I have read the very interesting and informative article of Hoover et al,1 which may open new avenues in treating imatinib-refractory and imatinib-sensitive chronic myeloid leukemia (CML). However, when testing the synergic potential of STI + farnesyl transferase inhibitor (FTI) combination on the STI571-sensitive Baf BCR-ABL-s cell line, a deficient statistical proof is shown.
In their Figure 2A, an analysis of variance result is provided with aP = .019 at 24 hours of treatment. The comparison, although, was made between 4 experimental conditions (dimethyl sulfoxide [DMSO], STI571, FTI, and combination), and no post hoc tests (as the Bonferroni one) are reported to compare the most relevant treatments, STI571, and the combination with FTI. So, with the kinetic cell-death data the paper shows, no additive or synergistic interaction can be stated.
Both assays, measuring the annexin V and caspase 3 levels,1(Fig 2F-G) also suggest an increased apoptosis with the combination, but again a lack of adequate statistic analysis hampers any estimation of increased therapeutic effect. A clear-cut evidence of a faster BCR-ABL+, STI571-sensitive cell apoptosis is a desirable goal, if the combined therapy is to be tested in vivo to treat imatinib-sensitive CML.
SCH66336/lonafarnib together with STI571/imatinib shows synergistic killing of BCR/ABL transformed leukemia cells
In our recent Blood manuscript1-1 we conclude that a combination of the farnesyl protein transferase inhibitor (FTI) SCH66336/lonafarnib together with the BCR/ABL kinase inhibitor STI571/imatinib shows synergistic killing of BCR/ABL–transformed leukemia cells. The combination would thus be useful for treating patients with chronic myeloid leukemia (CML). In his letter, Dr Brodsky argues that we use a deficient statistical proof to reach this conclusion. The statistical analysis in question relates to Figure 2A. In our paper we carefully avoided describing the results in this figure as showing a synergistic effect, but rather that “the drug combination showed enhanced killing of STI571-sensitive (s) cells relative to either drug alone.” 1(p1069) We believe this conclusion is adequately supported by the analysis of variance (ANOVA) performed between 2 groups of data: percent viabilities of cells treated with STI571 alone and percent viabilities of cells treated with the STI571/SCH66336 combination. Our conclusion that the drug combination shows synergistic killing is based on our consistent observation that SCH66336/lonafarnib alone has little effect on apoptosis (as measured by annexin V staining), while when given in combination with STI571/imatinib, SCH66336/lonafarnib typically doubles the cytocidal effect (Figure 2F).
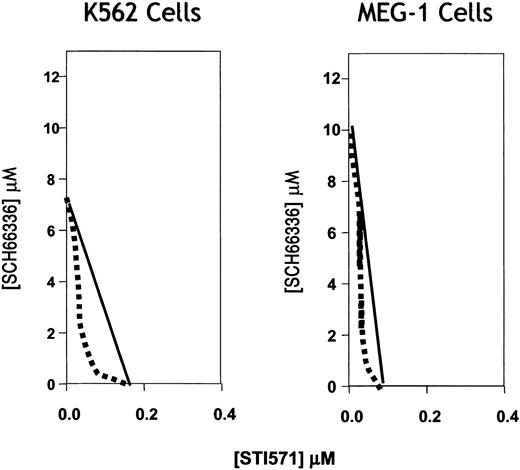
We agree that a more rigorous statistical demonstration of drug synergy is a desirable goal. We and our collaborators have now performed a suitable analysis of the effects on cell viability for combinations of the 2 compounds across a wide range of drug concentrations for K562 and MEG-1 cells, 2 different Philadelphia chromosome–positive cell lines from CML patients (Figure 1-1). Using an appropriate algorithm for isobologram analysis (G. Hajian, L. Hothorn, manuscript submitted), we conclude that indeed the 2 drugs show synergistic killing. The STI/FTI combination will be tested soon in clinical trials.
Representative isoboles for the Philadelphia chromosome–positive K562 and MEG-1 tumor cells treated with various concentrations of SCH66336/lonafarnib and STI571/imatinib.
Ten concentrations of FTI SCH66336 ranging from 20 μM to 0.03 μM and 6 concentrations of STI571 ranging from 2 μM to 0.05 μM were tested. Cell proliferation was quantified on days 2, 3, and 4 after compound addition by methyl-thiazol tetrazolium (MTT) assay. Cell proliferation data from drug interaction studies were analyzed using the Thin Plate Spline methodology (G. Hajian, L. Hothorn, manuscript submitted; O'Connell and Wolfinger1-2) as described previously.1-3 Data (day 3) reflect a left-shifted isobologram for both cell lines, consistent with a synergistic combination effect on cell viability.
Representative isoboles for the Philadelphia chromosome–positive K562 and MEG-1 tumor cells treated with various concentrations of SCH66336/lonafarnib and STI571/imatinib.
Ten concentrations of FTI SCH66336 ranging from 20 μM to 0.03 μM and 6 concentrations of STI571 ranging from 2 μM to 0.05 μM were tested. Cell proliferation was quantified on days 2, 3, and 4 after compound addition by methyl-thiazol tetrazolium (MTT) assay. Cell proliferation data from drug interaction studies were analyzed using the Thin Plate Spline methodology (G. Hajian, L. Hothorn, manuscript submitted; O'Connell and Wolfinger1-2) as described previously.1-3 Data (day 3) reflect a left-shifted isobologram for both cell lines, consistent with a synergistic combination effect on cell viability.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal