Systemic inflammation because of sepsis results in endothelial cell activation and microvascular injury. High-mobility group protein-1 (HMGB1), a novel inflammatory molecule, is a late mediator of endotoxin shock and is present in the blood of septic patients. The receptor for advanced glycation end products (RAGE) is expressed on endothelium and is a receptor for HMGB1. Here we examine the effects of HMGB1 on human endothelial cell function. Recombinant human HMGB1 (rhHMGB1) was cloned and expressed in Escherichia coli and incubated with human microvascular endothelium. rhHMGB1 caused a dose- and time-dependent increase in the expression of intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), and RAGE. rhHMGB1 induced the secretion of tumor necrosis factor-α (TNFα), interleukin 8 (IL-8), monocyte chemotactic protein-1 (MCP-1), plasminogen activator inhibitor 1 (PAI-1), and tissue plasminogen activator (tPA) (P < .01). rhHMGB1 stimulation resulted in transient phosphorylation of mitogen-activated protein (MAP) kinases, extracellular signal-related kinase (ERK), Jun N-terminal kinase (JNK), and p38, and in nuclear translocation of transcription factors NF-κB and Sp1. These effects are partially mediated by TNFα autocrine stimulation, as anti-TNFα antibodies significantly decrease chemokine and adhesion molecule responses (P ≤ .002). Thus, rhHMGB1 elicits proinflammatory responses on endothelial cells and may contribute to alterations in endothelial cell function in human inflammation.
Introduction
Systemic inflammation is one of the hallmarks of septic shock, and the microvascular endothelium provides an important site of regulation and amplification of these inflammatory responses.1 Endothelial cell activation leads to alterations in hemostasis, increases in vascular permeability, cell swelling and loss of barrier function, leukocyte adherence with cell clumping, and microthrombi formation.2,3 Microvascular injury is one of the characteristics of sepsis-associated tissue damage that may be manifested by single (eg, acute respiratory distress syndrome) or multiple organ failure syndromes.4 Many inflammatory mediators of sepsis contribute to the development of an activated endothelium (eg, tumor necrosis factor-α [TNFα] and interleukin 1 [IL-1]).5 Recently, high-mobility group protein-1 (HMGB1) has been identified as a novel inflammatory cytokine and a late mediator of endotoxin lethality in mice.6 The effects of HMGB1 on the endothelium have not been described.
HMGB1 is secreted by macrophages stimulated with endotoxin, TNFα, or IL-1β. Mice challenged with endotoxin have detectable blood levels of HMGB1 at 8 hours with a prolonged plateau for 16 to 32 hours. Administration of an anti-HMGB1 antibody improved survival after a lethal endotoxin challenge to mice even when given 2 hours after the challenge.7 Anti-HMGB1 antibody administration also ameliorates murine lung inflammation following intratracheal endotoxin challenge.8 The importance of HMGB1 in human disease is suggested by its detection in the serum of septic patients in which increased levels were associated with decreased survival.7
HMGB1 is a multifunctional protein; its earliest functions were described as a nonhistone DNA-binding nuclear protein. HMGB1 binds to DNA in a sequence-independent manner and modifies DNA structure to facilitate transcription, replication, and repair.9,10These functions are essential for survival, as HMGB1-deficient mice die of hypoglycemia within 24 hours after birth.11 In the developing nervous system, membrane-associated HMGB1 localizes to growth cones and promotes neurite outgrowth and extension by binding to the receptor for advanced glycation end products (RAGE).12,13 RAGE, a member of the immunoglobulin superfamily of receptors, is expressed on endothelium, smooth muscle cells, monocytes/macrophages, neurons, and in several malignant and transformed cells.14 RAGE interacts with a variety of ligands, including advanced glycation end products and HMGB1.15 HMGB1 binding to RAGE on neuroblastoma cells activates nuclear factor κB (NF-κB) and has been implicated in embryonic and malignant cell migration and invasion.16-18 The changes in cell mobility are mediated by the binding of membrane-associated HMGB1 to plasminogen and tissue plasminogen activator (tPA) with resultant enhanced local plasmin formation.19 HMGB1 is also exported to the platelet surface during platelet activation where it associates with the external surface of the plasma membrane.20
The presence of HMGB1 in the circulation of septic patients and the widespread expression of RAGE on endothelium suggests that HMGB1 might interact with endothelial cells and contribute to the inflammatory responses to infection. We demonstrate that human microvascular endothelial cells stimulated in vitro with HMGB1 increase their expression of intercellular and vascular adhesion molecules (ICAM-1 and VCAM-1) and RAGE receptor as well as secretion of a proinflammatory cytokine (TNFα), chemokines (IL-8 and monocyte chemotactic protein-1 [MCP-1]), and regulators of fibrinolysis (tPA and plasminogen activator inhibitor 1 [PAI-1]). This proinflammatory phenotype is mediated in part by early TNFα secretion and involves the activation of stress mitogen–activated protein kinase pathways and the transcription factors NF-κB and Sp1. These results describe a new function for circulating HMGB1 at the endothelial interface and suggest a role for HMGB1 in endothelial cell activation and injury in sepsis and systemic inflammation.
Materials and methods
Production of recombinant human HMGB1
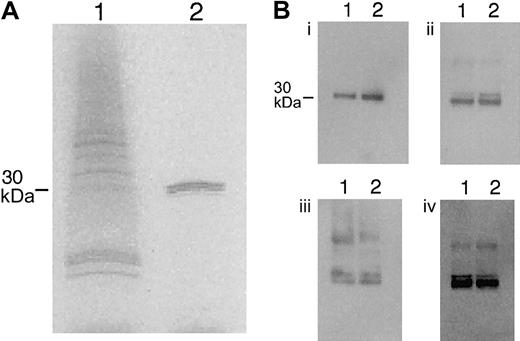
Peripheral blood mononuclear cells, collected from a single donor (Vacutainer CPT cell preparation tubes with sodium citrate; Becton Dickinson, Franklin Lakes, NJ), were plated on plastic flasks in RPMI-1640 with 10% fetal calf serum (FCS). After 4 hours, nonadherent cells were removed, and the remaining monocytes were stimulated with recombinant human TNFα (50 ng/mL; R&D Systems, Minneapolis, MN) for 18 hours. Cells were washed with phosphate-buffered saline (PBS), and total RNA was isolated (RNAqueous isolation kit; Ambion, Austin, TX). The reverse transcription of RNA to cDNA with amplification (GeneAmp; Applied Biosystems, Framingham, MA) was performed using the following primers for HMGB1: 5′-GCATGC ATG GGC AAA GGA GAT CCT-3′ (sense) and 5′-AAGCTT TAT TCA TCA TCA TCA TCA-3′ (antisense), corresponding to bases 53 to 70 and 682 to 699 of the published human HMGB1 DNA sequence (GenBank accession no.X12597). The polymerase chain reaction (PCR) product was ligated into pGEMT Easy vector (Promega, Madison, WI) and transformed into Escherichia coli JM109 (Qiagen, Valencia, CA) competent cells. Positive clones were selected and confirmed by DNA sequencing. The HMGB1 insert was digested with SphI andHindIII and subcloned into the SphI andHindIII cloning sites of the pQ30 vector (Qiagen) with a 6 × His tag at the N-terminus of the protein. The pQ30 construct was transformed into M15 E coli and was induced with 0.5 mM isopropyl-D-thiogalacto-pyranoside (IPTG; Sigma, St Louis, MO) for 90 minutes.21 The recombinant human HMGB1 (rhHMGB1) was extracted with 5% perchloric acid22 and purified with Ni-NTA agarose columns (Qiagen). The purified recombinant protein was detected by Coomassie staining (4%-20% sodium dodecyl sulfate–polyacrylamide gel electrophoresis [SDS-PAGE] under reducing conditions) as a single band with a molecular weight of approximately 30 kD (Figure 1A). Endotoxin was removed by detergent phase separation with Triton X-114 (Sigma).23 Endotoxin content after detergent phase separation was less than 1.5 pg/μg HMGB1 as assessed by the chromogenic Limulus amebocyte lysate assay (QCL-1000; BioWhittaker, Walkersville, MD). Both rhHMGB1 and HMGB1 extracted from A549 alveolar type-II–like cells were detected in Western blots by 3 affinity-purified rabbit antibodies against different segments of the protein (Research Genetics, Huntsville, AL) and a rabbit polyclonal antibody against the whole molecule (Figure 1B).
Purification of human recombinant HMGB1 and comparison with tissue-isolated HMGB1.
(A) Samples of bacteria cell lysate (lane 1) and purified rhHMGB1 (lane 2) resolved under denaturing conditions in a 4% to 20% SDS-PAGE and stained with Coomassie Blue. (B) Recombinant human HMGB1 (lane 1) and HMGB1 isolated from A549 cells (lane 2) were resolved in 4% to 20% SDS-PAGE, blotted onto nitrocellulose membranes, and incubated with the following rabbit polyclonal antibodies: (i) antisynthetic peptide gkgdpkkprgk (N-terminal sequence of human HMGB1, amino acids 2-13), (ii) antisynthetic peptide: kaekskkkkee (C-terminal sequence, amino acids 177-188), (iii) antisynthetic peptide kfkdpnapkrppsa (middle sequence, amino acids 88-101), and (iv) anti-HMGB1 (whole molecule). The antibodies were detected with horseradish-peroxidase antirabbit IgG.
Purification of human recombinant HMGB1 and comparison with tissue-isolated HMGB1.
(A) Samples of bacteria cell lysate (lane 1) and purified rhHMGB1 (lane 2) resolved under denaturing conditions in a 4% to 20% SDS-PAGE and stained with Coomassie Blue. (B) Recombinant human HMGB1 (lane 1) and HMGB1 isolated from A549 cells (lane 2) were resolved in 4% to 20% SDS-PAGE, blotted onto nitrocellulose membranes, and incubated with the following rabbit polyclonal antibodies: (i) antisynthetic peptide gkgdpkkprgk (N-terminal sequence of human HMGB1, amino acids 2-13), (ii) antisynthetic peptide: kaekskkkkee (C-terminal sequence, amino acids 177-188), (iii) antisynthetic peptide kfkdpnapkrppsa (middle sequence, amino acids 88-101), and (iv) anti-HMGB1 (whole molecule). The antibodies were detected with horseradish-peroxidase antirabbit IgG.
Cell culture
The human microvascular endothelial cell line HMEC-1 (kindly provided by F. J. Candal, Biological Products Branch of the Centers for Disease Control and Prevention, Atlanta, GA) is an SV-40–transformed microvascular endothelial cell line that retains the morphologic phenotype and functional characteristics of human microvascular endothelial cells.24 25 HMEC-1 cells were grown and maintained in endothelial basal medium (EBM; Clonetics, Walkersville, MD) supplemented with epidermal growth factor (10 ng/mL), hydrocortisone (1 μg/mL), penicillin (100 U/mL), streptomycin (100 μg/mL), amphotericin (250 ng/mL), and 10% FCS. Primary human microvascular lung endothelial cells (HMLECs; Clonetics) were maintained in EGM-2 MV Bullekit System (Clonetics). For all experiments, the cells were grown to confluence in collagen-coated flasks or collagen-coated 6-well plates (BD Biocoat, Bedford, MA). The HMLECs were used between passages 6 and 8.
Effects of rhHMGB1 on endothelial cells
HMEC-1 and HMLEC were incubated with rhHMGB1 (5-200 ng/mL) and TNFα (10 ng/mL) and medium alone were used as positive and negative control conditions, respectively. At different time points, cells and cell supernatants were harvested. To ensure that the effects of rhHMGB1 were not mediated by trace amounts of endotoxin present in the recombinant protein, rhHMGB1 was trypsinized and added to the HMEC-1 cultures as described previously.26 To assess the contribution of TNFα to the effects of rhHMGB1, cells were incubated 30 minutes prior to the addition of rhHMGB1 with a murine antihuman TNFα-neutralizing monoclonal antibody (BD Biosciences, San Diego, CA). Inhibition of HMGB1 interaction with RAGE was assessed using a mouse immunoglobulin G (IgG) monoclonal anti-RAGE antibody (Chemicon, Temecula, CA). HMEC-1 cells were incubated for 2 hours with increasing quantities of anti-RAGE antibody or mouse IgG prior to HMGB1 stimulation.
Quantitative flow cytometry
Endothelial cells were harvested with 0.25% trypsin and 1 mM EDTA (ethylenediaminetetraacetic acid; Clonetics), washed twice in PBS, and immediately fixed in 2% paraformaldehyde in PBS. After 15 minutes of incubation in 100 μL blocking solution (PBS, 0.1% bovine serum albumin [BSA], 10% normal mouse serum), 2 × 106 cells were incubated for 30 minutes with a phycoerythrin (PE)–mouse antihuman ICAM-1 monoclonal antibody (1 μg/mL; Caltag, Burlingame, CA), fluorescein isothiocyanate (FITC)–mouse antihuman VCAM-1 (2 μg/mL; Serotec, Raleigh, NC), or the corresponding immunoglobulin isotypes (IgG2a-PE and IgG1-FITC; Serotec). For detection of the RAGE, endothelial cells were blocked for 15 minutes in PBS with 0.1% BSA and 10% normal donkey serum and then incubated for 30 minutes with a goat antihuman polyclonal anti-RAGE antibody (1 μg/mL; Research Diagnostics) or an irrelevant goat IgG. Cells were washed, and an FITC-conjugated donkey antigoat polyclonal antibody (2 μg/mL; Molecular Probes, Eugene, OR) was added for 30 minutes. Cells were thoroughly washed and analyzed in a fluorescence-activated cell sorter scan (FACScan) system using CELLQuest software (Becton Dickinson, San Jose, CA). Fluorescence emission of 104 cells/sample was recorded as peak channel number on a logarithmic scale. To calculate the number of ICAM-1 and VCAM-1 receptors per cell, the peak channel of each cell population was transformed into antibody binding sites per cell using the Quantum Simply Cellular Microbeads and Quickcal software (Bangs Laboratories, Fishers, IN) as previously described.27,28 The number of antibody binding sites corresponding to the isotopic IgG was subtracted from each cell sample. For RAGE quantification, peak channel of each cell populations was transformed into molecules of equivalent soluble fluorochrome (MESFs) using the Quantum FITC MESF kit (Bangs Laboratories). MESFs corresponding to the irrelevant goat IgG were subtracted from each cell sample. Results are expressed as number of MESFs per cell.29
Secreted protein assays
Immunoreactive TNFα, IL-1β, MCP-1, IL-8, soluble ICAM-1 (sICAM-1), and soluble VCAM-1 (sVCAM-1), were measured in HMEC-1 and HMLEC supernatants by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions (R&D Systems). PAI-1 and tPA were measured by ELISA according to the manufacturer's instructions (American Diagnostica, Greenwich, CT).
Quantitative real-time PCR
Changes in mRNA levels for TNFα and IL-8 were quantified by real-time PCR (Taqman PCR detection; Applied Biosystems, Foster City, CA) in a 2-step reverse transcription–polymerase chain reaction (RT-PCR). Confluent monolayers of HMEC-1 cells were incubated with rhHMGB1 (100 ng/mL) or medium alone for 2 hours (n = 4), 6 hours (n = 4), and 24 hours (n = 3). Cells were lysed with RLT buffer and total RNA was isolated (Rneasy; Qiagen). The reverse transcription of RNA (2 μg/reaction) to cDNA was performed using random hexamers from the TaqMan reverse transcription reagents according to the manufacturer's instructions (Applied Biosystems). PCR products were synthesized from cDNA samples using the TaqMan universal PCR master mix and TaqMan predeveloped assay reagents for TNFα and IL-8 according to the manufacturer's instructions. RNase P (Applied Biosystems) was used as an internal control. Amplification and detection were carried out using the ABI PRISM 7900HT Sequence Detection System (Applied Biosystems). All values were normalized to levels of RNase P and were expressed as fold changes compared with unstimulated samples.30
Activation of mitogen-activated kinases
Mitogen-activated protein (MAP) kinase activation was determined by immunoblotting endothelial cell lysates. Confluent monolayers of HMEC-1 cells were stimulated with rhHMGB1 (100 ng/mL). At baseline, 5, 15, 30, 60, and 90 minutes, cells were washed with cold PBS and lysed (M-Per Mammalian Protein Extraction Reagent; Pierce, Rockford, IL). After centrifugation (14 000g for 10 minutes), supernatants were recovered, and the protein content was quantified by the Bradford method (BCA; Pierce). Total protein (10 or 20 μg) was fractionated by electrophoresis on 4% to 20% SDS-PAGE gels (Novex, Carlsbad, CA) under reducing and denaturing conditions and was transferred to nitrocellulose membranes. The double-phosphorylated forms of the MAP kinase were detected with the following antibodies: 0.5 μg/μL rabbit polyclonal anti-ERK1&2/MAPK (extracellular signal-related kinase 1 and 2; pTpY185/187) phosphospecific antibody, 0.5 μg/μL rabbit polyclonal anti-JNK1&2/SAPK (Jun N-terminal kinase 1 and 2 and stress-activated protein kinase; pTpY183/185) phosphospecific antibody, and 1 μg/μL rabbit polyclonal anti-p38 (pTpY180/182) phosphospecific antibody (all Biosource International, Camarillo, CA). Total MAP kinase proteins were detected with the following antibodies: 100 ng/mL rabbit polyclonal antibody anti-ERK1/2 (Promega), and 1/1000 dilution of rabbit polyclonal antibodies anti-SAPK/JNK and anti-p38 (Calbiochem, San Diego, CA). After incubation with peroxidase-conjugated donkey antirabbit secondary antibody (1/10 000 dilution; Jackson Immunoresearch, West Grove, PA), immunoreactive proteins were visualized with a chemiluminescence detection system (ECL+plus, Amersham Biotech). Band optical density corresponding to phosphorylated isoforms was analyzed by densitometry (Molecular Dynamics 301, Piscataway, NJ) using Image Quant software.
To assess the contribution of p38 MAPK and ERK pathways to HMGB1-stimulated cytokine responses, we used specific inhibitors of p38 MAPK (SB203580; Calbiochem) and ERK1/2 (PD98059; Calbiochem). The inhibitors were dissolved in dimethyl sulfoxide (DMSO) and added individually or together 2 hours prior to cell stimulation with HMGB1 (50 ng/mL). The final concentration of the inhibitors was SB203580, 10 μM dissolved in 0.1% DMSO, and PD98059, 25 μM dissolved in 0.04% DMSO.31 Cells were stimulated for 6 hours, and IL-8 and TNFα production was measured.
Electrophoretic mobility shift assay (EMSA)
Nuclear and cytoplasmic extracts from HMEC-1 cells were prepared (NE-PER Nuclear and Cytoplamic Extraction Reagents; Pierce) after 4-hour incubation with rhHMGB1 (100 ng/mL), TNFα (10 ng/mL), or control medium, and they were stored at −80°C until use. Protein content was assayed using the Bradford reagent and BSA as a standard (Pierce). End labeling of double-strand oligonucleotides containing the NF-κB binding motif (5′-AGT TGA GGG GAC TTT CCC AGC-3′) and the Sp1 consensus DNA binding motif (5′-ATT CGA TGC GGG CGG GGC GAG C-3′) was performed using γ32P-dATP (deoxyadenosine triphosphate; Amersham Pharmacia, Piscataway, NJ) and T4-polynucleotide kinase (Promega) at 37°C for 30 minutes. The removal of unincorporated nucleotide was done using G-25 Sephadex columns (Pharmacia Biotech, Piscataway, NJ). Equal amounts of nuclear extract protein (10 μg) were incubated in a 10-μL reaction mixture of 4% glycerol, 1 mM MgCl2, 0.5 mM EDTA, 0.5 mM DTT (dichlorodiphenyltrichloroethane), 50 mM NaCl, 10 mM Tris (tris(hydroxymethyl)aminomethane)–HCl, and 50 μg/mL poly (dI-dC) with or without a 100 × unlabeled oligonucleotide for 10 minutes at room temperature. 32P-labeled oligonucleotide probes were then added and incubated at room temperature for 20 minutes. In supershift assays, 1 μg antibody against NF-κB subunits p50 or p65 (Santa Cruz Biotechnology, Santa Cruz, CA) was added after the binding reaction and incubated for 1 hour at 4°C. Electrophoresis of samples was performed on 6% DNA retardation gels run in 0.5 × TBE buffer. Gels were vacuum-dried, and autoradiography was performed for 12 to 36 hours at −80°C.
Statistics
The data were analyzed using an analysis of variance (ANOVA) (SAS System 8.0; SAS Institute, Cary, NC).32 Depending on the experimental conditions, the model contained the following effects: time, cell, condition (HMGB1, negative and positive control), and level of antibody. All remaining interactions were pooled to form the error term. An estimate of the difference of the HMGB1 and negative control was made at the final time point. A linear and a quadratic model were used to examine the dose response curves, and the model with the best fit is reported. Variables were transformed where appropriate. Data are presented as mean ± standard error of the mean.
Results
rhHMGB1 induces the expression of the adhesion molecules ICAM-1 and VCAM-1 and its putative receptor RAGE on microvascular endothelial cells
ICAM-1 and VCAM-1 are adhesion receptors expressed on endothelial cells, and their up-regulation in response to proinflammatory cytokines is a sensitive marker of endothelial cell activation.1RAGE is a receptor for extracellular HMGB1,16 and its up-regulation might provide an amplification system for rhHMGB1 effects on endothelial cells. A preliminary study was designed to select an appropriate dose of hrHMGB1 for the endothelial cell stimulation experiments, based on HMGB1 levels detected in the serum of septic patients (approximately 30-150 ng/mL).7 Confluent cultures of HMEC-1 cells were incubated for 24 hours with doses of rhHMGB1, ranging from 5 to 200 ng/mL, and ICAM-1 and VCAM-1 expression was analyzed by quantitative flow cytometry. rhHMGB1 increased the expression of ICAM-1 and VCAM-1 in a dose-dependent manner (25 ng/mL, 1.6- and 3.7-fold increase; 50 ng/mL, 12.1- and 20.9-fold increase; 100 ng/mL, 13.4- and 24.2-fold increase; 200 ng/mL, 11.3- and 22.3-fold increase compared with control, respectively, bothP < .0001). HMGB1derived from A549 cells (100 ng/mL) increased ICAM-1 and VCAM-1 when incubated with the HMEC-1 cells (data not shown).
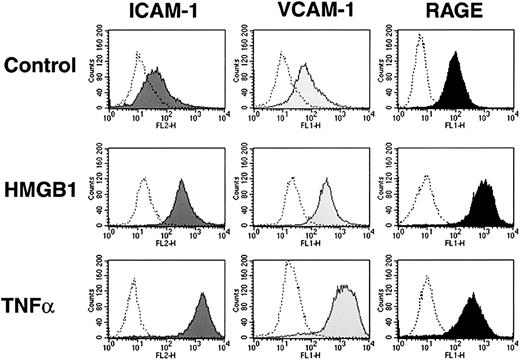
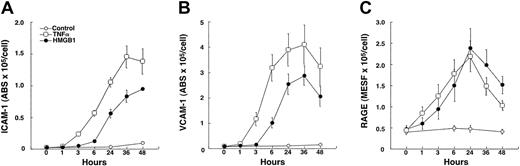
Incubation of confluent cultures of HMEC-1 cells with rhHMGB1 (50 ng/mL × 24 hours) or TNFα (10 ng/mL, positive control) resulted in increased expression of ICAM-1, VCAM-1, and RAGE compared with control medium (Figure 2). Similar responses occurred in primary endothelial cells; confluent cultures of HLMECs incubated with rhHMGB1 (50 ng/mL × 24 hours) had increased expression of ICAM-1 and VCAM-1 (Table 1). The time-course response of alterations in HMEC-1 surface receptors following stimulation with rhHMGB1 is shown in Figure 3A-C. ICAM-1 and VCAM-1 expression increased 3 hours after TNFα and 6 hours after rhHMGB1 stimulation. In contrast, rhHMGB1 and TNFα induction of RAGE had similar time courses, increasing within 1 hour of incubation. Increased levels of sICAM-1 and sVCAM-1 accompanied the induction of their respective cell surface markers (at 48 hours; sICAM in nonstimulated control 0.15 ± 0.03 versus rhHMGB1 2.92 ± 0.51 ng/mL,P < .0004 and sVCAM in nonstimulated control 0.05 ± 0.03 versus rhHMGB1 4.05 ± 0.73 ng/mL,P = .0002).
rhHMGB1 induces the expression of adhesion molecules ICAM-1 and VCAM-1, as well as RAGE receptor on HMEC-1 cells.
Flow cytometry histograms showing the expression of ICAM-1, VCAM-1, and RAGE after 24 hours' incubation with media alone, rhHMGB1 50 ng/mL, or TNFα 10 ng/mL. One representative example of 9 experiments is shown. The shaded histograms represent binding of anti–ICAM-1, anti–VCAM-1, and anti-RAGE antibodies, and the open histograms show binding of the respective isotype controls.
rhHMGB1 induces the expression of adhesion molecules ICAM-1 and VCAM-1, as well as RAGE receptor on HMEC-1 cells.
Flow cytometry histograms showing the expression of ICAM-1, VCAM-1, and RAGE after 24 hours' incubation with media alone, rhHMGB1 50 ng/mL, or TNFα 10 ng/mL. One representative example of 9 experiments is shown. The shaded histograms represent binding of anti–ICAM-1, anti–VCAM-1, and anti-RAGE antibodies, and the open histograms show binding of the respective isotype controls.
Effects of rhHMGB1 on human lung microvascular endothelial cells
| Response . | Condition after 24-hour incubation . | ||
|---|---|---|---|
| Media . | rhHMGB1 (50 ng/mL) . | TNFα (10 ng/mL) . | |
| ICAM-1 (abs × 103/cell) | 3.17 ± 0.32 | 41.32 ± 8.12* | 206.29 ± 81.31 |
| VCAM-1 (abs × 103/cell) | 7.46 ± 0.53 | 145.35 ± 9.08* | 584.16 ± 28.8 |
| TNFα (pg/mL) | 0 | 1238 ± 236* | 11 634 ± 3258 |
| IL-8 (ng/mL) | 0.83 ± 0.36 | 9.09 ± 1.20* | 89.23 ± 26.00 |
| MCP-1 (ng/mL) | 3.54 ± 1.53 | 10.94 ± 2.10* | 85.75 ± 13.71 |
| PAI-1 (ng/mL) | 1024 ± 276 | 2614 ± 552* | 3478 ± 552 |
| tPA (ng/mL) | 5.55 ± 0.85 | 6.71 ± 0.20 | 9.51 ± 0.32 |
| Response . | Condition after 24-hour incubation . | ||
|---|---|---|---|
| Media . | rhHMGB1 (50 ng/mL) . | TNFα (10 ng/mL) . | |
| ICAM-1 (abs × 103/cell) | 3.17 ± 0.32 | 41.32 ± 8.12* | 206.29 ± 81.31 |
| VCAM-1 (abs × 103/cell) | 7.46 ± 0.53 | 145.35 ± 9.08* | 584.16 ± 28.8 |
| TNFα (pg/mL) | 0 | 1238 ± 236* | 11 634 ± 3258 |
| IL-8 (ng/mL) | 0.83 ± 0.36 | 9.09 ± 1.20* | 89.23 ± 26.00 |
| MCP-1 (ng/mL) | 3.54 ± 1.53 | 10.94 ± 2.10* | 85.75 ± 13.71 |
| PAI-1 (ng/mL) | 1024 ± 276 | 2614 ± 552* | 3478 ± 552 |
| tPA (ng/mL) | 5.55 ± 0.85 | 6.71 ± 0.20 | 9.51 ± 0.32 |
Mean ± SEM of 3 experiments; abs indicates antibody binding sites/cell.
P < .05 rhHMGB1 compared with media alone.
rhHMGB1 induces the secretion of a proinflammatory cytokine (TNFα), chemokines (IL-8 and MCP-1) and regulators of fibrinolysis (tPA and PAI-1) by endothelium.
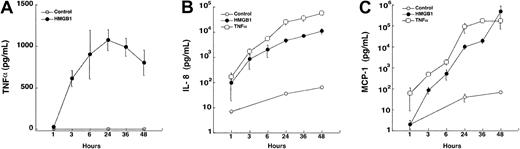
Within 3 hours of incubation of HMEC-1 cells with rhHMGB1, levels of TNFα, IL-8, and MCP-1 rose significantly compared with control conditions with plateau levels at 24 to 48 hours (allP < .0001, Figure 4A-C). We found different secretion patterns for TNFα and the chemokines. TNFα concentration rose sharply 3 hours after rhHMGB1 stimulation, reached a peak at 24 hours, and then slightly declined, whereas IL-8 and MCP-1 concentrations continued to increase. Similar induction of cytokine and chemokines release by HMGB1 was observed with HMLEC-1 (Table 1). Proteolysis of rhHMGB1 with trypsin abolished release of IL-8 by HMEC-1 cells (data not shown). No IL-1β was detected following rhHMBG1 or TNFα stimulation of the HMEC-1 or HLMEC cells (2 experiments in HMEC-1 and 2 experiments in HMLEC over 48 hours). rhHMBG1 also significantly increased the release of 2 pivotal regulators of fibrinolysis, tPA and PAI-1 (at 48 hours,P = .005 and .0006, respectively, Figure5 and Table 1). Incubation with an anti-RAGE antibody (80 μg/mL) diminished IL-8 production by 14% (5477 ± 1158 versus 4720 ± 1097 pg/mL,P = .02, t test) and TNFα production by 17% (479 ± 24 versus 365 ± 39 pg/mL,P = .11). Incubation with anti-RAGE antibody alone or murine IgG (alone or together with rhHMGB1) had no effect on IL-8 or TNFα production.
Quantitative PCR analysis of TNFα and IL-8 gene expression
Analysis of TNFα gene expression using TaqMan quantitative PCR revealed that TNFα mRNA was highly up-regulated 2 hours after treatment with rhHMGB1 (403.9- ± 96.7-fold increase from baseline,P < .001), sharply declined by 6 hours (56.2 ± 13.7), and returned to baseline levels at 24 hours (5.2 ± 1.9). IL-8 mRNA levels were up-regulated at 2 hours (182.6 ± 55.9,P = .03) and 6 hours (129. 2 ± 14.9,P = .02) and returned toward baseline levels at 24 hours (36.4 ± 10.8). These patterns change in parallel with the levels of TNFα and IL-8 protein found in the HMEC-1 supernatants.
Proinflammatory activity of HMGB1 is amplified by local TNF production
To investigate whether the proinflammatory activity of rhHMGB1 on endothelial cells is mediated in part by local release of TNFα, we incubated HMEC-1 cells with an anti-TNFα–neutralizing antibody (5 or 10 μg/mL) 30 minutes prior to HMGB1 (100 ng/mL) and TNFα (10 ng/mL) stimulation. Cells were incubated for 24 hours, IL-8 concentration was measured in the supernatants, and ICAM-1 and VCAM-1 expression was quantified by flow cytometry. Both concentrations of anti-TNFα antibody significantly decreased TNFα- and rhHMGB1-induced secretion of IL-8 and expression of ICAM-1 and VCAM-1 (Figure 6A-C). The suppressive effects of the anti-TNFα antibody were greater for cells stimulated with TNFα than with rhHMGB1 (10 μg/mL anti-TNFα antibody decreased IL-8 production by 91% ± 2% versus 72% ± 2%, ICAM-1 by 78% ± 4% versus 65% ± 3%, and VCAM-1 by 81% ± 3% versus 74% ± 3%, respectively, P = .004). The suppressive effects of the anti-TNFα antibody were incomplete; after coincubation with the anti-TNFα antibody, both TNFα- and rhHMGB1-stimulated cells had levels of IL-8 and adhesion molecules that remained greater than media alone (P < .0001).
rhHMGB1 interaction with endothelial cells induces MAP kinase activation
MAP kinase signaling pathways play a central role in endothelial cell activation in response to proinflammatory stimuli such as TNFα, IL-1β, and thrombin.33-35 Kinase activation was determined in HMEC-1 cells after rhHMGB1 stimulation by detection of the active, phosphorylated forms of 3 different MAP kinases. Representative blots of 5 independent experiments and densitometry analysis are shown in Figure 7. The phosphorylated form ERK1/2 was constitutively expressed in HMEC-1 cells, was strongly induced after 15 minutes of rhHMGB1 exposure, and remained up-regulated up to 60 minutes. Phosphorylation of JNK was detected at 5 minutes, peaked at 30 minutes of rhHMGB1 stimulation, and declined after 60 minutes. p38 phosphorylation was detectable at 5 minutes and remained present up to 60 minutes after incubation with HMGB1.
Inhibition of p38 MAPK with SB203580 decreased rhHMGB1-stimulated IL-8 release (at 6 hours) by nearly two thirds (P = .03), whereas ERK1/2 inhibition with PD98059 had no effect. The combination of both SB203580 and PD98059 decreased IL-8 release by 87% (P = .0003). TNFα secretion was unaltered by either p38 MAPK or ERK1/2 inhibition alone and was marginally decreased by the combination of both MAP kinase inhibitors (P = .13) (Table2).
Effects of p38 and ERK1/2 MAP kinase inhibition on HMGB1-stimulated cytokine release from HMEC-1 cells
| . | IL-8 (pg/mL) . | TNFα (pg/mL) . |
|---|---|---|
| p38 | ||
| Media | 37.5 ± 16.9 | 0.61 ± 0.4 |
| DMSO | 33.5 ± 15.2 | 2.8 ± 1.2 |
| SB203580 | 16.2 ± 8.1 | 6.1 ± 3.1 |
| HMGB1 | 2296.8 ± 140.2 | 502.7 ± 91.7 |
| DMSO + HMGB1 | 2165.9 ± 257.3 | 463.9 ± 76.3 |
| SB203580 + HMGB1 | 785.7 ± 63.2* | 444.4 ± 88.7 |
| ERK1 | ||
| Media | 57.2 ± 12.9 | 0.67 ± 0.4 |
| DMSO | 59.6 ± 11.3 | 0.99 ± 0.1 |
| PD90859 | 54.2 ± 14.3 | 0.82 ± 0.5 |
| HMGB1 | 1824.8 ± 465.2 | 228.1 ± 26.9 |
| DMSO + HMGB1 | 1850 ± 422.6 | 244.5 ± 36.7 |
| PD90859 + HMGB1 | 1843.2 ± 348.4 | 208 ± 42.4 |
| ERK2 | ||
| Media | 5.2 ± 0.2 | 5.7 ± 0.02 |
| DMSO | 4.1 ± 0.1 | 5.8 ± 0.2 |
| SB203580 + PD90859 | 2.8 ± 0.1 | 5.7 ± 0.1 |
| HMGB1 | 924.0 ± 181.0 | 292.4 ± 81.2 |
| DMSO + HMGB1 | 982.4 ± 22.5 | 223 ± 13.5 |
| SB203580 + PD90859 + HMGB1 | 127.6 ± 7.36† | 183.6 ± 11.9 |
| . | IL-8 (pg/mL) . | TNFα (pg/mL) . |
|---|---|---|
| p38 | ||
| Media | 37.5 ± 16.9 | 0.61 ± 0.4 |
| DMSO | 33.5 ± 15.2 | 2.8 ± 1.2 |
| SB203580 | 16.2 ± 8.1 | 6.1 ± 3.1 |
| HMGB1 | 2296.8 ± 140.2 | 502.7 ± 91.7 |
| DMSO + HMGB1 | 2165.9 ± 257.3 | 463.9 ± 76.3 |
| SB203580 + HMGB1 | 785.7 ± 63.2* | 444.4 ± 88.7 |
| ERK1 | ||
| Media | 57.2 ± 12.9 | 0.67 ± 0.4 |
| DMSO | 59.6 ± 11.3 | 0.99 ± 0.1 |
| PD90859 | 54.2 ± 14.3 | 0.82 ± 0.5 |
| HMGB1 | 1824.8 ± 465.2 | 228.1 ± 26.9 |
| DMSO + HMGB1 | 1850 ± 422.6 | 244.5 ± 36.7 |
| PD90859 + HMGB1 | 1843.2 ± 348.4 | 208 ± 42.4 |
| ERK2 | ||
| Media | 5.2 ± 0.2 | 5.7 ± 0.02 |
| DMSO | 4.1 ± 0.1 | 5.8 ± 0.2 |
| SB203580 + PD90859 | 2.8 ± 0.1 | 5.7 ± 0.1 |
| HMGB1 | 924.0 ± 181.0 | 292.4 ± 81.2 |
| DMSO + HMGB1 | 982.4 ± 22.5 | 223 ± 13.5 |
| SB203580 + PD90859 + HMGB1 | 127.6 ± 7.36† | 183.6 ± 11.9 |
HMGB1 (50 ng/mL), mean ± SEM of 3 experiments.
P = .03 by paired t test (DMSO + HMGB1 versus SB203580 + HMGB1).
P = .003 by paired t test (DMSO + HMGB1 versus SB203580 + PD90859 + HMGB1).
HMGB1 activates NF-κB and Sp1 transcriptional activity in endothelial cells
To further explore the regulatory mechanisms associated with endothelial cell activation by rhHMGB1, we examined changes in 2 nuclear transcription factors: NF-κB and Sp1. NF-κB is a multisubunit molecule that belongs to the Rel family of transcription factors and its activation is triggered by proinflammatory cytokine stimulation of endothelial cells.36 RAGE-HMGB1 binding in neuroblastoma cells enhances NF-κB–dependent transcription.16 Sp1 is a transcriptional factor that binds to GC boxes of various cellular promoters with sequence specificity.37 SP-1 binding elements in the RAGE promoter mediate HMGB1-induced RAGE expression in neuroblastoma cells.38 Nuclear extracts from HMEC-1 cells were incubated for 4 hours with rhHMGB1, TNFα, or medium alone and analyzed by EMSA. Nuclear extracts from HMEC-1 cells treated with rhHMGB1 and TNFα showed large increases in specific binding of the NF-κB oligonucleotide. Specificity was confirmed by competitive assay with a 100-fold molar excess of unlabeled oligonucleotide and a specific supershift with a monoclonal antibody against the p65 subunit (Figure8). Sp1 binding activity was present in control cells but was increased in rhHMGB1-stimulated cells (Figure 8). Thus, HMGB1 activates 2 nuclear transcription factors in the endothelium: NF-κB, a rapid-response factor for gene expression during inflammation, and Sp1, a binding factor associated with the RAGE promoter.
Discussion
HMGB1 is a novel inflammatory cytokine that contributes to the lethality of sepsis.7 We demonstrate that rhHMGB1 induces a proinflammatory phenotype in human microvascular endothelial cells in vitro characterized by up-regulation of leukocyte adhesion molecules (ICAM-1 and VCAM-1), secretion of the neutrophil and monocyte chemokines (IL-8 and MCP-1), production of a proinflammatory cytokine (TNFα), secretion of regulators of fibrinolysis (tPA and PAI-1), and enhanced expression of RAGE, a known receptor for HMGB1. These data suggest that HMGB1 can initiate and amplify inflammatory responses in the endothelium during infection or injury.
Endothelial activation is required for the transmigration and accumulation of inflammatory cells into tissues. We show that rhHMGB1 is a proinflammatory mediator and induces expression of 2 key adhesion molecules, ICAM-1 and VCAM-1. ICAM-1 mediates the firm adhesion of leukocytes to the endothelium and subsequent transmigration to the inflammatory sites. VCAM-1 contributes to the adhesion of activated lymphocytes and monocytes to endothelial cells in acute inflammatory tissues.39 Accompanying the up-regulation of adhesion molecules is the secretion of 2 potent neutrophil and monocyte chemokines, IL-8 and MCP-1. Thus, HMGB1 activates components that provide the necessary substrate for recruitment, adhesion, and transmigration of leukocytes across an activated endothelium and possibly to a nidus of inflammation.3
Tumor necrosis factor is a pivotal early mediator that regulates and amplifies the development of inflammation. Endothelial cells secrete TNFα under diverse inflammatory conditions, including ischemia, traumatic injury, allograft rejection, cytokine stimulation, and activation by bacterial components.40-44 We show that endothelial cell TNFα secretion occurs within 3 hours of rhHMGB1 stimulation (Figure 4A) and that TNFα-induced adhesion molecule expression occurs 3 hours before similar changes develop in rhHMGB1-stimulated cells (Figure 3A-B). The time course and the partial inhibition of rhHMGB1 responses (eg, IL-8 secretion, VCAM and ICAM expression) by an anti-TNFα–neutralizing antibody (Figure 6 A-C) suggests that local production of TNFα amplifies the proinflammatory effects of rhHMGB1 on endothelial cells.
Expression of cell surface markers on HMEC-1 over 48 hours following incubation with rhHMGB1 (50 ng/mL), TNFα (10 ng/mL), or media alone.
Significantly different time courses were observed for (A) ICAM-1 (P = .002), (B) VCAM-1 (P = .01), and (C) RAGE (P = .03) under the 3 conditions. Increased expression of ICAM-1 and VCAM-1 occurred within 3 hours after TNFα stimulation and was delayed until 6 hours after rhHMGB1 stimulation. Increased expression of RAGE occurred within 1 hour after either TNFα or rhHMGB1 stimulation and followed a similar time course. Data represent mean ± SEM of 4 separate experiments.
Expression of cell surface markers on HMEC-1 over 48 hours following incubation with rhHMGB1 (50 ng/mL), TNFα (10 ng/mL), or media alone.
Significantly different time courses were observed for (A) ICAM-1 (P = .002), (B) VCAM-1 (P = .01), and (C) RAGE (P = .03) under the 3 conditions. Increased expression of ICAM-1 and VCAM-1 occurred within 3 hours after TNFα stimulation and was delayed until 6 hours after rhHMGB1 stimulation. Increased expression of RAGE occurred within 1 hour after either TNFα or rhHMGB1 stimulation and followed a similar time course. Data represent mean ± SEM of 4 separate experiments.
Secretion of inflammatory mediators from HMEC-1 over 48 hours following incubation with rhHMGB1 (50 ng/mL), TNFα (10 ng/mL), or media alone (n = 4).
(A) rhHMGB1 induced early secretion of TNFα (P < .0001 compared with media alone), reaching a peak at 24 hours. (B) IL-8 and (C) MCP-1 rhHMGB1-mediated secretion (P < .0001 compared with medium alone) rose significantly after 3 hours and continued to accumulate in the supernatants up to 48 hours. Data represent mean ± SEM of 4 separate experiments.
Secretion of inflammatory mediators from HMEC-1 over 48 hours following incubation with rhHMGB1 (50 ng/mL), TNFα (10 ng/mL), or media alone (n = 4).
(A) rhHMGB1 induced early secretion of TNFα (P < .0001 compared with media alone), reaching a peak at 24 hours. (B) IL-8 and (C) MCP-1 rhHMGB1-mediated secretion (P < .0001 compared with medium alone) rose significantly after 3 hours and continued to accumulate in the supernatants up to 48 hours. Data represent mean ± SEM of 4 separate experiments.
rhHMGB1 stimulates the release of fibrinolytic regulators tPA and PAI-1 from HMEC-1 cells.
Incubation of HMEC-1 cells with rhHMGB1 (50 ng/mL) over a 48-hour period resulted in an increased secretion of (A) tPA (P = .005 at 48 hours compared with medium) and (B) PAI-1 (P = .0006 at 48 hours compared with medium). Both molecules were constitutively secreted by HMEC-1 cells. TNFα stimulation resulted in higher tPA and PAI-1 levels compared with HMGB1. Data represent mean ± SEM of 4 separate experiments.
rhHMGB1 stimulates the release of fibrinolytic regulators tPA and PAI-1 from HMEC-1 cells.
Incubation of HMEC-1 cells with rhHMGB1 (50 ng/mL) over a 48-hour period resulted in an increased secretion of (A) tPA (P = .005 at 48 hours compared with medium) and (B) PAI-1 (P = .0006 at 48 hours compared with medium). Both molecules were constitutively secreted by HMEC-1 cells. TNFα stimulation resulted in higher tPA and PAI-1 levels compared with HMGB1. Data represent mean ± SEM of 4 separate experiments.
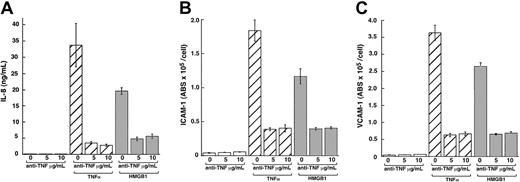
Anti-TNF antibody partially suppresses rhHMGB1-mediated endothelial cell activation.
Polyclonal neutralizing anti-TNF antibody (5 and 10 μg/mL) was incubated with HMEC-1, 30 minutes prior to stimulation with rhHMGB1 (100 ng/mL), TNFα (10 ng/mL), or media alone. (A) Anti-TNF antibody (10 μg/mL) treatment suppressed TNFα-induced IL-8 release by 91% ± 0.2% and rhHMGB1 IL-8 release by 72% ± 2 (P = .004). Suppression of the TNFα-induced production of IL-8 was greater than that of rhHMGB1 (P = .002). The antibody did not completely suppress the effects of either stimulus (TNFα with anti-TNF versus media alone or rhHMGB1 with anti-TNF versus media alone, P = .003). Similar responses were observed for ICAM-1 (B) and VCAM-1 (C) expression with 5 and 10 μg/mL anti-TNF antibody (P < .002). Data represent mean ± SEM of 3 separate experiments.
Anti-TNF antibody partially suppresses rhHMGB1-mediated endothelial cell activation.
Polyclonal neutralizing anti-TNF antibody (5 and 10 μg/mL) was incubated with HMEC-1, 30 minutes prior to stimulation with rhHMGB1 (100 ng/mL), TNFα (10 ng/mL), or media alone. (A) Anti-TNF antibody (10 μg/mL) treatment suppressed TNFα-induced IL-8 release by 91% ± 0.2% and rhHMGB1 IL-8 release by 72% ± 2 (P = .004). Suppression of the TNFα-induced production of IL-8 was greater than that of rhHMGB1 (P = .002). The antibody did not completely suppress the effects of either stimulus (TNFα with anti-TNF versus media alone or rhHMGB1 with anti-TNF versus media alone, P = .003). Similar responses were observed for ICAM-1 (B) and VCAM-1 (C) expression with 5 and 10 μg/mL anti-TNF antibody (P < .002). Data represent mean ± SEM of 3 separate experiments.
Microvascular coagulation and fibrinolysis are important pathophysiologic events in inflammation and sepsis. We show that rhHMGB1 induces endothelial cells to secrete 2 key components of the fibrinolytic system, tPA and PAI-1 in endothelial cells. Under physiologic conditions, tPA is released from the endothelium, binds to the endothelial cell surface, and converts plasminogen to plasmin. Plasmin degrades fibrin and prevents clotting. PAI-1 inactivates tPA and limits any exaggerated fibrinolysis that might lead to bleeding.2 An excess of PAI-1 compared with tPA is commonly found during acute inflammation because of endotoxin, sepsis, or injury.45 In neural tissue, membrane-associated HMGB1 binds both tPA and plasminogen via the lysine-rich kringle domain, resulting in plasmin activation that degrades HMGB1.19 An additional source of HMGB1 during hemostasis is activated platelets; HMGB1 is exported to the surface of platelets on activation by thrombin.20 Thus, HMGB1 has complex interactions with the hemostatic system that lead to modulation of coagulation and fibrinolysis. Further, these interactions may result in the generation of plasmin that limits HMGB1 effects by proteolytic degradation.19 Therapies limiting the effects of HMGB1 may be useful to alter the development of the coagulopathic state associated with sepsis.
Several lines of evidence demonstrate that the effects of HMGB1 are mediated by binding to RAGE. In neural tissue and malignant cells, RAGE activation by HMGB1 leads to MAP kinase activation and is associated with enhanced tumor growth, metastases, and release of matrix metalloproteinases.17 Rat smooth muscle cells undergo chemotaxis and cytoskeleton reorganization following HMGB1 binding to RAGE.46 We show that rhHMGB1 up-regulates RAGE on endothelial cells similar in degree to that of TNFα. Our use of a monoclonal anti-RAGE antibody decreased IL-8 secretion modestly but did not inhibit TNFα secretion. This may be due to the failure to completely block epitopes that are responsible for HMGB1 binding to RAGE. Only a small portion of the HMGB1 molecule (15-20 amino acids) is required for binding to the RAGE receptor.47 Polyclonal anti-RAGE antibodies decrease HMGB1 responses by almost two thirds (ie, HMGB1-induced permeability in Caco-2 cells and HMGB1-induced chemotaxis of smooth muscle cells).46,48 These data, however, leave open the possibility that other mediators or receptors may act in concert with RAGE activation to contribute to the development of an inflammatory phenotype. We show that a secondary mediator, TNFα, has an autocrine stimulatory effect on HMGB1-stimulated endothelium. HMGB1 binds to other receptors such as syndecan, which is expressed in epithelium and fibroblasts, as well as endothelium.49,50However, cell activation and signaling from this ligand-receptor interaction has not been characterized. It is conceivable that other scavenger receptors that bind polyanionic ligands such as the CD36 receptor may interact with HMGB1 and initiate cell activation.51 Further, HMGB1 has been used as a transfection agent, suggesting that a nonreceptor cell interaction may also occur.52
We evaluated 2 similar but functionally distinct MAP kinase pathways; ERK1/ERK2 kinase, activated by growth factors or mitogens, and the stress-activated protein kinases, JNK and p38, that are activated in response to diverse agonists (ie, cytokines, physical and chemical agents).53 Within 5 to 15 minutes, concentrations of rhHMGB1, similar to those found in the blood of septic patients, activate these 3 MAP kinase pathways in human microvascular endothelial cells. These results are similar to studies that demonstrate that several signaling pathways are activated by HMGB1 interaction with target cells. In neuronal tissue, HMGB1 binding to RAGE simultaneously activates 2 distinct downstream signaling pathways: Rac and Cdc42 (members of the Rho family of small guanosine triphosphatases [GTPases]) and Ras and NF-κB (members of the MAP kinase serine/threonine phosphorylation pathway). The former is responsible for cell motility and neurite outgrowth, whereas the latter is responsible for inflammation and stress responses.16 The inhibition of HMGB1 binding to RAGE by the use of soluble RAGE or tail-deletion RAGE transfectants suppresses the activation of ERK1/2, JNK, and p38 MAP kinases that are important signaling molecules in the growth and metastases of tumors.17
MAP kinase inhibitors have been used to characterize key cell signaling pathways resulting from HMGB1 cell activation. Increased smooth muscle migration following HMGB1 binding to RAGE is mediated through a pertussis toxin–sensitive pathway and blocked by PD98059, an inhibitor of ERK1/ERK2 activation.46 The use of the p38 MAP kinase inhibitor, SB203580, in our study significantly decreased IL-8 production and marginally decreased the release of TNF. Several factors may have contributed to the diminished effect of p38 MAP kinase inhibition on TNF secretion. SB203580 inhibits p38α and p38β isoforms but not p38γ or p38δ.54 If the latter 2 isoforms predominate in microvascular endothelial cells following HMGB1 stimulation, this inhibitor would not be effective. Because of complex interactions between the MAP kinase pathways, the effects of their inhibitors may be a graded adaptive response rather than an all or none response.53 Recent studies have shown that human umbilical vein endothelial cells stimulated by TNFα, activate ERK1/2, p38, and JNK MAP kinases. However, the secondary secretion of an inflammatory cytokine, granulocyte-macrophage colony-stimulating factor, was not altered by either SB203580 or PD98059 MAP kinase inhibitors.55 Others have shown that SB203580 inhibits Sephadex-induced lung edema in a dose-related manner but had no effect on lung tissue TNF levels, suggesting these are distinct cellular processes.56 Because neither SB203580 nor PD98059 affect JNK MAP kinase activation, this pathway may assume greater prominence when the p38 pathway is inhibited. Thus, TNF secretion and its effects on microvascular endothelial cells following HMGB1 stimulation may not be solely controlled by the p38 MAP kinase signaling pathway and may result from activation of other intracellular signals.
Little data are available that describe the signaling pathways associated with TNF secretion by endothelial cells. Human intestinal microvascular and umbilical vein endothelium constitutively express TNF and IL-8 mRNA.41 We show that following HMGB1 stimulation, the temporal sequence of IL-8 and TNF mRNA expression in endothelial cells is different; TNF mRNA returns to baseline levels at 6 hours, whereas IL-8 mRNA returns to baseline at 24 hours. Activated MAP kinases may contribute to these different profiles by affecting transcription rates through NF-κB activation or altering the translational rates by mRNA stabilization.53 57
We show that 2 nuclear transcription factors, NF-κB and Sp1 are activated by rhHMGB1. NF-κB is a rapidly inducible regulatory element and Sp1 is a transcriptional regulator that recognizes GC/GT boxes in a wide variety of genes.37,58,59 HMGB1-RAGE binding results in NF-κB nuclear translocation in neuroblastoma and malignant cells.16,17 RAGE activation also increases its own expression, creating a positive feedback loop after ligand binding.60 The RAGE promoter contains binding sites for NF-κB and Sp1 that contribute to this receptor up-regulation. In endothelium, TNFα and AGE enhance RAGE expression by activation of NFκB, whereas 17β estradiol activates RAGE expression through an Sp1/estrogen receptor complex.60 These data suggest that both mechanisms may be acting in rhHMGB1-stimulated endothelium to up-regulate the expression of RAGE.
rhHMGB1 is a potent proinflammatory molecule that activates endothelial cells and smooth muscle cells at low concentrations (25-100 ng/mL).46 At higher concentrations (10-20 μg/mL), HMGB1 stimulates migration and growth of embryonic or malignant cells.17,18,61 In vivo, 2 potential sources of circulating HMGB1 may occur; HMGB1 secreted from monocyte-macrophages in response to endotoxin, TNFα, or IL-1β and HMGB1 released from damaged or necrotic cells.46,62,63 Once released into the intravascular space, HMGB1 could potentially amplify local inflammatory responses by enhancing the release of cytokines and chemokines from monocytes-macrophages26 and interact with endothelial cells by up-regulating surface receptors and inducing the secretion of soluble proinflammatory mediators (Figure9). Modulating the activity of HMGB1 may provide a novel means of treating acute inflammatory conditions.
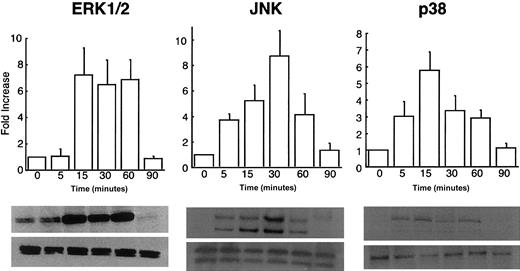
rhHMGB1-mediated induction of MAP kinases in endothelial cells.
Western blots of HMEC-1 cells treated with rhHMGB1 50 ng/mL for the indicated times were probed with antibodies against the phosphorylated (upper row) and common (lower row) forms of ERK1/2, JNK, and p38. Phospho-ERK 1/2 was mildly expressed at baseline and was strongly induced by rhHMGB1 at 15, 30, and 60 minutes. Phospho-JNK was detected within 5 minutes, reached a peak at 30 minutes, and returned to baseline levels by 90 minutes. Phospho-p38 was detected within 5 minutes, rose to maximum activity at 15 minutes, and remained detectable at 60 minutes. Summary data represents mean ± SEM of densitometry from 5 separate experiments.
rhHMGB1-mediated induction of MAP kinases in endothelial cells.
Western blots of HMEC-1 cells treated with rhHMGB1 50 ng/mL for the indicated times were probed with antibodies against the phosphorylated (upper row) and common (lower row) forms of ERK1/2, JNK, and p38. Phospho-ERK 1/2 was mildly expressed at baseline and was strongly induced by rhHMGB1 at 15, 30, and 60 minutes. Phospho-JNK was detected within 5 minutes, reached a peak at 30 minutes, and returned to baseline levels by 90 minutes. Phospho-p38 was detected within 5 minutes, rose to maximum activity at 15 minutes, and remained detectable at 60 minutes. Summary data represents mean ± SEM of densitometry from 5 separate experiments.
rhHMGB1 induces nuclear translocation and DNA binding of transcription factors NF-κB and SP-1.
Nuclear extracts were prepared from HMEC-1 cells after 4-hour incubation with control medium alone (lanes 1-2), TNFα 10 ng/mL (lanes 3-4), and rhHMGB1 100 ng/mL (lanes 5-8). The nuclear extracts were incubated with 32P-labeled NF-κβ or32P-labeled Sp1 consensus oligonucleotides and resolved by EMSA. Unlabeled oligonucleotide (100 ×; either NF-κβ or Sp1) was added to lanes 2, 4, and 6. Nuclear extracts from the rhHMGB1-treated HMEC-1 cells showed increased binding of the NF-κB (a) and Sp1 (b) oligonucleotides compared with untreated cells. Antibodies against NF-κB subunits p50 and p65 were added to lanes 7 and 8, respectively. The arrow (c) in lane 8 shows a supershift for the NF-κB p65 subunit. Representative results of 4 independent experiments for both NF-κB or Sp1 are shown.
rhHMGB1 induces nuclear translocation and DNA binding of transcription factors NF-κB and SP-1.
Nuclear extracts were prepared from HMEC-1 cells after 4-hour incubation with control medium alone (lanes 1-2), TNFα 10 ng/mL (lanes 3-4), and rhHMGB1 100 ng/mL (lanes 5-8). The nuclear extracts were incubated with 32P-labeled NF-κβ or32P-labeled Sp1 consensus oligonucleotides and resolved by EMSA. Unlabeled oligonucleotide (100 ×; either NF-κβ or Sp1) was added to lanes 2, 4, and 6. Nuclear extracts from the rhHMGB1-treated HMEC-1 cells showed increased binding of the NF-κB (a) and Sp1 (b) oligonucleotides compared with untreated cells. Antibodies against NF-κB subunits p50 and p65 were added to lanes 7 and 8, respectively. The arrow (c) in lane 8 shows a supershift for the NF-κB p65 subunit. Representative results of 4 independent experiments for both NF-κB or Sp1 are shown.
A model of cellular responses of HMGB1 stimulation of endothelial cells.
HMGB1 secreted from stimulated monocyte-macrophages or released from necrotic cells can activate endothelial cells through ERK1/2 and 2 stress-activated MAPK pathways (JNK and p38), resulting in NF-κB and Sp1 binding to cellular response elements. Cell activation results in the release of a proinflammatory cytokine (TNFα) and chemokines (IL-8 and MCP-1), up-regulation of adhesion molecules (ICAM-1 and VCAM-1), and an HMGB1 receptor, RAGE. TNFα acts locally to amplify responses initiated by HMGB1. Secretion of regulators of fibrinolysis (tPA and PAI-1) results in the activation of plasmin that may limit HMGB1 responses by proteolytic degradation of HMGB1.
A model of cellular responses of HMGB1 stimulation of endothelial cells.
HMGB1 secreted from stimulated monocyte-macrophages or released from necrotic cells can activate endothelial cells through ERK1/2 and 2 stress-activated MAPK pathways (JNK and p38), resulting in NF-κB and Sp1 binding to cellular response elements. Cell activation results in the release of a proinflammatory cytokine (TNFα) and chemokines (IL-8 and MCP-1), up-regulation of adhesion molecules (ICAM-1 and VCAM-1), and an HMGB1 receptor, RAGE. TNFα acts locally to amplify responses initiated by HMGB1. Secretion of regulators of fibrinolysis (tPA and PAI-1) results in the activation of plasmin that may limit HMGB1 responses by proteolytic degradation of HMGB1.
We thank Sura Alsaaty and Patricia Madara for their excellent technical assistance.
Prepublished online as Blood First Edition Paper, November 27, 2002; DOI 10.1182/blood-2002-05-1300.
Supported by National Institutes of Health intramural funds.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Anthony F. Suffredini, Building 10, Room 7D-43, CCMD/CC/NIH, 10 Center Dr, Bethesda, MD 20892-1662; e-mail:asuffredini@mail.cc.nih.gov.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal