Preconditioning with the nonmyeloablative regimen total lymphoid irradiation (TLI) before hematopoietic cell transplantation facilitates the establishment of mixed chimerism and protects against graft-versus-host disease. We reported that the development of mixed chimerism requires interleukin (IL)–4 and is associated with increased host anti-donor TH2 cells, but the effect of TLI on the differentiation of immunocompetent donor cells has not been investigated. To examine the extent to which TLI preconditioning influences donor T cells, we measured responses of transgenic CD4+ cells specific for ovalbumin peptide (OVA-Tg) following in vivo and in vitro antigen stimulation in a TLI-preconditioned environment. OVA-Tg cells that were adoptively transferred into TLI-preconditioned mice that express cross-reactive antigens produced more IL-4 and less interferon-γ and IL-2 than controls when stimulated with OVA peptide one week later. OVA-Tg primed in vitro with spleen from TLI-preconditioned mice generated more TH2 and fewer TH1 cells when stimulated in recall enzyme-linked immunosorbent spot (ELISPOT) assays with OVA peptide. Naive OVA-Tg up-regulated CD69 and CD25 normally following stimulation with OVA peptide in the presence of spleen from TLI-preconditioned mice, but proliferated less and secreted less IL-2 than controls. Surprisingly, naive OVA-Tg secreted IL-4 in primary cultures that were stimulated with OVA peptide in the presence of spleen from TLI-preconditioned mice. This response depends on CD4+ cells from TLI-spleen, which constitutively produce IL-4 and are composed primarily of CD4+–natural killer T (TNK) cells. Thus, TLI preconditioning enriches for IL-4–secreting and TNK-like CD4+ cells that may function in the protection from graft-versus-host disease by redirecting the differentiation of immunocompetent donor CD4+ cells toward TH2 and away from pathogenic TH1 cells.
Introduction
Total lymphoid irradiation (TLI)–preconditioned mice accept major histocompatibility complex (MHC)–disparate hematopoietic cell or bone marrow grafts if cells are given shortly after the completion of TLI treatment,1-3 but not at all if cell transfer is delayed beyond 7 days. When undergoing transplantation within this window, mice develop a state of bidirectional tolerance, which permits the engraftment of donor cells leading to stable mixed chimerism and inhibits the development of graft-versus-host disease (GVHD).4 5 This bidirectional tolerance can be achieved and maintained in the long term without the use of immunosuppressive therapy. Because TLI preconditioning combined with hematopoietic cell transplantation offers the potential for long-lived donor-specific tolerance and protection from GVHD without global immunosuppression, much interest has centered on understanding the mechanisms by which TLI induces acquired tolerance.
The development of mixed chimerism following hematopoietic cell transfer into TLI-preconditioned mice requires interleukin (IL)–4; treatment with anti–IL-4 at the time of hematopoietic cell transfer significantly reduces the incidence of mixed chimerism in TLI-preconditioned mice.6 IL-4 facilitates donor cell engraftment and the development of mixed chimerism by promoting the differentiation of host anti-donor TH2 CD4+cells and preventing the differentiation of host anti-donor TC1 CD8 cells.6 The protection from GVHD conferred by TLI preconditioning also requires IL-4.7 Whereas no TLI-preconditioned mice die of GVHD following infusion of allogeneic donor hematopoietic cells, all of the TLI-preconditioned mice die of GVHD when IL-4 is completely eliminated through the use of IL-4 knockout host and donor mice.7
The development of GVHD is related to the presence of immunocompetent donor CD4+ T cells in the inoculum,4,8 which presumably give rise to cells reactive against host antigens. However, the extent to which TLI preconditioning affects the activation and differentiation of immunocompetent donor T cells following encounter with their cognate antigen has not been extensively studied. We reasoned that TLI preconditioning protects against the development of GVHD in part by blocking the responses of “donor” CD4+ cells that are activated within the TLI environment. Indeed, we noted that normal CD4+ T cells that were stimulated with anti-CD3 in the presence of spleen cells from TLI-preconditioned mice proliferated less and produced less IL-2 compared with control stimulated cells.9 10
To begin characterizing how TLI preconditioning affects the antigen-specific immune responses of immunocompetent CD4+ T cells, we examined CD4+ cells from the DO11.l0 transgenic mouse following antigen stimulation in vivo and in vitro within a TLI-preconditioned environment. DO11.10 transgenic CD4+cells (OVA-Tg) express a clonotypic T-cell receptor (TCR) reactive against OVA peptide11 and can be distinguished from nontransgenic CD4+ cells using the monoclonal antibody (mAb) KJ1-26.12 OVA-Tg have been used to examine CD4+ T-cell differentiation.13 14 The present study shows that when OVA-Tg are primed either in vivo in a TLI-preconditioned mouse or in vitro with cells from a TLI-preconditioned mouse, they differentiate preferentially into IL-4–secreting OVA-Tg CD4+ cells. Host CD4+cells from TLI-preconditioned mice that constitutively secrete IL-4 promote the development of these OVA-Tg TH2 cells by providing endogenous IL-4 at the time of primary activation. The results of this study suggest that upon encountering antigen within TLI-treated mice, the activation and differentiation of immunocompetent CD4+ T cells are redirected away from the TH1 pathway and toward the TH2 pathway.
Materials and methods
Mice and TLI treatment
BALB/c (H-2d) and CB6F1(H-2d X H-2b) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). BALB/c IL-4−/− and DO11.10 OVA-Tg mice (a gift from K. Murphy)11 were bred at the University of Iowa Animal Care Facility. With use of the clonotypic mAb KJ1-26,12 70% to 80% of CD4+ cells from the DO11.10 transgenic mouse express the transgenic TCR, which recognizes peptide 323-339 of chicken OVA in the context of I-Ad 11 and cross-reacts with H-2balloantigen.15
Groups of BALB/c and CB6F1 mice were given TLI treatment as described previously.16 TLI treatment was started when mice reached 12 to 15 weeks of age and weighed approximately 25 to 30 g. Radiation treatment was given in daily 275-rad fractions, 5 days per week, for a total of 17 doses. For each radiation treatment, the mice were anesthetized and placed individually into a jig that allowed exposure of the peripheral lymphoid tissue while shielding vital organs. The accumulated radiation dose was 4675 rad.
In vivo priming of OVA-Tg cells using adoptive transfer
TLI-treated CB6F1 and BALB/c mice were injected intraperitoneally with 5 to 7 × 106 purified OVA-Tg cells one day after the completion of TLI. Seven days later, the mice were killed and the percentages and absolute numbers of OVA-Tg CD4+ cells were measured in the spleens using multiparameter fluorescence-activated cell sorting (FACS) analysis. Spleen cells were stained with anti-CD4–A594, CD8–fluorescein isothiocyanate (FITC; BD Pharmingen, San Diego, CA), and KJ1-26–R-phycoerythrin (RPE) (Caltag, Burlingame, CA). The anti-CD4 (American Type Culture Collection, Manassas, VA) mAb was purified and coupled to A594 (Molecular Probes, Eugene, OR). Stained cells were analyzed on the Coulter EPICS FACS, and the number of OVA-Tg CD4+ cells was determined by gating on double-positive cells. Spleen cells were then cultured in vitro with increasing amounts of OVA peptide, and cytokines were measured in the supernatant that was collected at 48 hours, as described in “Cytokine ELISA and ELISPOT assays.”
Cell preparation, antigen stimulation, and immunologic assays
Spleens were harvested from the following mice: adoptive transfer animals described above; TLI-preconditioned BALB/c wild-type (WT), BALB/c IL-4−/−, and CB6F1 mice; age-matched nonirradiated BALB/c WT and CB6F1 mice; and DO11.10 mice. Single-cell suspensions were prepared, and cells were resuspended at the appropriate concentration in complete medium (RPMI supplemented with 10% fetal calf serum, HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 2-mercaptoethanol, l-glutamine, penicillin/streptomycin), using trypan blue dye to exclude dead cells.
T-cell–depleted control antigen-presenting cells (APCs) were generated by passage through a CS depletion column (Miltenyi Biotec, Auburn, CA) using Thy1.2-conjugated microbeads (Miltenyi Biotec); the cells contained less than 5% T cells by FACS analysis. To isolate naive OVA-Tg CD4+ cells, we subjected splenocytes from DO11.10 mice to a 2-step magnetic-bead separation using the anti-FITC MultiSort Kit (Miltenyi Biotec) and anti-CD4–FITC (RM4-4; Pharmingen), followed by anti-CD62L microbeads (Miltenyi Biotec), according to the manufacturer's specifications. The preparations contained more than 95% CD4 cells, and more than 90% of the CD4+ cells were CD62L+ cells. CD4+ cells were removed from TLI-spleen using CD4+ depletion Miltenyi Biotec Microbeads and CS columns (Miltenyi Biotec), per the manufacturer's instructions. CD4-depleted TLI-spleen cells contained less than 1% CD4+cells.
Spleen cells from adoptive transfer mice (1 × 106cells/well) were cultured in complete RPMI medium in triplicate in 96-well round-bottom plates for 48 hours with increasing concentrations of OVA peptide 323-339 (American Peptide Company, Sunnyvale, CA) ranging from 0 to 1000 nM in a final volume of 200 μL/well.
For primary antigen stimulation, 2 × 105 purified naive OVA-Tg cells were cultured in complete RPMI medium in triplicate in 96-well round-bottom plates for 48 hours with increasing concentrations of OVA peptide 323-339 (0-1000 nM) and T-cell–depleted spleen from untreated BALB/c mice, unfractionated spleen from TLI-preconditioned mice, or a 1:1 mixture of both as a source of APCs in a final volume of 200 μL/well. TLI-preconditioned BALB/c WT or BALB/c IL-4−/− mice were killed 2 days after completion of TLI, and spleens were pooled from 4 to 6 TLI-preconditioned mice for each experiment because one TLI-preconditioned mouse typically yields 1 to 3 × 106 cells per spleen at 2 days after TLI. In some experiments, anti-CD28 (4 μg/mL; BD Pharmingen) or IL-2 (50 U/mL; R&D Systems, Minneapolis, MN) was added to the wells. This dose of anti-CD28 induced proliferation and IL-2 production from purified CD4+ cells that were stimulated with soluble anti-CD3.
To measure proliferation, we added 3H-thymidine in the final 6 hours of incubation, harvested the cells at 48 hours after stimulation, and measured 3H-thymidine incorporation in a scintillation counter (Beckmann LS 5000 TD). For measurement of IL-2, IL-4, and interferon (IFN)–γ cytokine production, plates were centrifuged at 48 hours, and supernatants were collected and frozen at −20°C for future cytokine-specific enzyme-linked immunosorbent assays (ELISAs).
In some experiments, cells were harvested at 24 hours after stimulation, and OVA-Tg cells were examined for the level of CD25 and CD69 surface expression using anti-CD25–RPE (BD Pharmingen), anti-CD69–FITC (BD Pharmingen), anti-CD4–Cy5 (in-house purification and conjugation to Cy5; Amersham, Piscataway, NJ), KJ1-26–biotin (in-house purification and conjugation to biotin; Sigma-Aldrich, St Louis, MO), and streptavidin-TR (Invitrogen Life Technologies, Carlsbad, CA), with 4-color FACS analysis (Coulter EPICS).
For antigen restimulation studies, purified naive OVA-Tg CD4+ cells (10 × 106 cells/well) were cultured in 6-well plates with equal numbers of T-cell–depleted spleen from untreated BALB/c mice, spleen from TLI-preconditioned mice, or a 1:1 mixture of both, plus 100 nM OVA peptide in 5 mL/well final volume of complete medium for 5 days. Cells from the primary cultures were centrifuged over a Lympholyte-M gradient (Cedar Lane Laboratories), and OVA-Tg cells were further purified using anti-CD4–phycoerythrin and KJ1-26–FITC and positive FACS sorting. Purified OVA-Tg CD4+ cells (greater than 99%) were replated in 2-fold dilutions (starting concentration 105/well) directly in anti–IL-4 or anti–IFN-γ–coated enzyme-linked immunosorbent spot (ELISPOT) plates along with T-cell–depleted BALB/c splenocytes (1.25 × 105 cells/well) and 100 nM OVA.
Cytokine ELISA and ELISPOT assays
Cytokine-specific ELISAs were performed using the following cytokine capture and binding antibodies: IL-2, JES6-1A12 (American Type Culture Collection; permission from DNAX, Palo Alto, CA), and JES6-5H4–biotin (American Type Culture Collection; permission from DNAX); IFN-γ, R4-6A2 (American Type Culture Collection), and rabbit anti-mouse IFN-γ (PeproTech, Rocky Hill, NJ); IL-4, BVD4-1D11 (American Type Culture Collection; permission from DNAX), and BVD6-24G2–horseradish peroxidase (HRP; American Type Culture Collection, permission from DNAX); and IL-10, JES5-2A5 (American Type Culture Collection, permission from DNAX), and SXC-1–biotin (American Type Culture Collection, permission from DNAX). The IFN-γ ELISA was developed using donkey anti-rabbit–HRP (Jackson ImmunoResearch, West Grove, PA), and the IL-2 ELISA used goat anti-biotin–HRP (Sigma). Detection limits for all assays were as follows: 10 to 28 pg/mL, IL-2; 5 to 38 pg/mL, IFN-γ; 1 to 4 pg/mL, IL-4; and 35 to 50 pg/mL, IL-10. Transforming growth factor (TGF)–β was detected using the TGF-β Emax Immunoassay system (Promega, Madison, WI) according to the manufacturer's specifications.
The cytokine ELISPOT assays were adapted from Taguchi et al,17 as described.18 The IL-4 ELISPOT used BVD4-1D11 for capture and BVD6-24G2–biotin and goat anti-biotin–alkaline phosphatase (Sigma-Aldrich) for detection. The IFN-γ ELISPOT used R4-6A2 for capture and rabbit anti-mouse IFN-γ antisera and donkey anti-rabbit–alkaline phosphatase for detection. The IL-2 ELISPOT used JES6-1A12 for capture and JES6-5H4–biotin and goat anti-biotin–HRP (Sigma-Aldrich) the detection. ELISPOTs were developed as described18 and counted using an inverted microscope.
Intracytoplasmic cytokine analysis
Cells from primary cultures were centrifuged over Lympholyte-M. Recovered cells were cultured in 24-well plates at 2 × 106 cells/mL with 50 ng/mL phorbol myristate acetate (PMA; Sigma-Aldrich) and 500 ng/mL ionomycin (Sigma-Aldrich) in the absence of APCs for 4 hours. Brefeldin A (10 μg/mL; Epicentre Technologies, Madison, WI) was added for the last 2 hours of culture. Cells were stained with anti-CD4+ and KJ1-26, then fixed and permeabilized using a commercial Fix & Perm kit (Caltag) according to the manufacturer's directions. Intracellular cytokine staining was performed with RPE-conjugated rat anti-mouse IFN-γ mAb (XMG-1.2; BD Pharmingen), APC-conjugated rat anti-mouse IL-4 mAb (11B11; BD Pharmingen), or conjugated rat immunoglobulin G1 (IgG1) isotype control mAb. Analysis was performed with a Coulter EPICS FACS.
Multiparameter FACS analysis
Splenocytes from TLI-treated and control mice were stained with anti-CD4–Cy5, anti–IL-2Rβ–FITC (TM-B1; BD Pharmingen), and anti-CD44–biotin (IM7; BD Pharmingen), followed by streptavidin-TXR (Invitrogen Life Technologies). Rat IgG2b, κ-FITC (BD Pharmingen) was used as an isotype control for anti–IL-2Rβ. In other experiments, splenocytes were stained with anti-CD4–RPE (H129.19; BD Pharmingen), anti-CD44–biotin (IM7; BD Pharmingen), and various antibodies against mouse Vβ TCRs-FITC followed by streptavidin TXR. The mouse antibodies were against the following: Vβ3 (KJ25); Vβ5 (MR9.4); Vβ6 (RR4-7); Vβ7 (TR310); Vβ8.1, 8.2, 8.3 (F23.1); Vβ8.1, 8.2 (KJ16.133.18); Vβ8.3 (IB3.3); Vβ9 (MR10-2); and Vβ11 (RR3-15). Vβ antibodies were from BD Pharmingen, except for Vβ8.1, 8.2, and 8.3 (F23.1) and Vβ8.1 and 8.2 (KJ16.133.18), which were obtained from P. Marrack (Howard Hughes Medical Institute, Denver, CO). All stained cells were resuspended in RPMI complete medium with 5 μg/mL propidium iodide to gate out dead cells. The stained cells were analyzed on a Coulter EPICS FACS.
Results
In vivo priming of OVA-Tg cells in TLI-preconditioned mice alters recall cytokine response
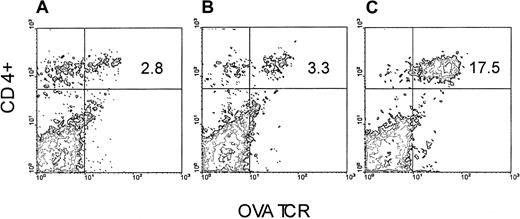
We took advantage of the observation that OVA-Tg cells, which exhibit cross-reactivity to H-2balloantigen,15 proliferate (not shown) and secrete cytokines (not shown) in a dose-dependent fashion following stimulation with CB6F1 cells. We therefore set up an adoptive transfer model in which we injected purified OVA-Tg T cells into either BALB/c or CB6F1 mice that had been preconditioned with TLI, and we measured the function of the cells one week later. OVA-Tg cells were detected in the spleens of TLI-preconditioned BALB/c and CB6F1 mice one week after adoptive transfer (Figure1). There was no difference in the percentages of OVA-Tg cells in the spleens of TLI-preconditioned BALB/c versus CB6F1 mice (mean ± SE: 2.5% ± 0.2%, BALB/c, n = 3; 3.1% ± 0.3%, CB6F1, n = 7;P = .33). However, compared with spleens of the BALB/c mice, spleens from the CB6F1 mice contained a higher absolute number of OVA-Tg CD4+ cells (mean ± SE: 1.14 ± 0.26 × 106, BALB/c; 3.29 ± 0.33 × 106, CB6F1;P = .004).
OVA-Tg are detected in the spleen following adoptive transfer into TLI-preconditioned mice.
OVA-Tg cells were adoptively transferred into TLI-preconditioned mice one day after completion of TLI. Seven days later, OVA-Tg cells were detected in the spleens of individual mice using multiparameter FACS analysis. Figure is a FACS contour plot depicting the percentage of OVA-Tg CD4+ cells in the spleen of a TLI-preconditioned BALB/c mouse (A) and a TLI-preconditioned CB6F1 mouse (B). (C) Control OVA-Tg mouse.
OVA-Tg are detected in the spleen following adoptive transfer into TLI-preconditioned mice.
OVA-Tg cells were adoptively transferred into TLI-preconditioned mice one day after completion of TLI. Seven days later, OVA-Tg cells were detected in the spleens of individual mice using multiparameter FACS analysis. Figure is a FACS contour plot depicting the percentage of OVA-Tg CD4+ cells in the spleen of a TLI-preconditioned BALB/c mouse (A) and a TLI-preconditioned CB6F1 mouse (B). (C) Control OVA-Tg mouse.
When spleen cells were examined one week after adoptive transfer for their ability to respond to OVA peptide (Figure2), OVA-Tg cells from TLI-preconditioned BALB/c mice secreted significantly more IL-2 and IFN-γ compared with cells from TLI-preconditioned CB6F1 mice, particularly at the higher concentrations of OVA peptide. In contrast, OVA-Tg cells from TLI-preconditioned CB6F1 mice secreted more IL-4 at high concentrations of OVA peptide than cells from TLI-preconditioned BALB/c mice.
Priming of OVA-Tg cells in TLI-preconditioned mice alters recall cytokine response.
OVA-Tg cells were adoptively transferred into TLI-preconditioned mice one day after completion of TLI. Seven days later, spleen cells were isolated from individual mice and cultured with increasing concentrations of OVA peptide for 48 hours, and cytokines were measured in the culture supernatant. Bars represent the means ± SE of cytokine responses from individual TLI-preconditioned CB6F1(black, n = 7) and BALB/c (hatched, n = 3) mice. The data are pooled from 3 separate experiments.
Priming of OVA-Tg cells in TLI-preconditioned mice alters recall cytokine response.
OVA-Tg cells were adoptively transferred into TLI-preconditioned mice one day after completion of TLI. Seven days later, spleen cells were isolated from individual mice and cultured with increasing concentrations of OVA peptide for 48 hours, and cytokines were measured in the culture supernatant. Bars represent the means ± SE of cytokine responses from individual TLI-preconditioned CB6F1(black, n = 7) and BALB/c (hatched, n = 3) mice. The data are pooled from 3 separate experiments.
In vitro priming of OVA-Tg with spleen from TLI-preconditioned mice promotes the maturation of OVA-Tg TH2 over TH1 cells
To examine whether the TLI-preconditioned environment skews the development of CD4+ cells toward TH2, we stimulated purified naive CD4+ OVA-Tg in vitro with 100 nM OVA peptide and APCs from either untreated or TLI-preconditioned BALB/c mice for 5 days. Purified OVA-Tg cells were then restimulated with PMA and calcium ionophore. OVA-Tg cells that were primed in the presence of control APCs gave rise to more IFN-γ–producing cells by intracytoplasmic staining (Figure 3), whereas OVA-Tg cells that were primed in the presence of TLI-spleen gave rise to 5-fold more IL-4–producing cells and 75% less IFN-γ–producing cells. We saw few dual-producing IL-4 and IFN-γ OVA-Tg cells, indicating that we were primarily detecting differentiation of either TH1 or TH2 cells. Thus, the presence of TLI-spleen during primary antigen activation promotes differentiation of TH2 OVA-Tg.
Priming of OVA-Tg in vitro with cells from TLI-preconditioned mice expands IL-4–producing OVA-Tg CD4+cells.
Purified naive OVA-Tg CD4+ cells were cultured for 5 days with 100 nM OVA peptide and APCs from either untreated BALB/c mice (A-B) or TLI-preconditioned BALB/c mice (C-D). Cells from these cultures were then restimulated with PMA/ionomycin as per “Materials and methods” and subsequently stained for CD4+ and Tg surface markers. The panels depict contour plots of gated OVA-Tg CD4+ T cells after PMA/ionomycin restimulation and show intracytoplasmic staining with rat IgG1 isotype control antibodies (A,C) or antibodies against IL-4 and IFN-γ (B,D).
Priming of OVA-Tg in vitro with cells from TLI-preconditioned mice expands IL-4–producing OVA-Tg CD4+cells.
Purified naive OVA-Tg CD4+ cells were cultured for 5 days with 100 nM OVA peptide and APCs from either untreated BALB/c mice (A-B) or TLI-preconditioned BALB/c mice (C-D). Cells from these cultures were then restimulated with PMA/ionomycin as per “Materials and methods” and subsequently stained for CD4+ and Tg surface markers. The panels depict contour plots of gated OVA-Tg CD4+ T cells after PMA/ionomycin restimulation and show intracytoplasmic staining with rat IgG1 isotype control antibodies (A,C) or antibodies against IL-4 and IFN-γ (B,D).
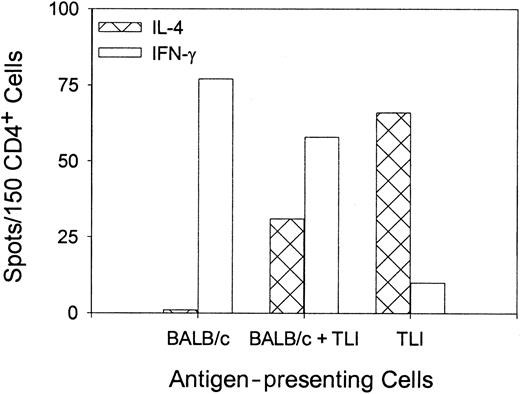
To confirm the differentiation of OVA peptide–reactive TH2 cells, we stimulated purified naive OVA-Tg in vitro as before with 100 nM OVA peptide plus control APCs, TLI-spleen, or a 1:1 combination in primary cultures for 5 days. OVA-Tg cells were purified from the primary cultures and restimulated with OVA peptide in secondary ELISPOT cultures in the presence of control APCs. Figure4 shows that most OVA-Tg cells from the control primary cultures matured into IFN-γ–producing TH1 cells following secondary antigen stimulation. In contrast, most OVA-Tg cells from primary cultures that contained TLI-spleen cells as the source of APCs matured into IL-4–producing TH2 cells following secondary antigen stimulation. Furthermore, the addition of TLI-spleen to the control APCs in primary cultures triggered the differentiation of OVA-Tg TH2 cells. Thus, the presence of TLI-spleen during the primary culture shifts the maturation of OVA-Tg CD4+ cells away from IFN-γ–producing TH1 cells and toward IL-4–producing TH2 cells.
Priming of OVA-Tg in vitro with cells from TLI-preconditioned mice enhances differentiation of antigen-specific TH2 cells.
Purified naive OVA-Tg CD4+ cells were cultured with 100 nM OVA peptide and APCs from untreated BALB/c mice (BALB/c), TLI-preconditioned BALB/c mice (TLI), or a 1:1 mixture of BALB/c/TLI for 5 days. OVA-Tg from these primary cultures then were purified and restimulated directly with OVA peptide and fresh APCs from untreated BALB/c mice in cytokine ELISPOT wells as per “Materials and methods.” Bars represent the number of IFN-γ–producing (open) or IL-4–producing (hatched) OVA-Tg cells 72 hours after secondary stimulation from one experiment. The figure depicts a single representative experiment of 3 separate experiments.
Priming of OVA-Tg in vitro with cells from TLI-preconditioned mice enhances differentiation of antigen-specific TH2 cells.
Purified naive OVA-Tg CD4+ cells were cultured with 100 nM OVA peptide and APCs from untreated BALB/c mice (BALB/c), TLI-preconditioned BALB/c mice (TLI), or a 1:1 mixture of BALB/c/TLI for 5 days. OVA-Tg from these primary cultures then were purified and restimulated directly with OVA peptide and fresh APCs from untreated BALB/c mice in cytokine ELISPOT wells as per “Materials and methods.” Bars represent the number of IFN-γ–producing (open) or IL-4–producing (hatched) OVA-Tg cells 72 hours after secondary stimulation from one experiment. The figure depicts a single representative experiment of 3 separate experiments.
TLI-spleen cells alter the proliferative response and the pattern of cytokines produced by OVA-Tg CD4+ cells following primary antigen activation
We then examined OVA-Tg responses in primary cultures. We first measured the ability of antigen-stimulated OVA-Tg to up-regulate surface CD69 and CD25, as an indirect indicator of TCR-ligand interaction. Purified naive OVA-Tg CD4+ cells were cultured for 24 hours with either no antigen or 100 nM OVA peptide and APCs from either untreated BALB/c mice or TLI-preconditioned BALB/c mice, and the levels of CD69 and CD25 expression were measured on OVA-Tg CD4+ cells by FACS. In 3 separate experiments, there were no differences in the percentages of OVA-Tg cells that coexpressed CD69 and CD25 (not shown), nor were there any differences in the levels of CD69 or CD25 expression (not shown) on OVA-Tg cells from primary cultures that contained TLI-spleen versus control APCs.
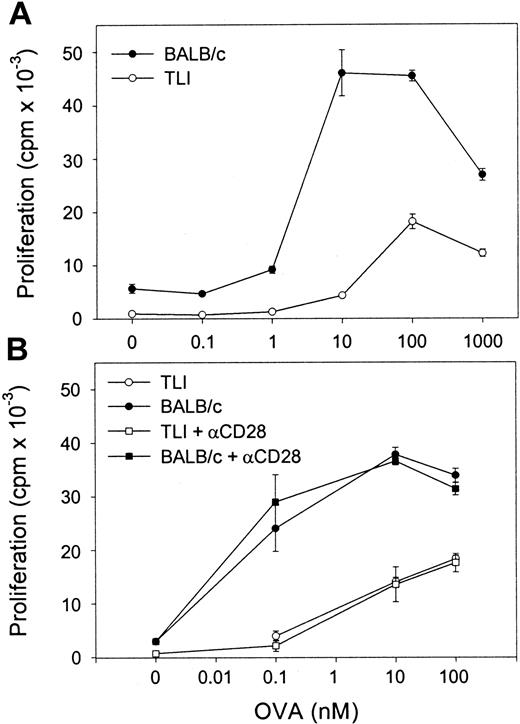
We next examined OVA-Tg proliferative responses to OVA peptide in primary cultures. OVA-Tg cells proliferated more when stimulated with OVA peptide in the presence of control APCs as compared with TLI-spleen (Figure 5A). The mean ± SE peak proliferative response of antigen-activated OVA-Tg cells was 43 568 ± 5136 counts per minute (cpm) when control APCs were used and 21 554 ± 3349 cpm when TLI-spleen cells were used in a total of 3 dose-response experiments. The addition of anti-CD28 did not enhance the proliferation of OVA-Tg cells whether they were cultured with control APCs or the TLI-spleen cells (Figure 5B). The addition of exogenous IL-2 did not restore the proliferative response of OVA-Tg cells from primary cultures with TLI-spleen cells to the control level (not shown).
OVA-Tg CD4+ cells show hyporesponsiveness following primary antigen stimulation in the presence of spleen from TLI-preconditioned mice.
(A) Purified naive OVA-Tg CD4+ were cultured with increasing concentrations of OVA peptide and APCs from either untreated BALB/c (BALB/c, ●) or TLI-preconditioned BALB/c mice (TLI, ○). After 48 hours, proliferation was measured by 3H-thymidine incorporation. (B) OVA-Tg cells were cultured as in (A) with OVA peptide and APCs from either untreated (BALB/c, solid symbols) or TLI-preconditioned (TLI, open symbols) BALB/c mouse spleen without (circles) or with (squares) anti-CD28. Proliferation was measured by3H-thymidine incorporation. Symbols represent the mean ± SD of triplicate wells for one representative experiment from 3 separate experiments. APCs alone did not proliferate in response to increasing OVA peptide (not shown).
OVA-Tg CD4+ cells show hyporesponsiveness following primary antigen stimulation in the presence of spleen from TLI-preconditioned mice.
(A) Purified naive OVA-Tg CD4+ were cultured with increasing concentrations of OVA peptide and APCs from either untreated BALB/c (BALB/c, ●) or TLI-preconditioned BALB/c mice (TLI, ○). After 48 hours, proliferation was measured by 3H-thymidine incorporation. (B) OVA-Tg cells were cultured as in (A) with OVA peptide and APCs from either untreated (BALB/c, solid symbols) or TLI-preconditioned (TLI, open symbols) BALB/c mouse spleen without (circles) or with (squares) anti-CD28. Proliferation was measured by3H-thymidine incorporation. Symbols represent the mean ± SD of triplicate wells for one representative experiment from 3 separate experiments. APCs alone did not proliferate in response to increasing OVA peptide (not shown).
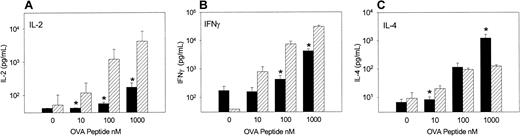
To examine the cytokine response of OVA-Tg in primary cultures, we stimulated OVA-Tg cells with increasing amounts of OVA peptide in the presence of control APCs, TLI-spleen cells, or a 1:1 combination of both, and determined cytokine production 48 hours after stimulation. OVA-Tg cells secreted significantly more IL-4 and less IL-2 when stimulated with OVA peptide in the presence of APCs from TLI-preconditioned mice, and this cytokine pattern persisted even if normal control APCs were added to the TLI-spleen cells (Figure6). TLI-spleen cells alone did not produce any IL-2, IFN-γ, or IL-4 cytokines in response to OVA peptide (not shown). The addition of anti-CD28 enhanced IL-4 production at higher antigen concentrations (Figure 7). There was no difference in the amount of IFN-γ secreted by OVA-Tg cells from primary cultures with APCs from either untreated or TLI-preconditioned mice (Figure 6). Although the addition of anti-CD28 increased the amount of IFN-γ secreted by OVA-Tg cells from primary cultures with control APCs at all concentrations, the addition of anti-CD28 did not affect IFN-γ production by OVA-Tg cells from cultures with TLI-spleen cells as APCs (Figure 7). Thus, in the presence of spleen cells from TLI-preconditioned mice, antigen-stimulated OVA-Tg cells produce more IL-4 and less IL-2 in antigen-driven primary cultures.
Spleen from TLI-preconditioned mice alters the pattern of cytokine secretion by OVA-Tg CD4+ cells following primary antigen activation.
Purified OVA-Tg CD4+ cells were cultured for 48 hours with APCs from untreated mice (BALB/c, n = 7), TLI-preconditioned BALB/c mice (TLI, n = 7), or a 1:1 mixture of both (BALB/c/TLI, n = 3) with either no antigen (open bars) or 100 nM OVA peptide (hatched bars), and cytokines were measured in the 48-hour supernatants by ELISA. Error bars represent the mean ± SE of the separate experiments. ELISA detection limits were 1 pg/mL (IL-4), 5 pg/mL (IFN-γ), and 10 pg/mL (IL-2). Statistics were performed to compare cytokine levels of restimulated cultures (hatched bars). *Significant difference compared with other groups, P < .05, analysis of variance and t test.
Spleen from TLI-preconditioned mice alters the pattern of cytokine secretion by OVA-Tg CD4+ cells following primary antigen activation.
Purified OVA-Tg CD4+ cells were cultured for 48 hours with APCs from untreated mice (BALB/c, n = 7), TLI-preconditioned BALB/c mice (TLI, n = 7), or a 1:1 mixture of both (BALB/c/TLI, n = 3) with either no antigen (open bars) or 100 nM OVA peptide (hatched bars), and cytokines were measured in the 48-hour supernatants by ELISA. Error bars represent the mean ± SE of the separate experiments. ELISA detection limits were 1 pg/mL (IL-4), 5 pg/mL (IFN-γ), and 10 pg/mL (IL-2). Statistics were performed to compare cytokine levels of restimulated cultures (hatched bars). *Significant difference compared with other groups, P < .05, analysis of variance and t test.
Naive OVA-Tg CD4+ cells secrete IL-4 following primary antigen activation in vitro in the presence of spleen from TLI-preconditioned mice.
Purified naive OVA-Tg CD4+ cells were cultured for 48 hours with increasing concentrations of OVA peptide and APCs from either untreated (BALB/c, solid symbols) or TLI-preconditioned (TLI, open symbols) BALB/c mouse spleen without (circles) or with (squares) anti-CD28. IL-4 (A) and IFN-γ (B) secretion was quantitated by ELISA of culture supernatants. Symbols represent the means of duplicate determinations of IL-4 and IFN-γ for one experiment. The ELISA detection limits were 1 pg/mL (IL-4) and 5 pg/mL (IFN-γ). The figure depicts one representative experiment of 3 separate experiments.
Naive OVA-Tg CD4+ cells secrete IL-4 following primary antigen activation in vitro in the presence of spleen from TLI-preconditioned mice.
Purified naive OVA-Tg CD4+ cells were cultured for 48 hours with increasing concentrations of OVA peptide and APCs from either untreated (BALB/c, solid symbols) or TLI-preconditioned (TLI, open symbols) BALB/c mouse spleen without (circles) or with (squares) anti-CD28. IL-4 (A) and IFN-γ (B) secretion was quantitated by ELISA of culture supernatants. Symbols represent the means of duplicate determinations of IL-4 and IFN-γ for one experiment. The ELISA detection limits were 1 pg/mL (IL-4) and 5 pg/mL (IFN-γ). The figure depicts one representative experiment of 3 separate experiments.
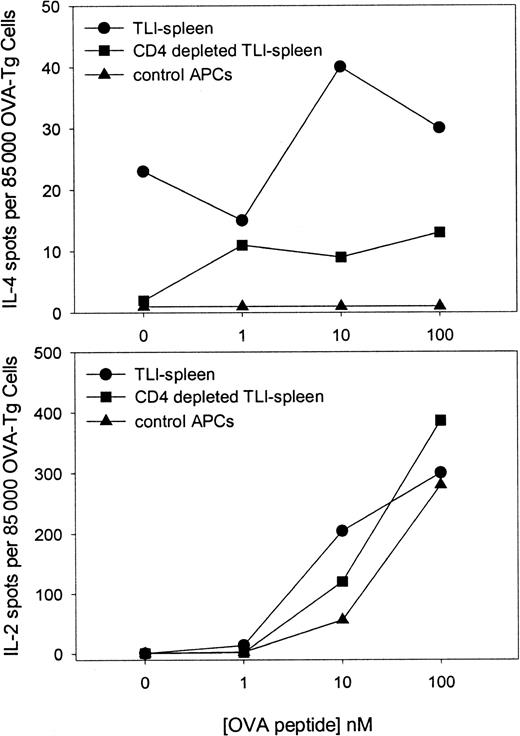
Secretion of IL-4 by OVA-Tg cells from primary in vitro cultures depends on CD4+ TLI-spleen cells that constitutively secrete IL-4
TLI treatment results in a relative enrichment of CD4+cells that produce IL-4.6 We examined whether the presence of the CD4+ cell within the TLI-spleen population had an effect on the responses of OVA-Tg cells in primary cultures. Highly purified naive OVA-Tg CD4+ cells were cultured with 100 nM OVA peptide and control APCs, unfractionated TLI-spleen cells, or TLI-spleen that had been depleted of CD4+ cells. The cultures were performed in either IL-4 (Figure8, upper panel) or IL-2 (Figure 8, lower panel) ELISPOT wells. No IL-4–producing cells were detected in primary cultures in which OVA-Tg cells were stimulated in the presence of control APCs. In sharp contrast, IL-4–producing cells were detected in primary cultures when OVA-Tg cells were stimulated in the presence of unfractionated TLI-spleen cells. Deletion of CD4+ cells from the TLI-spleen cell population decreased the amount of IL-4–producing cells detected in primary cultures, but did not alter the number of IL-2–producing cells.
Secretion of IL-4 by OVA-Tg cells in primary in vitro cultures depends on the presence of CD4+ cells from TLI-preconditioned mice.
Highly purified naive OVA-Tg cells were cultured as in Figure 6 with OVA peptide and APCs from untreated BALB/c mice (▴), whole spleen from TLI-preconditioned BALB/c mice (●), or CD4+-depleted spleen from TLI-preconditioned BALB/c mice (▪) in IL-4 (upper) and IL-2 (lower) ELISPOT plates. Cytokine-producing cells were detected as described in “Materials and methods.” The data represent a single experiment. The experiment depicted in the upper panel was repeated twice with similar results.
Secretion of IL-4 by OVA-Tg cells in primary in vitro cultures depends on the presence of CD4+ cells from TLI-preconditioned mice.
Highly purified naive OVA-Tg cells were cultured as in Figure 6 with OVA peptide and APCs from untreated BALB/c mice (▴), whole spleen from TLI-preconditioned BALB/c mice (●), or CD4+-depleted spleen from TLI-preconditioned BALB/c mice (▪) in IL-4 (upper) and IL-2 (lower) ELISPOT plates. Cytokine-producing cells were detected as described in “Materials and methods.” The data represent a single experiment. The experiment depicted in the upper panel was repeated twice with similar results.
Surprisingly, we detected IL-4–producing spots even in primary cultures that did not contain OVA peptide (Figure 8, upper panel; 0 nM peptide). This finding was consistent in 3 separate experiments and suggested that TLI-spleen contained cells that constitutively secreted IL-4. To examine whether this constitutive production of IL-4 triggered OVA-Tg cells to secrete IL-4 in the primary cultures, we cultured highly purified naive CD4+ OVA-Tg cells directly in IL-4 ELISPOT wells containing 100 nM OVA peptide and APCs from control, TLI-preconditioned WT mice, or TLI-preconditioned IL-4−/−mice (Figure 9). As expected, few OVA-Tg cells secreted IL-4 in primary cultures when stimulated with OVA peptide in the presence of APCs from control mice. In contrast, more IL-4–producing cells were detected in primary cultures when OVA-Tg cells were stimulated with OVA peptide in the presence of spleen cells from TLI-preconditioned WT mice, and the number of IL-4–producing cells increased in a dose-dependent fashion. Stimulation of OVA-Tg cells in the presence of spleen cells from TLI-preconditioned IL-4−/− mice reduced the number of IL-4–producing cells to background control levels.
Secretion of IL-4 by OVA-Tg cells in primary in vitro cultures depends on exogenous IL-4 from TLI-preconditioned mice.
Highly purified naive OVA-Tg CD4+ T cells were cultured for 16 hours with OVA peptide and APCs from untreated BALB/c WT (▴), TLI-preconditioned BALB/c WT (●), or TLI-preconditioned BALB/c IL-4−/− mice (▪) in IL-4 ELISPOT plates. Figure represents 1 of 2 experiments.
Secretion of IL-4 by OVA-Tg cells in primary in vitro cultures depends on exogenous IL-4 from TLI-preconditioned mice.
Highly purified naive OVA-Tg CD4+ T cells were cultured for 16 hours with OVA peptide and APCs from untreated BALB/c WT (▴), TLI-preconditioned BALB/c WT (●), or TLI-preconditioned BALB/c IL-4−/− mice (▪) in IL-4 ELISPOT plates. Figure represents 1 of 2 experiments.
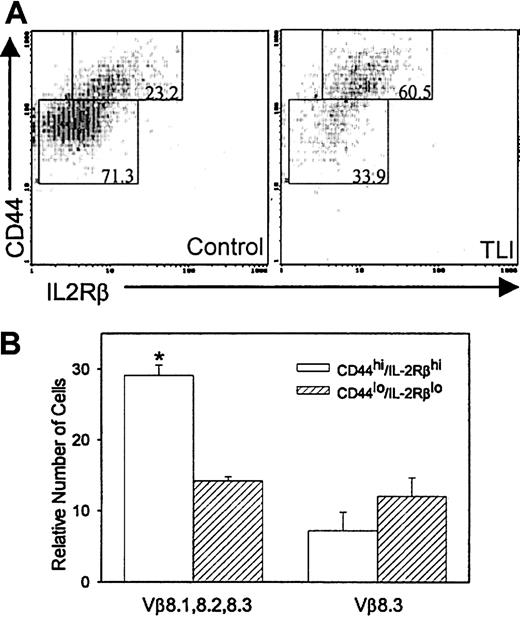
Increased CD4+–natural killer T (TNK) cells in TLI-treated mice
We previously reported that anti-CD3–stimulated spleen cells from 2-day post–TLI-treated mice secrete 20-fold greater amounts of IL-4 compared with IFN-γ and no measurable IL-10.6We also reported that the CD4+ cells and not the CD4−CD8− cells were the source of the IL-4.6 CD4+-TNK cells produce more IL-4 in relation to other cytokines19 and are capable of secreting high levels of IL-4 upon primary stimulation.20-22 To determine whether the CD4+ spleen cells in TLI-treated mice contained TNK cells, we measured CD44+ and IL-2Rβ expression in the CD4+ cells from the TLI-treated mice. We used coexpression of CD44hi/IL-2Rβ+ as a marker for CD4+-TNK cells20,21,23,24 because the BALB/c strain of mice does not express the standard NK marker, NK1.1. We did not measure coexpression of CD25. TLI-treated mice contained twice as many CD4+ spleen cells that coexpressed CD44hi/IL-2Rβ+ as did control unirradiated mice (57.2% ± 3.4%, TLI; versus 28.8% ± 4.7%, control; P = .0005). Figure10A depicts a typical example of CD4+/CD44hi/IL-2Rβ+cells from a TLI-treated BALB/c mouse and a BALB/c control mouse. To further confirm that the CD4+/CD44hi/IL-2Rβ+subset was TNK, we examined the Vβ usage of CD4+/CD44hi/IL-2Rβ+ cells and CD4+/CD44lo/IL-2Rβ−cells. CD4+-TNK cells preferentially express Vβ8 (especially 8.2), Vβ7, or Vβ2.21 25-28 We saw no difference in the percentages of CD4+/CD44hi/IL-2Rβ+ or CD4+/CD44lo/IL-2Rβ− populations that expressed Vβ3, 5, 6, 7, 9, or 11 (not shown). However, Figure10B demonstrates that CD4+/CD44hi/IL-2Rβ+ cells from TLI-treated mice expressed significantly more Vβ8.1, 8.2, and 8.3 compared with the CD4+/CD44lo/IL-2Rβ− cells (P = .00007). In contrast, Vβ8.3 expression was no different between the groups (Figure 10B), indicating that the CD4+/CD44hi/IL-2Rβ+ cells from TLI-treated mice are enriched for Vβ8.1- and/or Vβ8.2-expressing cells. Control mice did not show an increase in Vβ8.1-, 8.2-, or 8.3-expressing cells in the CD4+/CD44hi/IL-2Rβ+ subset as compared with the CD4+/CD44lo/IL-2Rβ−subset (P = .637, data not shown).
Enrichment of CD4+ TNK cells in TLI-treated mice.
(A) FACS contour plots of gated CD4+ spleen cells from a day-2 post–TLI-treated mouse (left) and a control mouse (right). The numbers in the boxed areas indicate the percentages of CD4+cells expressing either CD44hi/IL-2Rβ+ or CD44lo/IL-2Rβ−. Analysis was restricted to live cells by propidium iodine exclusion. Data are from 1 of 5 representative experiments. (B) Spleen cells from 2-day post–TLI-treated mice were analyzed for CD4, CD44, IL-2Rβ, and specific Vβ expression using 4-color FACS. Error bars represent the mean ± SE of the relative number of CD44hi/IL-Rβ+ (open bar) or CD44lo/IL-2Rβ− (hatched bars) CD4 cells that stain with Vβ 8.1, 8.2, 8.3, or Vβ 8.3 from 3 separate experiments. For each experiment, 3 to 5 spleens were pulled from TLI-treated mice.
Enrichment of CD4+ TNK cells in TLI-treated mice.
(A) FACS contour plots of gated CD4+ spleen cells from a day-2 post–TLI-treated mouse (left) and a control mouse (right). The numbers in the boxed areas indicate the percentages of CD4+cells expressing either CD44hi/IL-2Rβ+ or CD44lo/IL-2Rβ−. Analysis was restricted to live cells by propidium iodine exclusion. Data are from 1 of 5 representative experiments. (B) Spleen cells from 2-day post–TLI-treated mice were analyzed for CD4, CD44, IL-2Rβ, and specific Vβ expression using 4-color FACS. Error bars represent the mean ± SE of the relative number of CD44hi/IL-Rβ+ (open bar) or CD44lo/IL-2Rβ− (hatched bars) CD4 cells that stain with Vβ 8.1, 8.2, 8.3, or Vβ 8.3 from 3 separate experiments. For each experiment, 3 to 5 spleens were pulled from TLI-treated mice.
Discussion
We reported that TLI preconditioning alters the phenotype of host T cells, eliminating CD8+ cells and sparing a subset of radioresistant CD4+ T cells.6 The remaining T cells secrete IL-4 upon in vitro activation. When host CD4+cells are exposed to antigen during this early post-TLI phase, such as by adoptive transfer of semiallogeneic hematopoietic cells, they preferentially differentiate into anti-donor TH2 CD4+ cells.6 However, we had not investigated whether immunocompetent CD4+ cells behave similarly when exposed to antigen in a TLI-preconditioned environment.
The purpose of this study was to examine the extent to which TLI preconditioning affects the antigen-specific immune responses of immunocompetent CD4+ cells. We used OVA-Tg CD4+cells as a source of homogeneous immunocompetent “donor” T cells because they can be easily identified using anti-clonotypic mAbs. OVA-Tg cells cross-react to MHC alloantigens expressed on CB6F1 cells, in addition to their nominal antigen, OVA peptide. The property of cross-reactivity against CB6F1allowed us to examine the differentiation of OVA-Tg following in vivo priming by adoptive transfer into CB6F1 mice. The use of adoptive transfer of the parent → F1 combination allowed us to avoid the use of adjuvants for the in vivo priming.
Seven days after adoptive transfer, the TLI-preconditioned CB6F1 mice contained higher numbers of OVA-Tg cells in their spleens than TLI-preconditioned BALB/c mice. The spleens from the TLI-preconditioned CB6F1 mice were larger than those of the TLI-preconditioned syngeneic controls (1.36 ± 0.11 × 108 versus 0.57 ± 0.13 × 108; P < .003), but similar in size to the unmanipulated control CB6F1 mice (P = .2). This indicates in vivo expansion of OVA-Tg cells in response to the cross-reactive MHC antigen as well as expansion of non-Tg cells. OVA-Tg cells from TLI-preconditioned CB6F1mice differentiated primarily into TH2 cells (Figure 2). Fowler and Gress29 reported that donor TH2 cells do not cause acute GVHD and suppress GVHD following infusion of donor T cells without impairing engraftment. De Wit et al reported that although enhanced TH2-like cytokine responses have been associated with chronic GVHD,30 their presence is not sufficient for the development of chronic GVHD.31 We did not observe any features of acute or chronic GVHD, including splenomegaly, wasting, weight loss, hair loss, or death, in a subgroup of mice that were followed for up to 15 weeks (E.H.F., unpublished observations, August 2000).
OVA-Tg cells that were primed with cognate antigen OVA in vitro in the presence of APCs from TLI-preconditioned mice also preferentially differentiated into TH2-like cells (Figures 3-4). We were best able to demonstrate immunoredirection toward IL-4–producing cells using the ELISPOT assay, which readily detected the higher frequency of antigen-reactive IL-4–producing OVA-Tg cells and was more sensitive than intracytoplasmic staining. The ELISPOT assay has been reported as more sensitive for detecting IL-4–producing cells.32
Besides their propensity to differentiate into TH2 cells, OVA-Tg cells that were primed in vitro in the presence of spleen cells from TLI-preconditioned mice exhibited other properties that distinguished them from OVA-Tg cells that were primed with control APCs. The former proliferated less, produced less IL-2, but produced more IL-4. On the other hand, OVA-Tg cells from both cultures expressed the same levels of surface CD25 and CD69. We previously reported that anti-CD3–activated CD4+ cells from TLI-preconditioned mice secreted more IL-4 and less IL-2 than cells from nonirradiated control mice.18 CD4+ cells from untreated mice that were stimulated with anti-CD3 in the presence of spleen cells from TLI-preconditioned mice also proliferated less and secreted less IL-2, but up-regulated CD69 and CD25 normally.10 The results herein extend these previous observations to immunocompetent CD4+ cells that are stimulated with cognate antigen. Thus, OVA-Tg CD4+ cells that encounter antigen in vitro in the presence of TLI-spleen cells exhibit an immunosuppressive phenotype characterized by decreased IL-2 production and proliferation, normal up-regulation of CD25/CD69 expression, and increased production of IL-4.
The nature of the response of naive T cells to primary T-cell activation depends on several factors, including the strength of the TCR/Ag/MHC interaction (signal 1); the dose of peptide; the functional capacity of the APCs, which is largely dictated by costimulatory potential (signal 2); and the immediate cytokine environment. The up-regulation of both CD69 and CD25 depends on a strong TCR-ligand interaction33 and is relatively independent of costimulation.33-35 When the TCR-ligand interaction is weak, then up-regulation of CD69 and CD25 can occur in the presence of adequate costimulation.33 On the other hand, both IL-2 secretion and cell proliferation are relatively late events following cell activation and depend on B7 costimulation.33,36 37
Figures 5 through 7 show that naive OVA-Tg cells that are primed in the presence of spleen cells from TLI-preconditioned mice display a functional phenotype similar to that of naive CD4+ cells that are primed with altered peptide ligands. Naive T cells that are stimulated with altered peptide ligands proliferate less33,38,39 and produce less IL-2,33,40 but up-regulate CD25/CD69 normally if supplied with adequate costimulation.33 Furthermore, priming with altered peptide ligand induces naive CD4+ cells to produce IL-438,41 and promotes the differentiation of more TH2-like cells.38
For our in vitro studies, we used T-cell–depleted spleen as control APCs and unfractionated TLI-spleen for TLI APCs. In addition to the difference that the TLI-spleen contains 3% to 14% CD4+cells, TLI-spleen also has a lower percentage of B cells and a higher percentage of non-T/non-B cells.6 We did not compare expression of MHC class II. However, OVA-Tg cells that were primed with TLI-spleen showed normal up-regulation of CD25 and CD69 (not shown), indicating that TLI-spleen APCs were capable of delivering signal 1. In addition, although increasing the dose of antigen can reverse the hypoproliferative phenotype of cells stimulated with altered peptide ligand,38 39 increasing the antigen dose does not restore immune response to the levels detected in controls, nor does it prevent the production of IL-4 in the primary cultures (Figures 5 and 7). Therefore, it is unlikely that the results herein can be explained by an alteration in the strength of TCR/Ag/MHC signaling to the OVA-Tg cells.
We considered that the decreased ability of the naive OVA-Tg cells to fully proliferate or secrete IL-2 may be secondary to inadequate costimulation. OVA-Tg cells proliferate less and produce lower amounts of IL-2 if antigen stimulation is carried out in the presence of APCs that lack B7 molecules.37 However, the addition of anti-CD28 did not augment proliferation or IL-2 production by OVA-Tg cells that were primed with TLI-spleen (Figure 5 and data not shown) and did not affect IL-4 secretion in the primary cultures (Figure 7). Indeed, the results in Figure 7 argue that the B7/CD28 costimulatory pathway is intact in the TLI-spleen cells because naive CD4+ cells required B7/CD28 costimulation to produce IL-4 in primary cultures.37 42
The cytokine milieu at the time of initial antigen encounter greatly influences CD4+ subset maturation.43 IL-4 is absolutely required for the maturation of TH2 CD4+ cells,44 and the addition of IL-4 at the time of initial antigen exposure can enhance the differentiation of naive CD4+ cells into TH2 memory cells.34 When added to primary cultures, exogenous IL-4 stimulates OVA-Tg cells to transcribe IL-4 and to up-regulate IL-4 receptor.45 Exogenous IL-4 can also enhance IL-4R signaling,34 rendering the cell more sensitive to IL-4. Thus, naive OVA-Tg CD4+ cells can secrete low levels of IL-4 in primary cultures under the proper conditions, including in response to exogenous IL-4.34,41,46 47
Figures 8 and 9 demonstrate that spleen cells from TLI-preconditioned mice constitutively secreted IL-4; CD4+ cells within the TLI-spleen were the source of IL-4. When IL-4–secreting CD4+ cells were present in the TLI-spleen, OVA-Tg cells produced more IL-4 in the primary cultures in response to increasing doses of OVA peptide (Figure 8). The removal of this source of IL-4, by substituting TLI-spleen cells from IL-4−/− mice, abolished the ability of OVA-Tg cells to produce IL-4 in primary cultures (Figure 9). Thus, CD4+ cells within the spleen of TLI-preconditioned mice constitutively secrete IL-4, which in turn triggers the naive OVA-Tg cells to produce IL-4 upon antigen activation. We believe that naive OVA-Tg are secreting IL-4 following primary antigen activation in Figures 8 and 9. First, the purified naive OVA-Tg CD4 cells were more than 90% CD62L+. Second, we detected IL-4 cytokine at 24 hours after primary antigen activation, before naive cells could undergo 2 rounds of antigen stimulation.
The CD4+ cells in the TLI-treated BALB/c mice showed typical features of CD4+-TNK cells, including lower density of surface CD4 and CD319 (data not shown), coexpression of CD44hi/IL-2Rβ+ (Figure 10A),21and preference for Vβ8.1 and/or Vβ8.2 expression21,25-28 (Figure 10B). It is of interest to note that while the percentage of CD4+ cells in the spleens of 2-day post–TLI-treated mice is roughly 40% lower than that of control mice, the frequency of IL-4–producing CD4+ cells is nearly 5-fold greater in TLI-treated mice than in controls.6These results and the data herein indicate that TLI treatment enriches for CD4+-TNK cells that secrete IL-4. It is unlikely that the CD4+-TNK cells produced IL-10 because we measured no IL-10 when spleen cells were stimulated with anti-CD3 alone6 or with anti-CD3 plus anti-CD28 (not shown). TLI-spleen cells also secreted higher amounts of TGF-β constitutively compared with spleen from nonirradiated mice (864 ± 352 pg/mL versus 56.5 ± 17.1 pg/mL, TLI versus control; P < .05), and TGF-β levels were not further increased by stimulation with anti-CD3 or anti-CD3/anti-CD28 (not shown). Because TGF-β can inhibit the development of TH1 CD4+ cells,48studies are under way to determine the cellular source of the TGF-β and the role of TGF-β in inhibiting OVA-Tg proliferation and differentiation and GVHD in this transgenic system. One possible source is the CD4+-TNK cell described herein; NK cells have been reported to constitutively produce large amounts of TGF-β.49 Another possibility is a separate CD4+CD25+ regulatory cell, which is believed to be distinct from CD4+-TNK cells. CD4+CD25+ regulatory hybridomas that were isolated from mice with acquired MHC tolerance constitutively produced large amounts of TGF-β,50 and CD4+CD25+ cells have been reported to influence mortality from GVHD.51
The results of the experiments herein provide a mechanism by which TLI preconditioning promotes bidirectional tolerance. TLI preconditioning enriches for a population of CD4+-TNK cells whose constitutive production of IL-4 induces the differentiation of TH2 cells. The preferential development of TH2 cells from both host and donor CD4+ cells may prove to be one important aspect for the development of bidirectional tolerance in TLI-preconditioned mice that have undergone hematopoietic cell transplantation. Indeed, donor TH2 cells have been found to suppress GVHD following infusion of donor T cells.29 Lan et al7 reported similar findings, in that the TNK+ cells from TLI-treated mice protected the mice against the development of lethal GVHD and provided the source of IL-4. Although the authors did not directly examine cytokine production by CD4+ NK+ TCRαβ+ cells, they did note that 80% of the NK+ TCRαβ+ cells were CD4+ cells.7 Of note, Lan et al7examined a fully allogeneic combination, whereas we examined the semiallogeneic combination, raising the possibility that CD4+-TNK cells function in the TLI model of tolerance even in the most MHC-disparate combinations.
We thank the University of Iowa fluorescence-activated cell-sorting CORE facility and Justin Fishbaugh for assistance with flow cytometry, the Diabetes Endocrine Research Center CORE for reagents, the Radiation Biology Facility, and Jacqueline Capper for manuscript preparation.
Prepublished online as Blood First Edition Paper, October 24, 2002; DOI 10.1182/blood-2002-05-1513.
Supported by a Merit Review Grant from the Department of Veterans Affairs (E.H.F.), and by US Public Health Service Grants CA45541 (E.H.F.) and DK25295 (E.H.F.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Elizabeth H. Field, Department of Internal Medicine, University of Iowa Roy J. and Lucille A. Carver College of Medicine, 601 Highway 6, 6W33, Veterans Affairs Medical Center, Iowa City, IA 52246; e-mail: liz-field@icva.gov.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal