We established the molecular basis for pyruvate kinase (PK) deficiency in a white male patient with severe nonspherocytic hemolytic anemia. The paternal allele exhibited the commonPKLR cDNA sequence (c.) 1529G>A mutation, known to be associated with PK deficiency. On the maternal allele, 3 in cis mutations were identified in the erythroid-specific promoter region of the gene: one deletion of thymine −248 and 2 single nucleotide substitutions, nucleotide (nt) −324T>A and nt −83G>C. Analysis of the patient's RNA demonstrated the presence of only the 1529A allele, indicating severely reduced transcription from the allele linked to the mutated promoter region. Transfection of promoter constructs into erythroleukemic K562 cells showed that the most upstream −324T>A and −248delT mutations were nonfunctional polymorphisms. In contrast, the −83G>C mutation strongly reduced promoter activity. Site-directed mutagenesis of the promoter region revealed the presence of a putative regulatory element (PKR-RE1) whose core binding motif, CTCTG, is located between nt −87 and nt −83. Electrophoretic mobility shift assay using K562 nuclear extracts indicated binding of an as-yet-unidentified trans-acting factor. This novel element mediates the effects of factors necessary for regulation of pyruvate kinase gene expression during red cell differentiation and maturation.
Introduction
Pyruvate kinase (PK) catalyzes the final step of glycolysis in which phosphoenolpyruvate is converted to pyruvate with the concomitant generation of adenosine triphosphate (ATP). Pyruvate kinase deficiency is the most common cause of nonspherocytic hemolytic anemia due to defective glycolysis. The consequent lack of sufficient energy, which is required for normal functioning and cellular survival, shortens the life span of the mature PK-deficient erythrocyte. Consequently, PK-deficient patients display a phenotype of nonspherocytic hemolytic anemia albeit with variable clinical severity.1 PK deficiency is transmitted as an autosomal recessive disease and to date, more than 130 mutations inPKLR have been reported to be associated with PK deficiency.2 Most (70%) of these mutations are missense mutations affecting conserved residues in structurally and functionally important domains of PK.
The human gene for liver and red blood cell–specific PK (PKLR) is located on chromosome 1q213 where it directs tissue-specific transcription for both the liver-specific isozymes PK-L and the red blood cell–specific isozyme PK-R4-6 by the use of alternate promoters.7-9Functional analysis of the rat PK erythroid-specific promoter has indicated that nucleotides (nts) from −870 to +54, relative to the cap site, confer erythroid specificity to a reporter gene.10Within this region, a minimal promoter (nts −62 to +54), including a putative −50 CCACC/Sp1 element and a −20 GATA element, displayed erythroid-specific activity.10 Studies on the human promoter have, moreover, indicated that a region from −120 to −270, relative to the translational initiation codon, functions as a powerful enhancer.9 DNA sequence comparison between the rat and human erythroid-specific promoter of PKLR reveals 4 well-conserved elements, indicated in Figure1. Furthermore, 2 CAC boxes and 4 GATA motifs are present within the first 250 bp upstream region.9 So far only one mutation in the PK-R promoter has proven to be associated with PK deficiency—a single base substitution at nt −72 (−72A>G). The down-regulation of expression by this mutated promoter has been attributed to disruption of the consensus binding motif for GATA-1 at nts −69 to −74.11
Partial DNA sequence of the erythroid-specific promoter of PKLR.
A 469-bp region comprising the upstream regulatory domain and exon 1 down to the ATG codon as used in this study. Conserved elements (from Kanno et al9) between the human and rat PK-R promoter are depicted by dotted lines. The cytosine identified as the PK-R transcriptional start site5 is underlined. GATA-1, CAC/Sp1 motifs, and the novel regulatory element PKR-RE1, as reported in this study, in the upstream 270-bp region are shown in boxes (orientation indicated by arrows). The 3 in cis mutations, as identified in our patient, are indicated above their corresponding nucleotides (in capital letters) in the promoter sequence.
Partial DNA sequence of the erythroid-specific promoter of PKLR.
A 469-bp region comprising the upstream regulatory domain and exon 1 down to the ATG codon as used in this study. Conserved elements (from Kanno et al9) between the human and rat PK-R promoter are depicted by dotted lines. The cytosine identified as the PK-R transcriptional start site5 is underlined. GATA-1, CAC/Sp1 motifs, and the novel regulatory element PKR-RE1, as reported in this study, in the upstream 270-bp region are shown in boxes (orientation indicated by arrows). The 3 in cis mutations, as identified in our patient, are indicated above their corresponding nucleotides (in capital letters) in the promoter sequence.
Previously, we reported 2 in cis mutations in a severely PK-deficient Danish patient.12 We now report on the functional analysis of these mutations and show that the most proximal one, −83G>C, constitutes part of a core binding motif of a novel regulatory element (PKR-RE1) in the erythroid-specific promoter of PKLR, in close proximity to a regulatory GATA-1 binding site.
Patient, materials, and methods
The patient is a 6-year-old Danish boy who has suffered from severe, transfusion-dependent hemolytic anemia since birth. PK deficiency was diagnosed at the age of 1 year. Because of the continuous presence of transfused donor erythrocytes, we used a density gradient to separate reticulocytes from mature erythrocytes, in order to obtain an as-representative-as-possible patient-specific red cell population.13 PK activity and activity measurements of the red blood cell age-related enzymes, glucose-6-phosphate dehydrogenase (G6PD) and hexokinase (HK), were determined according to standardized procedures.14
DNA sequence analysis of PKLR
The coding region of PKLR was amplified using primers as previously described.15 Additional primers were used for part of the putative erythroid-specific promoter (GenBank accession number AB015984D13232) and 3′-untranslated region (3′-UTR; GenBank accession number D13243). Sense primer PKRP-ESF 5′-AGGTTACAGAGTGGTGAAGGC-3′ (nts −469 to −449, relative to the start codon) and antisense primer PKRP-ESR 5′-GCTTTCAGTGTGGGCCTGG-3′ (nts −20 to −1) amplify a 469-bp region immediately upstream of the initiator methionine. Sense primer PKRU-F 5′-TCTACGTTCTCCAGCCCACAC-3′ (nts +58 to +78, relative to the termination codon) and antisense primer PKRU-R 5′-GAGTGGGAAGGAATTTCTGGG-3′ (nts +689 to +669) amplify a 669-bp region of the 3′-UTR. Polymerase chain reactions (PCRs) were carried out with 200 ng DNA in 50-μL volumes containing 10 mM Tris-HCl, pH 8.3, 50 mM KCl, 1.5 mM MgCl2, 0.01% (wt/vol) gelatin, 0.2 mM of each deoxynucleotide triphosphate (dNTP), 0.3 μM of each primer, and 2.5 U AmpliTaq Gold DNA polymerase. All reagents were obtained from Applied Biosystems (Roche Molecular Systems, Branchburg, NJ). After initial incubation for 10 minutes at 95°C, samples were subjected to 35 cycles of amplification with denaturation at 94°C for 30 seconds, annealing for 30 seconds at 64°C (60°C for exon 9 and 3′-UTR) and extension at 72°C for 45 seconds, followed by an elongated extension time of 10 minutes after the last cycle. Automated DNA sequence analysis was performed with the ABI Prism dRhodamine Dye Terminator Cycle Sequencing Ready Reaction Kit (Applied Biosystems, Foster City, CA), according to the manufacturer's instructions. Sequencing reactions were all carried out in forward and reverse direction, and samples were analyzed on an Applied Biosystems ABI 310 Genetic Analyzer (Applied Biosystems). The PCR products were purified prior to DNA sequence analysis using the QIAquick PCR purification kit (Qiagen, Valencia, CA).
Restriction enzyme analysis
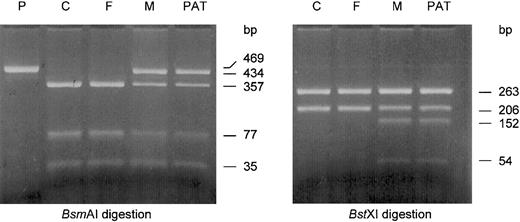
Mutations were confirmed by restriction enzyme analysis on newly amplified PCR product. The cDNA sequence (c.) 1529G>A mutation in exon 11 was confirmed by StyI digestion as described.15 The 2 promoter mutations, −324T>A and −83G>C, were confirmed by digestion of the PCR product withBstXI and BsmAI, respectively. The 469-bp PCR product from the wild-type allele, as amplified with PKRP-ESF and PKRP-ESR, contains one recognition site for BstXI that yields fragments of 206 bp and 263 bp after digestion. The −324T>A mutation creates a second recognition sequence for this enzyme, resulting in additional fragments of 152 bp and 54 bp. The −83G>C mutation abolishes one of 2 recognition sequences for BsmAI normally present in this PCR product. Consequently, fragments of 35 bp and 434 bp are produced after digestion of the mutant allele withBsmAI, whereas 35-bp, 357-bp, and 77-bp fragments are the result of digestion of the wild-type allele with this restriction enzyme. All enzymes were purchased from New England Biolabs, Beverly, MA.
Reverse transcription–PCR
Reverse transcription–PCR (RT-PCR) to detect the c.1529G>A mutation was performed using the GeneAmp RNA PCR Core Kit from Applied Biosystems (Roche Molecular Systems) according to the instructions of the manufacturer. Briefly, 1.0 μg of the patient's reticulocyte RNA was reverse transcribed using random hexamers as primers. After addition of 30 pmol of primers CDPK-11 (5′-CTCAGCCCAGCTTCTGTCTCG-3′, exon 11 nts 1437 to 1457) and PKr-6 (5′-GTGTGGGCTGGAGAACGTAGA-3′, exon 12 nts +78 to +58), the samples were subjected to 35 cycles of amplification with denaturation at 94°C for 30 seconds (5 minutes at 95°C prior to the first cycle), annealing at 58°C for 30 seconds, and extension at 72°C for 30 seconds, followed by an elongated extension time of 10 minutes after the last cycle. Total liver RNA was used as a positive control and controls without RNA as well as controls in which the reverse transcription step was omitted were included.
Allelic frequency determination
Allelic frequency of −248delT and population evidence regarding its physiologic effect were obtained by screening the DNA of 241 anonymized white control subjects and heterozygotes for the c.1529G>A mutation, respectively, for the −248delT mutation by allele-specific oligonucleotide hybridization (ASOH). Genomic DNA (100 ng) was amplified in the region of nt −248T in the PK-R promoter by PCR. The 25-μL system contained 34 mM Tris-HCl, pH 8.8, 8.3 mM NH4SO4, 1.5 mM MgCl2, 85 g/mL bovine serum albumin, 0.2 mM of each dNTP, 100 ng of sense (5′-CTCCCTGGATTCACTAGAGC-3′, nts −322 to −303) and antisense (5′-AGGATGGACTTTGCTAAGT-3′, nts 65 to 83) primers, and 1.5 UTaq DNA polymerase. After a 5-minute denaturation step at 98°C, 30 cycles of 93°C for 30 seconds, 58°C for 30 seconds, and 72°C for 30 seconds were performed followed by a 7-minute 72°C incubation. The 405-bp PCR product (4 μL) was then spotted on Nytran SuPerCharge membranes (Schleicher and Schuell, Keene, NH). The membranes were denatured, neutralized, and UV-crosslinked prior to hybridization. The membranes were hybridized with wild-type (5′-AAATATCTATTCACGTG-3′) and mutant (5′-AAAAATCTATTCACGTG-3′)32P-labeled oligonucleotide probes for the −248T position of the PK-R promoter. After hybridization the membranes were washed in 6 × SSC, 0.1% sodium dodecyl sulfate (SDS) at 50°C and developed with a Cyclone storage phosphor autoradiography system (Packard Instrument, Meriden, CT).
Promoter constructs and site-directed mutagenesis
The human PK promoter constructs from the patient and healthy controls were generated from a 469-bp PCR fragment comprising the upstream regulatory domain and exon 1, down to the ATG codon (Figure 1). The blunt-end PCR fragment was cloned into the pCR-Blunt vector (Invitrogen, Paisley, United Kingdom) before it was excised and inserted into the MluI and XhoI sites of pGL3-Basic (Promega, Madison, WI). Site-directed mutagenesis was performed with splicing by overlap extension as described.16 Using PK-R promoter reporter plasmid pGL3_PKRWT as the wild-type template, we generated the following mutants: pGL3_PKR91A (nt −91T>A), pGL3_PKR90T (nt −90C>T), pGL3_PKR89G (nt −89T>G), pGL3_PKR88G (nt −88T>G), pGL3_PKR87A (nt −87C>A), pGL3_PKR86G (nt −86T>G), pGL3_PKR85A (nt −85C>A), pGL3_PKR84G (nt −84T>G), pGL3_PKR83C (nt −83G>C), pGL3_PKR82G (nt −82T>G), pGL3_PKR81A (nt −81C>A), pGL3_PKR80G (nt −80T>G), pGL3_PKR79T (nt −79C>T) and pGL3_PKR78T (nt −78C>T). Briefly, 2 PCR products were generated that harbored the desired mutation using primers PKRP-ESF and PKRP-ESR in combination with the applicable mutant antisense primers and sense primers, respectively (primer sequences are available on request). Fragments were electrophoresed and purified from the agarose gel using the QIAquick Gel Extraction Kit (Qiagen). Subsequently, for each mutant promoter construct 12.5 μL of each of both fragments obtained by the first PCR reaction were combined and subjected to a second round of amplification with primers PKRP-ESF and PKRP-ESR. Finally, the blunt-end mutated PCR fragment was cloned into the pCR-Blunt vector (Invitrogen), and subcloned into the XhoI and MluI sites of the pGL3-Basic vector, as described above. All constructs were verified by DNA sequence analysis. There were 3 additional mutant promoter constructs prepared as described that harbored the −248delT polymorphism (pGL3_PKR248delT), the −324T>A mutation (pGL3_PKR324A) and, by using the patient's DNA as a template, both the −324T>A and −83G>C mutations in cis (pGL3_PKR324A/83C).
Cell culture and transient DNA transfections
K562 cells were cultured in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal calf serum, 1% streptomycin, and 1% penicillin in 10% CO2 at 37°C. Cells were transiently transfected with Lipofectamine (Life Technologies, Paisley, United Kingdom) according to the manufacturer's instructions. Briefly, 30 000 cells/cm2 were seeded in 24-well plates 24 hours prior to transfection. The cells were transfected with 2 μg reporter plasmid DNA and 100 ng RL-SV40 plasmid (Promega) that was used as internal control. After 48 hours, luciferase activity was measured with the Dual Luciferase Assay kit (Promega) and normalized to renilla luciferase activity. The promoterless pGL3-Basic Luciferase Reporter Vector (Promega) was used as a negative control.
Electrophoretic mobility shift assays
Electrophoretic mobility shift assays (EMSAs) were performed essentially as described17 with K562 nuclear extracts, prepared according to Dignam et al.18 Wild-type and mutant double-stranded oligonucleotide probes were obtained by annealing the following single-stranded primers: PKWT, sense 5′-TTCTCTTCTCTGTCTCCCTT-3′ and antisense 5′AAGGGAGACAGAGAAGAGAA-3′; and PKmut, sense 5′-TTCTCTTCTCgGTCTCCCTT-3′ and antisense 5′-AAGGGAGACcGAGAAGAGAA-3′, respectively. PKmut contains the −84T>G mutation (in lower case). Competitors (excess of unlabeled probe oligonucleotide or corresponding mutant oligonucleotide) were included as described in the figure legends.
Results
Glycolytic enzyme activities
The results from the measurement of peripheral blood glycolytic enzyme activities in the patient and his parents are summarized in Table 1. In the patient, PK activity was only just below the lower reference value, whereas the HK and G6PD values were high, indicating that the red cell population was relatively young. Consequently, we interpreted the PK activity as too low. To exclude the interference of donor cells, we isolated the low-density, reticulocyte-rich fraction of the patient by Percoll-density centrifugation. Subsequent glycolytic enzyme activity measurements in this fraction showed an even lower PK activity. In contrast, the G6PD and HK activity remained unaltered, thus underscoring the presence of PK deficiency in the patient's red blood cells. The PK activity measured in peripheral blood of the father was normal, whereas the erythrocyte PK activity of the mother was low, relative to that of G6PD and HK.
Glycolytic enzyme activities in the patient and his parents
| . | PK (U/gHb) . | G6PD (U/gHb) . | HK (U/gHb) . |
|---|---|---|---|
| Peripheral blood | |||
| Control | 8.4-14.4 | 9.5-15.0 | 1.05-1.81 |
| Patient | 7.1 | 21.4 | 4.30 |
| Father | 14.2 | 21.0 | 1.90 |
| Mother | 8.3 | 18.0 | 2.30 |
| Reticulocyte-rich fraction | |||
| Patient | 3.8 | 21.7 | 4.22 |
| . | PK (U/gHb) . | G6PD (U/gHb) . | HK (U/gHb) . |
|---|---|---|---|
| Peripheral blood | |||
| Control | 8.4-14.4 | 9.5-15.0 | 1.05-1.81 |
| Patient | 7.1 | 21.4 | 4.30 |
| Father | 14.2 | 21.0 | 1.90 |
| Mother | 8.3 | 18.0 | 2.30 |
| Reticulocyte-rich fraction | |||
| Patient | 3.8 | 21.7 | 4.22 |
DNA sequence analysis of PKLR
By DNA sequence analysis of PKLR, the patient was found to be heterozygous for the common c.1529G>A mutation in exon 11 (Figure 2A). This mutation was confirmed by StyI digestion and subsequent restriction enzyme analysis of his parents revealed that the patient had inherited this allele from his father (data not shown). Apart from heterozygosity for the well-established polymorphisms2c.1705A>C, c.1738C>T, and c.1992T>C in exon 12, no other mutations were detected in PKLR exons and splice junctions. However, 3 previously undescribed base alterations were indentified in the PK-R promoter compared with the healthy control individual. Of these, 2 were single nucleotide substitutions of, respectively, thymine to adenine at nt −324 (−324T>A) and guanine to cytosine at nt −83 (−83G>C). Both mutations were confirmed in the patient byBstXI and BsmAI digestion, respectively, and also found to be present in the patient's mother, whereas they were absent in the patient's father (Figure 3), thereby demonstrating that both mutations were present in cis. Neither allele was detected in a healthy control population (n = 100). A third sequence variation was observed around nt −248 in both the patient and his mother but not in the patient's father and the control. Its characterization, however, was hampered because of an apparent concomitant variation in the number of adenines between nts −249 and −258 in all subjects. Since the latter was likely to be a PCR artifact due to slippage of Taq DNA polymerase at this homonucleotide run,19 we cloned this promoter fragment and characterized its DNA sequence context (see “Polymorphic deletion of thymine −248”).
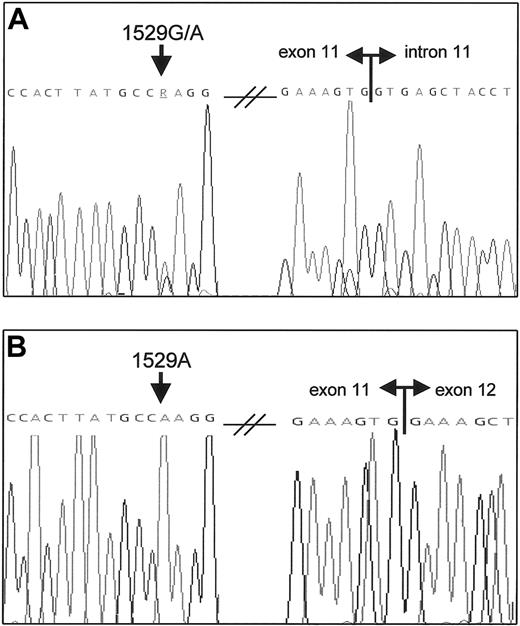
Heterozygous PKLR c.1529G>A missense mutation and sole expression of the 1529A allele in the patient.
(A) PKLR DNA sequence analysis of exon 11 in the patient shows a heterozygous G>A substitution (arrow). The splice donor site of intron 11 is indicated. (B) RT-PCR analysis of the patient's RNA yielded only one transcript that contained the 1529A mutation (arrow), thereby indicating a severely reduced transcription of the in trans 1529G allele. Double horizontal arrows denote boundaries between exons.
Heterozygous PKLR c.1529G>A missense mutation and sole expression of the 1529A allele in the patient.
(A) PKLR DNA sequence analysis of exon 11 in the patient shows a heterozygous G>A substitution (arrow). The splice donor site of intron 11 is indicated. (B) RT-PCR analysis of the patient's RNA yielded only one transcript that contained the 1529A mutation (arrow), thereby indicating a severely reduced transcription of the in trans 1529G allele. Double horizontal arrows denote boundaries between exons.
Heterozygosity for 2 novel mutations in the PK-R promoter at nt −83 (−83G>C) and nt −324 (−324T>A) in the patient and his mother.
A 469-bp fragment was amplified as described and subjected to restriction enzyme digestion. The obtained pattern for each reaction is indicated by arrows (see “Patient, materials, and methods”). Heterozygosity for both the −83G>C mutation (BsmAI digestion) and the −324T>A mutation (BstXI digestion) was confirmed in the patient (PAT) and also detected in his mother (M), whereas they were absent in the patient's father (F). P indicates uncut PCR product; and C, healthy control.
Heterozygosity for 2 novel mutations in the PK-R promoter at nt −83 (−83G>C) and nt −324 (−324T>A) in the patient and his mother.
A 469-bp fragment was amplified as described and subjected to restriction enzyme digestion. The obtained pattern for each reaction is indicated by arrows (see “Patient, materials, and methods”). Heterozygosity for both the −83G>C mutation (BsmAI digestion) and the −324T>A mutation (BstXI digestion) was confirmed in the patient (PAT) and also detected in his mother (M), whereas they were absent in the patient's father (F). P indicates uncut PCR product; and C, healthy control.
Polymorphic deletion of thymine −248
DNA sequence analysis of a number of cloned promoter fragments from the patient and the control confirmed the presence of the −324T>A and −83G>C mutations in cis in the patient. The third mutation in the patient constituted the deletion of thymine −248 (−248delT).
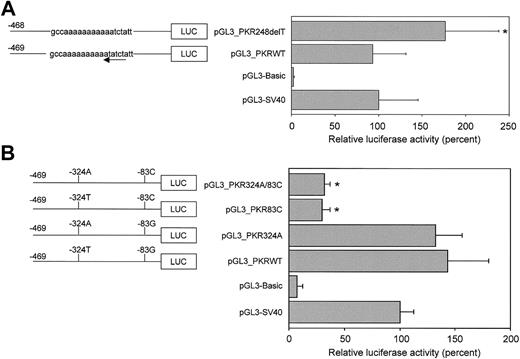
Since nt −248T constitutes part of an inverted consensus binding site for GATA-1, (A/T)GATA(A/G),20 the −248delT mutation potentially disrupts binding of GATA-1 (Figure 1). Therefore, constructs containing the wild-type (pGL3_PKRWT) and the −248delT polymorphic allele (pGL3_PKR248delT) were transiently transfected in K562 cells to determine the effect of the −248delT mutation on promoter activity. A comparison between the 2 alleles showed a statistically significant (P < .05) increase in promoter activity upon −248delT (Figure 4A).
The −83G>C mutation in the PK-R promoter strongly down-regulates promoter activity in vitro.
Luciferase reporter gene constructs containing 469 bp of the wild-type or mutated PK-R promoter were transiently transfected in K562 erythroleukemic cells. Luciferase activities were expressed relative to control pGL3-SV40 and pGL3-Basic was included as a negative (promoterless) control. (A) Constructs pGL3_PKRWT and pGL3_PKR248delT contained the wild-type or polymorphic −248delT allele, respectively. The latter mutation disrupts an inverted GATA-1 binding site (arrow) but no down-regulation of promoter strength is observed. In contrast, an increase in promoter activity was observed upon removal of thymine −248. (B) Individual and combined effects of the −83G>C and −324T>A missense mutations were studied using constructs that contained only the −83G>C mutation (pGL3_PKR83C) or the −324T>A mutation (pGL3_PKR324A), or both mutations in cis (pGL3_PKR324A/83C). The −324T>A mutation had no effect on promoter activity as compared with the wild-type (pGL3_PKRWT). In contrast, the −83G>C mutation is capable of strongly reducing in vitro promoter activity and is unaffected by the concomitant presence of the −324T>A substitution in cis. * Statistically significant (P < .05).
The −83G>C mutation in the PK-R promoter strongly down-regulates promoter activity in vitro.
Luciferase reporter gene constructs containing 469 bp of the wild-type or mutated PK-R promoter were transiently transfected in K562 erythroleukemic cells. Luciferase activities were expressed relative to control pGL3-SV40 and pGL3-Basic was included as a negative (promoterless) control. (A) Constructs pGL3_PKRWT and pGL3_PKR248delT contained the wild-type or polymorphic −248delT allele, respectively. The latter mutation disrupts an inverted GATA-1 binding site (arrow) but no down-regulation of promoter strength is observed. In contrast, an increase in promoter activity was observed upon removal of thymine −248. (B) Individual and combined effects of the −83G>C and −324T>A missense mutations were studied using constructs that contained only the −83G>C mutation (pGL3_PKR83C) or the −324T>A mutation (pGL3_PKR324A), or both mutations in cis (pGL3_PKR324A/83C). The −324T>A mutation had no effect on promoter activity as compared with the wild-type (pGL3_PKRWT). In contrast, the −83G>C mutation is capable of strongly reducing in vitro promoter activity and is unaffected by the concomitant presence of the −324T>A substitution in cis. * Statistically significant (P < .05).
In vivo evidence regarding the functional consequences on transcriptional activity of the −248delT deletion was obtained from a study of carriers of c.1529G>A and −248delT in trans. If the −248T mutation abolished or severely impaired transcription, such compound heterozygous patients should be PK deficient and anemic or at least have macrocytosis because of increased erythropoiesis. In a previous study,21 several thousand DNA samples from a general population were screened for the c.1529G>A mutation and 11 heterozygotes were detected. Among these, 4 individuals also carried the −248delT mutation. Since 37 of 37 c.1529G>A alleles carried the wild-type promoter (data not shown), it is reasonable to assume that these individuals were compound heterozygotes for c.1529G>A and −248delT. If the −248delT mutation prevented transcription, then the patient should be PK deficient. Enzyme activities were not available, but we compared the average hemoglobin of 3 female compound heterozygotes (12.6 g/dL), which was no different than the 12.1 g/dL of the 3 female single heterozygotes for c.1529G>A. The male patient had a hemoglobin level of 15.2 g/dL. All had normal mean corpuscular volume (MCV) values. We infer that the −248delT mutation is a benign polymorphism and is not associated with reduced promoter activity and, consequently, with PK deficiency. Subsequent determination of the allelic frequency of this mutation was performed by screening 241 control subjects of a general white population for this mutation by allele-specific oligonucleotide hybridization. There were 206 wild-type subjects (−248T/T), 34 heterozygous subjects (−248T/delT) and one homozygous control (−248delT/delT). Consequently, the allelic frequency for this novel polymorphism in the PK-R promoter is 0.075.
Functional characterization of nt −324T>A and nt −83G>C promoter mutations
To assess the functional consequences of the mutated PK-R promoter region, we first performed RT-PCR analysis on the patient's RNA with primers spanning exons 11 and 12 to determine the relative expression of the 2 alleles. As shown in Figure 2B, only the 1529A allele could be detected in the patient, strongly indicating that transcription is severely reduced by the mutated promoter.
To study the individual and combined effects of the −324T>A and −83G>C mutations on PK-R promoter activity, we transfected constructs pGL3_PKRWT (wild-type), pGL3_PKR324A (nt −324T>A), pGL3_PKR83C (nt −83G>C), and pGL3_PKR324A/83C (nt −324T>A and nt −83G>C in cis) in K562 cells and compared their relative luciferase activities. Figure 4B shows that the −83G>C substitution is capable of down-regulating promoter activity (pGL3_PKR83C and pGL3_PKR324A/83C). This effect was achieved either with (pGL3_PKR324A/83C) or without (pGL3_PKR83C) the concomitant presence of the −324T>A mutation. In contrast, promoter activity was not altered, with regard to the wild-type promoter, in case only the −324T>A mutation was present (pGL3_PKR324A). We infer that the −83G>C mutation disrupts transcription.
Delineation of a putative regulatory element comprising the nt −83G>C mutation
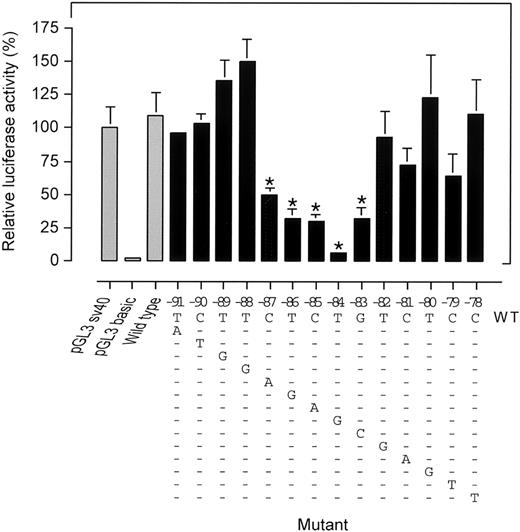
Since the proximal erythroid-specific promoter region ofPKLR previously was reported to dictate basal promoter activity,9 we hypothesized nt −83 to be part of a previously unrecognized trans-acting factor binding element. To unravel the sequence of the putative cis-element we generated a series of consecutive promoter mutants from nts −91 to −78 and determined their activity in K562 cells. Figure5 shows that substitution of nts −87 to −83 leads to a decreased promoter activity. In particular, the −84T>G mutation exhibited a profound reduction in transcription. Substitutions further upstream (nts −88 to −91) or downstream (nts −82 to −78) did not significantly alter promoter activity. Thus, we defined the existence of a novel regulatory element in the PK-R promoter PKR-RE1 whose core binding motif is confined to nts −87 to −83.
Site-directed mutagenesis of a region of the PK-R promoter spanning nts −91 to −78 identifies the core motif CTCTG of a novel PK-R regulatory element (PKR-RE1).
PK-R promoter reporter gene constructs harboring mutations from nts −91 to −78 were expressed in K562 cells. The applicable mutation is depicted in the lower part of the figure. Luciferase activities were calculated relative to control pGL3-SV40. pGL3-Basic was included as a negative (promoterless) control. The decreased promoter activity as a result of the introduced mutations at nts −87 to −83 revealed a single pentanucleotide motif CTCTG. * Statistically significant (P < .05).
Site-directed mutagenesis of a region of the PK-R promoter spanning nts −91 to −78 identifies the core motif CTCTG of a novel PK-R regulatory element (PKR-RE1).
PK-R promoter reporter gene constructs harboring mutations from nts −91 to −78 were expressed in K562 cells. The applicable mutation is depicted in the lower part of the figure. Luciferase activities were calculated relative to control pGL3-SV40. pGL3-Basic was included as a negative (promoterless) control. The decreased promoter activity as a result of the introduced mutations at nts −87 to −83 revealed a single pentanucleotide motif CTCTG. * Statistically significant (P < .05).
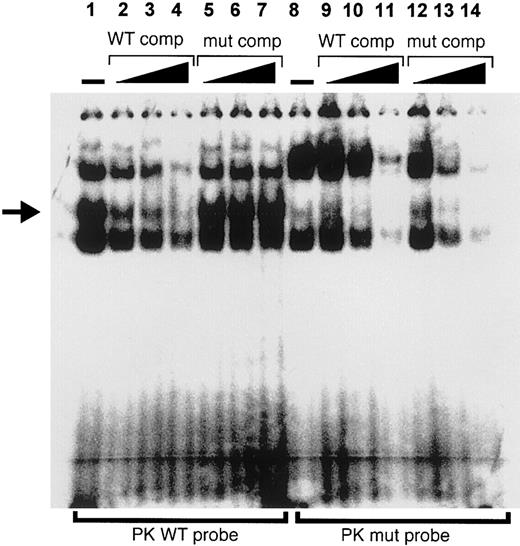
To further explore the involvement of PKR-RE1 in binding trans-acting factors, we performed EMSA with K562 nuclear extract and oligonucleotide probes designed according to the native core binding motif (PKWT) and mutated PKR-RE1 (PKmut; −84T>G). Figure6 demonstrates the formation of 3 distinct DNA-protein complexes upon incubation with labeled PKWT (lane 1). One of these bands (arrow) could be competed off successfully by increasing amounts of excess unlabeled PKWT (lanes 2-4), but remained unaffected by the addition of increasing amounts of excess unlabeled PKmut as a competitor (lanes 5-7), thereby establishing its specificity. This band was absent when lysates were incubated with radiolabeled PKmut (lane 8), thereby suggesting that the particular protein-DNA interaction was abolished in case PKR-RE1 was disrupted. Instead, another band appeared that could, however, be competed off successfully by increasing amounts of both unlabeled PKWT and PKmut (lanes 9-11 and 12-14, respectively). We infer that the −83C is part of a putative trans-acting factor binding element, characterized by a CTCTG core motif.
PKR-RE1 is involved in DNA-protein interaction.
Electrophoretic mobility shift assay was performed with K562 nuclear extract and oligonucleotide probes designed according to the native core binding motif (PKWT; lanes 1-7) and mutated PKR-RE1 (PKmut; lanes 8-14). Absence (−) and increasing amounts of unlabeled wild-type and mutant competitor are indicated. The figure shows 3 distinct DNA-protein complexes upon incubation with labeled PKWT (lane 1). One of these bands (arrow) could be competed off successfully by increasing amounts of excess unlabeled PKWT (lanes 2-4), but remained unaffected by the addition of increasing amounts of excess unlabeled PKmut as a competitor (lanes 5-7). In contrast, this band was absent when lysates were incubated with radiolabeled PKmut (lane 8), whereas the extra band that appeared upon incubation with PKmut could be competed off succesfully by increasing amounts of both unlabeled PKWT and PKmut (lanes 9-11 and 12-14, respectively).
PKR-RE1 is involved in DNA-protein interaction.
Electrophoretic mobility shift assay was performed with K562 nuclear extract and oligonucleotide probes designed according to the native core binding motif (PKWT; lanes 1-7) and mutated PKR-RE1 (PKmut; lanes 8-14). Absence (−) and increasing amounts of unlabeled wild-type and mutant competitor are indicated. The figure shows 3 distinct DNA-protein complexes upon incubation with labeled PKWT (lane 1). One of these bands (arrow) could be competed off successfully by increasing amounts of excess unlabeled PKWT (lanes 2-4), but remained unaffected by the addition of increasing amounts of excess unlabeled PKmut as a competitor (lanes 5-7). In contrast, this band was absent when lysates were incubated with radiolabeled PKmut (lane 8), whereas the extra band that appeared upon incubation with PKmut could be competed off succesfully by increasing amounts of both unlabeled PKWT and PKmut (lanes 9-11 and 12-14, respectively).
Discussion
We established the molecular basis for PK deficiency in a 6-year-old boy of Danish ancestry who suffered from severe hemolytic anemia. On the paternal allele of this patient we detected a guanine-to-adenine substitution at nt 1529 in PKLR. The Arg510Gln encoded by this frequently occurring mutation and the consequent structural changes in PK that lead to PK deficiency have been well documented.15 22 The fact that the father had normal PK activity (Table 1), in spite of being heterozygous, emphasizes the difficulty of accurately identifying heterozygotes based on enzyme activity alone. On the maternal allele of the patient, we detected 3 novel mutations in the erythroid-specific promoter ofPKLR. Two of these mutations were single-base substitutions, −324T>A and −83G>C, relative to the initiator adenine. The third change consisted of the deletion of thymine at nt −248.
RT-PCR analysis of the patient's RNA demonstrated sole expression of the 1529A allele (Figure 2B). Therefore, it was conceivable that the mutated promoter caused effective down-regulation of transcription from the affected allele. Analysis of the various promoter mutations in K562 cells showed that −83G>C alone was capable of inducing a drastically reduced promoter activity in vitro, whereas the −324T>A and −248delT mutations exerted no such effect (Figure 4). The latter mutation represents a nonfunctional polymorphic substitution (allele frequency 0.075), whereas −324T>A is a nonfunctional mutation.
In vivo evidence regarding the lack of functional consequences on transcriptional activity of the −248delT deletion came from a study among individuals who were compound heterozygous for the c.1529G>A and −248delT in trans. If transcription was hampered by the −248delT mutation, such patients should be PK deficient and anemic or at least have macrocytosis because of increased erythropoiesis. However, the average hemoglobin level of 3 female compound heterozygotes (12.6 g/dL) showed no differences when compared with the average hemoglobin level of 3 female single heterozygotes for c.1529G>A (12.1 g/dL). One male patient had a hemoglobin level of 15.2 g/dL and all individuals had normal MCV values.
Because −248delT disrupts the GATA-1 binding motif at nts −244 to −249, we also investigated whether this mutation was able to influence promoter activity in vitro. Luciferase activities reflecting the relative promoter strength of these constructs showed no decline in promoter activity as a result of −248delT (Figure 4A). As this would have been suggestive of a functional stimulatory GATA-1 binding site in vitro, it is unlikely that the latter is the case. Thus, both in vitro and in vivo evidence support the presence of a nonfunctional GATA-1 binding site at nts −244 to −249 in the erythroid-specific promoter of PKLR. Interestingly, we found that the wild-type allele conferred an approximately 2-fold increase in promoter activity upon deletion of thymine −248 (Figure 4A). Although the effect is relatively small, it is possible that −248delT modulates the phenotypic expression of PK deficiency, as previously shown for a polymorphic dinucleotide repeat at the UDP-glucuronosyltransferase 1 promoter23 that contributes to the clinical phenotype in G6PD-deficient neonates.24
Only one mutation in PKLR is known to date that is associated with a reduced transcription from its erythroid-specific promoter and consequent quantitative reduction in PK-R. A markedly reduced amount of PK-R mRNA was detected, 20% by semiquantitative RT-PCR analysis, as a result of a single nucleotide substitution at nt −72.11 The involvement of GATA-1 was presumed since the mutation was located in the core of the binding motif of this erythroid-specific transcription factor.20,25,26Initially, a −249delA mutation in the PK-R promoter was reported in association with PK deficiency.27 However, reinvestigation of the patient's DNA revealed the presence of the same 3 in cis mutations we report here.27 Kugler has allowed us to examine a DNA sample from his patient, and we have confirmed his results (data not shown).
Most current data on the function of the PKLR gene indicate that the proximal promotor region is essential for transcriptional initiation. The distal region upstream position −300, where nt −324T is located, appears to be dispensable for transcriptional regulation as shown by Kanno et al, who found that removal of nts −513 to −304 had no effect on promoter activity.9 In the same study, the proximal 120 bp, containing 2 CAC boxes and one GATA binding motif, were shown to direct a basal promoter activity, whereas the 150 bp upstream acted as an enhancer.9 From studies on the rat PK-R promoter, this region was also found to be functionally important.10,28,29 Lacronique et al demonstrated that a proximal 320-bp PK-R promoter fragment is able to direct erythroid-specific transcription in a rat fetal liver cell-free transcription system and interacts both in vitro and in vivo in an erythroid-specific manner with GATA-1, CACC-binding proteins, and unidentified factors recognizing G/C-rich motifs.28Furthermore, Max-Audit et al demonstrated the specific binding of GATA-1 and members of the CACC/Sp1 family to the proximal GATA binding site of a minimal promoter spanning nts −62 to +54.10GATA-1 and Sp1, 2 factors known to physically interact,30have also been shown to cooperatively activate transcription from a minimal (nts −62 to +43) rat PK-R promoter in Drosophila S2 cells.29
Because of the effect of the −83G>C mutation and its location in a well-conserved region between rat and humans (Figure 1), we anticipated that nt −83G was an essential part of a trans-acting factor binding element. Systematic mutagenesis of the region disclosed the presence of a cis-element, whose core CTCTG extends from nts −87 to −83 (Figure 5). We designated this novel regulatory element in the PK-R promoter, PKR-RE1. Subsequent EMSA using K562 nuclear extract demonstrated in vitro DNA-protein interaction at the core of PKR-RE1 involving an as-yet-unidentified protein (Figure 6). In agreement with these data, Lacronique et al previously showed by in vitro DNAse I footprinting that the corresponding conserved sequence in the rat PK-R promoter encompassing nts −64 to −58 was involved in DNA-protein interaction.28 In gel shift assays, the labeled probe containing the CTCTG sequence and spanning nts −80 to −57 formed 2 retarded complexes which, on the basis of mobility shift assays, were suggested to correspond to GATA-1–containing complexes, although GATA-1 later proved to bind more upstream.28 Since the CTCTG motif is located just 10 bp upstream of the GATA-1 binding site, an appealing model would be that GATA-1 formed a complex with the trans-acting factor binding to PKR-RE1, similar to the previously described erythroid ternary complex between GATA-1, the helix-loop-helix factor TAL1, and the bridging LIM-only protein Lmo2.31 Although PKR-RE1 does not constitute an E-box, we tested whether GATA-1– and FOG-mediated trans activation of the PK-R promotor was affected by the −83G>C mutation, but have so far not observed any difference between the wild-type sequence and the mutant (R.v.W., W.W.v.S., C.N., and F.C.N., unpublished results, November 2000). Based on current knowledge, we therefore propose that the PKR-RE1 functions independently of GATA-1.
Since the CTCTG motif resembles no known transcription factor elements,32 PKR-RE1 may be involved in a novel mechanism of erythroid-specific trans activation. Future identification of the putative trans-acting factor(s) may provide important leads to our understanding of erythroid-specific transcriptional regulation involved in red cell differentiation and maturation.
The authors are most grateful for the technical assistance of Joan Christiansen and Annet van Wesel and want to express their thanks to Karen de Vooght for helpful discussion of the manuscript.
Prepublished online as Blood First Edition Paper, September 26, 2002; DOI 10.1182/blood-2002-07-2321.
Supported in part by National Institutes of Health grants HL25552 and RR00833, the Toyota Foundation, the Danish Research Counsil, and the NOVO-Nordisk Foundation.
R.v.W. and W.W.v.S. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Wouter W. van Solinge, Department of Clinical Chemistry, G03.550 University Medical Center, Postbus 85500, 3508 GA, Utrecht, The Netherlands; e-mail:wsolinge@lab.azu.nl.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal