Activating mutations of the protein tyrosine kinase (PTK) FLT3 can be found in approximately 30% of patients with acute myeloid leukemia (AML), thereby representing the most frequent single genetic alteration in AML. These mutations occur in the juxtamembrane (FLT3 length mutations; FLT3-LMs) and the second tyrosine kinase domain of FLT3-TKD and confer interleukin 3 (IL-3)–independent growth to Ba/F3 cells. In the mouse bone marrow transplantation model, FLT3-LMs induce a myeloproliferative syndrome stressing their transforming activity in vivo. In this study, we analyzed the pro-proliferative and antiapoptotic potential of FLT3 in FLT3-LM/TKD-mutation–transformed Ba/F3 cells and AML-derived cell lines. The PTK inhibitor SU5614 has inhibitory activity for FLT3 and selectively induces growth arrest, apoptosis, and cell cycle arrest in Ba/F3 and AML cell lines expressing a constitutively activated FLT3. In addition, the compound reverts the antiapoptotic and pro-proliferative activity of FLT3 ligand (FL) in FL-dependent cells. No cytotoxic activity of SU5614 was found in leukemic cell lines that express a nonactivated FLT3 or no FLT3 protein. At the biochemical level, SU5614 down-regulated the activity of the hyperphosphorylated FLT3 receptor and its downstream targets, signal transducer and activator of (STAT) 3, STAT5, and mitogen-activated protein kinase (MAPK), and the STAT5 target genes BCL-XL and p21. Our results show that SU5614 is a PTK inhibitor of FLT3 and has antiproliferative and proapoptotic activity in AML-derived cell lines that endogenously express an activated FLT3 receptor. The selective and potent cytotoxicity of FLT3 PTK inhibitors support a clinical strategy of targeting FLT3 as a new molecular treatment option for patients with FLT3-LM/TKD-mutation+ AML.
Introduction
FLT3 is a member of the class III protein receptor tyrosine kinases (RTKs) that are characterized by 5 immunoglobulinlike domains, a juxtamembrane (JM), and 2 protein tyrosine kinase (PTK) domains separated by an interkinase domain.1-3 The binding of FLT3 ligand (FL) induces dimerization and autophosphorylation of the receptor and subsequent activation of downstream signaling pathways.4 FLT3 is expressed at the surface of immature hematopoietic cells and plays an important role in the survival and expansion of multipotent progenitors. Furthermore, primary AML blasts express functional FLT3 protein and stimulation with FL induces their proliferation and inhibits apoptosis by induction of BCL-2.5 FLT3 length mutations (FLT3-LMs), which consist of in-frame internal tandem duplications (ITDs) and insertions in the JM domain of FLT3, occur in 20% to 25% of patients with acute myeloid leukemia (AML).6-8 These mutations have a variable length, result in an elongated FLT3 protein with constitutive tyrosine kinase activity, and are associated with higher leukocyte counts at diagnosis.7 9
In vitro studies show that an FLT3-LM and a TEL-FLT3 construct transform interleukin 3 (IL-3)–dependent cell lines to factor independence and constitutively activate signal transducer and activator of transcription 5 (STAT5) and mitogen-activated protein kinase (MAPK).10-13 In addition, enforced expression of FLT3-LM14 and a TEL-FLT3 fusion protein15 induce a myeloproliferative disease in the mouse bone marrow transplantation (BMT) model and a myeloproliferation in transgenic mice, respectively. Interestingly, FLT3-LM found as secondary genetic alterations in acute promyelocytic leukemia (APL) cooperate with promyelocytic leukemia/retinoic acid receptor α (PML/RARα) in the development of the murine APL phenotype.16
Primary AML blasts harboring FLT3-LMs were selectively sensitive to the growth inhibitory activity of the PTK inhibitor AG1295 compared with FLT3-LM− blasts.17 These findings underline the pathogenetic relevance of the transforming FLT3 mutations for the malignant phenotype of AML blasts. The clinical relevance of the FLT3-LM has been demonstrated in several studies in which the presence of the FLT3-LM was associated with a worse clinical outcome.6,7,18,19 In addition, a recent Cancer and Leukemia Group B study shows that genomic loss of the residual FLT3 wild-type (FLT3WT) allele occurring in 30% to 35% of patients with FLT3-LMs further impairs the prognosis of FLT3-LM+AML.20 Recently, mutations at codon 835/836 in the activation loop of FLT3 were described in 7% of patients with AML.21-23 The position Asp835 of FLT3 corresponds to position Asp816 of KIT that is mutated in patients with mastocytosis and AML. In addition to these mutations, we have identified a new recurrent FLT3 mutation with transforming potential in patients with AML that is generated by an insertion of 2 amino acids between codons 840 and 841 (FLT3-840GS).24
In the present study, we identified the small molecule PTK inhibitor SU5614 as an inhibitor of the mutated and wild-type FLT3 RTK that induces growth arrest and apoptosis in FLT3-transformed cells and AML-derived cell lines expressing a constitutively activated FLT3. These findings have important implications for clinical studies with this class of PTK inhibitors in AML.
Materials and methods
Screening for the LM and the mutations at codon 835/836 in theFLT3 gene
Analysis of Asp835/836 mutations was performed using the restriction fragment gene length polymorphism at codon 835/836 exactly as described previously.21 After amplification of a 114-bp fragment from exon 20 using gDNA, polymerase chain reaction (PCR) products were digested by EcoRV and separated on an 8% polyacrylamide gel. Undigested bands were cut out from the gel, purified with a Qiaquick gel extraction kit (Qiagen, Hilden, Germany), and directly sequenced on a DNA sequencer (ABI PRISM 310 Genetic Analyzer; Perkin Elmer; obtained from Applied Biosystems, Weiterstadt, Germany) using a BigDye terminator cycle sequencing kit (Applied Biosystems, Darmstadt, Germany). Screening for FLT3-LM was performed as described by Nakao et al.25
Reagents and cell lines
Recombinant murine IL-3 and recombinant human FL were purchased from Biosource International (Camarillo, CA) and R & D Systems (Minneapolis, MN), respectively. Restriction enzymes were obtained from New England Biolabs (Beverly, MA) and reagents for PCR were purchased from Applied Biosystems (Foster City, CA). All cell lines were obtained from the German Collection of Microorganisms and Cell Cultures (DSMZ; Braunschweig, Germany) or the American Type Culture Collection (Manassas, VA) and were cultured according to the supplier's instructions. Low-passage murine Ba/F3 cells were obtained from the DSMZ and were maintained in RPMI 1640 medium with 10% fetal bovine serum (FBS) and 10% WEHI-conditioned medium as a source of murine IL-3 when indicated. The PTK inhibitors SU5614 and SU1498 were obtained from Calbiochem (Calbiochem-Novabiochem, Bad Soden, Germany), dissolved in dimethyl sulfoxide (DMSO) at stock concentrations of 10 mM and 50 mM, aliquoted, and stored at −20°C. Imatinib mesylate (STI571; Gleevec) was kindly provided by Novartis (Basel, Switzerland). Final concentrations of DMSO in growth medium did not exceed 0.1%. MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromid) assays were performed with Cell Proliferation Kit I obtained from Roche Diagnostics (Mannheim, Germany).
Cell proliferation of Ba/F3 cells
Cells were seeded at a density of 4 × 104/mL growth medium in the presence or absence of IL-3 as indicated. Viable cells were counted for 3 days in a standard Neubauer chamber after staining with trypan blue. For experiments using the PTK inhibitor SU5614, cells were seeded at a density of 4 × 104/mL in RPMI 1640 containing 10% FBS and recombinant IL-3 as indicated. Experiments were performed in 48-well plates with 1 mL cell suspension per well.
Cell proliferation of human leukemic cells
The cell lines were washed with phosphate-buffered saline (PBS) and cultured in 1 mL RPMI 1640 medium containing 10% FBS with the exception of MV4-11 cells, which were cultured in RPMI 1640 containing 20% FBS. Cells were seeded at a density of 1 × 105cells/mL in 24-well plates for 72 hours in the presence or absence of PTK inhibitors as indicated. For inhibition of FL-induced antiapoptotic effects by SU5614, OCI-AML5 cells were starved in RPMI 1640 medium containing 0.25% FBS for 16 hours. Cells were washed twice and seeded in 1 mL RPMI without FBS at a density of 2 × 105cells/mL in 48-well plates. FL and SU5614 were added where indicated and cells were cultured for 96 hours. Viable cells were counted in a standard Neubauer chamber after staining with trypan blue. Figures show mean values and SDs from 3 independent experiments unless otherwise indicated.
For MTT assays cells were counted and split daily to obtain cells in logarithmic growth phase. Experiments were performed in 96-well plates in triplicate with 100 μL medium/well. Marginal wells were not used. Cells were seeded at a density of 2 × 105 cells/mL in RPMI 1640 containing 10% FBS and different concentrations of SU5614. After 84 hours, cells were processed according to the manufacturer's instructions. After 96 hours, absorbance at 570 nm was measured using 690 nm as reference wavelength in an OptiMax microplate reader (Molecular Devices, Ismaning/Munich, Germany).
Cell cycle and apoptosis analysis
Cell cycle analyses were performed by determination of the DNA content of cell nuclei with propidium iodide (PI) as described previously.26 Assessment of apoptotic cells was carried out by annexin V/7-amino-actinomycin D (7-AAD) staining as recommended by the manufacturer (annexin V–phycoerythrin apoptosis detection kit, Becton Dickinson, Heidelberg, Germany) using a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA).
Antibodies
The following antibodies were used: anti-FLT3/flk2 (S18, sc-480; Santa Cruz, Heidelberg, Germany); anti-PY (PY99; Santa Cruz); antiphospho-Thr202/Tyr204-p42/p44 MAPK-Ab, antiphospho-AKT-Ser473, antiphospho-STAT3-Tyr705, antiphospho-STAT5-Tyr694 (all from New England Biolabs, Frankfurt, Germany); anti-STAT3 (C20; Santa Cruz); and anti-STAT5 (sc-835; Santa Cruz).
DNA constructs and vectors
The FLT3ITD-NPOS construct contains a 28-amino acid duplicated sequence (CSSDNEYFYVDFREYEYDLKWEFPRENL) inserted between amino acids 610/611 and the FLT3ITD-W51 construct contains a 6-amino duplicated sequence (REYEDL) inserted between amino acids 601/602 of human FLT3WT. All FLT3 constructs were subcloned in the MSCV-IRES-EYFP/EGFP retroviral expression vector (kindly provided by R. K. Humphries, The Terry Fox Laboratory, University of British Columbia, Vancouver, BC, Canada).
In vitro mutagenesis
The FLT3 D835Y and V592A mutations were introduced into the full-length human FLT3WT cDNA using the QuikChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA) according to the manufacturer's instructions. The correct sequence of all constructs was confirmed by nucleotide sequencing.
Transient transfection of BOSC23 cells
One day prior to transfection BOSC23 cells were seeded into 10-cm dishes at a density of 1.0 × 105/mL. Transient transfections were then carried out using the calcium-phosphate coprecipitation method with a total of 6 μg pDNA/10-cm dish. Then, 18 hours after transfection 10 mL fresh medium was added, the cells were allowed to grow for another 30 hours, and the retroviral supernatant was used for transduction of Ba/F3 cells.
Transduction of Ba/F3 cells
Ba/F3 cells (2 × 105) were seeded in 1 mL growth medium and subsequently transduced once with 200 μL retroviral supernatant in the presence of polybrene (8 μg/mL). The FACS-Vantage system equipped with a Turbo-Sort device (Becton Dickinson, San Jose, CA) was used to highly purify EGFP/EYFP+ pool cells 48 hours after transduction.
Expression of CD135 and CD117 by flow cytometry
Leukemic cells were incubated for 30 minutes on ice with a mouse phycoerythrin (PE)–labeled isotype-matched control antibody (Becton Dickinson, Heidelberg, Germany), CD117-PE, or CD135-PE (Becton Dickinson, Heidelberg, Germany) antibody. Viable cells were analyzed using a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA).
Immunoprecipitation and Western blot analysis were performed as described previously.27
Statistical analysis
Data were statistically tested using the t test for dependent or independent samples (Excel; Microsoft, Redmond, WA). Differences with P < .05 were considered statistically significant.
Results
FLT3ITD and FLT3D835Y have a direct transforming potential in Ba/F3 cells
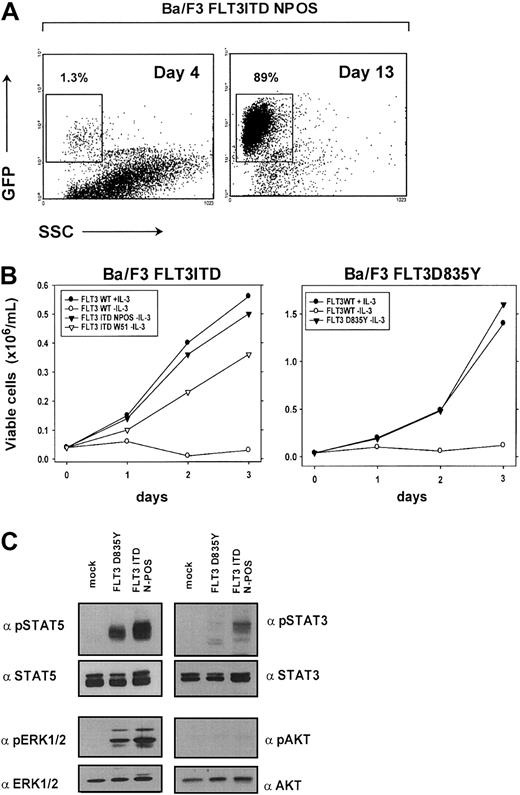
We first generated FLT3ITD-NPOS/W51 and FLT3D835Y mutant expressing Ba/F3 cells to confirm the transforming potential of the FLT3ITD and FLT3D835Y constructs in this cell line model. To achieve selection of successfully transduced cells, IL-3–dependent Ba/F3 cells were transduced with the pMSCV-IRES-EGFP/EYFP retroviral expression vector that contains an internal ribosome entry site (IRES) and allows transcription of enhanced green fluorescent protein/enhanced yellow fluorescent protein (EGFP/EYFP) and the FLT3 construct from the same transcript. The percentage of EGFP+ cells was determined at different time points after transduction of Ba/F3 cells with FLT3ITD-NPOS retrovirus. After IL-3 was withdrawn, we observed a substantial competitive growth advantage of FLT3 mutant expressing EGFP+ cells compared with nontransduced control cells. EGFP+ cells expressing the FLT3ITD-NPOS mutant receptor expanded from 1.3% 4 days after transduction to 89% of all cells after 13 days of culture (Figure 1A). After prolonged in vitro culture EGFP+ cells counted for more than 99% (data not shown), which directly demonstrated the ability of the FLT3ITD mutant to induce IL-3–independent growth in Ba/F3 cells. In addition, the FLT3ITD and FLT3D835Y mutant conferred long-term IL-3–independent growth to Ba/F3 cells (Figure 1B). In contrast, the FLT3WT construct was unable to induce factor independence when stably expressed in Ba/F3 cells (Figure 1B). Identical expression levels of all FLT3 constructs was confirmed by flow cytometry and by Western blot after immunoprecipitation with a FLT3-specific antibody (data not shown).
FLT3ITD and FLTD835Y have transforming potential in Ba/F3 cells and activate STAT5 and MAPK.
(A) FLT3ITD mutants confer a competitive growth advantage to IL-3–dependent Ba/F3 cells. EGFP expression was determined by flow cytometry in Ba/F3 cells transduced with pMSCV-EGFP-IRES-FLT3ITD-NPOS retrovirus 4 days and 13 days after transduction and IL-3 withdrawal. (B) FLT3ITD-NPOS or FLT3ITD-W51, but not FLT3WT constructs, confer IL-3–independent growth to Ba/F3 cells. Ba/F3 cells stably expressing FLT3ITD-NPOS, FLT3ITD-W51, and FLT3WT were seeded at a density of 4 × 104 cells/mL in the absence or presence of IL-3 (10 ng/mL). Viable cells were counted for up to 3 days by trypan blue exclusion. (C) FLT3ITD and FLT3D835Y activate STAT5 and MAPK1/2. Protein lysates from Ba/F3 cells transformed either by FLT3ITD-NPOS and FLT3D835Y or vector-transduced cells (mock) grown in the presence of IL-3 were analyzed by Western blot. No activation of AKT and only weak activation of STAT3 could be detected.
FLT3ITD and FLTD835Y have transforming potential in Ba/F3 cells and activate STAT5 and MAPK.
(A) FLT3ITD mutants confer a competitive growth advantage to IL-3–dependent Ba/F3 cells. EGFP expression was determined by flow cytometry in Ba/F3 cells transduced with pMSCV-EGFP-IRES-FLT3ITD-NPOS retrovirus 4 days and 13 days after transduction and IL-3 withdrawal. (B) FLT3ITD-NPOS or FLT3ITD-W51, but not FLT3WT constructs, confer IL-3–independent growth to Ba/F3 cells. Ba/F3 cells stably expressing FLT3ITD-NPOS, FLT3ITD-W51, and FLT3WT were seeded at a density of 4 × 104 cells/mL in the absence or presence of IL-3 (10 ng/mL). Viable cells were counted for up to 3 days by trypan blue exclusion. (C) FLT3ITD and FLT3D835Y activate STAT5 and MAPK1/2. Protein lysates from Ba/F3 cells transformed either by FLT3ITD-NPOS and FLT3D835Y or vector-transduced cells (mock) grown in the presence of IL-3 were analyzed by Western blot. No activation of AKT and only weak activation of STAT3 could be detected.
The FLT3ITD and D835Y receptor mutants activate STAT5 and MAPK
To identify common signaling pathways that specifically transduce the transforming signals of the FLT3ITD and FLT3-TKD mutated receptor, we analyzed the activity of these mutants to activate STAT3, STAT5, and MAPK. Using phosphospecific antibodies, we found that both the FLT3ITD and D835Y mutants induced a strong activation of STAT5 and MAPK, but only weak activation of STAT3, and no detectable activation of AKT (Figure 1C). These data demonstrate that STAT5 and MAPK are common targets of the FLT3ITD and FLT3-TKD mutants.
SU5614 is a potent PTK inhibitor of FLT3 and induces growth arrest in FLT3ITD- and FLT3D835Y-transformed Ba/F3 cells
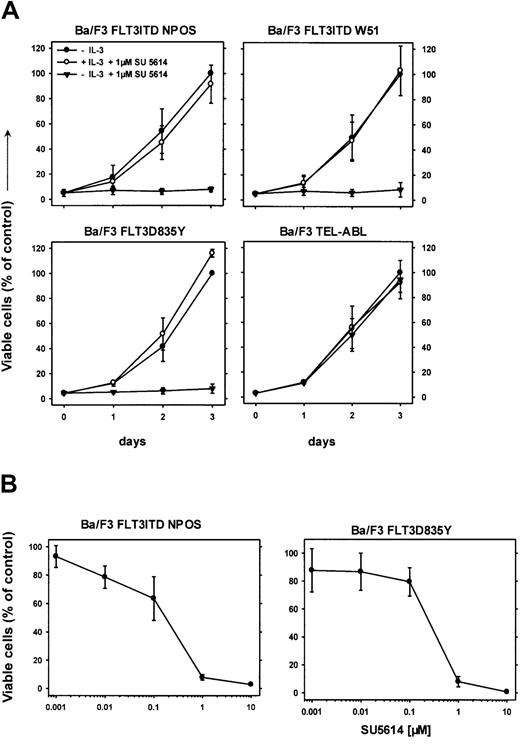
We have previously reported that the PTK inhibitor SU5614 inhibits the split domain RTK vascular endothelial growth factor receptor 2 (VEGFR-2; KDR) and KIT, and induces growth arrest and apoptosis in KIT-expressing AML cell lines.28 We hypothesized that SU5614 might also be an inhibitor of FLT3. To test this hypothesis, Ba/F3 cells transformed by 2 FLT3ITD mutants and the FLT3D835Y construct were grown in the presence or absence of 1 μM SU5614. As shown in Figure 2, SU5614 induced a growth inhibition of all FLT3-transformed Ba/F3 lines.
SU5614 induces growth arrest in Ba/F3 cells transformed by FLT3ITD and FLT3D835Y mutants in the absence but not in the presence of IL-3.
(A) SU5614 induces growth arrest in Ba/F3 cells transformed by FLT3ITD-NPOS, FLT3ITD-W51, FLT3D835Y, but not by TEL-ABL. Mean values and SDs were calculated from 3 independent experiments. The growth of cells in the absence of inhibitor at 72 hours was defined as 100%. (B) Dose-response curve of the inhibitory activity of SU5614 on Ba/F3 cells transformed by FLT3ITD-NPOS and FLT3D835Y after 72 hours of incubation. The growth of cells that were incubated without inhibitor was defined as 100%. Values represent means and SDs from 3 independent experiments.
SU5614 induces growth arrest in Ba/F3 cells transformed by FLT3ITD and FLT3D835Y mutants in the absence but not in the presence of IL-3.
(A) SU5614 induces growth arrest in Ba/F3 cells transformed by FLT3ITD-NPOS, FLT3ITD-W51, FLT3D835Y, but not by TEL-ABL. Mean values and SDs were calculated from 3 independent experiments. The growth of cells in the absence of inhibitor at 72 hours was defined as 100%. (B) Dose-response curve of the inhibitory activity of SU5614 on Ba/F3 cells transformed by FLT3ITD-NPOS and FLT3D835Y after 72 hours of incubation. The growth of cells that were incubated without inhibitor was defined as 100%. Values represent means and SDs from 3 independent experiments.
This growth inhibitory activity was completely abolished when cells were grown in the presence of IL-3. The inhibitory concentration of 50% (IC50) of SU5614 for inducing growth arrest in FLT3ITD-NPOS– and FLT3D835Y-transformed cells was 175 nM and 250 nM, respectively (Figure 2B). The selectivity of SU5614 was demonstrated by testing inhibitor activity in Ba/F3 cells transformed by other constitutively activated tyrosine kinases.
In contrast to FLT3ITD- or FLT3D835Y-expressing Ba/F3 cells, cells transformed by TEL-ABL (Figure 2A), TEL-JAK2, and BCR-ABL (data not shown) were resistant to the inhibitory activity of SU5614 at concentrations up to 10 μM.
SU5614 induces cell cycle arrest and apoptosis in FLT3-transformed Ba/F3 cells
To further characterize the mechanisms of growth inhibition by SU5614, we first analyzed induction of apoptosis after inhibitor treatment of FLT3ITD-transformed Ba/F3 cells. In the absence of IL-3, FLT3ITD-transformed cells underwent rapid apoptotic cell death after exposure to SU5614 as measured by the expression of annexin V/7-AAD (Figure 3A) and the appearance of DNA fragmentation identified by DNA laddering on agarose gel electrophoresis (Figure 3B). The addition of IL-3 rescued FLT3-transformed cells from the apoptosis-inducing activity of SU5614, at concentrations up to 10 μM (Figure 3C).
SU5614 induces apoptosis and cell cycle arrest in Ba/F3 cells transformed by the FLT3ITD-NPOS mutant.
(A,C) Ba/F3 cells transformed by FLT3ITD-NPOS were stained with annexin V–PE and 7-AAD and analyzed by flow cytometry. Representative dot plots from 1 of 3 independently performed experiments are shown. Values represent the means ± SDs of 3 independent experiments. (B) DNA fragmentation identified by DNA laddering on agarose gel electrophoresis in Ba/F3 FLT3ITD-NPOS. Cells were incubated with SU5614 in the presence or absence of recombinant murine IL-3 (10 ng/mL) for the indicated time intervals. gDNA was analyzed in a 2% agarose gel. (D) Cell cycle analyses were performed by determination of the DNA content with PI. Cells were stained with PI and cell nuclei were analyzed by flow cytometry. *P < .05; **P < .01; ***P < .001 according to Student t test comparing the percentage of cells in G0/G1, S, and G2/M phases in untreated versus inhibitor-treated samples.
SU5614 induces apoptosis and cell cycle arrest in Ba/F3 cells transformed by the FLT3ITD-NPOS mutant.
(A,C) Ba/F3 cells transformed by FLT3ITD-NPOS were stained with annexin V–PE and 7-AAD and analyzed by flow cytometry. Representative dot plots from 1 of 3 independently performed experiments are shown. Values represent the means ± SDs of 3 independent experiments. (B) DNA fragmentation identified by DNA laddering on agarose gel electrophoresis in Ba/F3 FLT3ITD-NPOS. Cells were incubated with SU5614 in the presence or absence of recombinant murine IL-3 (10 ng/mL) for the indicated time intervals. gDNA was analyzed in a 2% agarose gel. (D) Cell cycle analyses were performed by determination of the DNA content with PI. Cells were stained with PI and cell nuclei were analyzed by flow cytometry. *P < .05; **P < .01; ***P < .001 according to Student t test comparing the percentage of cells in G0/G1, S, and G2/M phases in untreated versus inhibitor-treated samples.
Cell cycle analysis of inhibitor treated cells after staining with PI confirmed the induction of apoptosis as measured by the frequency of nuclei with a hypodiploid DNA content (data not shown). Furthermore, in the absence of IL-3 SU5614 induced a dose-dependent cell cycle arrest by increasing the percentage of cells in G0/G1from 65.4% ± 0.8% (0 μM inhibitor) to 79.9% ± 3.3% (2.5 μM inhibitor) and a parallel reduction of the percentage of cells in S phase and G2/M phase (Figure 3D).
In summary, these data indicate that SU5614 has a direct cytotoxic effect in FLT3-transformed cells by inducing cell cycle arrest and apoptosis.
The FLT3 receptor, MAPK, and STAT5 are direct targets of SU5614 in Ba/F3 cells
The inhibitory activity of SU5614 against FLT3ITD and FLT3D835Y allowed us to analyze the signaling pathways activated by FLT3 mutants. For this purpose, FLT3ITD-transformed Ba/F3 cells were incubated with SU5614 at various doses and for different time intervals.
The activation status of the FLT3 receptor, STAT5, and MAPK was analyzed after immunoprecipitation with FLT3 antibody and blotting with antiphosphotyrosine (PY) antibody and phospho-specific antibodies in crude lysates. SU5614 strongly inhibited the hyperphosphorylation of the FLT3ITD receptor (Figure 4A) as well as the FLT3D835Y construct (data not shown). In addition, the inhibitor induced a rapid and sustained time- and dose-dependent down-regulation of STAT5 and MAPK activity, which was already evident after 3 hours of treatment (Figure 4B-C).
SU5614 inhibits the tyrosine phosphorylation of FLT3, STAT5, and MAPK in a time- and dose-dependent manner.
(A) Ba/F3 cells expressing FLT3ITD-NPOS were incubated for 6 hours with the indicated concentrations of SU5614 and protein lysates were immunoprecipitated (IP) with FLT3 antibody. Precipitates were analyzed by Western blot. (B) Ba/F3 cells expressing FLT3ITD-NPOS were incubated for the indicated time intervals with SU5614 at a concentration of 1 μM. Protein lysates were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and after blotting, membranes were incubated with the indicated antibodies. (C) Lysates were prepared as described in panel A and membranes were probed with phospho-specific antibodies against STAT5 and MAPK followed by specific STAT5 and MAPK antibody.
SU5614 inhibits the tyrosine phosphorylation of FLT3, STAT5, and MAPK in a time- and dose-dependent manner.
(A) Ba/F3 cells expressing FLT3ITD-NPOS were incubated for 6 hours with the indicated concentrations of SU5614 and protein lysates were immunoprecipitated (IP) with FLT3 antibody. Precipitates were analyzed by Western blot. (B) Ba/F3 cells expressing FLT3ITD-NPOS were incubated for the indicated time intervals with SU5614 at a concentration of 1 μM. Protein lysates were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and after blotting, membranes were incubated with the indicated antibodies. (C) Lysates were prepared as described in panel A and membranes were probed with phospho-specific antibodies against STAT5 and MAPK followed by specific STAT5 and MAPK antibody.
These results show that SU5614 inhibits the activation of FLT3 and its downstream targets STAT5 and MAPK in a time- and dose-dependent manner.
The AML cell lines MV4-11, MonoMac1, and MonoMac6 express an activated FLT3 receptor
To further evaluate the inhibitory activity of SU5614 in FLT3-transformed AML cell lines, we screened a panel of 10 cell lines for expression and activation of FLT3 and their sensitivity toward the growth inhibitory activity of this compound. Protein expression of FLT3 was detected on the surface of 5 of a panel of 10 representative leukemic cell lines using a PE-conjugated anti-CD135 antibody by flow cytometry and by immunoprecipitation with FLT3-specific antibodies (Figure 5; summarized in Table1). In contrast to primary AML blasts, FLT3ITD and FLT3-TKD mutations were rarely found in AML cell lines and could be detected only in the AML MV4-11 cell line, which carried an FLT3ITD mutation (Table 1). In addition to the FLT3ITD mutation, a loss/deletion of the FLT3WT allele was present in MV4-11 cells after amplification of exon 17 and visualization of the product after agarose gel electrophoresis (data not shown). Sequencing of exon 17 confirmed the presence of a 30-bp length mutation that resulted in a duplication of 10 amino acids (VDFREYEYDH) inserted between amino acids 591/592 of FLT3 (data not shown).
Expression and activation of FLT3 in leukemic cell lines—the FLT3V592A mutant expressed in MM1 and MM6 cells induces IL-3–independent growth in Ba/F3 cells.
(A) Expression of FLT3 on the surface of leukemic cells analyzed by flow cytometry after staining with an anti-CD135 antibody. Open histograms represent isotype control; filled histograms show fluorescence intensity of CD135. (B-C) Activation of FLT3 in leukemic cell lines analyzed by immunoprecipitation (IP) and blotting by anti-PY antibody with (+) or without (−) stimulation with FL. Blots were stripped and reprobed with a specific FLT3 antibody. As a positive control, Ba/F3 cells stably expressing an FLT3ITD mutant are shown in lane 1 (panel B). (D) Ba/F3 cells stably expressing FLT3ITD-W51 and FLT3V592A were seeded at a density of 4 × 104 cells/mL in the absence or presence of IL-3 (10 ng/mL). Viable cells were counted for up to 3 days by trypan blue exclusion. A representative result of 3 experiments with similar results is shown.
Expression and activation of FLT3 in leukemic cell lines—the FLT3V592A mutant expressed in MM1 and MM6 cells induces IL-3–independent growth in Ba/F3 cells.
(A) Expression of FLT3 on the surface of leukemic cells analyzed by flow cytometry after staining with an anti-CD135 antibody. Open histograms represent isotype control; filled histograms show fluorescence intensity of CD135. (B-C) Activation of FLT3 in leukemic cell lines analyzed by immunoprecipitation (IP) and blotting by anti-PY antibody with (+) or without (−) stimulation with FL. Blots were stripped and reprobed with a specific FLT3 antibody. As a positive control, Ba/F3 cells stably expressing an FLT3ITD mutant are shown in lane 1 (panel B). (D) Ba/F3 cells stably expressing FLT3ITD-W51 and FLT3V592A were seeded at a density of 4 × 104 cells/mL in the absence or presence of IL-3 (10 ng/mL). Viable cells were counted for up to 3 days by trypan blue exclusion. A representative result of 3 experiments with similar results is shown.
Summary of expression and activation of FLT3 in relation to the sensitivity of leukemic cell lines to the growth inhibitory activity of SU5614
| Cell line . | CD135 . | FLT3 protein (IP-WB) . | FLT3-PY (IP-WB) . | FLT3 mutation . | IC50SU5614, μM . |
|---|---|---|---|---|---|
| MM6 | +++ | +++ | +++ | + | 0.45 |
| MM1 | +++ | +++ | + | + | 0.60 |
| MV4-11 | + | + | − | + | 0.30 |
| THP-1 | ++ | + | − | − | > 10 |
| OA5 | ++ | ++ | − | − | > 10 |
| HL60 | − | ND | − | − | > 10 |
| PLB985 | − | ND | − | − | > 10 |
| K562 | − | ND | − | − | > 10 |
| NB4 | − | − | − | − | > 10 |
| U937 | − | − | − | − | > 10 |
| Cell line . | CD135 . | FLT3 protein (IP-WB) . | FLT3-PY (IP-WB) . | FLT3 mutation . | IC50SU5614, μM . |
|---|---|---|---|---|---|
| MM6 | +++ | +++ | +++ | + | 0.45 |
| MM1 | +++ | +++ | + | + | 0.60 |
| MV4-11 | + | + | − | + | 0.30 |
| THP-1 | ++ | + | − | − | > 10 |
| OA5 | ++ | ++ | − | − | > 10 |
| HL60 | − | ND | − | − | > 10 |
| PLB985 | − | ND | − | − | > 10 |
| K562 | − | ND | − | − | > 10 |
| NB4 | − | − | − | − | > 10 |
| U937 | − | − | − | − | > 10 |
CD135 expression was analyzed by flow cytometry and the fluorescence intensities were expressed as follows: +++, strong; ++, intermediate; +, weak; −, absent. The expression (FLT3IP-WB) and activation (FLT3-PY) were determined by immunoprecipitation and blotting with αPY antibodies as shown in Figure 5. The results were quantified according to the intensity of the signal from − (absent) to +++ (strong). FLT3 mutation indicates the presence (+) or absence (−) of an FLT3-LM, FLT3-TKD, or FLT3V592A mutation. The concentration of SU5614 that inhibits 50% growth (as determined by cell counting) after an incubation period of 72 hours was determined (IC50).
ND indicates not determined.
To analyze the activation status of FLT3 in leukemic cell lines, we performed immunoprecipitations with a FLT3-specific antibody and assessed autophosphorylation as a surrogate for activation by blotting with a PY antibody (Figure 5B-C; Table 1). In the absence of FL stimulation, autophosphorylation of FLT3 was found in 2 cell lines expressing FLT3 protein (MonoMac1 [MM1] and MonoMac6 [MM6]). The autophosphorylation level of FLT3 in MM6 cells could be slightly enhanced by exogenous FL (Figure 5B). In cell lines that express an unphosphorylated FLT3, stimulation with FL resulted in a strong activation of FLT3 indicating a functional FLT3 receptor (eg, THP1 in Figure 5B and OCI-AML5 in Figure 5C).
To further clarify the mechanisms of constitutive FLT3 activation in MM6 and MM1 cells, the complete FLT3 cDNA was sequenced. Both MM1 and MM6 cells carry a point mutation in the JM domain, which result in the substitution of valine by alanine at position 592 (V592A). Overexpression of this mutant in IL-3–dependent Ba/F3 cells resulted in factor-independent growth, thereby showing the transforming activity of this mutant (Figure 5D). Compared with FLT3ITD-expressing cells, FLT3V592A-Ba/F3 cells showed a slightly slower growth rate.
AML cell lines expressing an activated FLT3 receptor are selectively sensitive to the growth inhibitory activity of the FLT3 PTK inhibitor SU5614
To analyze the growth inhibitory activity of SU5614 in AML-derived cell lines, cells were incubated for up to 72 hours with inhibitor concentrations ranging from 0.001 μM to 10 μM. In proliferation assays, the growth of MM1, MM6, and MV4-11 cells was selectively inhibited by SU5614 in a dose-dependent manner, whereas all other cell lines were completely resistant to the compound (Figure6; Table 1). The IC50 for the growth inhibitory activity was 300 to 600 nM in sensitive cells, which was slightly higher compared with the IC50 in Ba/F3-FLT3ITD/D835Y cells (Table 1). To precisely calculate the IC50 of SU5614 against the most commonly found type of FLT3 mutation in clinical AML samples, we analyzed the MV4-11 cell line expressing a FLT3ITD mutant in detail over a wide range of inhibitor concentrations. These analyses showed that SU5614 inhibits the FLT3ITD mutant with an IC50 of 150 nM (Figure 6C).
The FLT3 PTK inhibitor SU5614 induces growth arrest in AML cell lines expressing an activated FLT3, but not in cells that express a nonactive FLT3 or no FLT3 protein.
(A) Cells were seeded at a density of 1.0 × 105 cells/mL in the absence or presence of different concentrations of SU5614. Viable cells were counted for up to 3 days by trypan blue exclusion. Mean values and SDs were calculated from 3 independent experiments for THP-1 and OCI-AML 5 cells. The growth curve of MM6 cells shows a representative result of 3 experiments with similar results. (B) Dose-response curve of the inhibitory activity of SU5614 in AML cell lines after 72 hours of incubation. The growth of cells that were incubated without inhibitor was defined as 100%. Values represent means and SDs from 3 independent experiments. (C) Dose-response curve of the inhibitory activity of SU5614 in MV4-11 cells after 84 hours of incubation. The OD was measured at 570 nm using an MTT assay (see “Materials and methods”). (D) Growth inhibitory activity of SU5614, SU1498, and imatinib mesylate in MV4-11 and MM6 cells. Cells were seeded at a density of 1 × 105/mL and incubated for 72 hours with 5 μM inhibitor. The growth of cells that were incubated without inhibitor was defined as 100%. Values represent means and SDs from 3 independent experiments.
The FLT3 PTK inhibitor SU5614 induces growth arrest in AML cell lines expressing an activated FLT3, but not in cells that express a nonactive FLT3 or no FLT3 protein.
(A) Cells were seeded at a density of 1.0 × 105 cells/mL in the absence or presence of different concentrations of SU5614. Viable cells were counted for up to 3 days by trypan blue exclusion. Mean values and SDs were calculated from 3 independent experiments for THP-1 and OCI-AML 5 cells. The growth curve of MM6 cells shows a representative result of 3 experiments with similar results. (B) Dose-response curve of the inhibitory activity of SU5614 in AML cell lines after 72 hours of incubation. The growth of cells that were incubated without inhibitor was defined as 100%. Values represent means and SDs from 3 independent experiments. (C) Dose-response curve of the inhibitory activity of SU5614 in MV4-11 cells after 84 hours of incubation. The OD was measured at 570 nm using an MTT assay (see “Materials and methods”). (D) Growth inhibitory activity of SU5614, SU1498, and imatinib mesylate in MV4-11 and MM6 cells. Cells were seeded at a density of 1 × 105/mL and incubated for 72 hours with 5 μM inhibitor. The growth of cells that were incubated without inhibitor was defined as 100%. Values represent means and SDs from 3 independent experiments.
The IC50 for SU5614 calculated from the growth-response curve using an MTT assay (150 nM) differed slightly from the IC50 calculated from cell counting experiments (300 nM). This difference might be due to a more precise estimation of the IC50 in the MTT assay in which inhibitor concentrations closely covering the range between 0.01 μM and 1 μM were used. However, it might also be attributable to the different assay systems.
The growth of cells expressing a nonactivated FLT3 protein (THP-1, O-A5) or cells lacking FLT3 expression (NB4, U937, K562, HL60, PLB985) was completely unaffected by SU5614. To exclude that the inhibitory activity against KIT and VEGFR-2 might contribute to the growth arrest induced by SU5614 in our study, we analyzed the protein and RNA levels of these PTKs in sensitive cell lines. Neither MM1, MM6, nor MV4-11 expressed KIT protein (CD117 expression by flow cytometry) or VEGFR-2 RNA (reverse transcription–PCR; data not shown).
To further exclude that the inhibition of other kinases than FLT3 by SU5614 might contribute to the cytotoxic activity in AML cell lines, we analyzed the growth inhibitory effects of other PTK inhibitors known not to inhibit FLT3 in these cells. For this purpose we used the PTK inhibitors imatinib mesylate and SU1498, which inhibit platelet-derived growth factor receptor (PDGFR), KIT, ABL, ARG,29 and VEGFR-2, respectively.30 As shown in Figure 6D, SU5614 but not imatinib mesylate or SU1498 has significant growth inhibitory activity in MV4-11 and MM6 cells. In addition, neither SU1498 nor imatinib mesylate significantly inhibited the growth of Ba/F3 cells expressing an FLT3ITD mutant (data not shown).
To allow a direct comparison of the inhibitory activity of SU5614 against a broader range of protein tyrosine kinases, the IC50 values calculated for FLT3 were compared with the IC50 for receptor and nonreceptor tyrosine kinases (Table2). These data clearly demonstrate that SU5614 has strong inhibitory activity against FLT3, KIT, VEGFR-2, and the PDGFR with IC50 values ranging from 0.07 μM to 0.65 μM. Despite this broad inhibitory activity, we could clearly show that the effects of SU5614 on AML cell lines were caused by the inhibitory activity of SU5614 against FLT3. The following findings strongly support our hypothesis: (1) selective growth inhibition of cell lines expressing a constitutively active FLT3 by SU5614; (2) very similar IC50 of SU5614 concerning the growth inhibitory activity, apoptosis inducing activity, and dephosphorylation of FLT3; (3) lack of growth inhibitory activity of other PTK inhibitors with activity against KIT, PDGFR (imatinib mesylate), and VEGFRs (SU1498) in SU5614-sensitive cell lines.
Inhibitory activity of SU5614 against different protein tyrosine kinases
| Protein tyrosine kinase . | IC50, μM . | |
|---|---|---|
| Kinase assay . | Biologic assay . | |
| FLT3 | ND | — |
| Growth arrest | — | 0.45 |
| Apoptosis | — | 0.65 |
| Dephosphorylation of FLT3 | — | 0.15 |
| KIT | 0.03 | 0.2 |
| VEGFR-2/VEGF | 0.46 | 0.07 |
| PDGFRβ/PDGF | 0.36 | 0.5 |
| FMS | 13 | ND |
| EGFR/EGF | > 100 | > 100 |
| FGFR-1/FGF | > 100 | > 50 |
| TEL-ABL/BCR-ABL | ND | > 10 |
| TEL-JAK2 | ND | > 10 |
| Protein tyrosine kinase . | IC50, μM . | |
|---|---|---|
| Kinase assay . | Biologic assay . | |
| FLT3 | ND | — |
| Growth arrest | — | 0.45 |
| Apoptosis | — | 0.65 |
| Dephosphorylation of FLT3 | — | 0.15 |
| KIT | 0.03 | 0.2 |
| VEGFR-2/VEGF | 0.46 | 0.07 |
| PDGFRβ/PDGF | 0.36 | 0.5 |
| FMS | 13 | ND |
| EGFR/EGF | > 100 | > 100 |
| FGFR-1/FGF | > 100 | > 50 |
| TEL-ABL/BCR-ABL | ND | > 10 |
| TEL-JAK2 | ND | > 10 |
In biologic assays, the concentration of SU5614 that inhibits 50% growth of MM6 or TEL-ABL/TEL-JAK2–transduced Ba/F3 cells after an incubation period of 72 hours was determined (IC50). The concentration of SU5614 that induces apoptosis in 50% of MM6 cells after a 48-hour incubation period or that induces a 50% dephosphorylation of FLT3 in MM6 cells after an incubation time of 3 hours was defined as the IC50. The autophosphorylation of FLT3 was measured in intact cells by antiphosphotyrosine antibody after immunoprecipitation with FLT3 antibody and the IC50was calculated by densitometry. To determine the IC50 of SU5614 against KIT, Kasumi-1 cells that express high levels of KIT and show a cytokine-dependent growth were used. The concentration of SU5614 that inhibits 50% growth in Kasumi-1 cells after an incubation period of 72 hours was determined (IC50) as described previously.28 The IC50 values of SU5614 against VEGFR-2, KIT, PDGFR, FMS, EGFR, and FGFR as determined in kinase assays and in biologic assays have been described previously and are reproduced with the permission of the publisher and the authors.46
FGF indicates fibroblast growth factor; FGFR, fibroblast growth factor receptor; ND, not determined; —, not applicable.
SU5614 induces apoptotic cell death and cell cycle arrest in AML cell lines expressing an activated FLT3 receptor
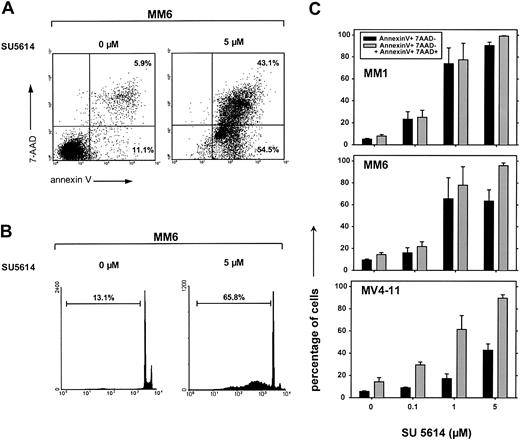
To further evaluate the mechanisms of SU5614-induced growth arrest, we analyzed the potential of the compound to directly induce apoptotic cell death. The exposure of MM1, MM6, and MV4-11 cells to SU5614 for 48 hours resulted in the rapid induction of apoptotic cell death as determined by annexinV/7-AAD staining and the appearance of hypodiploid DNA after staining of cell nuclei with PI. A typical experiment is shown in Figure 7A (annexin V/7-AAD staining) and in Figure 7B (PI staining) showing apoptosis in 97.6% (panel A) and 65.8% (panel B) of MM6 cells after exposure to 5 μM SU5614 for 48 hours. The percentage of total apoptotic cells (ie, early apoptotic [annexinV+/7AAD−] plus late apoptotic [annexinV+/7-AAD+]) at a concentration of 5 μM SU5614 was similar in all sensitive cell lines and ranged from 90% to 98% (Figure 7C). According to the results in FLT3 mutant transduced Ba/F3 cells (Figure 3D), the induction of apoptosis was paralleled by an arrest of cells in G0/G1 phase and a significant reduction of cells in S phase and G2/M phase (data not shown).
Inhibition of FLT3 by the PTK inhibitor SU5614 induces apoptosis in AML-derived cell lines expressing a constitutive active FLT3.
(A) MM6 cells were treated with 5 μM SU5614 for 48 hours and were analyzed by flow cytometry after staining with annexin V–PE and 7-AAD. Representative dot plots from 1 of 3 independently performed experiments are shown. (B) MM6 cells were treated as described in panel A with SU5614 and the percentage of cell nuclei showing a hypodiploid DNA content was analyzed after staining with PI by flow cytometry. A representative histogram of 3 independent experiments is shown. (C) Dose-response curve of the apoptosis-inducing activity of SU5614 in AML cell lines after 72 hours of incubation. Black bars represent early apoptotic cells (annexin V+/7-AAD−) and gray bars represent early and late apoptotic cells (annexin V+/7-AAD− and annexin V+/7-AAD+). Cells were treated as described in panel A. Values represent means and SDs from 3 independent experiments.
Inhibition of FLT3 by the PTK inhibitor SU5614 induces apoptosis in AML-derived cell lines expressing a constitutive active FLT3.
(A) MM6 cells were treated with 5 μM SU5614 for 48 hours and were analyzed by flow cytometry after staining with annexin V–PE and 7-AAD. Representative dot plots from 1 of 3 independently performed experiments are shown. (B) MM6 cells were treated as described in panel A with SU5614 and the percentage of cell nuclei showing a hypodiploid DNA content was analyzed after staining with PI by flow cytometry. A representative histogram of 3 independent experiments is shown. (C) Dose-response curve of the apoptosis-inducing activity of SU5614 in AML cell lines after 72 hours of incubation. Black bars represent early apoptotic cells (annexin V+/7-AAD−) and gray bars represent early and late apoptotic cells (annexin V+/7-AAD− and annexin V+/7-AAD+). Cells were treated as described in panel A. Values represent means and SDs from 3 independent experiments.
Inhibition of FLT3 activity in AML cell lines down-regulates the activity of the STAT3/5 and MAPK pathways and STAT5 target genes
We used the MM6 cell line as a model to analyze the signaling pathways downstream from the FLT3 receptor. As shown in Figures 5B and 8A, MM6 cells express a strongly activated FLT3 receptor whose activity is completely down-regulated by the FLT3 PTK inhibitor SU5614 after an incubation period of 3 hours (IC50 = 0.15 μM; Figure 8A; Table 2). In addition, constitutive activation of MAPK, STAT3, and STAT5 was found in untreated MM6 cells. The exposure of MM6 cells to concentrations of 0.1 μM to 5 μM SU5614 resulted in a dose-dependent down-regulation of STAT3, STAT5 (Figure 8B), and MAPK (data not shown) activity.
SU5614 inhibits the tyrosine phosphorylation of FLT3, STAT3, and STAT5, and down-regulates STAT5 target genes in a dose-dependent manner.
(A) MM6 cells expressing a constitutive active FLT3 were incubated for 3 hours with the indicated concentrations of SU5614, and protein lysates were immunoprecipitated (IP) with FLT3 antibody. Precipitates were subjected to SDS-PAGE and after blotting, membranes were incubated with αPY antibody. Blots were stripped and probed with αFLT3 antibody. (B) MM6 cells were incubated for 3 hours with SU5614. Protein lysates were subjected to SDS-PAGE and after blotting, membranes were probed with phospho-specific antibodies against STAT3 and STAT5 followed by reblotting with specific STAT3 and STAT5 antibodies. (C) Ba/F3 cells expressing the FLT3ITD NPOS mutant were incubated for up to 72 hours with the indicated concentrations of SU5614. Protein lysates were analyzed by SDS-PAGE as described in panel B and blots were probed with antibodies against BCL-XL, p21, and β-actin.
SU5614 inhibits the tyrosine phosphorylation of FLT3, STAT3, and STAT5, and down-regulates STAT5 target genes in a dose-dependent manner.
(A) MM6 cells expressing a constitutive active FLT3 were incubated for 3 hours with the indicated concentrations of SU5614, and protein lysates were immunoprecipitated (IP) with FLT3 antibody. Precipitates were subjected to SDS-PAGE and after blotting, membranes were incubated with αPY antibody. Blots were stripped and probed with αFLT3 antibody. (B) MM6 cells were incubated for 3 hours with SU5614. Protein lysates were subjected to SDS-PAGE and after blotting, membranes were probed with phospho-specific antibodies against STAT3 and STAT5 followed by reblotting with specific STAT3 and STAT5 antibodies. (C) Ba/F3 cells expressing the FLT3ITD NPOS mutant were incubated for up to 72 hours with the indicated concentrations of SU5614. Protein lysates were analyzed by SDS-PAGE as described in panel B and blots were probed with antibodies against BCL-XL, p21, and β-actin.
To identify target genes that might be responsible for the growth inhibition of FLT3-transformed cell lines by SU5614, we analyzed 2 STAT5-regulated genes, BCL-XL andp21 in Ba/F3 FLT3ITD-transformed cells. At a concentration of 1 μM, SU5614 induced a time-dependent and significant down-regulation of BCL-XL and p21 protein levels whose expression was strong in untreated cells, but was barely detectable after 72 hours of inhibitor treatment (Figure 8C). Equal loading of all lanes was confirmed after reblotting with an antibody against β-actin.
SU5614 reverts the antiapoptotic activity of FL in serum-starved FLT3WT-expressing OCI-AML5 cells
To characterize the activity of SU5614 against the FLT3WT kinase, we analyzed the human AML cell line OCI-AML5. Previous work from Meyer and Drexler has shown that stimulation with FL inhibits apoptosis and induces a slight proliferative response under serum-free culture conditions in these cells.31 Our results show that the growth of OCI-AML5 cells was completely unaffected by SU5614 at concentrations up to 10 μM in the presence of 10% FBS (Table 1; Figure 9B). In the absence of FL and serum, OCI-AML5 cells rapidly undergo apoptotic cell death, whereas FL at a concentration of 100 ng/mL significantly increased cell viability (Figure 9A). The parallel incubation of OCI-AML5 cells with FL and SU5614 completely inhibited the pro-proliferative and antiapoptotic activity of FL in these cells over a period of 4 days. As shown in Figure 9C, the stimulation of serum-starved OCI-AML5 cells with FL strongly induces tyrosine phosphorylation of FLT3, which is completely inhibited by SU5614 at a concentration of 1 μM.
Inhibition of wild-type FLT3 by SU5614 reverts the antiapoptotic effect of FL in serum-starved OCI-AML5 cells.
(A) After a 16-hour starvation period in RPMI 1640 with 0.25% FBS, cells were washed twice and seeded at a density of 2.0 × 105 cells/mL in RPMI 1640 with 0% FBS and with 100 ng/mL FL and 5 μM SU5614 as indicated. Viable cells were counted for 4 days by trypan blue exclusion. Values represent means and SDs from 3 independent experiments. (B) Cells were seeded at a density of 1.0 × 105/mL in the absence or presence of 10 μM SU5614. Viable cells were counted for 3 days by trypan blue exclusion. Mean values and SEs were calculated from 3 independent experiments. (C) OCI-AML5 cells were starved in RPMI 1640 with 0.3% FBS 16 hours prior to incubation with the indicated concentrations of SU5614. After 1 hour of inhibitor incubation, cells were stimulated with FL for 5 minutes and lysed. Protein lysates were immunoprecipitated (IP) with FLT3 antibody and precipitates were analyzed by Western blot.
Inhibition of wild-type FLT3 by SU5614 reverts the antiapoptotic effect of FL in serum-starved OCI-AML5 cells.
(A) After a 16-hour starvation period in RPMI 1640 with 0.25% FBS, cells were washed twice and seeded at a density of 2.0 × 105 cells/mL in RPMI 1640 with 0% FBS and with 100 ng/mL FL and 5 μM SU5614 as indicated. Viable cells were counted for 4 days by trypan blue exclusion. Values represent means and SDs from 3 independent experiments. (B) Cells were seeded at a density of 1.0 × 105/mL in the absence or presence of 10 μM SU5614. Viable cells were counted for 3 days by trypan blue exclusion. Mean values and SEs were calculated from 3 independent experiments. (C) OCI-AML5 cells were starved in RPMI 1640 with 0.3% FBS 16 hours prior to incubation with the indicated concentrations of SU5614. After 1 hour of inhibitor incubation, cells were stimulated with FL for 5 minutes and lysed. Protein lysates were immunoprecipitated (IP) with FLT3 antibody and precipitates were analyzed by Western blot.
Taken together, these data clearly show that in addition to FLT3ITD and FLT3D835Y, SU5614 has potent inhibitory activity against the FLT3WT kinase.
Discussion
Activating mutations in theFLT3 gene represent the most common single genetic alterations in AML. We have investigated the functional impact of these mutations on the growth of AML-derived cell lines.
We report here that the PTK inhibitor SU5614 has inhibitory activity against constitutively activated FLT3 mutants in hematopoietic cells and against the wild-type FLT3 kinase. SU5614 at submicromolar concentrations selectively induces growth arrest, apoptosis, and cell cycle arrest in IL-3–dependent cells transformed with the AML-specific mutants FLT3D835Y/FLT3ITD and in AML-derived cell lines expressing a constitutive active FLT3. At the biochemical level, SU5614 inhibits the constitutive activation of FLT3, STAT3, STAT5, and MAPK and down-regulates the expression of the STAT5 target genes p21and BCL-XL. Our data show that the growth of AML cell lines expressing a constitutive active FLT3 is dependent on the antiapoptotic and pro-proliferative activity of FLT3. We suggest that pharmacologic inhibition of the FLT3-induced mitogenic pathways by FLT3 PTK inhibitors represents a promising therapeutic strategy for the treatment of FLT3-LM/TKD mutation+ AML.
SU5614 and its related compound SU5416 are small-molecule inhibitors of PTKs that have been previously characterized in detail, and have selective inhibitory activity for several RTKs, including VEGFR-2 and KIT.28,32 33 We report that SU5614 has inhibitory activity against FLT3 and induces growth arrest and apoptosis in AML blasts endogenously expressing an activated FLT3 receptor. Moreover, the compound reverts the antiapoptotic and pro-proliferative activity of FL in FL-dependent cells.
Although SU5614 has a broader spectrum of inhibitory activity for other PTKs, the specificity of SU5614 against FLT3 in our study is supported by the following findings: (1) selective cytotoxic activity of SU5614 in Ba/F3 cells and AML blasts expressing an activated FLT3 receptor, but lack of growth inhibition in cells expressing a nonactivated FLT3 or no FLT3 protein, or other constitutively activated tyrosine kinases, including TEL/ABL, BCR/ABL, and TEL/JAK2; (2) absence of expression of other PTKs that are known targets of SU5614 in sensitive cell lines (KIT, VEGFR-2); (3) demonstration of a direct inhibition of the activity of FLT3 and FLT3 target genes by SU5614; and (4) absence of growth inhibitory activity of other PTK inhibitors without activity against FLT3, but with activity against KIT, PDGFR, and VEGFR-2 in SU5614-sensitive cell lines.
Although our data do not completely exclude the possibility that inhibition of other targets by SU5614 might contribute to the growth inhibitory activity of the compound, the evidence presented here strongly supports a specific activity of SU5614 against FLT3. Imatinib mesylate inhibits BCR/ABL, but also has inhibitory activity for other tyrosine kinases including KIT and PDGFβR. It is not known to what extent broader spectrum of specificity of PTK inhibitors might contribute to efficacy.
Using the PTK inhibitor AG1295, Levis et al have shown that the pharmacologic inhibition of FLT3 can induce growth arrest selectively in FLT3-LM expressing primary AML cells.17 The data presented here are in line with these observations and demonstrate that, despite the accumulation of multiple genetic events, AML-derived cell lines expressing an activated FLT3 are dependent on the antiapoptotic and pro-proliferative potential of FLT3. In contrast to the MV4-11 cell line that carries a known FLT3ITD mutation, a new activating point mutation in the JM domain of FLT3 was found in MM1 and MM6 cells. Although the transforming activity in Ba/F3 cells was clearly demonstrated, further studies have to estimate the frequency of this mutation in patients with AML.
The signal transduction of the activated FLT3 receptor in AML blasts has been analyzed in transformed Ba/F3 and 32D cells, but has not been characterized in human AML cells.10 11 By using a specific FLT3 PTK inhibitor in MM6 cells we could show that STAT3, STAT5, and MAPK are direct target genes of the activated FLT3 receptor in human AML cell lines. Although we have not performed direct functional studies to address this topic, several findings presented in this paper support a fundamental role for STAT5 and MAPK as mediators of the transforming potential of FLT3 in AML: (1) STAT5 and MAPK are selectively activated in several FLT3ITD/D835Y-transformed IL-3–dependent cell lines and also in AML cell lines expressing an activated FLT3; (2) the treatment of FLT3-transformed cells with a specific FLT3 PTK inhibitor induces a rapid and sustained down-regulation of STAT5/ERK activity; and (3) the cytotoxic effects of FLT3 PTK inhibitors in Ba/F3 cells and AML cell lines and the inactivation of FLT3-MAPK/STAT5 pathway have very similar IC50 values and time kinetics.
Direct evidence for a transforming capacity of a constitutively active STAT3/STAT5 pathway was provided by previous studies from our own34 and other groups35 showing that constitutive active mutants of STAT3 and STAT5 have a direct transforming potential in IL-3–dependent cell lines and primary mouse bone marrow cells. In contrast, the mitogenic potential of an activated MAPK pathway in AML cells is more controversial. Whereas Ajenjo et al found no effects of MAPK inhibitors on the growth of AML cell lines at all,36 other groups provided evidence for an important antiapoptotic37 function of MAPK in several AML cell lines.
Target genes of STAT5 and STAT3, which mediate their transforming potential include BCL-XL, cyclin D1, and p21.38 Our results clearly indicate that the disruption of the FLT3-STAT3/5 signaling pathway by FLT3 PTK inhibitors results in down-regulation of the STAT5 target genes BCL-XL and p21. In accordance with the observation of Lisovsky et al that BCL-2is a direct target gene of FLT3 in FL-stimulated AML blasts,5 the up-regulation of BCL-XL by active PTK probably plays an important role in the antiapoptotic phenotype in AML. The cell cycle inhibitor p21 induces G0/G1arrest in epithelial cells, but is strongly up-regulated by IL-3 and BCR-ABL in Ba/F3 cells39 and is involved in the resistance of BCR-ABL–expressing cells against cytotoxic agents.40
The findings of a direct cytotoxic activity of FLT3 PTK inhibitors in AML-derived cell lines expressing an activated FLT3 receptor have profound clinical implications. Although the transforming potential of FLT3 has been shown in previous studies, our data clearly indicate that an endogenous activated FLT3 receptor provides a critical and nonredundant pro-proliferative and antiapoptotic signal in AML blasts. These data provide the basis for a therapeutic approach of targeting FLT3 in patients with AML carrying activating FLT3 mutations.
Recently published data have shown that FLT3ITD mutations in the mouse BMT model and the TEL-FLT3 fusion protein in transgenic mice induce a myeloproliferative syndrome.14,15 Although FLT3 mutations alone were not sufficient to induce an AML phenotype, the FLT3-induced phenotype is in good accordance with the in vitro data and supports an important role for the mutant FLT3 receptor to induce an antiapoptotic and pro-proliferative signal. The data presented here provide important additional insights into the function of the activated FLT3 receptor and show that the disruption of the constitutive active FLT3 receptor signaling is sufficient to induce apoptotic cell death in AML cell lines. Our results are in line with very recently published data showing that 3 new PTK inhibitors, CT53518,41 PKC412,42 and CEP-70143 have inhibitory activity against FLT3-transformed cells in vitro and in vivo.
Due to its FLT3-inhibitory activity (IC50 = 150 nM for the FLT3ITD mutant), SU5614 or related PTK inhibitors represent promising compounds for clinical studies in FLT3-LM/TKD-mutation+ AML. In addition, SU5614 inhibits at least 2 other PTKs whose activity is deregulated in AML blasts, KIT, and VEGFR-2 with an IC50 of 70 nM (VEGFR-2) and 200 nM (KIT). Preliminary data from case reports have shown that the pharmacologic inhibition of each of these PTKs by specific inhibitors may also have therapeutic value in AML.44 45 The combined inhibitory activity of SU5614 against the VEGFR-2 and FLT3 in vivo might therefore have a synergistic cytotoxic potential by combining the antiangiogenic activity (VEGFR-2) and the FLT3 inhibitory activity in patients with FLT3-LM/TKD-mutation+ AML.
The authors thank Mrs K. Schmieja for excellent technical assistance and Stefan Bohlander for critical reading of the manuscript. We also thank W. Kern for his support in performing the FACS analyses.
Prepublished online as Blood First Edition Paper, October 24, 2002; DOI 10.1182/blood-2002-04-1045.
Supported by a grant from the Deutsche Krebshilfe 10-1562 and the Deutsche Forschungsgemeinschaft (DFG Sp556/1-3).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Karsten Spiekermann, Department of Internal Medicine III, University Hospital Grosshadern, CCG Leukemia, GSF National Research Center for Environment and Health, Marchioninistrasse 25, 81377 Munich, Germany; e-mail:spiekermann@gsf.de.







This feature is available to Subscribers Only
Sign In or Create an Account Close Modal