P-selectin is an adhesion molecule expressed on activated platelets and endothelium. It is known to play an important role in atherosclerosis. P-selectin also circulates in plasma in a soluble form (sP-selectin), which induces procoagulant microparticle formation. We investigated the role of platelet versus endothelial P-selectin in generating sP-selectin and in the formation of atherosclerotic lesions in the apolipoprotein E (apoE)–deficient mouse model. For this we transplanted apoE−/−P-selectin−/− and apoE−/−P-selectin+/+ lethally irradiated mice with bone marrow of either genotype. Seven months after transplantation, we determined from the chimeric animals that the majority of circulating sP-selectin was of endothelial origin. Thus, in atherosclerosis, the procoagulant sP-selectin reflects endothelial rather than platelet activation. We found that endothelial P-selectin was crucial for the promotion of atherosclerotic lesion growth because in its absence only relatively small lesions developed. However, platelet P-selectin also contributed to the lesion development because lesions in wild-type recipients receiving transplants with wild-type platelets were 30% larger than those receiving P-selectin-deficient platelets (P < .008) and were more frequently calcified (80% versus 44%). In comparison with P-selectin wild-type animals, absence of either endothelial or platelet P-selectin inhibited migration of smooth muscle cells into the lesion. Thus, in addition to endothelium, platelets and their P-selectin also actively promote advanced atherosclerotic lesion development.
Introduction
Atherosclerosis and other inflammatory diseases are characterized by the infiltration of leukocytes into foci of inflammation. In atherosclerosis, the cells recruited are primarily monocytes.1 P-selectin, an adhesion receptor found in storage granules of platelets and endothelial cells,2mediates rolling of monocytes on activated endothelium,3the first step in the cell adhesion cascade.4,5 This interaction allows chemokines, such as monocyte chemoattractant protein 1 (MCP-1) or RANTES, presented on the surface of endothelial cells, to activate the monocytes, which then can bind to other endothelial cell adhesion molecules such as intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1). The monocytes thereby attach firmly to the endothelium and finally transmigrate into the arterial intima. Targeted disruption of the P-selectin gene in the mouse results in marked inhibition of leukocyte rolling along a stimulated vessel wall and in delayed recruitment of monocytes into sites of inflammation.6,7 Furthermore, absence of P-selectin in atherosclerosis-prone apolipoprotein E (apoE)-deficient mice delays and reduces atherosclerotic lesion formation.8,9 However, P-selectin is not only expressed on activated endothelial cells but also on activated platelets and it mediates rosetting of the platelets with monocytes and neutrophils.10 This interaction could contribute to monocyte recruitment11 and could bring platelets, with all their biologic activities, into lesions. Quite some time ago, Ross and Harker proposed the “response to injury” model of atherosclerosis and suggested platelet involvement in this process. However, a direct contribution of platelets to atherosclerotic lesion growth or maturation has not been demonstrated experimentally except when arteriosclerosis was induced by mechanical12 or chemical damage to the endothelium.13
P-selectin, released from the cell surface, circulates as a soluble molecule in the plasma.14 It was recently shown that soluble P-selectin (sP-selectin) can exert procoagulant activity15 and it might therefore play an important role in thrombosis and acute coronary events. sP-selectin is often used as a marker of platelet activation,16 although its origin has not been established with certainty.
We performed bone marrow transplantation experiments using apoE-deficient and apoE/P-selectin double-deficient mice to evaluate both the contribution of endothelial and platelet P-selectin to the development of atherosclerotic lesions and to the production of sP-selectin.
Materials and methods
Mice and bone marrow transplantation
P-selectin–deficient (P-sel−/−) mice were backcrossed 4 times to C57BL/6J and interbred with apolipoprotein E–deficient (apoE−/−) mice that themselves were backcrossed 8 times to the C57BL/6J background.17Littermates from the F2 generation of the intercross were used to establish apoE−/−P-sel+/+ and apoE−/−P-sel−/− matings.8 For the preparation of bone marrow, donor mice were killed; femur and tibia bones were isolated under sterile conditions and the bone marrow cells were harvested by flushing the bones with medium (RPMI, 5% fetal bovine serum [FBS]). The cells were washed and resuspended in Hanks balanced salt solution (HBSS). Recipient 6-week-old male mice were lethally irradiated by exposure to a cesium source, 2 doses of 6 Gy, 3 hours apart. Immediately after the second dose of irradiation, the mice were injected with 1 × 107 freshly prepared donor bone marrow cells. For the first 4 weeks after transplantation, recipient mice were kept in a sterile unit and given sterile water and sterile food. Mice were kept on a normal chow diet containing 5% fat (wt/wt; Harlan/Teklad, Madison, WI) until 30 weeks after transplantation. Thirty-six-week-old, nonirradiated apoE−/−P-sel+/+ and apoE−/−P-sel−/− mice were used for reference. Before being killed, mice were fasted overnight and blood was collected through puncture of the retro-orbital venous plexus. Experimental procedures were approved by the Animal Care and Use Committee of the Center for Blood Research.
Determination of bone marrow reconstitution
Platelet-rich plasma (PRP) was obtained from ACD-anticoagulated blood (38 mM citric acid, 75 mM trisodium citrate, 100 mM dextrose; 10% final concentration) by centrifugation at 200g for 7 minutes and recentrifugation of the plasma and buffy coat for another 5 minutes at 180g. Platelets were washed with washing buffer (129 mM NaCl, 13.6 mM trisodium citrate, 11.1 mM dextrose, 1.6 mM KH2PO4, pH 6.8), centrifuged at 850gfor 12 minutes, and resuspended in resuspension buffer (137 mM NaCl; 4 mM KCl; 0.5 mM MgCl2; 0.5 mM sodium phosphate, 11.1 mM dextrose; 10 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid]; pH 7.4). Platelets were activated with 0.5 U/mL thrombin (Sigma, St Louis, MO) for 10 minutes at 37°C, incubated for 40 minutes at room temperature with fluorescein isothiocyanate (FITC)-labeled antimouse P-selectin antibody clone RB40.34 (Pharmingen, San Diego, CA), and analyzed by fluorescence-activated cell sorting (FACS) on a Becton Dickinson FACSCalibur (Franklin Lakes, NJ). A complete blood cell count of EDTA (ethylenediaminetetraacetic acid)-anticoagulated blood was performed using an automatic cell counter (Coulter, Miami, FL).
Cholesterol determination
Total cholesterol concentrations in plasma were measured using an enzymatic assay kit (Infinity Cholesterol Reagent, Sigma).
Quantification of aortic sinus lesions
Mice were deeply anesthetized by intraperitoneal injection of avertin. After cutting open the right atrium, the heart and the vascular system were perfused in situ for 5 to 10 minutes with phosphate-buffered saline (PBS) until the outflow from the right atrium was clear. The hearts were collected, placed immediately in optimum cutting temperature (OCT) compound, and frozen on dry ice. Cryostat sections (10 μm) were cut at the level of the aortic sinus, collected on Superfrost microscopic glass slides, and stored at −20°C until further use. Sections were stained with oil red-O and hematoxylin (Sigma) and counterstained with light green (Sigma). Within the aortic sinus, lesions from 5 sections, each 80 μm apart, were measured by a blinded observer, using a Leica Q500MC image analysis program. Values reported represent the mean lesion area (± SEM) for each animal. Presence or absence of calcifications was evaluated for each section.
Immunohistochemical analysis
Sections 10-μm thick were fixed in cold acetone for 6 minutes. Sections were incubated for 10 minutes with 3% hydrogen peroxide to block endogenous peroxidase. After washing with PBS, unspecific binding sites were blocked by 30 minutes of incubation with 10% normal rabbit serum (for monoclonal) or goat serum (for polyclonal first antibodies) just prior to incubation with the following primary antibodies: antihuman smooth muscle α-actin, horseradish peroxidase (HRP) coupled (no. U7033; Dako, Carpinteria, CA); monoclonal rat antimouse platelet–endothelial cell adhesion molecule (PECAM), clone Mec13.3 (Pharmingen); monoclonal rat antimouse Mac-3 antigen, present on mouse mononuclear phagocytes, clone M3/84 (Pharmingen); polyclonal rabbit antimouse P-selectin (generous gift from Dr M. C. Berndt, Baker Medical Research Institute, Melbourne, Victoria, Australia); monoclonal rat antimouse glycoprotein (GP) IIbIIIa (generous gift from Dr A. K. Ng, University of Southern Maine, Portland). Biotinylated rabbit-antirat or goat antirabbit secondary antibodies and streptavidin-linked HRP (all from Vector Laboratories, Burlingame, CA) were used. 3-amino-9-ethylcarbazole (AEC; Vector Laboratories) served as substrate for HRP and sections were counterstained with hematoxylin (Sigma). For each animal smooth muscle cells (SMCs) were counted on the section immediately next to those that were used to quantify lesion size.
sP-selectin ELISA
Enzyme-linked immunosorbent assay (ELISA) plates were coated overnight with monoclonal antimouse P-selectin antibodies, clone RB40.34 (Pharmingen), at 4°C. Plates were washed 3 times with PBS/0.1% Triton X-100/0.5% bovine serum albumin (BSA) and incubated for 45 minutes with blocking solution (PBS/0.1% Triton X-100/1% BSA). After removing the blocking solution, plasma diluted 1:15 in “diluent solution” from the soluble P-selectin ELISA kit (BEE 6; R & D Systems, Minneapolis, MN), was applied and incubated for 30 minutes. The “sP-selectin conjugate,” containing a polyclonal antihuman P-selectin antibody cross-reacting with mouse P-selectin, was then added and incubated for an additional 80 minutes. Plates were washed 3 times with 300 μL “wash buffer” and incubated for 15 minutes with 100 μL “substrate.” The reaction was stopped by adding 100 μL “stop solution” (reagents in quotation marks were all from the BEE 6 kit). A DYNEX ELISA-reader (Thermo Labsystems, Helsinki, Finland), set to 450 nm, with wavelength correction set to 650 nm, determined the optical density (OD).
Statistical analysis
In most instances, several group of animals with transplants were compared; ANOVA and Fisher protected least significant difference (PLSD) post hoc tests were performed. When only 2 groups were compared (Figure 2B), unpaired t test was applied. Statview software (SAS Institute, Cary, NC) was used and data are presented as mean ± SEM.
Results
Characteristics of apoE-deficient mice receiving bone marrow transplants
At age 6 weeks, male apoE−/−P-sel−/−and apoE−/−P-sel+/+ mice were lethally irradiated and reconstituted with bone marrow of either genotype to obtain 4 groups of animals: apoE−/−P-sel+/+bone marrow transplanted into apoE−/−P-sel+/+recipient mice (wt-wt), apoE−/−P-sel−/−bone marrow transplanted into apoE−/−P-sel+/+mice (ko-wt), apoE−/−P-sel+/+ bone marrow transplanted into apoE−/−P-sel−/− mice (wt-ko), and apoE−/−P-sel−/− bone marrow transplanted into apoE−/−P-sel−/− mice (ko-ko). Thus, for clarity, the nomenclature of the chimeric mice denotes first the P-selectin genotype of platelets and second that of endothelium. Characteristics of the different groups of mice, before death at the end of the study, are listed in Table1.
Characteristics of the transplanted apoE−/−animals
| P-selectin genotype . | Plts . | ECs . | Cholesterol level, mg/dL . | WBC count, × 103 cells/μL . | Plt count, × 103 cells/μL . | Spleen weight, g . |
|---|---|---|---|---|---|---|
| wt-wt | wt | wt | 507.3 ± 38.8 | 9.6 ± 1.7 | 1389 ± 81 | 0.087 ± 0.012 |
| ko-wt | ko | wt | 512.8 ± 65 | 8.5 ± 1.2 | 1216 ± 112 | 0.138 ± 0.006 |
| wt-ko | wt | ko | 411.1 ± 17 | 5.4 ± 1.9 | 1159 ± 77 | 0.094 ± 0.013 |
| ko-ko | ko | ko | 474.2 ± 48.1 | 6.0 ± 1.1 | 1152 ± 143 | 0.178 ± 0.022 |
| P-selectin genotype . | Plts . | ECs . | Cholesterol level, mg/dL . | WBC count, × 103 cells/μL . | Plt count, × 103 cells/μL . | Spleen weight, g . |
|---|---|---|---|---|---|---|
| wt-wt | wt | wt | 507.3 ± 38.8 | 9.6 ± 1.7 | 1389 ± 81 | 0.087 ± 0.012 |
| ko-wt | ko | wt | 512.8 ± 65 | 8.5 ± 1.2 | 1216 ± 112 | 0.138 ± 0.006 |
| wt-ko | wt | ko | 411.1 ± 17 | 5.4 ± 1.9 | 1159 ± 77 | 0.094 ± 0.013 |
| ko-ko | ko | ko | 474.2 ± 48.1 | 6.0 ± 1.1 | 1152 ± 143 | 0.178 ± 0.022 |
There were no significant differences due to the P-selectin genotype in serum total cholesterol, white blood cell (WBC) or platelet counts among the 4 groups of transplanted animals.
In contrast, the genotype of the chimeric animals affected splenic weights. Platelet P-selectin appeared to be the most important factor determining the weight of the organ (P determined by ANOVA and Fisher PLSD post-hoc test: wt-wt, ko-ko < .0001; wt-wt, ko-wt < .02; wt-ko, ko-wt < .04; wt-wt, wt-ko = .74; ko-wt, ko-ko < .06). Thus, the presence of platelet P-selectin appears to prevent the appearance of the enlarged spleen seen in apoE−/−P-sel−/− mice.8
Plts indicates platelets; ECs, endothelial cells; WBC, white blood cell; n = 6-10.
Cholesterol values, WBC count, and platelet count were not significantly different among transplanted animals. ApoE−/−P-sel−/− animals were previously described as displaying splenomegaly with a large infiltrate of macrophages.8 In this study, the ko-ko animals had larger spleens than wt-wt mice (Table 1). The transplantation procedure did not affect spleen size significantly when compared to age-matched controls without transplants. Interestingly, reconstituting the P-sel–deficient animals with wild-type platelets (wt-ko) appeared to normalize the splenic weights so that they became similar to those of wt-wt mice (P = .74) and smaller than those of ko-ko mice (P < .0002). At this time, it is not clear how platelet P-selectin prevents splenomegaly nor whether it interferes with recruitment/retention of macrophages in the spleen.
Flow cytometer analysis of P-selectin expression on activated platelets at 36 weeks showed that more than 95% of the platelets in all mice chimeric for P-selectin were of donor origin, indicating that the bone marrow transplantation was successful (Figure1). ApoE−/−P-sel−/− mice bred poorly and showed significantly higher mortality than apoE−/−mice.8 18 Thus, the mice receiving bone marrow transplants presented in this study were generated in many small groups over a period of 2 years avoiding cage effects of housing.
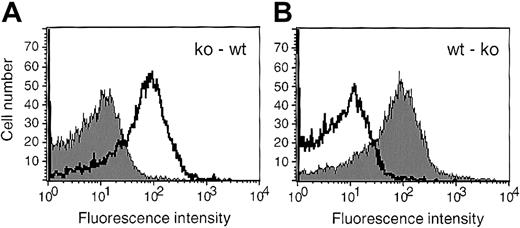
Platelets in mice that received bone marrow transplants were of donor genotype.
Platelets isolated from the mice receiving transplants were activated by thrombin, incubated with FITC-labeled anti P-selectin antibodies, and analyzed by flow cytometry. A representative histogram for each set of chimeric animals is shown; ko-wt (A), wt-ko (B). The shaded curve represents P-selectin expression on activated platelets from an animal receiving a bone marrow transplant, whereas the nonshaded curve was obtained by staining activated platelets of a control mouse without transplant of the same genotype as the recipient.
Platelets in mice that received bone marrow transplants were of donor genotype.
Platelets isolated from the mice receiving transplants were activated by thrombin, incubated with FITC-labeled anti P-selectin antibodies, and analyzed by flow cytometry. A representative histogram for each set of chimeric animals is shown; ko-wt (A), wt-ko (B). The shaded curve represents P-selectin expression on activated platelets from an animal receiving a bone marrow transplant, whereas the nonshaded curve was obtained by staining activated platelets of a control mouse without transplant of the same genotype as the recipient.
Role of endothelial and platelet P-selectin in atherosclerotic lesion formation
After transplantation, mice were maintained on regular mouse chow for 30 weeks before collection of the hearts for analysis. We found that although the lesions at the aortic sinus were well developed at this stage, there were too few lesions in the rest of the aortic tree for comparative analysis. There are 2 likely reasons for this. First, in the absence of atherogenic diet, lesions in the aortic tree develop slowly in the apoE−/− mouse. We observed only 12% of the surface covered with lesions at 15 months and no lesions at 4 months of age.8 Second, it was reported that irradiation/bone marrow transplantation increases lesion growth in the aortic sinus while delaying lesion formation in the rest of the aorta.19 Absence of endothelial P-selectin alone produced a significant reduction (P < .0001) in lesion size (wt-ko: 0.334 ± 0.038 mm2, n = 8) compared with wt-wt lesions (wt-wt: 0.669 ± 0.047 mm2, n = 10). In the absence of endothelial P-selectin, the lesions were relatively small whether or not platelets were expressing P-selectin (ko-ko: 0.271 ± 0.032 mm2, n = 6; wt-ko: 0.334 ± 0.038 mm2, n = 8; P = .33), indicating that endothelial P-selectin is very important for lesion growth. Interestingly, the mean atherosclerotic lesions of ko-wt mice (0.513 ± 0.034 mm2, n = 9) were significantly smaller than the lesions of wt-wt mice (0.669 ± 0.047 mm2, n = 10; P < .008), demonstrating that, when endothelial P-selectin is present, platelets and platelet P-selectin also play a major role in mediating atherosclerotic lesion formation. Lesions of wt-wt animals (0.669 ± 0.047 mm2, n = 10) had a tendency to be slightly larger than those of apoE−/− mice without transplants (0.554 ± 0.041 mm2, n = 5;P = 0.14) as was reported for the aortic sinus lesions of irradiated low-density lipoprotein (LDL) receptor-deficient mice with transplants.19 Lesions of apoE−/−P-sel−/− mice without transplants (0.290 ± 0.018 mm2, n = 4) were much smaller than apoE−/−P-sel+/+ mice and similar in size to lesions of ko-ko animals (0.271 ± 0.032 mm2, n = 6;P = .68) likely due to P-selectin deficiency in these animals (Figure 2).
Endothelial and platelet P-selectin both affect the size of atherosclerotic lesions formed in apoE−/− animals.
Lesions at the aortic sinus were stained with oil red-O and measured. Dots represent the mean lesion size per animal. The bar indicates the mean lesion area ± SEM of the corresponding group. (A) Animals that received bone marrow transplants. Wt-wt and ko-wt animals had significantly larger lesions than wt-ko and ko-ko animals, establishing that endothelial P-selectin is crucial in atherosclerosis. Wt-wt animals had larger lesions than ko-wt animals, showing that also platelet P-selectin is important in atherosclerotic lesion growth. (B) Mice that did not receive transplants. The lesions in apoE−/−P-sel+/+ (wt) and apoE−/−P-sel−/− (ko) mice were not significantly different from those of wt-wt and ko-ko mice, respectively (shown in panel A).
Endothelial and platelet P-selectin both affect the size of atherosclerotic lesions formed in apoE−/− animals.
Lesions at the aortic sinus were stained with oil red-O and measured. Dots represent the mean lesion size per animal. The bar indicates the mean lesion area ± SEM of the corresponding group. (A) Animals that received bone marrow transplants. Wt-wt and ko-wt animals had significantly larger lesions than wt-ko and ko-ko animals, establishing that endothelial P-selectin is crucial in atherosclerosis. Wt-wt animals had larger lesions than ko-wt animals, showing that also platelet P-selectin is important in atherosclerotic lesion growth. (B) Mice that did not receive transplants. The lesions in apoE−/−P-sel+/+ (wt) and apoE−/−P-sel−/− (ko) mice were not significantly different from those of wt-wt and ko-ko mice, respectively (shown in panel A).
Composition of atherosclerotic lesions of the mice with transplants
Lesions, stained with oil red-O and counterstained with light green, showed extensive lipid deposition (Figure3). Immunohistochemical staining for macrophages confirmed that most of the cells within the lesions were macrophages (> 90%). We also evaluated the presence of SMCs cells in the lesions with antibodies to α-actin. In all 4 groups of mice most of the lesions reached an early fibro-fatty stage, that is, contained SMCs and some lesions were calcified (Table2).
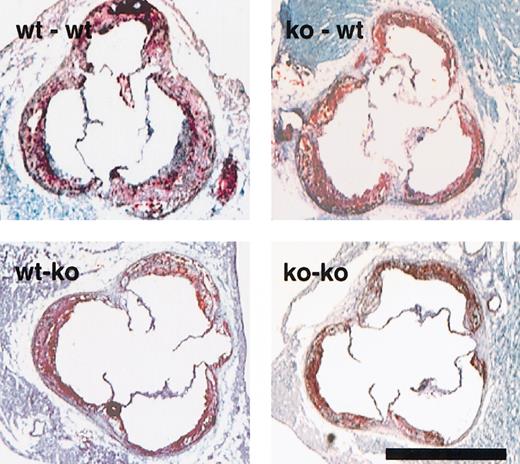
Photographs of the aortic sinus of the animals with bone marrow transplants depict the atherosclerotic lesions formed.
Representative sections of the 4 different sets of animals, stained with oil red-O and counterstained with hematoxylin and light green are as indicated in upper left corner. Lesions of all genotypes show extensive foam cell accumulation. Bar = 1 mm.
Photographs of the aortic sinus of the animals with bone marrow transplants depict the atherosclerotic lesions formed.
Representative sections of the 4 different sets of animals, stained with oil red-O and counterstained with hematoxylin and light green are as indicated in upper left corner. Lesions of all genotypes show extensive foam cell accumulation. Bar = 1 mm.
Characteristics of lesions
| Genotype . | wt-wt . | ko-wt . | wt-ko . | ko-ko . |
|---|---|---|---|---|
| % animals showing calcification | 80 (10) | 44.4 (9) | 37.5 (8) | 33.3 (6) |
| SMCs/section | 15.5 ± 3.5 (9) | 10.2 ± 2.4 (8) | 5.4 ± 1.6 (8) | 1.9 ± 0.5 (6) |
| SMC density SMCs/mm2 | 23.7 ± 6.2 (9) | 20.0 ± 4.9 (8) | 13.3 ± 3.3 (8) | 9.2 ± 3.1 (6) |
| Genotype . | wt-wt . | ko-wt . | wt-ko . | ko-ko . |
|---|---|---|---|---|
| % animals showing calcification | 80 (10) | 44.4 (9) | 37.5 (8) | 33.3 (6) |
| SMCs/section | 15.5 ± 3.5 (9) | 10.2 ± 2.4 (8) | 5.4 ± 1.6 (8) | 1.9 ± 0.5 (6) |
| SMC density SMCs/mm2 | 23.7 ± 6.2 (9) | 20.0 ± 4.9 (8) | 13.3 ± 3.3 (8) | 9.2 ± 3.1 (6) |
Numbers in parentheses indicate number of animals analyzed.
Interestingly, the total number of SMCs in the lesions was a function of both platelet and endothelial P-selectin, with highest SMC numbers in lesions expressing P-selectin on both cell types (15.5 ± 3.5 SMCs/lesion, n = 9), medium number in lesions expressing P-selectin on one cell type only (7.8 ± 1.5 SMCs/lesion, n = 16), and the lowest in animals without P-selectin (1.9 ± 0.5 SMCs/lesion, n = 6; Figure 4). The density of the SMCs in the lesions also apparently depended on both platelet and endothelial P-selectin, although this did not reach statistical significance (Table 2). Compared with wt-wt mice, the percentage of animals showing calcifications in lesions was markedly reduced in chimeras missing either endothelial or platelet P-selectin (Table 2). Immunohistochemical staining for GPIIbIIIa showed that platelets were present in atherosclerotic lesions. However, numbers of platelets were low and no significant differences among the 4 groups could be established. Staining for PECAM-1 indicated that the observed platelets were not within atherosclerotic neo-vessels.
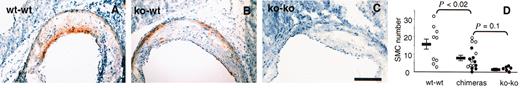
Immunohistochemical staining for α-actin shows positive SMCs in atherosclerotic lesions.
(A) wt-wt; (B) ko-wt; (C) ko-ko animals; bar = 0.2 mm; (D) α-actin–positive SMCs within lesions were counted on 5 aortic sinus sections 80 μm apart. Dots represent the mean number of SMCs per section for each animal. Black dots (●) correspond to animals that lack endothelial P-selectin (ie, wt-ko, ko-ko). Bars show the mean ± SEM for each group. The atherosclerotic lesions of the chimeric animals appear to have an intermediate content of SMCs.
Immunohistochemical staining for α-actin shows positive SMCs in atherosclerotic lesions.
(A) wt-wt; (B) ko-wt; (C) ko-ko animals; bar = 0.2 mm; (D) α-actin–positive SMCs within lesions were counted on 5 aortic sinus sections 80 μm apart. Dots represent the mean number of SMCs per section for each animal. Black dots (●) correspond to animals that lack endothelial P-selectin (ie, wt-ko, ko-ko). Bars show the mean ± SEM for each group. The atherosclerotic lesions of the chimeric animals appear to have an intermediate content of SMCs.
Hematopoietic progenitor cells, released from the bone marrow, were shown to participate in angiogenesis20 21 and could potentially also replace endothelium over damaged atherosclerotic areas. By immunochemistry, we did not detect P-selectin–positive endothelial cells in the aortic sinus section of wt-ko animals. In addition, the fact that there was no significant difference in lesion size between wt-ko and ko-ko animals indicates that bone marrow–derived endothelialization did not play a significant role in promoting atherosclerosis in the apoE−/− mice. We cannot conclude that no angioblasts were recruited to the lesion from the transplanted bone marrow, only that the large majority of the endothelium within and covering the lesion was of the recipient genotype.
Origin of soluble P-selectin in plasma of apoE−/− mice
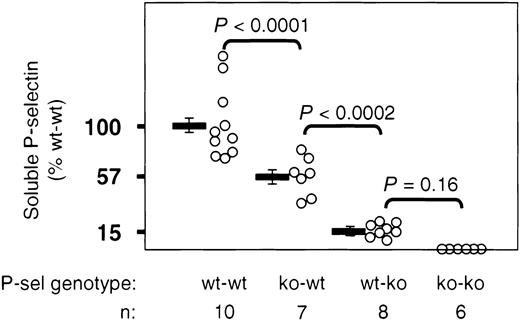
P-selectin on the cell surface of activated platelets or endothelial cells can be shed and circulates in the soluble form in plasma. Because sP-selectin has procoagulant activity that could play an important role during plaque rupture, we were interested in establishing the cellular origin of this molecule. We measured sP-selectin by a sandwich ELISA. As in clinical studies,22we did not ultracentrifuge the plasma and therefore we did not differentiate between microparticle-associated and free-in-plasma P-selectin.14 23 Measurements of plasma from all ko-ko animals gave background OD readings of zero. When we considered the mean OD reading of wt-wt animals as 100%, soluble P-selectin levels in ko-wt animals represented 57.6% of this value, whereas levels of wt-ko animals represented only 15.1%. The small increment of sP-selectin coming from the platelets of wt-ko mice did not reach statistical significance when compared with mice not expressing P-selectin (ko-ko). This indicates that the majority of the soluble P-selectin in the apoE−/− mice was of endothelial origin (Figure5).
sP-selectin in apoE−/− mice originates predominantly from endothelium.
Plasma sP-selectin levels of mice that received transplants were measured by ELISA at the end of the study. OD readings from plasma of wt-wt animals were considered as 100%. Bars represent the mean OD ± SEM.
sP-selectin in apoE−/− mice originates predominantly from endothelium.
Plasma sP-selectin levels of mice that received transplants were measured by ELISA at the end of the study. OD readings from plasma of wt-wt animals were considered as 100%. Bars represent the mean OD ± SEM.
Discussion
It is becoming increasingly clear that P-selectin is a pivotal component in cardiovascular disease and therefore is a potential therapeutic target. The absence of P-selectin delays and reduces atherosclerotic lesion formation in mice.8,9,24,25 In several animal models of restenosis, deficiency or blocking of P-selectin with antibodies or recombinant soluble P-selectin glycoprotein ligand 1 (PSGL-1) inhibits neointimal growth after arterial injury.18,26-29 P-selectin mediates monocyte rolling ex vivo on dissected atherosclerotic arteries3 and thus could be involved in leukocyte recruitment into the lesions. Although the role of endothelial P-selectin in monocyte recruitment to the growing atherosclerotic lesion seems intuitive,30until now it was not directly demonstrated as the gene targeting and inhibition studies described affect both endothelial and platelet P-selectin. Similarly, it has not been shown whether platelets with their P-selectin contribute to lesion development. In the case of plaque rupture, soluble P-selectin may help to promote thrombus formation and fibrin deposition,15 eventually leading to a myocardial infarction. In the present study we have evaluated the separate contributions of platelets versus endothelial P-selectin in the development of atherosclerosis and in the generation of plasma sP-selectin.
sP-selectin, either endogenously generated through proteolytic cleavage of surface-expressed P-selectin or infused intravenously in its recombinant form, stimulates the release of procoagulant microparticles in mice, some of which contain tissue factor.15 Thus high levels of sP-selectin induce a procoagulant state. This observation may explain why high levels of sP-selectin in apparently healthy women are associated with a higher risk of future cardiovascular events.22 The origin of sP-selectin in blood remains an open question. Positive correlation of sP-selectin levels with the platelet count31 and with released platelet proteins, as well as lack of correlation with von Willebrand factor, an endothelial cell activation marker,32 led to the assumption that sP-selectin originates predominantly from platelets. Because high levels of sP-selectin might contribute to the severity of many thrombotic and cardiovascular diseases such as unstable angina,33 we felt it would be clinically relevant to determine the cellular origin of sP-selectin to eventually devise ways to inhibit its generation in patients. Our bone marrow transplantation experiment provided an excellent opportunity to establish the origin of sP-selectin. To our surprise we observed that, in atherosclerotic apoE−/− mice, endothelial cells released almost 4 times more sP-selectin than platelets (Figure 5). Thus, sP-selectin levels reflected more endothelial than platelet activation and we therefore question the use of sP-selectin as a platelet activation marker in the context of atherosclerosis. High levels of fibrinogen and coagulation factors are well-known risk factors for coronary artery disease.34 35 Because sP-selectin through its procoagulant activity could stimulate deposition of fibrin, it could also promote atherosclerosis. Because platelets contributed relatively little sP-selectin in this model of atherosclerosis, we believe that their significant contribution to lesion development extends beyond their share of sP-selectin.
Our study documents that endothelial P-selectin plays a prominent role in atherosclerosis. ApoE−/− mice in which endothelial P-selectin was present (wt-wt and ko-wt) had significantly larger lesions than mice that were deficient for endothelial P-selectin (ko-ko and wt-ko). Platelets release many growth factors, chemokines, and small molecules promoting hemostasis. Whether platelets are involved in atherosclerosis before plaque rupture occurs was not clear. In this study, we provide evidence that platelets play an important role in atherosclerotic lesion formation because we observed that the lesions of wt-wt animals were 30% larger than lesions of ko-wt animals (Figure2). How could platelets expressing P-selectin promote atherosclerosis? There are several possibilities, which are not mutually exclusive: (1) P-selectin expressed on activated platelets causes formation of platelet-neutrophil36 and platelet-monocyte aggregates in blood.37,38 Platelet microparticles, containing P-selectin, were also shown to support leukocyte-leukocyte interaction under flow.39 These associations could augment infiltration of monocytes into atherosclerotic lesions,11,28 similar to L-selectin promoting secondary capture of leukocytes at sites of inflammation.40 (2) It was recently reported that platelet-derived microparticles associate with PSGL-1–expressing hematopoietic stem cells and facilitate their homing to the bone marrow.41 By a similar mechanism, P-selectin on microparticles could coat monocytes and contribute to their recruitment into atherosclerotic lesions. Interestingly, monocytoid cells were shown to roll and adhere to endothelium in vitro at a much higher shear rate when complexed with activated platelets than alone.11 This could help homing of the monocytes in unfavorable shear stress conditions. (3) Platelets and platelet microparticles were shown to deposit chemokines, such as RANTES42 or other agents,43 on endothelium, which can attract and activate monocytes, thus possibly promoting their recruitment into the lesions. (4) Platelets or substances released from activated platelets were shown to induce in vitro macrophage foam cell formation and thereby could contribute to lesion growth.44-46
P-selectin also plays a role in maturation of atherosclerotic lesions. It was previously shown that most (70%) of 4-month-old apoE−/− mice already had SMC-containing fibro-fatty lesions, whereas none of the apoE−/−P-sel−/− mice had SMCs in their lesions at that age.8 We now show that both endothelial and platelet P-selectin apparently contribute to lesion maturation. Lesions expressing either endothelial or platelet P-selectin showed an intermediate level of maturity in regard to SMC content, that is, they were less mature than lesions where both cell types expressed P-selectin but showed a tendency to be more mature than those where no P-selectin was expressed (Figure 4; Table 2). This indicates that the recruitment of SMCs might be influenced by the amount of P-selectin expressed. Contact with P-selectin, presented either by platelets or endothelium, might activate monocytes entering or within the atherosclerotic lesion to secrete chemokines that attract or stimulate proliferation of SMCs.47 Similarly, in comparison with wt-wt animals, we observed a striking reduction in the number of animals that showed calcification in their lesions if either endothelial or platelet P-selectin were missing (Table 2).
In conclusion, we have shown that most of the procoagulant sP-selectin in atherosclerotic apoE−/− mice came from endothelium rather than platelets. We further demonstrated that endothelial P-selectin is crucial for atherosclerotic lesion growth and more unexpectedly that platelets and platelet P-selectin also play an important role in atherosclerotic lesion progression. Thus, agents inhibiting P-selectin or preventing platelet activation could become effective tools in reducing atherosclerotic lesion development and preventing acute cardiovascular events.
We thank Ali Hafezi-Moghadam for critical reading of the manuscript and Hongwei Wang for help with statistical analysis; Simin Saffaripour, Maria Economopoulos, Patrick André, and Christine Livingston for advice and help; and Lesley Cowan for assistance in preparing the manuscript. We also thank Dr M. C. Berndt, Baker Medical Research Institute, Melbourne, Victoria, Australia, and Dr A. K. Ng, University of Southern Maine, Portland, for providing antibodies.
Prepublished online as Blood First Edition Paper, December 12, 2002; DOI 10.1182/blood-2002-07-2209.
Supported by National Heart, Lung and Blood Institute grant R01 HL 53756 (D.D.W.). P.C.B. was supported by a stipend from the Swiss National Science Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Denisa D. Wagner, Center for Blood Research, 800 Huntington Ave, Boston, MA 02115; e-mail:wagner@cbr.med.harvard.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal