Single-agent thalidomide (THAL) at “conventional” doses (> 100 mg/d) has been evaluated in myelofibrosis with myeloid metaplasia (MMM) based on its antiangiogenic properties and the prominent neoangiogenesis that occurs in MMM. THAL monotherapy at such doses produces approximately a 20% response rate in anemia but is poorly tolerated (an adverse dropout rate of > 50% in 3 months). To improve efficacy and tolerability, we prospectively treated 21 symptomatic patients (hemoglobin level < 10 g/dL or symptomatic splenomegaly) with MMM with low-dose THAL (50 mg/d) along with a 3-month oral prednisone (PRED) taper (beginning at 0.5 mg/kg/d). THAL-PRED was well tolerated in all enrolled patients, with 20 patients (95%) able to complete 3 months of treatment. An objective clinical response was demonstrated in 13 (62%) patients, all improvements in anemia. Among 10 patients who were dependent on erythrocyte transfusions, 7 (70%) improved and 4 (40%) became transfusion independent. Among 8 patients with thrombocytopenia (platelet count < 100 × 109/L), 6 (75%) experienced a 50% or higher increase in their platelet count. In 4 of 21 patients (19%), spleen size decreased by more than 50%. Responses observed were mostly durable after discontinuation of the PRED. The dose of THAL in this study (50 mg/d) was better tolerated than the higher doses used in previous studies. Adverse events associated with corticosteroid therapy were mild and transient. Clinical responses did not correlate with improvements in either intramedullary fibrosis or angiogenesis. THAL-PRED is well tolerated and preliminarily appears to be a promising drug regimen for treating cytopenias in patients with MMM.
Introduction
Myelofibrosis with myeloid metaplasia (MMM) is a clonal hematopoietic stem cell disorder characterized by progressive anemia, leukoerythroblastosis, splenomegaly, progression to acute leukemia, and premature death.1 Median survival in patients afflicted with MMM is 3 to 5 years2 and has not yet been improved by currently available medical therapy. Allogeneic hematopoietic stem cell transplantation is potentially curative in a subset of young patients with MMM,3 yet it carries significant risks of transplantation-related mortality and graft-versus-host disease. Autologous hematopoietic stem cell transplantation is also of potential palliative benefit in the management of MMM,4 but it lacks a clear survival benefit and may be too rigorous for many patients with MMM to endure. Currently, only modest benefits have been reported with medical therapies for the palliation of either MMM-associated anemia (erythropoietin,5 androgens6,7) or splenomegaly (hydroxyurea,8interferon-α2b9). Unfortunately, none of these regimens confers a survival benefit or demonstrable change in the intramedullary manifestations of the disease. The lack of effective therapy for MMM has led to intense study into the pathogenetic mechanisms of the disease and the use of targeted therapeutic approaches.
The current pathogenetic hypothesis in MMM is that clonal proliferation of megakaryocytes and monocytes is accompanied by an abnormal cytokine release (transforming growth factor-beta [TGF-β],10,11basic fibroblast growth factor [bFGF],12 and platelet-derived growth factor [PDGF]13) that mediates a detrimental bone marrow stromal reaction.14 The resultant secondary processes occurring in the marrow include polyclonal proliferation of fibroblasts and osteoblasts associated with collagen fibrosis and new bone formation, respectively.15 In addition, the progressive neoangiogenesis has been demonstrated in 98% of patients and is prognostically detrimental in MMM.16Indeed, progression of angiogenesis appears to accompany the acceleration of essential thrombocythemia into the myelofibrotic state (ie, postthrombocythemic myeloid metaplasia).17 The potential pathogenetic role of angiogenesis and associated proangiogenic cytokines led to the interest in piloting the use of the cytokine/angiogenesis inhibitor thalidomide in patients with MMM.
Thalidomide (THAL), initially developed in the 1950s as a sedative hypnotic agent, exhibits potent antiangiogenic18properties through the inhibition of bFGF and possibly vascular endothelial growth factor (VEGF).18,19 Clinically, THAL has activity in patients with another angiogenesis-associated20 intramedullary clonal disorder, multiple myeloma.21 Subsequent pilot trials using THAL were conducted in MMM based on the agent's antiangiogenic and anticytokine properties.22-27 To date, 79 patients with MMM in these studies have been treated with escalating THAL daily doses of 100 to 800 mg orally. Palliative benefit was observed in MMM-associated anemia (0%-50%) and thrombocytopenia (25%-80%), with minimal impact on disease-associated splenomegaly. Unfortunately, although THAL was active, it was also poorly tolerated at the doses used. Up to 91% of patients with MMM discontinued therapy because of side effects.22 In addition, undesired exuberant myeloproliferation was observed with adverse clinical consequences in some MMM patients.28 However, in our later trial, we found that in 3 patients who had their doses of THAL reduced secondary to side effects (200 mg down to 50 mg), the agent was better tolerated and responses were maintained at the lower dose.24
We hypothesized that a low daily dose of THAL (50 mg/d) might be preferred in the treatment of MMM, because tolerability of THAL appears to be dose dependent and because the therapeutic benefit of THAL in MMM was maintained with dose reduction. Furthermore, we felt that a better therapeutic benefit might be achieved if THAL were combined with corticosteroids. The combination of THAL and corticosteroids results in an improved response in multiple myeloma.29,30Corticosteroids, alone or in combination, occasionally have palliative benefit in managing the anemia associated with MMM,1presumably via the effects on the excess cytotoxic (CD3+/CD56+) T lymphocytes.31 We speculate that because corticosteroids are occasionally helpful in MMM, their combination with low-dose THAL may augment each of these agents' individual activities in this disease, and be better tolerated than higher doses of either agent.
Patients, materials, and methods
Patients
After obtaining approval from the Mayo Clinic Institutional Review Board, 21 patients meeting standard diagnostic criteria for MMM1 were enrolled in the study. All subtypes of MMM were eligible (agnogenic myeloid metaplasia [AMM], postpolycythemic myeloid metaplasia [PPMM], and postthrombocythemic myeloid metaplasia [PTMM]). All patients underwent pretreatment physical examination, baseline laboratory assessment of serum chemistries and circulating hematologic parameters, and bone marrow examination with cytogenetic and fluorescent in situ hybridization (FISH) studies to exclude occult chronic myeloid leukemia. Lymphocyte numbers and phenotypic subsets were measured serially to assess any effect of therapy on these parameters. Effect on extramedullary hematopoiesis was assessed by a whole-body technetium sulfur colloid radionuclide scan (99mTc-SC).
THAL was administered by rigorously adhering to the Celgene STEPS program for THAL safety; patients received an oral 50-mg daily dose. For the first 3 months, a tapering regimen of prednisone (PRED) was also administered (0.5 mg/kg/d for 1 month; 0.25 mg/kg/d for the second month; 0.125 mg/kg/d for the third month). Patients showing any evidence of response after 3 months of combination therapy were treated for an additional 3 months with only 50 mg THAL daily. Primary end points for response were improvements in anemia, thrombocytopenia, or hepatosplenomegaly. Secondary end points of response included improvement in either constitutional symptoms (fatigue, night sweats, fever, bone pain), bone marrow features of MMM (reticulin fibrosis, osteosclerosis, angiogenesis, karyotype), or radiographic evidence of extramedullary hematopoiesis (EMH) (99mTc-SC scans). The National Cancer Institute Common Toxicity Criteria (version 2.0) were used to evaluate side effects.
Estimation of effects of therapy on the in vitro myeloid progenitor growth
Assessment of THAL-PRED on the quantity and subtypes of circulating myeloid progenitors were made. Peripheral blood mononuclear cells (PBMCs) were isolated from 10 mL EDTA (ethylenediaminetetraacetic acid)–anticoagulated peripheral blood by double-layer Ficoll-Hypaque density centrifugation according to the method described by English and Andersen.32 The top layer representing PBMCs was removed, washed in Dulbecco phosphate-buffered saline, and counted on a hemocytometer. PBMCs were then resuspended in Iscove modified Dulbecco medium (Life Technology, Rockville, MD) with 20% heat-inactivated fetal bovine serum (Biosource, Walkersville, MD). Isolated PBMCs were then plated in growth-factor–containing methylcellulose medium Methocult (Stem Cell Technologies; Vancouver, BC, Canada). After incubation for 14 days at 37°C in 5% CO2, myeloid (granulocyte-macrophage colony-forming units [CFU-GMs]) and erythroid (erythrocyte colony-forming units [CFU-Es], erythroid burst-forming units [BFU-Es]) colonies were counted by light microscopy using morphologic criteria established by Stem Cell Technologies.
Estimation of antiangiogenic effect of therapy on patients with MMM
To assess the effect of THAL-PRED on angiogenesis, 2 methods were used: immunohistochemistry and cytokine analyses. First, bone marrow trephines were subjected to immunohistochemistry with an antibody to CD34 and graded in a semiquantitative fashion consistent with our previously published method.16 Estimations of intramedullary angiogenesis were performed at the time of registration and after 6 months of therapy by 2 of the authors in a blinded fashion (R.A.M. and C.-Y.L.). The second method of assessing the effects of THAL-PRED on angiogenesis was by serial measurements of proangiogenic cytokines in the urine of the enrolled patients with MMM. Enzyme-linked immunosorbent assays (ELISAs) were performed on the urine of the enrolled patients at the time of registration and after 3 and 6 months of therapy with THAL-PRED, respectively, for the cytokines VEGF, bFGF, and thrombospondin 1 (TSP-1). Cytokine measurements were then corrected for urinary creatinine measurements as has been previously been reported.33
Estimation of effect of therapy on EMH by 99mTc-SC scans
Technetium 99m sulfur colloid (99mTc-SC), a radiopharmaceutical that is phagocytosed by reticuloendothelial cells, is an accepted method for imaging both bone marrow and EMH.3499mTc-SC (14-16 mCi [518-592 MBq]) scans were performed at the time of registration and after 3 months of therapy and were reviewed in a blinded fashion by an experienced nuclear medicine specialist. Uptake in the spleen, liver, bone marrow (volume and distribution), and lungs were graded from −2 (markedly decreased) to 0 (normal) to +2 (markedly increased). Scans were then serially assessed for radiographic changes in EMH as a result of the THAL-PRED therapy.
Statistical analysis
Twenty-one patients were enrolled in a one-stage phase 2 clinical trial to test the null hypothesis that the true response probability is 10% or less versus the hypothesis that the true response probability is more than 10%. The final decision rule was that at least 6 of the first 20 patients needed to experience a response to conclude that the drug combination shows evidence of promising activity. An exact 95% binomial confidence interval was used to estimate the true response rate.
To determine whether there is an association between response and various bone marrow features of MMM or response and radiographic evidence of EMH (99mTc-SC scans), the following analyses were conducted. Fisher exact test was used to assess the significance of the difference between distributions of categorical data. The Wilcoxon rank sum test or the Kruskal-Wallis test was used to assess whether continuous variables differ significantly between categories. All data were analyzed using SAS software (SAS, Cary, NC). Corrections were made for multiple comparisons.
Results
Patients and drug toxicity
Twenty-one patients meeting the entry criteria enrolled in this trial. The patients exhibited a typical patient demographic and symptomatic distribution for the disorder. The median age at the time of enrollment was 63 years (range, 46-74 years), and 5 of the patients (24%) were women. Fifteen of the patients (47%) had AMM, with the remainder having developed myelofibrosis following pre-existing myeloproliferative disorders (PPMM and PTMM). At registration, 10 patients were dependent on erythrocyte transfusions, and 8 patients had clinically relevant thrombocytopenia (baseline platelet count < 100 × 109/L). In addition, the patients had active disease, with 16 patients (76%) having an adverse Dupriez35 MMM prognostic score (≥ 1). Additional baseline clinical characteristics are summarized in Table1. The patients were begun on oral THAL, 50 mg/d, and the tapering dose of PRED.
Characteristics of 21 patients with MMM treated with THAL-PRED
| Patient characteristic . | . |
|---|---|
| Type of MMM, no. (%) | |
| AMM | 15 (72) |
| PPMM | 3 (14) |
| PTMM | 3 (14) |
| Sex, no. (%) | |
| Male | 16 (76) |
| Female | 5 (24) |
| Age, y, mean (range) | |
| At diagnosis | 61 (38-73) |
| At time of trial | 63 (46-74) |
| Dupriez MMM prognostic score,35 no. (%) | |
| 0 | 5 (24) |
| 1 | 10 (48) |
| 2 | 6 (28) |
| MMM-related symptoms, no. (%) | |
| Weight loss (> 20% baseline) | 5 (24) |
| Fevers | 3 (14) |
| Night sweats | 6 (29) |
| Bone pain | 3 (14) |
| Laboratory studies/physical exam at enrollment, mean (range) | |
| Hemoglobin level, g/dL | 9 (7.2-12.9) |
| Leukocyte count, × 109/L | 7.7 (1.4-25.6) |
| Absolute neutrophil count, × 109/L | 3.6 (0.7-14.8) |
| Immature myeloid (nonblasts) | 7% (0%-21%) |
| Myeloblasts | 1% (0%-19%) |
| Nucleated erythrocytes/100 leukocytes | 1.0% (0%-23%) |
| Peripheral blood CD34+ count/μL, n = 6 | 84 (3-1890) |
| Platelet count, × 109/L | 157 (23-448) |
| Lactate dehydrogenase, U/L; 112-257 normal range | 515 (170-766) |
| Alkaline phosphatase, U/L; 98-251 normal range | 232 (117-802) |
| Aspartate transaminase, U/L | 26 (9-107) |
| Creatinine, mg/dL | 1.1 (0.8-2.0) |
| Spleen size, cm below left costal margin | 14 (0-26) |
| Patient characteristic . | . |
|---|---|
| Type of MMM, no. (%) | |
| AMM | 15 (72) |
| PPMM | 3 (14) |
| PTMM | 3 (14) |
| Sex, no. (%) | |
| Male | 16 (76) |
| Female | 5 (24) |
| Age, y, mean (range) | |
| At diagnosis | 61 (38-73) |
| At time of trial | 63 (46-74) |
| Dupriez MMM prognostic score,35 no. (%) | |
| 0 | 5 (24) |
| 1 | 10 (48) |
| 2 | 6 (28) |
| MMM-related symptoms, no. (%) | |
| Weight loss (> 20% baseline) | 5 (24) |
| Fevers | 3 (14) |
| Night sweats | 6 (29) |
| Bone pain | 3 (14) |
| Laboratory studies/physical exam at enrollment, mean (range) | |
| Hemoglobin level, g/dL | 9 (7.2-12.9) |
| Leukocyte count, × 109/L | 7.7 (1.4-25.6) |
| Absolute neutrophil count, × 109/L | 3.6 (0.7-14.8) |
| Immature myeloid (nonblasts) | 7% (0%-21%) |
| Myeloblasts | 1% (0%-19%) |
| Nucleated erythrocytes/100 leukocytes | 1.0% (0%-23%) |
| Peripheral blood CD34+ count/μL, n = 6 | 84 (3-1890) |
| Platelet count, × 109/L | 157 (23-448) |
| Lactate dehydrogenase, U/L; 112-257 normal range | 515 (170-766) |
| Alkaline phosphatase, U/L; 98-251 normal range | 232 (117-802) |
| Aspartate transaminase, U/L | 26 (9-107) |
| Creatinine, mg/dL | 1.1 (0.8-2.0) |
| Spleen size, cm below left costal margin | 14 (0-26) |
Therapy with THAL-PRED was very well tolerated. Twenty patients (95%) were able to complete the first 3 months of treatment without dose modification. One patient (patient no. 10) experienced blastic transformation of his disease shortly after beginning the THAL-PRED therapy and discontinued therapy. Adverse events associated with THAL-PRED were mild and included constipation (38%), leukocytosis (38%), mild neuropathy (≤ grade 2; 29%), and mild sedation (29%; Table 2). Corticosteroid-associated adverse events including hyperglycemia (24%), mild visual changes (19%), and anxiety (19%) were all mild and transient.
Comparison of toxicity between single-agent THAL and low-dose THAL-PRED therapy in MMM
| Toxicity . | No. of patients affected (%) . | ||
|---|---|---|---|
| THAL + PRED . | Grade 2/grade 3 or higher . | THAL,24 100-400 mg . | |
| Leukocytosis* | 8 (38) | 6/2 (29) | 2 (13) |
| Thrombocytosis | 4 (19) | 3/2 (14/10) | 3 (20) |
| Constipation | 8 (38) | 3 (14) | 9 (60) |
| Edema | 5 (24) | 2 (10) | 3 (20) |
| Visual disturbance | 4 (19) | 2 (10) | 2 (13) |
| Fatigue | 2 (10) | 2 (10) | 10 (67) |
| Thrombosis | 1 (5) | 1/1 (5/5) | 0 (0) |
| Paresthesias | 6 (29) | 1 (5) | 4 (33) |
| Sedation | 6 (29) | 1 (5) | 3 (20) |
| Neutropenia | |||
| Less than 1500/μL | 5 (24) | 1 (5) | 6 (40) |
| Less than 1000/μL | 4 (19) | 4 (19) | 4 (27) |
| Anxiety | 4 (19) | 0 (0) | 3 (20) |
| Rash | 3 (14) | 0 (0) | 4 (33) |
| Orthostatic symptoms | 0 (0) | 0 (0) | 5 (33) |
| Tremor | 0 (0) | 0 (0) | 3 (20) |
| Myeloproliferative reaction | 0 (0) | 0 (0) | 3 (20) |
| Dry mouth | 0 (0) | 0 (0) | 2 (13) |
| Sinus bradycardia | 0 (0) | 0 (0) | 2 (13) |
| Abdominal pain | 0 (0) | 0 (0) | 2 (13) |
| Decreased hearing | 0 (0) | 0 (0) | 2 (13) |
| Anorexia | 0 (0) | 0 (0) | 1 (7) |
| Confusion | 0 (0) | 0 (0) | 1 (7) |
| Dry eyes | 0 (0) | 0 (0) | 1 (7) |
| Depression | 0 (0) | 0 (0) | 1 (7) |
| Toxicity . | No. of patients affected (%) . | ||
|---|---|---|---|
| THAL + PRED . | Grade 2/grade 3 or higher . | THAL,24 100-400 mg . | |
| Leukocytosis* | 8 (38) | 6/2 (29) | 2 (13) |
| Thrombocytosis | 4 (19) | 3/2 (14/10) | 3 (20) |
| Constipation | 8 (38) | 3 (14) | 9 (60) |
| Edema | 5 (24) | 2 (10) | 3 (20) |
| Visual disturbance | 4 (19) | 2 (10) | 2 (13) |
| Fatigue | 2 (10) | 2 (10) | 10 (67) |
| Thrombosis | 1 (5) | 1/1 (5/5) | 0 (0) |
| Paresthesias | 6 (29) | 1 (5) | 4 (33) |
| Sedation | 6 (29) | 1 (5) | 3 (20) |
| Neutropenia | |||
| Less than 1500/μL | 5 (24) | 1 (5) | 6 (40) |
| Less than 1000/μL | 4 (19) | 4 (19) | 4 (27) |
| Anxiety | 4 (19) | 0 (0) | 3 (20) |
| Rash | 3 (14) | 0 (0) | 4 (33) |
| Orthostatic symptoms | 0 (0) | 0 (0) | 5 (33) |
| Tremor | 0 (0) | 0 (0) | 3 (20) |
| Myeloproliferative reaction | 0 (0) | 0 (0) | 3 (20) |
| Dry mouth | 0 (0) | 0 (0) | 2 (13) |
| Sinus bradycardia | 0 (0) | 0 (0) | 2 (13) |
| Abdominal pain | 0 (0) | 0 (0) | 2 (13) |
| Decreased hearing | 0 (0) | 0 (0) | 2 (13) |
| Anorexia | 0 (0) | 0 (0) | 1 (7) |
| Confusion | 0 (0) | 0 (0) | 1 (7) |
| Dry eyes | 0 (0) | 0 (0) | 1 (7) |
| Depression | 0 (0) | 0 (0) | 1 (7) |
Single-agent THAL treatment consisted of 200 mg/d or higher. The THAL dose in the combined therapy was 50 mg/d, plus prednisone in tapered doses as described in “Patients, materials, and methods.” All toxicities were graded according to the Common Toxicity Criteria Version 2.0 of the National Cancer Institute. For a diagnosis of thrombocytosis, the platelet increases must be above normal limits. For leukocytosis, the leukocyte increases must be above normal limits.
One patient transformed to acute myeloid leukemia.
Although undesired leukocytosis and thrombocytosis were observed in 38% and 19% of the patients, respectively, a clinical consequence was observed in only one patient (deep venous thrombosis). In the patient with thrombosis, the platelet count at registration was 446 × 109/L and rose to 570 × 109/L at the time of the thrombotic event. Exuberant and undesirable myeloproliferation was a noted adverse event in the prior trials with THAL as a single agent at higher doses. To assess for the development of any undesired EMH, serial assessments of 99mTc-SC scans were performed. After blinded review by an experienced nuclear medicine specialist, there was no demonstrable increase in uptake of the radiocolloid in either the liver, spleen, or any other anatomic site as a result of the THAL-PRED regimen.
THAL-PRED improves cytopenias
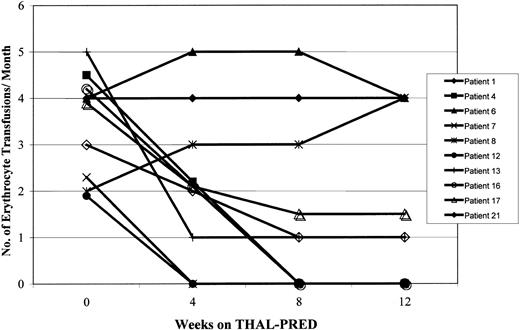
THAL-PRED was very active in alleviating MMM-associated cytopenias. An objective and sustained clinical response occurred in the anemia of 13 patients (62%; 95% exact CI, 38%-82%). Overall, the median hemoglobin value increased by 1.8 g/dL (range, 0.1-5.1 g/dL). Seven of the 10 patients who were dependent on erythrocyte transfusions at study entry had a clinical response; 4 (40%) became transfusion independent (Figure 1), and the rest had a meaningful decrease in transfusion requirements (≥ 50%). Among those not dependent on transfusions (n = 11; 7 [64%] with hemoglobin level < 10 g/dL), the hemoglobin value increased by 2.1 g/dL (range, 0.1-5.1 g/dL). All 8 patients who had clinically relevant thrombocytopenia on study entry (platelet count < 100 × 109/L) had an increase in their platelet count, of whom 6 (75%) had a more than 50% increase in their platelet count (Table 3; Figure2).
Effects of THAL-PRED on erythrocyte transfusion requirements.
The effects of THAL-PRED on the erythrocyte transfusion requirements are shown for the 10 patients with transfusion-dependent MMM at study onset.
Effects of THAL-PRED on erythrocyte transfusion requirements.
The effects of THAL-PRED on the erythrocyte transfusion requirements are shown for the 10 patients with transfusion-dependent MMM at study onset.
Clinical effects of THAL-PRED on 21 patients with MMM
| Demographics . | THAL-PRED effects . | Responses . | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient . | Age . | Sex . | MMM type . | Cell . | RF . | OS . | AG . | Karyotype . | Hb start . | Hb max . | PLT start . | PLT max . | Spleen start . | Spleen min . | CD34+ at start . | CD34+ at 3 mo . | Anemia . | Platelet . | Spleen . |
| 1 | 72 | F | AMM | 30 | 2.5 | 0 | 4 | Normal | 9.73-150 | 10.73-150 | 24 | 35 | 9 | 9 | 851 | 1288 | N | N | N |
| 2 | 55 | M | PPMM | 95 | 1.5 | 0 | 4 | (18p+) | 12.9 | 15.2 | 303 | 477 | 15 | 13 | 24 | 17 | Y | NA | N |
| 3 | 78 | M | AMM | 70 | 2.0 | 0 | 4 | Normal | 8.4 | 11.3 | 154 | 389 | 22 | 13 | 32 | 41 | Y | NA | Y |
| 4 | 55 | M | AMM | 80 | 2.5 | 0 | 3 | Normal | 9.63-150 | 10.8 | 127 | 214 | 10 | 6 | 14 | 12 | Y | NA | N |
| 5 | 73 | F | AMM | 5 | 3.0 | 0 | 3 | t(1;10) | 7.9 | 13 | 95 | 228 | 5 | 0 | 104 | 13 | Y | Y | Y |
| 6 | 66 | M | PTMM | 30 | 2.5 | 0 | 3 | t(3;4) | 7.23-150 | 8.73-150 | 157 | 323 | 12 | 12 | 447 | 4437 | N | NA | N |
| 7 | 74 | M | AMM | 50 | 2.0 | 0 | 4 | Normal | 93-150 | 9.8 | 448 | 890 | 20 | 17 | 63 | 371 | Y | NA | N |
| 8 | 64 | M | AMM | 95 | 1.0 | 0 | 4 | (+21) | 7.53-150 | 9.63-150 | 138 | 167 | 20 | 14 | 1211 | 3913 | N | NA | N |
| 9 | 60 | M | AMM | 80 | 2.0 | 2 | 4 | (13q−) | 11 | 11.1 | 32 | 37 | 26 | 26 | 43 | 744 | N | N | N |
| 10 | 73 | M | AMM | 15 | 2.0 | 2 | 3 | t(1;7) | 9.8 | 11.5 | 38 | 79 | 8 | 8 | 1891 | NA | N | Y | N |
| 11 | 64 | F | PPMM | 30 | 2.0 | 2 | 3 | (20q−) | 10.9 | 13 | 186 | 391 | 8 | 4 | 84 | 100 | Y | NA | Y |
| 12 | 69 | M | AMM | 40 | 1.5 | 0 | 4 | Normal | 8.33-150 | 10.4 | 32 | 96 | 0 | 0 | 3 | 3 | Y | Y | N |
| 13 | 74 | M | AMM | 40 | 2.0 | 0 | 4 | (20q−) | 8.33-150 | 9.73-150 | 37 | 101 | 20 | 18 | 15 | 13 | Y | Y | N |
| 14 | 46 | M | AMM | 30 | 1.5 | 3 | 3 | Normal | 9 | 10.6 | 275 | 540 | 14 | 6 | 206 | 132 | Y | NA | Y |
| 15 | 49 | M | MMM | 95 | 2.0 | 0 | 4 | Normal | 8.3 | 11 | 192 | 390 | 15 | 11 | 119 | 73 | Y | NA | N |
| 16 | 66 | F | AMM | 5 | 2.0 | 0 | 3 | Complex | 8.83-150 | 11.2 | 74 | 192 | 0 | 0 | 83 | 1010 | Y | Y | N |
| 17 | 62 | M | AMM | 5 | 2.5 | 0 | 3 | NA | 9.43-150 | 11.23-150 | 162 | 316 | 8 | 8 | 354 | 427 | Y | NA | N |
| 18 | 43 | M | AMM | 30 | 2.0 | 3 | 4 | Normal | 10.7 | 11.7 | 179 | 415 | 18 | 15 | 273 | 271 | N | NA | N |
| 19 | 75 | M | PTMM | 70 | 2.0 | 1 | 4 | (20q−) | 8.3 | 10.7 | 231 | 328 | 20 | 17 | 42 | 61 | N | NA | N |
| 20 | 63 | M | PPMM | 80 | 1.5 | 0 | 4 | Normal | 12 | 13.4 | 446 | 569 | 25 | 25 | 151 | 166 | N | NA | N |
| 21 | 73 | F | PTMM | 5 | 2.0 | 0 | 2 | Normal | 8.63-150 | 10.43-150 | 23 | 44 | 6 | 5 | 103 | 28 | Y | Y | N |
| Demographics . | THAL-PRED effects . | Responses . | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient . | Age . | Sex . | MMM type . | Cell . | RF . | OS . | AG . | Karyotype . | Hb start . | Hb max . | PLT start . | PLT max . | Spleen start . | Spleen min . | CD34+ at start . | CD34+ at 3 mo . | Anemia . | Platelet . | Spleen . |
| 1 | 72 | F | AMM | 30 | 2.5 | 0 | 4 | Normal | 9.73-150 | 10.73-150 | 24 | 35 | 9 | 9 | 851 | 1288 | N | N | N |
| 2 | 55 | M | PPMM | 95 | 1.5 | 0 | 4 | (18p+) | 12.9 | 15.2 | 303 | 477 | 15 | 13 | 24 | 17 | Y | NA | N |
| 3 | 78 | M | AMM | 70 | 2.0 | 0 | 4 | Normal | 8.4 | 11.3 | 154 | 389 | 22 | 13 | 32 | 41 | Y | NA | Y |
| 4 | 55 | M | AMM | 80 | 2.5 | 0 | 3 | Normal | 9.63-150 | 10.8 | 127 | 214 | 10 | 6 | 14 | 12 | Y | NA | N |
| 5 | 73 | F | AMM | 5 | 3.0 | 0 | 3 | t(1;10) | 7.9 | 13 | 95 | 228 | 5 | 0 | 104 | 13 | Y | Y | Y |
| 6 | 66 | M | PTMM | 30 | 2.5 | 0 | 3 | t(3;4) | 7.23-150 | 8.73-150 | 157 | 323 | 12 | 12 | 447 | 4437 | N | NA | N |
| 7 | 74 | M | AMM | 50 | 2.0 | 0 | 4 | Normal | 93-150 | 9.8 | 448 | 890 | 20 | 17 | 63 | 371 | Y | NA | N |
| 8 | 64 | M | AMM | 95 | 1.0 | 0 | 4 | (+21) | 7.53-150 | 9.63-150 | 138 | 167 | 20 | 14 | 1211 | 3913 | N | NA | N |
| 9 | 60 | M | AMM | 80 | 2.0 | 2 | 4 | (13q−) | 11 | 11.1 | 32 | 37 | 26 | 26 | 43 | 744 | N | N | N |
| 10 | 73 | M | AMM | 15 | 2.0 | 2 | 3 | t(1;7) | 9.8 | 11.5 | 38 | 79 | 8 | 8 | 1891 | NA | N | Y | N |
| 11 | 64 | F | PPMM | 30 | 2.0 | 2 | 3 | (20q−) | 10.9 | 13 | 186 | 391 | 8 | 4 | 84 | 100 | Y | NA | Y |
| 12 | 69 | M | AMM | 40 | 1.5 | 0 | 4 | Normal | 8.33-150 | 10.4 | 32 | 96 | 0 | 0 | 3 | 3 | Y | Y | N |
| 13 | 74 | M | AMM | 40 | 2.0 | 0 | 4 | (20q−) | 8.33-150 | 9.73-150 | 37 | 101 | 20 | 18 | 15 | 13 | Y | Y | N |
| 14 | 46 | M | AMM | 30 | 1.5 | 3 | 3 | Normal | 9 | 10.6 | 275 | 540 | 14 | 6 | 206 | 132 | Y | NA | Y |
| 15 | 49 | M | MMM | 95 | 2.0 | 0 | 4 | Normal | 8.3 | 11 | 192 | 390 | 15 | 11 | 119 | 73 | Y | NA | N |
| 16 | 66 | F | AMM | 5 | 2.0 | 0 | 3 | Complex | 8.83-150 | 11.2 | 74 | 192 | 0 | 0 | 83 | 1010 | Y | Y | N |
| 17 | 62 | M | AMM | 5 | 2.5 | 0 | 3 | NA | 9.43-150 | 11.23-150 | 162 | 316 | 8 | 8 | 354 | 427 | Y | NA | N |
| 18 | 43 | M | AMM | 30 | 2.0 | 3 | 4 | Normal | 10.7 | 11.7 | 179 | 415 | 18 | 15 | 273 | 271 | N | NA | N |
| 19 | 75 | M | PTMM | 70 | 2.0 | 1 | 4 | (20q−) | 8.3 | 10.7 | 231 | 328 | 20 | 17 | 42 | 61 | N | NA | N |
| 20 | 63 | M | PPMM | 80 | 1.5 | 0 | 4 | Normal | 12 | 13.4 | 446 | 569 | 25 | 25 | 151 | 166 | N | NA | N |
| 21 | 73 | F | PTMM | 5 | 2.0 | 0 | 2 | Normal | 8.63-150 | 10.43-150 | 23 | 44 | 6 | 5 | 103 | 28 | Y | Y | N |
Age indicates patient's age at registration; cell, bone marrow cellularity; RF, reticulin fibrosis grade 0-4; OS, osteosclerosis grade 0-4; AG, angiogenesis grade 1-4; Karyotype, G-banded karyotype on 20 metaphases from bone marrow aspirate at time of registration; Hb, hemoglobin in g/dL; PLT, platelet count (× 109/L); Spleen, spleen size (cm below the left costal margin by palpation); CD34+, CD34+ cell enumeration in peripheral blood by flow cytometry (cells/μL); Y, yes; N, no; NA, not applicable.
Dependent on erythrocyte transfusions.
Effects of THAL-PRED on platelet counts.
The effects of THAL-PRED on the untransfused platelet counts are shown for the 8 patients with MMM who were thrombocytopenic (baseline platelet count < 100 × 109/L) at study onset.
Effects of THAL-PRED on platelet counts.
The effects of THAL-PRED on the untransfused platelet counts are shown for the 8 patients with MMM who were thrombocytopenic (baseline platelet count < 100 × 109/L) at study onset.
Additional effects of THAL-PRED
Only modest palliative benefits with the THAL-PRED therapy occurred with respect to splenomegaly. Four patients (19%) developed a more than 50% decrease in palpable splenomegaly (Table 3). No significant or persistent decrease in the amount of splenic EMH occurred as measured by serial assessments of splenic uptake of99mTc-SC radiocolloid. No consistent decrease in constitutional symptoms was observed; however, improvements in constitutional symptoms are subjective and improvements are difficult to quantify.
Little appreciable change in intramedullary manifestations of disease occurred among MMM patients who responded to THAL-PRED therapy (Table4). No significant changes occurred in the bone marrow cellularity, reticulin fibrosis, or osteosclerosis among responders. In addition, karyotype abnormalities did not change during the treatment course; however, no new karyotypic abnormalities developed. No appreciable changes in the markers of intramedullary angiogenesis occurred as assessed by microvessel density grading by light microscopy of marrow trephines subjected to immunohistochemistry for CD34, urinary excretion of the proangiogenic cytokines VEGF or bFGF, or plasma TSP-1 levels (Table 3). Furthermore, no consistent changes in the in vitro growth of immature myeloid progenitors in the methylcellulose cultures occurred in response to therapy. This included a lack of change in erythroid progenitors (BFU-Es or CFU-Es) in patients with an anemic response, or myeloid colony growth (ie, granulocyte-erythrocyte-macrophage-megakaryocyte colony-forming units [CFU-GEMMs], CFU-GMs, or other colony type) in patients with leukocytosis. Changes that occurred in lymphocyte number or subtype were inconsistent and did not correlate with either response or adverse events.
Changes in bone marrow and urinary manifestations of angiogenesis and myelofibrosis in 10 patients with MMM completing 6 months of THAL-PRED therapy
| Patient no. . | Responses . | Cellularity, % . | RF . | Osteosclerosis . | Angiogenesis . | Karyotype . | Urinary VEGF . | Urinary bFGF . | Urinary TSP . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hb . | PLT . | Spleen . | Time 0 . | 6 mo . | Time 0 . | 6 mo . | Time 0 . | 6 mo . | Time 0 . | 6 mo . | Time 0 . | 6 mo . | Time 0 . | 6 mo . | Time 0 . | 6 mo . | Time 0 . | 6 mo . | |
| 2 | Y | NA | N | 95 | 90 | 1.5 | 1.5 | 0 | 0 | 4 | 4 | 18p+ | 18+ | NA | 224.33 | NA | 2650 | NA | 32 087 |
| 3 | Y | NA | Y | 70 | 80 | 2.0 | 2 | 0 | 0 | 4 | 4 | Normal | Normal | 82.44 | 75.72 | 2121 | 5906 | 37 802 | 44 194 |
| 4 | Y | NA | N | 80 | 80 | 2.5 | 2.5 | 0 | 0 | 3 | 3 | Normal | Normal | 63.74 | 15.49 | 2039 | 1609 | 46 902 | 33 135 |
| 5 | Y | Y | Y | 5 | 5 | 3.0 | 3 | 0 | 0 | 3 | 2 | t(1;10) | NA | 58.94 | 89.18 | 4037 | 4520 | 56 000 | 57 213 |
| 7 | Y | NA | N | 50 | 70 | 2.0 | 1.5 | 0 | 0 | 4 | 3 | Normal | Normal | 21.04 | 12.18 | 1647 | 2988 | 27 299 | 32 246 |
| 11 | Y | NA | Y | 30 | 35 | 2.0 | 1.5 | 2 | 2 | 3 | 3 | 20q− | 20q− | 32.90 | 60.43 | 2972 | 4030 | 63 200 | 70 189 |
| 12 | Y | Y | N | 40 | 40 | 1.5 | 1 | 0 | 0 | 4 | 4 | Normal | Normal | 55.77 | 28.83 | 5062 | 2359 | 58 519 | 35 742 |
| 13 | Y | Y | N | 40 | 55 | 2.0 | 2 | 0 | 0 | 4 | 4 | 20q− | 20q− | 190.16 | 155.97 | 2430 | 3264 | 53 153 | 54 597 |
| 14 | Y | NA | Y | 30 | 50 | 1.5 | 2.5 | 3 | 2.5 | 3 | 4 | Normal | Normal | 132.89 | 37.78 | 1118 | 939 | 35 862 | 13 944 |
| 21 | Y | Y | N | 5 | 10 | 2.0 | 2 | 0 | 0 | 2 | 1 | Normal | Normal | 26.88 | 68.52 | 4606 | 3692 | 49 375 | 66 872 |
| Patient no. . | Responses . | Cellularity, % . | RF . | Osteosclerosis . | Angiogenesis . | Karyotype . | Urinary VEGF . | Urinary bFGF . | Urinary TSP . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hb . | PLT . | Spleen . | Time 0 . | 6 mo . | Time 0 . | 6 mo . | Time 0 . | 6 mo . | Time 0 . | 6 mo . | Time 0 . | 6 mo . | Time 0 . | 6 mo . | Time 0 . | 6 mo . | Time 0 . | 6 mo . | |
| 2 | Y | NA | N | 95 | 90 | 1.5 | 1.5 | 0 | 0 | 4 | 4 | 18p+ | 18+ | NA | 224.33 | NA | 2650 | NA | 32 087 |
| 3 | Y | NA | Y | 70 | 80 | 2.0 | 2 | 0 | 0 | 4 | 4 | Normal | Normal | 82.44 | 75.72 | 2121 | 5906 | 37 802 | 44 194 |
| 4 | Y | NA | N | 80 | 80 | 2.5 | 2.5 | 0 | 0 | 3 | 3 | Normal | Normal | 63.74 | 15.49 | 2039 | 1609 | 46 902 | 33 135 |
| 5 | Y | Y | Y | 5 | 5 | 3.0 | 3 | 0 | 0 | 3 | 2 | t(1;10) | NA | 58.94 | 89.18 | 4037 | 4520 | 56 000 | 57 213 |
| 7 | Y | NA | N | 50 | 70 | 2.0 | 1.5 | 0 | 0 | 4 | 3 | Normal | Normal | 21.04 | 12.18 | 1647 | 2988 | 27 299 | 32 246 |
| 11 | Y | NA | Y | 30 | 35 | 2.0 | 1.5 | 2 | 2 | 3 | 3 | 20q− | 20q− | 32.90 | 60.43 | 2972 | 4030 | 63 200 | 70 189 |
| 12 | Y | Y | N | 40 | 40 | 1.5 | 1 | 0 | 0 | 4 | 4 | Normal | Normal | 55.77 | 28.83 | 5062 | 2359 | 58 519 | 35 742 |
| 13 | Y | Y | N | 40 | 55 | 2.0 | 2 | 0 | 0 | 4 | 4 | 20q− | 20q− | 190.16 | 155.97 | 2430 | 3264 | 53 153 | 54 597 |
| 14 | Y | NA | Y | 30 | 50 | 1.5 | 2.5 | 3 | 2.5 | 3 | 4 | Normal | Normal | 132.89 | 37.78 | 1118 | 939 | 35 862 | 13 944 |
| 21 | Y | Y | N | 5 | 10 | 2.0 | 2 | 0 | 0 | 2 | 1 | Normal | Normal | 26.88 | 68.52 | 4606 | 3692 | 49 375 | 66 872 |
Urinary angiogenesis markers were done by ELISA and corrected for urinary creatinine. VEGF values in are in ng/g creatinine; normal value is 140 ng/g creatinine. bFGF values are in pg/g creatinine; normal value is 11 512 pg/g creatinine. TSP values are in ng/g creatinine; normal value is 57 732 pg/g creatinine. Hb indicates hemoglobin response; PLT, platelet count response; Spleen, spleen response; RF, reticulin fibrosis grade 0-4; Karyotype, G-banded karyotype on 20 metaphases from bone marrow aspirate at time of registration; N, no; NA, not available; Y, yes.
Results after discontinuation of PRED
Among the 13 patients who experienced a clinical benefit with the THAL-PRED therapy, 12 continued on the second phase of the trial (THAL 50 mg/d only). The one patient who had responded to THAL-PRED but did not continue on the study stopped the drugs due to progression of hypercatabolic symptoms, which were not alleviated by the THAL-PRED therapy. Ten of the MMM patients completed both the THAL-PRED phase and subsequent THAL only (3 months 50 mg/d) phase of the trial. After discontinuation of the PRED, clinical responses were maintained in 8 of 13 (62%), 4 of 6 (66%), and 2 of 4 (50%) patients in terms of anemia, thrombocytopenia, and splenomegaly, respectively.
Prognostic indicators of clinical response
Improvements in anemia correlated with lower pretherapy CD34+ cell counts in the peripheral blood (median, 81.2 CD34+ cells/μL versus 554 CD34+ cells/μL [nonresponders]; P = .03) as well as lower numbers of circulating blasts (median, 0.8% versus 4.7% in nonresponders;P = .03; Table 5). Individuals with less neoangiogenesis in the marrow were more likely to respond (P = .03). Improvements in clinically significant thrombocytopenia were associated with older ages (median, 71.4 versus 61.7 years; P = .04), smaller pretreatment spleens (median, 6.5 cm versus 16.1 cm below the left costal margin;P < .01), and hypocellular marrows (median cellularity, 18% versus 58% for nonresponders; P < .01). Thrombocytopenic responders had more splenic uptake on their baseline99mTc-SC scans, as well as higher levels of the antiangiogenic cytokine TSP-1 in the urine (P = .03; Table5). Splenic responses were infrequent (n = 4); however, they did correlate with erythrocyte transfusion dependence (P = .03) as well as increased uptake of the radiocolloid into the lungs and marrow on the 99mTc-SC scans (P = .02 and .03, respectively; Table 5). Responses in either splenomegaly or thrombocytopenia were strongly correlated with a concurrent response in anemia (P < .01).
Correlations between responses to THAL-PRED and patient characteristics
| . | Hemoglobin response, P . | Platelet response, P . | Spleen response, P . |
|---|---|---|---|
| Demographic characteristics | |||
| Age | .77 | .045-150 | .85 |
| Sex | .89 | .08 | .18 |
| MMM subtype | .67 | .58 | .71 |
| Duration of disease | .79 | .72 | .69 |
| Dupriez MMM score35 | .15 | .22 | .58 |
| Spleen size | .32 | < .015-150 | .75 |
| Laboratory values | |||
| Hemoglobin | .74 | .18 | .71 |
| Erythrocyte Tx Dep | .81 | .12 | .035-150 |
| Leukocyte count | .12 | .25 | .89 |
| Platelet count | .69 | < .015-150 | .76 |
| CD34+ cell count | .035-150 | .57 | .39 |
| Blast, % | .035-150 | .48 | .33 |
| LDH | .12 | .23 | .72 |
| Bone marrow results | |||
| Marrow cellularity | .84 | < .015-150 | .39 |
| RF grade | .63 | .60 | .55 |
| OS grade | .23 | .43 | .24 |
| Marked AG, grade 4 | .035-150 | .12 | .22 |
| Abnormal karyotype | .39 | .11 | .63 |
| Ancillary assays | |||
| Spleen grade,99mTc-SC | .15 | .035-150 | .19 |
| Lung EMH,99mTc-SC | .62 | .82 | .025-150 |
| Bone marrow,99mTc-SC uptake | .26 | .36 | .035-150 |
| Urinary VEGF | .45 | .31 | .10 |
| Urinary bFGF | .72 | .50 | .81 |
| Urinary TSP | .90 | .035-150 | .23 |
| . | Hemoglobin response, P . | Platelet response, P . | Spleen response, P . |
|---|---|---|---|
| Demographic characteristics | |||
| Age | .77 | .045-150 | .85 |
| Sex | .89 | .08 | .18 |
| MMM subtype | .67 | .58 | .71 |
| Duration of disease | .79 | .72 | .69 |
| Dupriez MMM score35 | .15 | .22 | .58 |
| Spleen size | .32 | < .015-150 | .75 |
| Laboratory values | |||
| Hemoglobin | .74 | .18 | .71 |
| Erythrocyte Tx Dep | .81 | .12 | .035-150 |
| Leukocyte count | .12 | .25 | .89 |
| Platelet count | .69 | < .015-150 | .76 |
| CD34+ cell count | .035-150 | .57 | .39 |
| Blast, % | .035-150 | .48 | .33 |
| LDH | .12 | .23 | .72 |
| Bone marrow results | |||
| Marrow cellularity | .84 | < .015-150 | .39 |
| RF grade | .63 | .60 | .55 |
| OS grade | .23 | .43 | .24 |
| Marked AG, grade 4 | .035-150 | .12 | .22 |
| Abnormal karyotype | .39 | .11 | .63 |
| Ancillary assays | |||
| Spleen grade,99mTc-SC | .15 | .035-150 | .19 |
| Lung EMH,99mTc-SC | .62 | .82 | .025-150 |
| Bone marrow,99mTc-SC uptake | .26 | .36 | .035-150 |
| Urinary VEGF | .45 | .31 | .10 |
| Urinary bFGF | .72 | .50 | .81 |
| Urinary TSP | .90 | .035-150 | .23 |
P corrected for effects of multiple comparisons. Reticulin fibrosis and osteosclerosis were graded 0 to 4; angiogenesis was graded 1 to 4.
Statistically significant.
Discussion
THAL-PRED is better tolerated than high-dose THAL
Based on the results of several prospective, unblinded, single-arm clinical trials, THAL monotherapy appears to have clinical activity with MMM,22-27 but unfortunately the doses used in published trials have been poorly tolerated. In the later trials (Table6), the starting dose of THAL ranged from 100 to 200 mg, with a goal of achieving a dose of 600 to 1000 mg. These higher THAL doses were poorly tolerated. Of the 79 patients reported in these trials, 48 (61%) were intolerant of THAL and up to 91% discontinued therapy.22 However, in our previous trial,24 reduction of the THAL dose to 50 mg/d in a subset of patients improved drug tolerability without loss of activity. This current report confirms the impression of our previous work, because 95% of patients treated with THAL, 50 mg/d, were able to complete the first 3 months of therapy with the THAL-PRED regimen. The only discontinuation in the first 3 months was secondary to blastic transformation. Although blastic transformation is a potential occurrence in 10% to 15% of patients with MMM,1 only 2 cases of acute leukemia (including this latter patient) have occurred in the 100 MMM patients (2%) who have taken THAL. Therefore, these latter 2 cases of acute leukemia are within the expected range of transformation and are probably not related to the therapy.
Published clinical trials of single-agent THAL in MMM
| Authors and reference no. . | No. of patients . | Min . | Max . | Dropout/DM . | Anemia response . | Platelet response . | Spleen response . |
|---|---|---|---|---|---|---|---|
| Elliott et al24 | 15 | 506-150 | 400 | 12 (80%) | 3/15 (20%) | 4/5 (80%) | 1/15 (7%) |
| Barosi et al22 | 21 | 100 | 400 | 19 (91%) | 3/7 (43%) | 2/3 (66.7%) | 4/20 (20%) |
| Canepa et al23 | 10 | 200 | 800 | 0 | 3/10 (30%) | 3/10 (30%) | 3/10 (30%) |
| Pozzato et al25 | 6 | 100 | NR | NR | 3/6 (50%) | NR | 0/6 |
| Piccaluga et al26 | 12 | 100 | 600 | 11/12 (92%) | 3/12 (25%) | 3/12 (25%) | 2/12 (16.7%) |
| Merup et al27 | 15 | 200 | 800 | 6/15 (40%) | 0 | 0 | 0 |
| Authors and reference no. . | No. of patients . | Min . | Max . | Dropout/DM . | Anemia response . | Platelet response . | Spleen response . |
|---|---|---|---|---|---|---|---|
| Elliott et al24 | 15 | 506-150 | 400 | 12 (80%) | 3/15 (20%) | 4/5 (80%) | 1/15 (7%) |
| Barosi et al22 | 21 | 100 | 400 | 19 (91%) | 3/7 (43%) | 2/3 (66.7%) | 4/20 (20%) |
| Canepa et al23 | 10 | 200 | 800 | 0 | 3/10 (30%) | 3/10 (30%) | 3/10 (30%) |
| Pozzato et al25 | 6 | 100 | NR | NR | 3/6 (50%) | NR | 0/6 |
| Piccaluga et al26 | 12 | 100 | 600 | 11/12 (92%) | 3/12 (25%) | 3/12 (25%) | 2/12 (16.7%) |
| Merup et al27 | 15 | 200 | 800 | 6/15 (40%) | 0 | 0 | 0 |
Min indicates minimum THAL dose; Max, maximum THAL dose; Dropout/DM, number (percentage) of patients in the trial with early dropout or necessitating dose modification; NR, not reported.
The 50-mg dose was given only as a dose modification after initial toxicity.
The types and severity of the adverse events in the remaining 20 patients who completed the 3 months of THAL-PRED were significantly less than our prior trial of THAL monotherapy that used higher doses (Table 2).24 Specifically, fatigue, constipation, depression, bradycardia, and orthostatic symptoms were significantly reduced by the THAL-PRED regimen. In addition, the exuberant myeloproliferative reactions observed in the prior THAL trials in MMM were significantly diminished. Indeed, our serial assessments of EMH by99mTc-SC scans confirmed the lack of significant myeloproliferation at the THAL dose used. Furthermore, we did not see any increase in the in vitro growth of myeloid progenitors among the patients with MMM after THAL-PRED therapy. One patient in the THAL-PRED trial experienced a deep venous thrombosis (leg) at the time of an increase in the platelet count. Although the combination of THAL and corticosteroids has been associated with thrombogenic potential,36 our patient had multiple potential contributing risk factors for thrombosis (myeloproliferative disorder, thrombocytosis, immobility) making it impossible to determine the exact etiology of this patient's thrombotic event.
THAL-PRED has significant activity for anemia and thrombocytopenia in MMM
THAL-PRED was not only better tolerated than higher dose THAL monotherapy, but it also appeared to have greater efficacy for palliation of both the anemia and thrombocytopenia associated with MMM. In the current study, we observed a meaningful improvement in anemia in 62% of the patients as compared to the 0% to 50% responses described with the THAL monotherapy trials (Table 6). The degree of palliative benefit for anemia compares favorably with other palliative measure for MMM, including autologous stem cell transplantation (58%),4 erythropoietin (33%),5 and androgens (20%-57%).6,7 The latter therapies are often toxic, precluding their common implementation (ie, autologous transplant—toxicity, applicability, and expense) or frequently ineffective (erythropoietin—usually only works if serum erythropoietin level is inadequate for degree of anemia; and androgens—not traditionally useful if patient has chromosomal abnormalities). The data on the use of corticosteroids as single-agent therapy in MMM is very limited and suggests a possible response rate of 26% with steroids alone for MMM-associated anemia.37 Of particular note is the 70% response rate we observed among individuals in the THAL-PRED trial who were dependent on erythrocyte transfusions. This latter observation is especially significant because transfusion dependence adversely affects quality of life and can lead to iatrogenic iron overload, alloimmunization, and exposure to infectious agents. Patients who responded with improvement in anemia displayed features of MMM characteristic of an earlier stage of the disease, with decreased angiogenesis, decreased peripheral blood CD34 count, and less advanced angiogenesis in the marrow compared with nonresponders. Canepa and colleagues23 also reported a trend for patients with cellular phase MMM to have a more favorable response to THAL. Combined with our present observations, we suggest that THAL-PRED should be considered even in earlier phases of symptomatic MMM.
THAL increased the platelet counts both in our current trial and the previously reported monotherapy trials. A meaningful platelet response occurred in 75% of patients with thrombocytopenia treated with THAL-PRED; this response rate is as good or better than the platelet responses seen in the previous trials (response rate, 25%-80%; Table6). Indeed, thrombocytopenia is a known adverse prognostic factor in MMM,38 and in the past has been successfully palliated only with autologous stem cell transplantation.4
THAL monotherapy and THAL-PRED appear to raise the platelet count in patients with MMM regardless of their baseline platelet count. Accordingly, patients with pre-existing thrombocytosis should have adequate platelet control prior to initiation of therapy. Although we saw less undesired thrombocytosis with THAL-PRED than thalidomide at higher doses, one patient had a thrombotic event on the combination therapy, so diligent control of thrombocytosis while on THAL-PRED is advised.
THAL-PRED does not appear to decrease EMH
Decreases in splenomegaly were rare and not durable with THAL-PRED. Even the higher THAL doses previously used resulted in only modest reductions in splenomegaly (0%-30%; Table 6). The lack of decrease in palpable splenomegaly correlated with the lack of benefit we observed in decreasing hepatosplenic EMH by the 99mTc-SC scans. Concordant with a lack of effect of THAL-PRED on EMH (manifest as splenomegaly), we did not see significant improvement in the intramedullary disease manifestations. THAL-PRED did not change karyotypic abnormalities, nor did it normalize the marrow cellularity or decrease the amount of reticulin fibrosis or osteosclerosis. Additionally, THAL-PRED did not decrease the aberrantly high number of circulating myeloid progenitors even in patients with a clear clinical response to therapy. In fact, there was no evidence from our correlative studies that THAL-PRED has a direct effect on the overt intramedullary manifestations of MMM, yet changes may have occurred in the fibrogenic cytokine milieu, adhesion molecules, or prostaglandins not adequately reflected in our ancillary studies.
THAL-PRED does not significantly affect angiogenesis in MMM
Angiogenesis is markedly increased in the marrows of patients with MMM,16 perhaps to as great a degree as any hematologic malignancy.39 However, whether the increases in microvasculature are causal in the pathogenesis of MMM is unclear. Because various cytokines that are increased in MMM patients (bFGF,12 TGF-β,10,11 VEGF40) share both profibrotic and proangiogenic capabilities, it is possible that the increased angiogenesis in MMM is not causal, but instead incidental to the fibrosing process.
Although THAL is antiangiogenic in in vitro systems,18 it is unclear whether this is the predominant mechanism for its activity in MMM. In our previous study of THAL monotherapy in MMM, we observed a decrease in angiogenesis only in 2 patients (13%). Variable decreases in angiogenesis (by intramedullary light microscopy) and decreases in serum bFGF and VEGF were reported by Piccaluga and colleagues26 in some of their responders. However, in our current trial we did not observe a clear decrease in intramedullary angiogenesis in any patient, even for patient 5 who had a very favorable response to the therapy. We also measured the effect of therapy on the level of proangiogenic cytokines in the urine of treated patients. Neoangiogenesis in malignancy is felt to arise from an imbalance between proangiogenic (ie, VEGF, bFGF) and antiangiogenic cytokines (TSP-1).41 In this trial the lack of change in immunohistochemical evidence of angiogenesis correlated with a lack of change in the angiogenic cytokines.
Several possibilities may explain the apparent lack of effect of THAL-PRED on the angiogenesis in MMM. First, the therapy may inhibit the formation of new vasculature and therefore not produce a reduction in baseline microvasculature proliferation that was already present. Second, urinary assays of angiogenic cytokines may not adequately reflect changes in the bone marrow microenvironment for these cytokines. Also, THAL may either have a variable effect on angiogenesis in MMM patients or THAL has an alternative mechanism of action in MMM.
Other potential mechanisms of action for THAL-PRED
THAL-PRED is clinically active in alleviating the cytopenias associated with this disorder; however, no effect of THAL-PRED on karyotype, intramedullary fibrosis, or angiogenesis could be determined. THAL is also active in multiple myeloma, yet the mechanism for this is unclear.42 THAL inhibits tumor necrosis factor α (TNF-α) expression43 by destabilizing its mRNA, which might be potentially relevant to both conditions. We have recently reported clinical activity of the TNF-α inhibitor, etanercept, in MMM.44 In contrast to the present trial, however, clinical benefit was manifested by palliation of constitutional symptoms, and a major effect on cytopenias was not seen in the etanercept trial.
Corticosteroids might be synergistic with THAL in MMM by their direct inhibitory effect on interleukin 6 (IL-6) production.45 This property, coupled with thalidomide's effects on IL-1β, IL-2, IL-10, IL-12, and adhesion molecules,46 47 might result in the increased therapeutic response at doses of both drugs that are well tolerated.
Conclusions
The combination of low-dose THAL and PRED is well tolerated and appears promising for the alleviation of disease-associated cytopenias in patients with MMM. Further follow-up will be necessary to determine the durability of the responses observed. Although the current trial was not designed to determine a possible survival advantage of THAL-PRED over supportive care, it is conceivable that a significant reduction in the detrimental effects of anemia and thrombocytopenia on patients with MMM may well lead to better long-term outcomes. The mechanism of action of THAL-PRED in MMM still remains unclear, but it does not appear to involve a direct improvement in intramedullary fibrosis, angiogenesis, or EMH. Future trials in MMM should look at extending the activity seen in THAL-PRED by the inclusion of therapeutic agents, which may have an effect against the aberrant clonal process.
The authors acknowledge Celgene for providing thalidomide for this clinical trial.
Prepublished online as Blood First Edition Paper, November 27, 2002; DOI 10.1182/blood-2002-09-2928.
Supported by research funding from Celgene, Warren, NJ.
One of the authors (J.B.Z.) is Chief Medical Officer and Vice President for Medical Affairs for Celgene, whose product was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Ayalew Tefferi, Division of Hematology and Internal Medicine, Mayo Clinic, 200 First St, SW, Rochester, MN 55905; e-mail: tefferi.ayalew@mayo.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal