | — | — | — | — | 4 | 0.39 |
| 12 | 10.0 | 47 | 6 | 4 | 3 | 35 | 2 | 3 | 0.06 |
| 13 | 4.1 | 0 | 1 | 18 | 18 | 54 | 8 | 1 | 0.09 |
| Granulocyte control | 0.27 | 0 | — | — | 1 | 92 | 2 | 5 | 22.6 |
Pro indicates promyelocyte; myelo, myelocyte; meta, metamyelocyte; gran, granulocyte; mono, monocyte; lymph, lymphocyte; and —, < 1% WBCs.
Total collected WBCs by leukocytopheresis.
WBC differential of the collected product.
Ratio of platelets (PLT) to WBCs in the collected product prior to centrifugation and lipid extraction.
Includes monoblasts and promonocytes.
Glycosphingolipid isolation
Prior to lipid extraction, all leukocyte concentrates were initially diluted with isotonic ammonium bicarbonate (NH4HCO3, 154 mM) to osmotically lyse contaminating erythrocytes and then centrifuged (350g, 15 minutes). After carefully decanting the supernatant containing platelets and RBC ghosts,49,50 leukocytes were washed thrice more with NH4CO3, centrifuged (4500g, 30 minutes), frozen, and lyophilized dry. Platelets and RBCs were isolated as described.49 50
The total GSL fraction of each tissue was isolated using the procedure of Ledeen and Yu with modification.49-51 Briefly, lyophilized cell powders were extracted with chloroform-methanol (C/M 1:1 [vol/vol]; 100 mL/g dry weight) to obtain a total lipid extract. Neutral lipids were isolated by anion exchange chromatography (A-25 DEAE-Sephadex; Sigma, St Louis, MO), saponified with 0.3 N methanolic NaOH to remove phospholipids, followed by dialysis against distilled water. The dialysis retentate was lyophilized dry, dissolved in chloroform, and applied to a silicic acid column (40 mesh; Mallinckrodt-Baker, Phillipsburg, NJ). After washing with chloroform and ethyl acetate, the column was stripped of neutral GSLs with acetone-methanol 9:1 and 7:3 (vol/vol). To remove alkali-resistant phospholipids, the latter 2 fractions were pooled and then acetylated as described.52 The deacetylated, purified neutral GSL was resuspended in C/M 1:1 (vol/vol) to a final concentration of 10 mg/mL. The total neutral GSL/leukocyte sample was determined by direct measurement of dry weight GSL (in triplicate).
Immunologic reagents
Monoclonal antibody (MoAb) Pk002 was purchased from Accurate (San Diego, CA).53 MoAb MC631 was purchased as a hybridoma supernatant from the Developmental Studies Hybridoma Bank maintained by the Department of Biology at the Univiversity of Iowa, Iowa City, under contract no. N01-HD-2-3144.54 MoAbs 1B2 and SH34 were purchased from American Type Culture Collection (ATCC, Manassas, VA) as hybridomas.55,56 MoAb TE5 was a kind gift from Dr Eric Holmes, Pacific Northwest Research Foundation, Seattle, WA.57 Rabbit polyclonal anti-Gg3 and MoAb Gal 01 were purchased from Matreya (Pleasant Gap, PA). Recombinant B19 empty capsids were a kind gift from MedImmune (Gaithersburg, MD).58 Anti-B19 (MoAb 829) was purchased from Chemicon International (Temecula, CA). Stx holotoxin from Shigella dysenteriae and polyclonal rabbit anti–shiga toxin antibody were a kind gift from Dr Donohue-Rolfe, Tufts University (Medford, MA).59 All MoAbs used were mouse immunoglobulin M (IgM) except anti-B19 (mouse IgG). The antibody specificity of MoAbs and Stx is shown in Table4.
Immunologic reagents
| Reagent . | Epitope . | Family . | Specificity . | Reference no. . |
|---|---|---|---|---|
| MoAb TE5 | GlcNAcβ1-R | Lacto/neolacto | Lc3, Lc5, etc | 57 |
| MoAb 1B2 | Galβ1-4GlcNAcβ1-R | Neolacto | Lactosaminyl | 55 |
| MoAb Pk002 | Galα1-4Galβ1-4Glc/GlcNAc-R | Globo | Gb3, P1 | 53 |
| MoAb MC631 | (R)-GalNAcβ1-3Galα1-4Gal-R | Globo | Gb4 | 54 |
| MoAb Gal-01 | Galβ1-1Cer | Gala | GalCer | 4-150 |
| Stx | Galα1-4Gal-R | Gala/globo | Gal2Cer, Gb3, P1 | 21,59 |
| Anti-Gg3 | GalNAcβ1-4Galβ1-4Glc-R | Ganglio | Gg3 | 4-150 |
| MoAb SH34 | Galβ1-3GalNAcβ1-4Gal-R | Ganglio | Gg4 | 56 |
| B19 capsid | (R)-GalNAcβ1-3Galα1-4Gal-R | Globo | Gb44, Forssman | 18,58 |
| Galβ1-4GlcNAc-R | Neolacto | Lactosaminyl | 25 |
| Reagent . | Epitope . | Family . | Specificity . | Reference no. . |
|---|---|---|---|---|
| MoAb TE5 | GlcNAcβ1-R | Lacto/neolacto | Lc3, Lc5, etc | 57 |
| MoAb 1B2 | Galβ1-4GlcNAcβ1-R | Neolacto | Lactosaminyl | 55 |
| MoAb Pk002 | Galα1-4Galβ1-4Glc/GlcNAc-R | Globo | Gb3, P1 | 53 |
| MoAb MC631 | (R)-GalNAcβ1-3Galα1-4Gal-R | Globo | Gb4 | 54 |
| MoAb Gal-01 | Galβ1-1Cer | Gala | GalCer | 4-150 |
| Stx | Galα1-4Gal-R | Gala/globo | Gal2Cer, Gb3, P1 | 21,59 |
| Anti-Gg3 | GalNAcβ1-4Galβ1-4Glc-R | Ganglio | Gg3 | 4-150 |
| MoAb SH34 | Galβ1-3GalNAcβ1-4Gal-R | Ganglio | Gg4 | 56 |
| B19 capsid | (R)-GalNAcβ1-3Galα1-4Gal-R | Globo | Gb44, Forssman | 18,58 |
| Galβ1-4GlcNAc-R | Neolacto | Lactosaminyl | 25 |
Specificity per manufacturer (Matreya).
High-performance thin-layer chromatography
HPTLC was performed using aluminum-backed HPTLC plates (E. Merck, Darmstadt, Germany).50 Approximately 40 μg neutral GSL was spotted per lane and then developed in a solvent of chloroform-methanol-water 60:30:5 (C/M/W, vol/vol). GSLs were detected by spraying with diphenylamine reagent (DPA) or by immunostaining as described below.50 51 GSL bands were characterized by intensity (percent total staining density) and relative mobility (Rf) by scanning densitometry at 580 nm (Shimadzu Instruments, Columbia, MD). Error in mobility measurements (SD) was less than ± 0.10 unless stated otherwise. GSL standards were purchased from Matreya and Sigma.
HPTLC immunostaining
Air-dried, solvent-developed plates were dipped in a hexane solution of 0.2% polyisobutyl-methacrylate (Polysciences, Warrington, PA) for 60 seconds and air dried. HPTLC immunostaining with MoAbs was performed as described using an avidin-linked alkaline phosphatase detection method (Vector Laboratories, Burlingame, CA).21,60 HPTLC immunostaining with B19 capsids and Stx was performed as described.21 25 All experiments were performed in triplicate.
High-performance liquid chromatography
Underivatized neutral GSLs from an ANLL M1 was separated by HPLC (Shimadzu LC-610) using evaporative laser light scattering detection (Varex ELSD II, Burtonsville, MD).49 61 The sample (30.0 mg) was applied to a preparative silica gel column (1.0 × 25 cm, 8 μm; Ranin, Woburn, MA) and eluted with a gradient of C/M (95:5) to C/M/W (60:40:20, vol/vol), applied at a flow rate of 5 mL/min over 2 hours. Fractions were collected every 2 minutes and analyzed by DPA and HPTLC immunostaining. Because 2 major GSL bands coeluted, fractions 31 to 36 were pooled and rechromatographed over an analytic column (5 × 100 mm, Spherisob SI-80, 3 μm; ES Industries, West Berlin, NJ) using a gradient of hexane-isopropanol-water 68:30:2 to 5:90:5 (vol/vol) at a flow rate of 1 mL/min over 25 minutes.
Characterization of carbohydrate structure
For [1H]nuclear magnetic resonance spectroscopy (NMR), underivatized GSL samples were exchanged against D2O by repeated lyophilization and then dissolved in dimethylsulfoxide-d6 containing 2% D2O and 1% tetramethylsilane (Aldrich, Milwaukee, WI).62,63 NMR spectra were acquired at 600 MHz on a Bruker AMX-600 spectrometer (Karlsruhe, Germany; Department of Chemistry, University of Iowa) in the Fourier-transformed mode with quadrature detection at a probe temperature of 303 °K. Integrated 1-D spectra were typically obtained with a sweep width of 6000 Hz (10 ppm), 4.01-second cycle time, and 200 to 400 scans. Homonuclear 2-D NMR (COSY, TOCSY, NOESY) spectra were obtained essentially as described.63-65 For COSY experiments, 256 to 400 time proportional phase increments (TPPIs) were collected with 32 transients per t1, a pulse delay of 4 seconds, and a sweep width of 6000 Hz over 4000 data points in t2. For TOCSY and NOESY experiments, 400 TPPIs were collected with 48 transients per t1, a pulse delay of 4 seconds, and a mixing time of 100 milliseconds and 500 milliseconds, respectively.
Fast atom bombardment–mass spectroscopy (FAB-MS) was performed on an Autospec mass spectrometer (Micromass, Beverly, MA; Department of Chemistry, University of Iowa) using triethanolamine/tetramethylurea as the matrix.66 For compositional analysis, GSLs were hydrolyzed with dry methanolic hydrochloric acid, followed by trimethylsialylation and gas chromatography (Shimadzu) over a DB-5 column (J&W Scientific, Folsom, CA) at 150°C for 2 minutes and then 4°C/min to maximum 230°C.67 Individual sugars were identified by their retention time and peak areas relative to trimethylsialyl standards (Sigma).
Statistics
Statistical correlations were performed using the Pearson product moment correlation and Student ttest.68P < .05 was considered statistically significant.
Results
Decreased neutral GSL expression in ANLL
Neutral GSL content was determined by direct measurement of the dried, purified, neutral GSLs and reported as micrograms of dry weight GSL per 100 million leukocytes collected (Table5). In general, the total neutral GSL yield from mature granulocytes and CML cells was comparable to those obtained by other investigators (114 to 139 μg/108cells).24,30,37,69 In contrast, the neutral GSL content of ANLL cells was 8-fold to 10-fold less then mature neutrophils, ranging from 6.2 to 19.4 μg/108 cells (mean 9.8 ± 4 μg/108 cells). Among monocytic leukemias (M5), the neutral GSL yield was very similar to mature peripheral blood monocytes (11 μg/108 cells).24
Distribution of neutral GSLs extracted from ANLL, CML, and normal peripheral blood granulocytes5-150
| Neutral Size . | GSL bands5-150Rf . | Band . | Relative distribution of neutral GSLs (% total) per sample5-151 . | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | 9 . | 10 . | 11 . | 12 . | 13 . | Gran . | |||
| GSL1 | 0.72 | a | 9.5 | 9.8 | 10.2 | 8.4 | 11.5 | 12.8 | 7.7 | 45.5 | 15.3 | 27.9 | 19.3 | 0.8 | 3.8 | 1.2 |
| GSL2 | 0.57 | b | 51.4 | 54.5 | 42.8 | 56.8 | 58.2 | 55.4 | 48.5 | 28.9 | 56.1 | 42.5 | 42.4 | 8.6 | 80.6 | 79.8 |
| Ratio | ||||||||||||||||
| GSL2/GSL1 | 5.4 | 5.6 | 4.2 | 6.8 | 5.1 | 4.3 | 6.3 | 0.6 | 3.7 | 1.5 | 2.2 | 10.7 | 21.2 | 66.5 | ||
| GSL3 | 0.45 | c, d | 4.2 | 6.1 | 7.6 | 14.0 | 4.3 | 4.8 | 16.9 | 5.1 | 12.2 | 4.8 | 4.3 | 8.0 | 2.7 | 0.9 |
| GSL4 | e, f | 29.4 | 25.2 | 34.5 | 12.8 | 23.6 | 21.6 | 24.1 | 17.5 | 9.3 | 22.1 | 33.9 | 65.2 | 11.2 | 15.2 | |
| 0.27 | e | 17.0 | 10.7 | 17.6 | 4.4 | 3.6 | 7.8 | 10.3 | <1.0 | 2.0 | 12.5 | 17.7 | 3.6 | 1.2 | 1.2 | |
| % GSL45-152 | 56.5 | 42.4 | 51.0 | 34.4 | 15.4 | 36.2 | 42.7 | 4.4 | 21.5 | 56.6 | 44.5 | 5.1 | 7.9 | 8.5 | ||
| 0.23 | f | 13.1 | 14.5 | 16.9 | 8.4 | 20.0 | 13.8 | 13.8 | 16.7 | 7.3 | 9.6 | 18.4 | 61.9 | 10.2 | 14.0 | |
| % GSL45-152 | 43.5 | 57.3 | 49.0 | 65.6 | 84.6 | 63.8 | 57.3 | 95.6 | 78.5 | 43.4 | 55.5 | 94.9 | 92.1 | 91.5 | ||
| Total GSLs, μg/108cells | 8.8 | 19.4 | 9.4 | 7.2 | 8.8 | 14.9 | 10.1 | 8.2 | 7.7 | 6.8 | 6.2 | 6.3 | 128.1 | 155.0 | ||
| Neutral Size . | GSL bands5-150Rf . | Band . | Relative distribution of neutral GSLs (% total) per sample5-151 . | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | 9 . | 10 . | 11 . | 12 . | 13 . | Gran . | |||
| GSL1 | 0.72 | a | 9.5 | 9.8 | 10.2 | 8.4 | 11.5 | 12.8 | 7.7 | 45.5 | 15.3 | 27.9 | 19.3 | 0.8 | 3.8 | 1.2 |
| GSL2 | 0.57 | b | 51.4 | 54.5 | 42.8 | 56.8 | 58.2 | 55.4 | 48.5 | 28.9 | 56.1 | 42.5 | 42.4 | 8.6 | 80.6 | 79.8 |
| Ratio | ||||||||||||||||
| GSL2/GSL1 | 5.4 | 5.6 | 4.2 | 6.8 | 5.1 | 4.3 | 6.3 | 0.6 | 3.7 | 1.5 | 2.2 | 10.7 | 21.2 | 66.5 | ||
| GSL3 | 0.45 | c, d | 4.2 | 6.1 | 7.6 | 14.0 | 4.3 | 4.8 | 16.9 | 5.1 | 12.2 | 4.8 | 4.3 | 8.0 | 2.7 | 0.9 |
| GSL4 | e, f | 29.4 | 25.2 | 34.5 | 12.8 | 23.6 | 21.6 | 24.1 | 17.5 | 9.3 | 22.1 | 33.9 | 65.2 | 11.2 | 15.2 | |
| 0.27 | e | 17.0 | 10.7 | 17.6 | 4.4 | 3.6 | 7.8 | 10.3 | <1.0 | 2.0 | 12.5 | 17.7 | 3.6 | 1.2 | 1.2 | |
| % GSL45-152 | 56.5 | 42.4 | 51.0 | 34.4 | 15.4 | 36.2 | 42.7 | 4.4 | 21.5 | 56.6 | 44.5 | 5.1 | 7.9 | 8.5 | ||
| 0.23 | f | 13.1 | 14.5 | 16.9 | 8.4 | 20.0 | 13.8 | 13.8 | 16.7 | 7.3 | 9.6 | 18.4 | 61.9 | 10.2 | 14.0 | |
| % GSL45-152 | 43.5 | 57.3 | 49.0 | 65.6 | 84.6 | 63.8 | 57.3 | 95.6 | 78.5 | 43.4 | 55.5 | 94.9 | 92.1 | 91.5 | ||
| Total GSLs, μg/108cells | 8.8 | 19.4 | 9.4 | 7.2 | 8.8 | 14.9 | 10.1 | 8.2 | 7.7 | 6.8 | 6.2 | 6.3 | 128.1 | 155.0 | ||
Solvent was C/M/W 65:30:5 (vol/vol). Results reported are the mean of 7 independent sample determinations; SD less than 3.0%. GSL1 indicates monoglycosylceramide; GSL2, diglycosylceramide; GSL3, triglycosylceramide; and GSL4, tetraglyosylceramide.
GSLs were identified by the size of the oligosaccharide moiety, relative mobility (Rf), and immunostaining characteristics (Figure 2).
Percent distribution of major neutral GSL species as determined by scanning densitometry of DPA-stained HPTLC plates.
The percentage total GSL4 as band e (Figure 2A, Rf 0.27) or band f (doublet, Rf 0.23).
Relative expression of neutral GSLs in myeloid leukemia
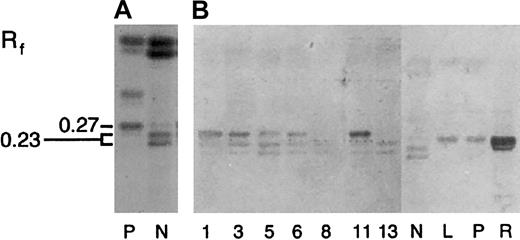
To examine the expression of individual GSL species, neutral GSL samples were separated by HPTLC and then chemically stained with DPA reagent. Based on relative mobilities (Rf, Table 5 and Figure 2A), leukemic and normal peripheral blood cells expressed predominantly monoglycosylceramides (GSL1, Rf 0.72), diglycosylceramides (GSL2, Rf0.57), triglycosylceramides (GSL3, Rf 0.45), and tetraglycosylceramides (GSL4, Rfs 0.23 and 27).24,25 On densitometry, GSL2 was the major GSL species expressed by most myeloid cells except sample 12, where GSL4 constituted nearly 65% of the total. In general, neutral GSL expression in ANLL differed from normal granulocytes by a relative increase in GSL1 and decreased GSL2/GSL1 ratio. In addition, most ANLL samples appeared to express at least 2 GSL4 species, with Rfs similar to Gb4 (Rf 0.27, lanes R, L, P) and nLc4 (Rf 0.23, lane N; Figure2A).25
Immunologic detection of neolacto- and globo-family GSLs, including Gb3, in acute myeloid leukemia.
Neutral GSLs from ANLL (lanes 1-11), CML (lanes 12 and 13), and peripheral blood neutrophils (N), lymphocytes (L), platelets (P), and red cells (R) were separated by HPTLC and chemically stained with DPA or immunostained with carbohydrate-specific MoAbs and lectins (Table4). (A) DPA spray, (B) MoAb TE5, (C) MoAb IB2, (D) MoAb Pk002, (E) MoAb MC631, (F) MoAb Gal01, and (G) Stx. Lane numbers for ANLL and CML refer to individual sample numbers shown in Tables 2, 3, and 5. Numbers to left of figures refer to the relative mobility (Rf) of specific GSLs. GSL controls included galactosylceramide (GalC, panel F) and kidney neutral GSLs (K, panel G). Bracketed bands (E, lane 12) represent nonspecific binding. Solvent was C/M/W 60:30:5 (vol/vol).
Immunologic detection of neolacto- and globo-family GSLs, including Gb3, in acute myeloid leukemia.
Neutral GSLs from ANLL (lanes 1-11), CML (lanes 12 and 13), and peripheral blood neutrophils (N), lymphocytes (L), platelets (P), and red cells (R) were separated by HPTLC and chemically stained with DPA or immunostained with carbohydrate-specific MoAbs and lectins (Table4). (A) DPA spray, (B) MoAb TE5, (C) MoAb IB2, (D) MoAb Pk002, (E) MoAb MC631, (F) MoAb Gal01, and (G) Stx. Lane numbers for ANLL and CML refer to individual sample numbers shown in Tables 2, 3, and 5. Numbers to left of figures refer to the relative mobility (Rf) of specific GSLs. GSL controls included galactosylceramide (GalC, panel F) and kidney neutral GSLs (K, panel G). Bracketed bands (E, lane 12) represent nonspecific binding. Solvent was C/M/W 60:30:5 (vol/vol).
Myeloid leukemia cells express neolacto-series GSLs
Neolacto-series GSLs are a characteristic feature of mature granulocytes and have been reported in ANLL and CML.24,27-31,37,38 To screen for neolacto-family GSLs, samples were immunostained with MoAbs TE5 and IB2 (Table 4; Figure 2B-C).55,57 As shown in Figure 2B, MoAb TE5 recognized Lc3 (GSL3, Rf 0.45) in all myeloid cells. In samples 1, 8, and 12, a second TE5+ band (Rf 0.18) was also identified, which may represent nLc5 (Table1).57 Likewise, MoAb 1B2 binding was observed to nLc4 (GSL4, Rf 0.23) in granulocytes and all myeloid leukemias. Additional 1B2+ GSL bands (Rfs 0.08, 0.01) were noted in samples 1, 2, 3, 5, 6, 8, 12, and 13 (Figure 2C) and may represent the extended neolacto-series GSLs, nLc6, and nLc8, respectively.37
Globo-series expression in ANLL
Globo GSLs (Gb3, Gb4; Table 1; Figure 1) are major neutral GSLs in red cells, platelets, lymphocytes, and monocytes but are reportedly absent from mature peripheral blood neutrophils.24,25,37,38 To screen for Gb3 and Gb4, samples were immunostained with MoAbs Pk002 and MC631, respectively (Figure 2D,E).25,53 54MoAb Pk002 binding to Gb3 (Rf 0.45) was observed in all 3 positive controls (lanes R, P, L; Figure 2D) and 9 of 11 ANLLs, although staining was weak in 2 of 3 ANLL M5 samples. Faint or no binding was observed in ANLL M4, CML, and mature neutrophils. Likewise, MoAb MC631 binding to Gb4 (Rf 0.27) was observed in 9 of 11 ANLLs. A faint MC631 band was also observed in the CML-B/M2 sample (lane 12; Figure 2E). No Gb4 was identified in samples 8, 9, 13, and normal granulocytes. Nonspecific binding to nLc4 was noted in sample 12 (brackets; Figure 2E).
Gala-series GSLs are not expressed in myeloid leukemia
The gala-series GSL, galactosylceramide (GalCer), is reportedly expressed by the myeloid leukemia cell lines K562 and KG127,70 and is a receptor for HIV.17 A second gala-series GSL, galabiosylceramide (Gal2Cer), is a receptor for Stx's and has been reported to be a marker of mature neutrophils.21,29,38 To screen for GalCer and Gal2Cer, samples were immunostained with MoAb Gal 01 and Stx from Shigella dysenteriae, respectively (Figure2F,G).21,59 Positive controls included GalCer (Rf 0.72) and human kidney GSLs (Gal2Cer and Gb3).21 Faint GalCer was identified in one ANLL (M5b, lane 11; Figure 2F) and platelets. Stx binding to Gal2Cer (triplet, Rf 0.57) was observed only in the kidney control (Figure 2G, lane K). Stx also recognized a GSL3 band (Rf 0.45), consistent with Gb3, in 9 of 11 ANLL samples, platelets, red cells, lymphocytes, and kidney. Stx binding was also observed to Gb4 in many samples and is an artifact associated with polyisobutylmethacrylate fixation.71
Myeloid leukemias do not express ganglio-series neutral GSLs
Ganglio-series GSLs have been reported in the promyelocytic cell line HL-60, K562 cells, murine myelogenous leukemia, and some human acute lymphoblastic leukemias.23,69,72,73 To screen for ganglio-series GSLs, GSL samples were immunostained with a polyclonal anti-Gg3 and MoAb SH34, an anti-Gg4.56 Authentic Gg3 and Gg4 were included as positive controls. Neither Gg3 (Rf 0.40) nor Gg4(Rf 0.21) was identified in any sample tested (data not shown).
Isolation and characterization of major neutral GSLs in ANLL
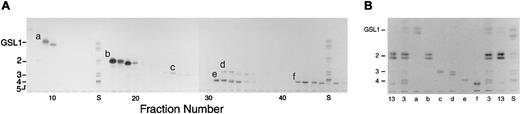
To verify that ANLL blasts express globo- and neolacto-series GSLs, the total neutral GSL fraction from an ANLL M1 (sample 3) was separated by HPLC. Isolated GSLs were identified by HPTLC based on their mobility and immunologic reactivity (Figure3A). A total of 6 major DPA bands (a-f) were identified and tentatively identified as GlcCer (band a), LacCer (b), Gb3 (c, Stx+, Pk002+), Lc3 (d, TE5+), Gb4 (e, MC631+), and nLc4 (f, IB2+). Two bands (d and e) coeluted in our initial separation and were subsequently isolated by rechromatography on an analytic HPLC column (Figure3B).
HPLC isolation and purification of the 6 major neutral GSLs in ANLL.
(A) The total neutral GSL fraction from an ANLL M1 (sample 3) was separated by HPLC, followed by HPTLC of individual fractions (fraction numbers 7 through 47). Six major neutral GSLs (bands a-f) were identified. (B) HPTLC of the isolated and purified major neutral GSLs (bands a-f) in ANLL. The total neutral GSL fractions from an ANLL (sample 3) and CML (sample 13) were included as controls. Lane S, commercial GSL size standards ranging from monoglycosylceramides (GSL1, GlcCer), diglycosylceramides (GSL2, LacCer), triglycosylceramides (GSL3, Gb3), tetraglycosylceramides (GSL4, Gb4), and pentaglycosylceramides (GSL5, Forssman). HPTLC solvent, C/M/W 65:25:4 (vol/vol). Stain, DPA reagent.
HPLC isolation and purification of the 6 major neutral GSLs in ANLL.
(A) The total neutral GSL fraction from an ANLL M1 (sample 3) was separated by HPLC, followed by HPTLC of individual fractions (fraction numbers 7 through 47). Six major neutral GSLs (bands a-f) were identified. (B) HPTLC of the isolated and purified major neutral GSLs (bands a-f) in ANLL. The total neutral GSL fractions from an ANLL (sample 3) and CML (sample 13) were included as controls. Lane S, commercial GSL size standards ranging from monoglycosylceramides (GSL1, GlcCer), diglycosylceramides (GSL2, LacCer), triglycosylceramides (GSL3, Gb3), tetraglycosylceramides (GSL4, Gb4), and pentaglycosylceramides (GSL5, Forssman). HPTLC solvent, C/M/W 65:25:4 (vol/vol). Stain, DPA reagent.
Isolated GSLs were further characterized by [1H]NMR.62,63 Characteristic proton signals for the ceramide lipid moiety were identified in all 6 bands (Table 6; Figure4).63 Signature proton resonances for the oligosaccharide moiety were identified in the anomeric proton region at 4 to 5 ppm (Figure5). Based on the number, chemical shift (parts per million), and J1,2 coupling constants of the anomeric protons,63 bands a to f were identified as GlcCer, LacCer, Gb3, Lc3, Gb4, and nLc4 (Table 6; Figure 4).62-65,74,75The chemical shifts of the remaining methine and methylene protons were also consistent for GlcCer, LacCer, Lc3, Gb4,and nLc4 (data not shown).62-65 75 Band c was not subjected to 2D-NMR due to insufficient sample.
Proton chemical shifts and J1,2coupling constants (Hz) of bands a-f
| Band . | VI . | V . | IV . | III . | II . | I . | R1a, R1b . | R2 . | R3 . | R4 . | R5 . | NH− . | −CH3 . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a | — | — | — | — | — | 4.06 | 3.39, 3.94 | 3.77 | 8.87 | 5.35 | 5.52 | 8.30 | 0.84 |
| J1,2 | — | — | — | — | — | 9.8 | — | — | — | — | — | — | — |
| b | — | — | — | — | 4.18 | 4.15 | 3.40, 3.96 | 3.76 | 3.86 | 5.31 | 5.52 | 8.28 | 0.84 |
| J1,2 | — | — | — | — | 7.1 | 7.8 | — | — | — | — | — | — | — |
| c6-150 | — | — | — | 4.80 | 4.25 | 4.16 | 6-150, 3.96 | 3.75 | 3.87 | 5.33 | 5.52 | 8.30 | 0.84 |
| d | — | 4.62 | — | — | 4.25 | 4.16 | 3.40, 3.96 | 3.75 | 3.86 | 5.33 | 5.52 | 8.48 | 0.84 |
| J1,2 | — | 7.7 | — | — | 7.7 | 7.7 | — | — | — | — | — | — | — |
| e | — | — | 4.50 | 4.79 | 4.25 | 4.16 | 3.40, 3.96 | 3.77 | 3.85 | 5.32 | 5.52 | 8.27 | 0.84 |
| J1,2 | — | — | 8.4 | 3.6 | 7.7 | 8.2 | — | — | — | — | — | — | — |
| f | 4.20 | 4.67 | — | — | 4.26 | 4.12 | 3.39, 3.98 | 3.77 | 3.88 | 5.34 | 5.53 | 8.31 | 0.85 |
| J1,2 | 6.9 | 8.2 | — | — | 7.0 | 7.9 | — | — | — | — | — | — | — |
| Band . | VI . | V . | IV . | III . | II . | I . | R1a, R1b . | R2 . | R3 . | R4 . | R5 . | NH− . | −CH3 . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a | — | — | — | — | — | 4.06 | 3.39, 3.94 | 3.77 | 8.87 | 5.35 | 5.52 | 8.30 | 0.84 |
| J1,2 | — | — | — | — | — | 9.8 | — | — | — | — | — | — | — |
| b | — | — | — | — | 4.18 | 4.15 | 3.40, 3.96 | 3.76 | 3.86 | 5.31 | 5.52 | 8.28 | 0.84 |
| J1,2 | — | — | — | — | 7.1 | 7.8 | — | — | — | — | — | — | — |
| c6-150 | — | — | — | 4.80 | 4.25 | 4.16 | 6-150, 3.96 | 3.75 | 3.87 | 5.33 | 5.52 | 8.30 | 0.84 |
| d | — | 4.62 | — | — | 4.25 | 4.16 | 3.40, 3.96 | 3.75 | 3.86 | 5.33 | 5.52 | 8.48 | 0.84 |
| J1,2 | — | 7.7 | — | — | 7.7 | 7.7 | — | — | — | — | — | — | — |
| e | — | — | 4.50 | 4.79 | 4.25 | 4.16 | 3.40, 3.96 | 3.77 | 3.85 | 5.32 | 5.52 | 8.27 | 0.84 |
| J1,2 | — | — | 8.4 | 3.6 | 7.7 | 8.2 | — | — | — | — | — | — | — |
| f | 4.20 | 4.67 | — | — | 4.26 | 4.12 | 3.39, 3.98 | 3.77 | 3.88 | 5.34 | 5.53 | 8.31 | 0.85 |
| J1,2 | 6.9 | 8.2 | — | — | 7.0 | 7.9 | — | — | — | — | — | — | — |
— indicates not applicable.
Due to small sample, J1,2 coupling constant and R1a not resolved.
GSL structure and numbering of glycosyl anomeric and ceramide protons.
Correlation between structure and observed proton shifts (1H NMR) for bands a-f (Table 6; Figure 5).
[1H]NMR 1-D spectra between 4.0 and 5.0 ppm, showing the anomeric protons for ANLL GSL bands a-f.
1-D NMR spectrum for GSL bands a to f (A-F), respectively. The size and composition of the carbohydrate moiety of each GSL band was determined by the number and chemical shift (ppm) of their anomeric or H-1 protons, which are present as split peaks in the NMR spectrum between 4.0 and 5.0 ppm. The anomeric linkage (α, β) of each carbohydrate was determined by the coupling constant or difference in frequency (J1,2; Hz) between each set of H-1 peaks.64The numbering of individual anomeric protons (I-1, II-1, III-1, IV-1, V-1, VI-1) and their subsequent identification, based on published standards and 2-D NMR, is shown in Table 6 and Figure 4. Note that the H-1 proton of glucose (I-1; Table 6; Figure 4) in bands c and e is adjacent or split by the H-5 proton of galactose (III-5), which is consistent with the published NMR spectra of Gb3 and Gb4, respectively.64,74 75
[1H]NMR 1-D spectra between 4.0 and 5.0 ppm, showing the anomeric protons for ANLL GSL bands a-f.
1-D NMR spectrum for GSL bands a to f (A-F), respectively. The size and composition of the carbohydrate moiety of each GSL band was determined by the number and chemical shift (ppm) of their anomeric or H-1 protons, which are present as split peaks in the NMR spectrum between 4.0 and 5.0 ppm. The anomeric linkage (α, β) of each carbohydrate was determined by the coupling constant or difference in frequency (J1,2; Hz) between each set of H-1 peaks.64The numbering of individual anomeric protons (I-1, II-1, III-1, IV-1, V-1, VI-1) and their subsequent identification, based on published standards and 2-D NMR, is shown in Table 6 and Figure 4. Note that the H-1 proton of glucose (I-1; Table 6; Figure 4) in bands c and e is adjacent or split by the H-5 proton of galactose (III-5), which is consistent with the published NMR spectra of Gb3 and Gb4, respectively.64,74 75
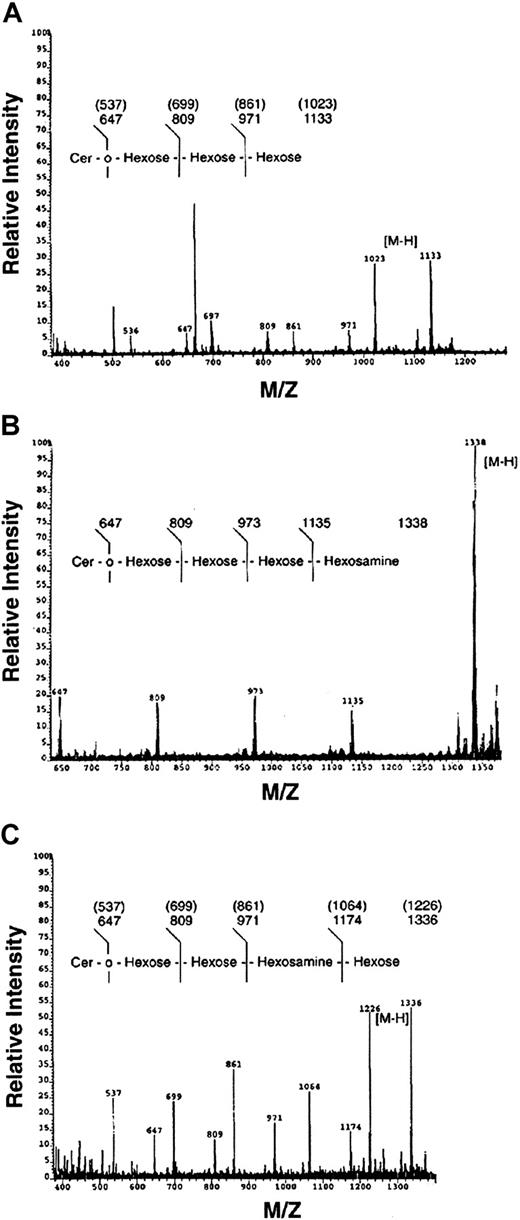
Bands c, e, and f were also analyzed by mass spectrometry (FAB-MS) and compositional analysis by gas chromatography (data not shown). As shown in Figure 6, band c possessed a trihexose oligosaccharide, composed of galactose and glucose (ratio 2.3:1.0), consistent with a Gal-Gal-Glc-Cer or Gb3. Similarly, band e was a HexNAc-Hex-Hex-Hex-Ceramide, composed of Gal/Glc/GalNAc (ratio 2.3:1.0:0.74), consistent with Gb4. In contrast, band f possessed a Hex-HexNAc-Hex-Hex-Ceramide sequence, composed of Gal/Glc/GlcNAc (ratio 1.73:1.0:0.51), consistent with the neolacto GSL, nLc4. In summary, the major myeloid neutral GSLs in ANLL were identified and confirmed as GlcCer, LacCer, Gb3, Lc3, Gb4, and nLc4 by immunologic, chromatographic, and spectroscopic methods.
Confirmation of Gb3, Gb4, and nLc4 by fast atom bombardment–mass spectrometry.
FAB-MS of ANLL bands c (A), e (B), and f (C) showing molecular (M-H) and sequence ions. Congenic peaks, representing heterogeneity in ceramide fatty acid content, are identified in samples C and F. Note bands c and e both possess a trihexosylceramide sequence characteristic of all globo GSLs. The FAB-MS of bands c, e, and f is consistent with the structures of Gb3, Gb4, and nLc4, respectively (Table 1).
Confirmation of Gb3, Gb4, and nLc4 by fast atom bombardment–mass spectrometry.
FAB-MS of ANLL bands c (A), e (B), and f (C) showing molecular (M-H) and sequence ions. Congenic peaks, representing heterogeneity in ceramide fatty acid content, are identified in samples C and F. Note bands c and e both possess a trihexosylceramide sequence characteristic of all globo GSLs. The FAB-MS of bands c, e, and f is consistent with the structures of Gb3, Gb4, and nLc4, respectively (Table 1).
ANLL characteristics associated with the expression of globo GSLs
The expression of globo GSLs and the percent Gb4(percent total GSL4) were compared by FAB subclass, cytogenetic, and lineage-specific immunophenotypic markers (Tables 2 and 5). In general, there was no association between the expression of globo GSLs and a specific FAB subclass. Globo GSLs were identified in all myeloblastic ANLLs (FAB M0-M2) and 4 of 5 “differentiated” ANLLs (FAB M3 and M5) but not ANLL M4, CML, and mature granulocytes. There was no association between GSL expression and cytogenetic abnormalities: The t(15:17) and inv(16) in samples 7 and 8 are common chromosomal abnormalities in ANLL M3 and M4, respectively.76 There was a slight positive correlation between globo GSL expression and expression of CD13 (P < .05). A weak association was also noted between increased Gb4 (percent total) and expression of lymphoid (P < .10) or early hematopoietic markers (CD34 and terminal deoxynucleotidyl transferase [TdT],P < .10; Pearson product moment correlation).
Parvovirus B19 binds both Gb4 and nLc4 on myeloid blasts
Gb4 is the receptor for parvovirus B19 on red cells and platelets.18,23 Parvovirus B19 is also reported to bind mature neutrophils, which lack Gb4, possibly through binding nLc4 on neutrophils.25,77 To investigate whether B19 recognized either Gb4 and/or nLc4 on myeloblasts, GSL samples were subjected to HPTLC immunostaining with recombinant B19 empty capsids as described.18 25 B19 bound Gb4 in red cells, platelets, lymphocytes, and 5 of 6 ANLLs (Figure7). In addition to Gb4, B19 also recognized nLc4 in all myeloid cells tested. A comparison of B19 binding to Gb4 and nLc4 was compared using the ratio of B19 binding to DPA staining as determined by scanning densitometry (B19/DPA, square millimeters). In general, B19 binding to Gb4 was twice that observed for nLc4(B19/DPA ratio: 0.81 ± 0.28 vs 0.37 ± 0.06, n = 6;P < .01, t test).
Parvovirus B19 binds both Gb4 and nLc4 in ANLL.
(A) The total neutral GSL fraction from human platelets (P) and normal granulocytes (N), separated by HPTLC and chemically stained with DPA. (B) Neutral GSLs immunostained with parvovirus B19 capsids. GSL samples include the total neutral GSL fractions from ANLL (samples 1, 3, 5, 6, 8, 11; Tables 2 to 4), CML (sample 13), neutrophils (N), lymphocytes (L), platelets (P), and red cells (R). Numbers on left indicate the mobility of Gb4 (Rf 0.27) and nLc4(Rf 0.23). Solvent, C/M/W 65:30:5 (vol/vol).
Parvovirus B19 binds both Gb4 and nLc4 in ANLL.
(A) The total neutral GSL fraction from human platelets (P) and normal granulocytes (N), separated by HPTLC and chemically stained with DPA. (B) Neutral GSLs immunostained with parvovirus B19 capsids. GSL samples include the total neutral GSL fractions from ANLL (samples 1, 3, 5, 6, 8, 11; Tables 2 to 4), CML (sample 13), neutrophils (N), lymphocytes (L), platelets (P), and red cells (R). Numbers on left indicate the mobility of Gb4 (Rf 0.27) and nLc4(Rf 0.23). Solvent, C/M/W 65:30:5 (vol/vol).
Discussion
The last decade has seen increased interest in the expression, differentiation, regulation, and biologic role of cell surface carbohydrates. On granulocytes and monocytes, carbohydrate antigens have been shown to facilitate vascular adhesion, cell activation, and phagocytosis.14-16,78,79 Cell surface carbohydrates may also play a role in myelopoiesis, as evidenced by changes inN-glycan and ganglioside expression with neutrophilic and monocytic differentiation.8,23,80-83 In HL60 cells, granulocytic and monocytic differentiation are accompanied and induced by distinct changes in ganglioside synthesis and expression.8,82 83 Our studies suggest that myelomonocytic differentiation, particularly granulocytopoiesis, may also be accompanied by changes in the expression of neutral GSLs.
Using ANLL cells as a model for neutral GSL differentiation in early myelopoiesis, we observed both quantitative and qualitative differences in the expression of neutral GSLs. Quantitatively, myeloblasts expressed significantly less neutral GSLs than mature granulocytes or CML cells. Similar findings have been reported by others and suggest a dramatic increase in total neutral GSL content with neutrophilic maturation.30,31,81 Neutrophilic maturation also appears to be associated with a relative and absolute increase in LacCer content, as evidenced by the percent LacCer and LacCer/GlcCer ratio. Biologically, high LacCer expression on mature neutrophils may play a role in inflammation and infection. A receptor for several bacteria and fungi,3,84,85 LacCer may facilitate microbial adhesion to neutrophils. In addition, LacCer activates neutrophils, stimulating NADPH oxidase activity and up-regulating CD11b/CD18 and intercellular adhesion molecule (ICAM) expression on neutrophil membranes.14,15 86
ANLL samples were also screened for qualitative differences in globo-, gala-, neolacto-, and ganglio-family GSL expression. Contrary to earlier reports, we found no evidence that Gal2Cer is a marker of neutrophilic maturation.29,38 With one exception, neither GalCer nor Gal2Cer was identified in any myeloid cell tested. Nor did we find evidence of ganglio-series GSLs in human myeliod cells. Early reports of Gg4 expression on HL60 cells might reflect the known cross-reactivity of anti-Gg4 antibodies with LacCer and neolacto GSLs.56,72 In agreement with our findings, Yohe et al87 recently reported an absence of ganglio-series gangliosides in monocytes. In contrast, both ANLL and CML expressed neolacto-series GSLs, a hallmark feature of mature peripheral blood neutrophils.24,25,36,37 Several extended neolacto-series GSLs, including an unusual intermediate (nLc5), were also identified in ANLL, CML, and mature neutrophils. Earlier studies have suggested that ANLL cells lack the necessary “elongating” glycosyltransferases necessary for synthesis of long-chain neolacto GSLs.57 Our data, however, indicate that early myeloid cells are capable of synthesizing a host of neolacto GSLs, including GSLs with Lex activity (data not shown). Neolacto GSLs (nLc4, nLc6, nLc8) are reported to serve as receptors for Neisseria meningitidis andHaemophilus influenzae, whereas Lex and sialyl-Lex GSL may serve as potential receptors for human granulocytic ehrlichiosis and E-selectin.74,88 89
Unlike CML or mature neutrophils, most ANLL samples also expressed globo-family GSLs. Gb3 and Gb4 were identified in 9 of 11 ANLL samples by immunologic and physical methods and did not appear to reflect RBC, platelet, or lymphocyte contamination. As shown in Table 3, platelet contamination in ANLL samples was minimal relative to our normal granulocyte control. In addition, all samples were differentially centrifuged and washed to remove residual platelets prior to lipid extraction. Likewise, lymphocyte contamination was less than 5%, with leukemic blasts accounting for more than 85% of all nucleated cells extracted. We also found no correlation between the percent Gb4, percent lymphocytes, and platelet-leukocyte ratios in our ANLL samples (P > .10, Pearson product moment correlation). To eliminate RBC contamination, all leukocyte samples were repeatedly washed with NH4CO3 to osmotically lyse residual RBCs prior to lipid extraction. If Gb3 or Gb4 were the result of residual RBCs in our leukocyte pellets, they should be uniformly present in all leukocyte samples, including the normal granulocyte control, which was not observed.
The common expression of globo GSLs in our ANLL samples agrees with several small early studies. Previously, Macher et al31reported Gb4 in 6 of 6 clinical ANLLs, with Gb4accounting for 20% to 75% of the total GSL4 present. Gb3and/or Gb4 was also identified in KG1, THP-1, and K526 cells, 3 human ANLL cell lines.27,32,39 Finally, Gb4 has been reported on the membranes of some clinical ANLL samples by immunofluorescence microscopy and flow cytometry.53,90 In contrast, Kyogashima et al83 failed to observe either Gb3 or Gb4 in 3 of 3 ANLLs, including 2 cases of acute monocytic leukemia, which might be expected to express globo GSLs.24,32 Overall, 17 of 20, or 85%, of all clinical ANLL samples published to date have been shown to express globo GSLs, suggesting that globo GSLs are commonly expressed in ANLL. In general, globo GSLs are expressed by myeloblastic (M0-M2) and monoblastic (M5) leukemias and, in our study, weakly associated with the expression of either early hematopoietic or lymphoid markers. In contrast, globo GSLs are weak or absent on more “differentiated” neutrophilic leukemias (HL60, CML) and mature granulocytes.37,38,72,81 The unusually strong expression of globo GSLs in our hypogranular ANLL M3, and a second clinical hypogranular M3 sample reported by Macher et al,31 may indicate phenotypic and maturational differences between the hypogranular and hypergranular (HL60) variants of ANLL M3.
Based on our results and those of others,24,27-32 we propose an updated model of neutral GSL differentiation during human myelopoiesis (Figure 8). As originally suggested by Buehler et al,27 early myeloblasts express GlcCer, LacCer, and globo- and neolacto-series GSLs. With increasing neutrophilic maturation, there is a marked increase in total neutral GSLs with increased LacCer and neolacto GSL synthesis, accompanied by a loss in globo GSL expression. Although it is unclear at what point globo GSL synthesis ceases, the absence of globo GSLs in HL60 cells suggests it may occur at or after the promyelocytic stage of differentiation.8,27,81 Interestingly, trace Gb3 was recently identified in neutrophil secretory granules but not azurophilic and primary granules, which arise later in neutrophilic maturation.69
Model of neutral GSL expression in human myelomonocytic differentiation.
Globo and neolacto GSLs are expressed by CFU-GMs, early myeloblasts, and monoblasts. During granulocytic maturation, globo GSL synthesis ceases, accompanied by a marked increase in total neutral GSL content. In contrast, globo and neolacto GSLs are expressed by early and late monocytes, with no apparent increase in total neutral GSL content with increasing monocytic differentiation. Adapted with permission fromBiochemistry.27 Copyright 1985, American Chemical Society.
Model of neutral GSL expression in human myelomonocytic differentiation.
Globo and neolacto GSLs are expressed by CFU-GMs, early myeloblasts, and monoblasts. During granulocytic maturation, globo GSL synthesis ceases, accompanied by a marked increase in total neutral GSL content. In contrast, globo and neolacto GSLs are expressed by early and late monocytes, with no apparent increase in total neutral GSL content with increasing monocytic differentiation. Adapted with permission fromBiochemistry.27 Copyright 1985, American Chemical Society.
Unlike granulocytes, monocytic differentiation does not appear to be associated with either an increase or dramatic shift in neutral GSL synthesis. Both monoblasts and mature circulating monocytes express globo and neolacto GSLs, averaging 6 to 11 μg per neutral GSLs per 108 cells.24 Monocytes, therefore, maintain a GSL pattern very similar to that observed in early myeloblasts and possibly even committed progenitor cells such as CFU-GM, CFU-GEMM, and megakaryocyte-erythroid colony-forming unit (CFU-Meg/E)26: GlcCer, LacCer, and globo and neolacto GSLs have been identified in HEL cells, an erythroblastic cell line; HUT7, an erythromegakaryoblastic cell line; and K562 cells, a pleuripotent cell line capable of erythroid, monocytic, granulocytic, and megakaryocytic differentiation.25-28 Overall, neutral GSL differentiation in human myelopoiesis differs significantly from human lymphopoiesis, which is characterized by neolacto expression in early pro– and pre–B cells, followed by a switch to globo GSLs with increasing lymphoid maturation.39,40 It also differs from murine myelopoiesis, which is characterized by globo- and ganglio-series expression in early myeloid cells, ganglio GSLs in mature neutrophils, and increased globo GSL expression in monocytes.23 41
The expression of globo GSLs on early myeloid precursors and other blood cells may have important implications for autologous stem cell transplantation.20,22 Recently, LaCasse et al20 proposed using Stx1 for ex vivo purging of autologous stem cell collections. In preliminary studies, Stx1 binding and cytotoxicity was demonstrated against lymphoma, multiple myeloma, and breast cancer but not HPCs, with no discernable loss in HPC numbers and normal hematopoietic differentiation in vitro. Likewise, Stx1 effectively purged lymphoma cells from murine marrow prior to transplantation in severe combined immunodeficiency (SCID) mice.22 In fact, Stx1 could be used as an immunotoxin for a large number of malignancies: Globo GSLs are commonly expressed on tissues arising from embryonic mesoderm and are highly expressed on many epithelial tumors, including those demonstrating P-glycoprotein–mediated multidrug resistance.7,25 91
Given the potential clinical applications for Stx1 in transplantation, it is imperative to discern the expression and regulation of globo and other GSLs in human hematopoiesis. Our data and those of other investigators suggest that globo GSLs might be expressed by early myelomonocytic precursors and possibly late committed pleuripotent progenitors, such as the CFU-GM, CFU-GEMM, and CFU-Meg/E. As a consequence, Stx treatment could potentially delay early cellular recovery following autologous transplantation. This may explain the dose-dependent decrease in CFUs (CFU-GM and CFU-Meg/E) observed after Stx treatment of murine bone marrow, which expresses globo- and ganglio-series GSLs.22,23 In humans, Stx1 treatment also decreased early CFU formation but only at high Stx1 doses.22 The latter may reflect the relatively low levels of Gb3 in early myeloid cells when compared with lymphoma and other Stx-sensitive tissues.21 In contrast, myelomonocytic precursors might be quite sensitive to Stx2e, which recognizes Gb4 or P blood group antigen.19Stx1 and Stx2e binding and toxicity have been shown for THP-1, a Gb3-positive ANLL cell line. Interestingly, THP-1 differentiation is associated with a 50% decrease in Gb3and resistance to Stx1.92
Globo GSL expression on normal peripheral blood elements may also have major implications for Stx1-mediated tumor purging. Gb3 is a major GSL on human red cells, platelets, and lymphocytes, representing a natural “sink” for Stx1 in stem cell and marrow collections. As a result, significantly higher Stx1 concentrations may be required, possibly approaching levels toxic to late committed progenitors.20,22 This has not been a problem in murine transplantation models because murine erythrocytes, lymphocytes, and neutrophils express predominantly ganglio GSLs.23,93 94Because GSL expression and differentiation is often tissue- and species-specific, transplantation protocols using Stx1 for ex vivo purging in animal models may require considerable modification prior to use in human transplantation. For example, a combination of CD34 selection, followed by ex vivo purging with Stx1 (or Stx2e), could minimize Stx toxicity of CD34+ myeloid progenitors by removing peripheral blood elements, thus decreasing the final Stx concentration necessary for tumor purging.
Finally, Gb4 expression on myeloblasts may explain the neutropenia sometimes observed after parvovirus B19 infection. The etiologic agent of “fifth disease,” parvovirus B19 frequently causes a mild to severe anemia, often accompanied by mild decreases in platelets and leukocytes.35 B19 has also been linked to chronic neutropenia of childhood and aplastic anemia.33,34Several studies have shown that both B19-induced anemia and thrombocytopenia reflect primary viral infection of marrow erythroblasts and megakaryocytes, which express Gb4, the B19 receptor.25,95,96 Our data suggest that early myeloid precursors also express Gb4 and are potentially susceptible to B19 infection. Although myeloid cells are reportedly resistant to B19 in vitro,95 B19 infection of early myeloid precursors with decreased myeloid progenitors and inhibition of CFU-GM formation has been demonstrated in vivo.32-35 As previously reported, weak B19 binding to nLc4 may indicate a second, lectin-binding site on the B19 capsid.25 B19 binding to nLc4 and structurally similar glycans on myeloid glycoproteins may explain why B19 viral replicative forms are preferentially isolated in the neutrophilic fraction of blood.25,77 97
In summary, human myeloid differentiation appears to involve changes in both neutral GSL and ganglioside expression. Changes in the expression and concentration of specific GSLs may play a role in neutrophil function by binding microorganisms3,84,85,88 as well as potentiating neutrophil activation, endothelial adhesion, and phagocytosis.14-16,86 Globo GSL expression on myeloblasts also suggests the potential susceptibility of early myeloid precursors to parvovirus B19 and Stx1. With the recent cloning of several GSL glycosyltransferases, including Gb3- and Gb4-synthase,98 99 it may become possible to study the physiologic role and regulation of GSLs during hematopoiesis.
The authors thank Dr Lynn Teesh, Director of the High Resolution Mass Spectrometry Facility, and John Snyder of the High Field Nuclear Magnetic Resonance Facility, Department of Chemistry, The University of Iowa, for their help in FAB-MS and NMR analysis.
Prepublished online as Blood First Edition Paper, September 5, 2002; DOI 10.1182/blood-2002-03-0718.
Supported by grants from the College of American Pathology, National Blood Foundation, and National Institutes of Health (grants R01 HL55447 and R29 HL42395).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Laura Cooling, Clinical Assistant Professor, Department of Pathology, The University of Michigan Medical School, 2F226 University Hospital, Blood Bank, Box 0054, 15000 East Medical Center Dr, Ann Arbor, MI 48109-0054; e-mail:lcooling@med.umich.edu.




![Fig. 5. [1H]NMR 1-D spectra between 4.0 and 5.0 ppm, showing the anomeric protons for ANLL GSL bands a-f. / 1-D NMR spectrum for GSL bands a to f (A-F), respectively. The size and composition of the carbohydrate moiety of each GSL band was determined by the number and chemical shift (ppm) of their anomeric or H-1 protons, which are present as split peaks in the NMR spectrum between 4.0 and 5.0 ppm. The anomeric linkage (α, β) of each carbohydrate was determined by the coupling constant or difference in frequency (J1,2; Hz) between each set of H-1 peaks.64The numbering of individual anomeric protons (I-1, II-1, III-1, IV-1, V-1, VI-1) and their subsequent identification, based on published standards and 2-D NMR, is shown in Table 6 and Figure 4. Note that the H-1 proton of glucose (I-1; Table 6; Figure 4) in bands c and e is adjacent or split by the H-5 proton of galactose (III-5), which is consistent with the published NMR spectra of Gb3 and Gb4, respectively.647475](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/2/10.1182_blood-2002-03-0718/4/m_h80233651005.jpeg?Expires=1765897406&Signature=oMCt~VWG0Q2la-DEGNcb53beziKF9yj0giLNN-o2TpyP4JN7ByUh9XlPCZ9cqzpAT4IXhxQeEza6Uz4xcGjWIfEhwKFOkxp75JzER~Lixa6oEA8zKOlWFQ7pVku45Tbh9Ni7bxXESpYDY0GJxmDl0UAE6lu9c2XwOPWPmRXe11TxD5TlcjkldPEC6yl1Rz9pfpas2aSOCo3n5dTFgsMRyPy8ftYah7gDKXj3MhjmLE3LvYP3JwuWT-3lMjO5H0euKhrszFiLhGzjXK6ZYURzS2zSMzj7GyiLJiYYOPFTl8tbeqCUsvprc~9Kn~HK99yIKSs40dxI9cVC6HTAEp5NaA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal