The correlates of protective immunity to disease-inducing viruses in humans remain to be elucidated. We determined the kinetics and characteristics of cytomegalovirus (CMV)–specific CD4+ and CD8+ T cells in the course of primary CMV infection in asymptomatic and symptomatic recipients of renal transplants. Specific CD8+ cytotoxic T lymphocyte (CTL) and antibody responses developed regardless of clinical signs. CD45RA−CD27+CCR7− CTLs, although classified as immature effector cells in HIV infection, were the predominant CD8 effector population in the acute phase of protective immune reactions to CMV and were functionally competent. Whereas in asymptomatic individuals the CMV-specific CD4+ T-cell response preceded CMV-specific CD8+T-cell responses, in symptomatic individuals the CMV-specific effector-memory CD4+ T-cell response was delayed and only detectable after antiviral therapy. The appearance of disease symptoms in these patients suggests that functional CD8+ T-cell and antibody responses are insufficient to control viral replication and that formation of effector-memory CD4+ T cells is necessary for recovery of infection.
Introduction
The outcome of viral infections is determined by tropism and virulence of the virus, its ability to manipulate the immune system, and, importantly, the effectiveness of the host's immune response in retaining the virus.1-3 In animal models, insight has been obtained into the development of primary antiviral responses, but detailed information on this subject in humans is lacking. Still, knowledge on the correlates of relevant human protective immune responses is of prime importance for effective vaccination strategies and immunotherapeutic interventions.
In controlling viral disease, neutralizing antibodies and effective cytotoxic T-lymphocyte (CTL) responses are believed to be the main effector arms of the adaptive immune system. Both responses are critically dependent on CD4+ T-cell help,4-6and deficiency of helper cells leads to persistence of virus in the presence of activated but functionally unresponsive CD8+ T cells.7
In models of human T-cell differentiation, CD8+ T-cell memory is established by either a linear differentiation pathway, where memory cells are generated from a primary effector cell pool, which, after clearance of antigen, gives rise to a population of memory cells, or by a divergent pathway, where memory and effector cells each derive from a common precursor as 2 distinct lineages.8 Variants on these differentiation models propose the existence of a stem cell–like memory cell or a central memory cell, giving rise to effector cells on antigenic stimulation.9,10 Functional differentiation within the CD8+ T-cell compartment can be determined by combined analysis of surface and intracellular markers.11,12 In healthy individuals, 2 populations of primed T cells can be distinguished; cytotoxic effector cells are marked by the absence of secondary lymphoid homing receptors such as CD62L and CCR7, and of costimulatory molecules such as CD28 and CD27, and by expression of CD45RA and abundance of cytotoxic effector molecules as granzyme B, perforin, and CD95 ligand memory cells.11 Recent literature on virus-specific CD8+ T cells, however, contradicts the sole restriction of lytic capacity to one particular subset or marker.13,14During acute viral infection, a remarkable uniform phenotype of proliferating virus-specific T cells with effector function is found, that is, CD8+CD45R0+CD27+(Ki-67+).15,16As infection resolves, CD8+ T cells, as a consequence of differentiation processes, first lose CD28 and then CD27.17 In latently infected persons, memory CD8+ T cells specific for asymptomatic latent viruses as such as Epstein-Barr virus (EBV) and cytomegalovirus (CMV) show phenotypic and functional heterogeneity,18-20 and the factors determining the phenotype of memory cells in latent infection are as yet unresolved. Possible determinants are initial viral load and clonal T-cell burst, virulence of individual virus strains, and tropism of the virus, the latter requiring different homing properties of virus-specific cells, as is shown in EBV infection, where EBV-specific cells to latent epitopes express CD62L and CCR7, a chemokine receptor able to bind EBV-induced molecule 1 (ELC), which is expressed on lymph node and tonsillar tissue, enabling them to circulate to B-cell sites of infection.18 21
In pathologic conditions of viral persistence, as in HIV infection, it has been suggested that impairment of both maturation and effector function of CD8+ T cells is a major cause for the inability of the immune system to control viral replication and subsequent disease.10,22 In these studies, virus-specific CD8+ T cells accumulate in the CD45R0+CD27+CCR7− subset and display none of the features normally associated with cytotoxic effector function. The finding that a direct correlation exists between number and phenotype of virus-specific CD8+ T cells and the level of plasma viral load23 24 corroborates that functional maturation of CD8+ T cells is of prime importance in maintaining viral latency.
We here document the development of a human immune response to a clinically relevant virus from primary infection until the latent stage in CMV-seronegative recipients of a CMV-harboring allotransplant.25 In most renal transplant recipients CMV infection resolves without clinically apparent disease despite immunosuppressive medication. In some patients, however, CMV infection leads to severe clinical disease symptoms warranting antiviral drug therapy. To document the development of protective and nonprotective primary immune responses in humans, we performed a longitudinal quantitative and qualitative analysis of the CMV-specific CD4+ and CD8+ T-cell responses during primary infection in asymptomatic and symptomatic recipients of renal transplants. Our data show that although competent CD8+effector cells develop both in asymptomatic and symptomatic infection, for protective immunity and containment of viral replication, interferon γ (IFN-γ)–secreting CD4+ T cells are indispensable.
Patients, materials, and methods
Patients
Three HLA-A2+ and 6 HLA-B7+CMV-seronegative renal transplant recipients of a CMV-seropositive kidney were longitudinally studied (Table1). All patients received a first graft from either a postmortem donor or a living related donor. Basic immunosuppressive therapy consisted of cyclosporin A, blood trough levels aimed at 150 ng/mL, and prednisone 10 mg daily. In case of living related transplantation (patients 2 and 7), mycophenolate mofetil (MMF) was added at a dosage of 1000 mg twice daily. Rejection was treated with methylprednisolone 500 mg for 6 days, and in case of ongoing rejection (patient 7) with monoclonal antibody (mAb) against CD3 (OKT3) for 10 days. The cumulative dose of corticosteroids did not differ between patients experiencing asymptomatic or symptomatic CMV infection. No relationship was observed between CMV infection and the time points of rejection episodes. Antiviral treatment consisted of ganciclovir (5 mg/kg intravenously twice daily, adjusted for renal function) and was initiated when visceral CMV disease was diagnosed on the basis of organ involvement confirmed by tissue biopsy. Heparinized peripheral blood samples were collected before transplantation and weekly during 17 weeks after transplantation. To analyze the latent state, as defined by quantitative polymerase chain reaction (PCR) viral load below the detection limit of 80 copies/mL, a blood sample was taken more than 30 weeks after infection. Peripheral blood mononuclear cells (PBMCs) were isolated using standard density gradient centrifugation techniques and subsequently cryopreserved. All patients gave written informed consent and the study was approved by the local medical ethical committee.
Patient characteristics
| Patients . | Days to first positive PCR . | Maximum viral load, copies/mL . | Duration of infection, duration of positive PCR in days . | Days to CD4 from first positive PCR . | Days to CD8 from first positive PCR . | First antibody appearance, days from first positive PCR . | Clinical symptoms . | Start of antiviral therapy, days from transplantation . | Rejection episodes, number, days from transplantation . | Rejection therapy . |
|---|---|---|---|---|---|---|---|---|---|---|
| Asymptomatic patients | ||||||||||
| 1 | 26 | 22 000 | 28 | 10 | 21 | 14 | NA | NA | 0 | NA |
| 2 | 46 | 14 000 | 59 | 17 | 31 | 31 | NA | NA | 1, d 9 | MPNS |
| 3 | 18 | 22 000 | 42 | 14 | 28 | 21 | NA | NA | 0 | NA |
| 4 | 27 | 133 000 | 48 | 0 | 10 | 7 | NA | NA | 0 | NA |
| 5 | 26 | 32 000 | 66 | 10 | 24 | 17 | NA | NA | 1, d 19 | MPNS |
| Median | 26 | 22 000 | 48 | 10* | 24 | 17 | NA | NA | NA | NA |
| Symptomatic patients | ||||||||||
| 6 | 26 | 92 000 | 88 | 28 | 18 | 16 | Gastrointestinal | 33 | 0 | NA |
| 7 | 28 | 140 000 | 50 | 35 | 24 | 28 | Gastrointestinal | 49 | 2, d 57, 69 | MPNS, OKT 3 |
| 8 | 28 | 124 000 | 74 | 53 | 32 | 4 | Pancytopenia | 49 | 1, d 27 | MPNS |
| 9 | 22 | 140 000 | 35 | 42 | 14 | 14 | Gastrointestinal | 39 | 1, d 45 | MPNS |
| Median | 27 | 132 000 | 62 | 39* | 21 | 15 | NA | 44 | NA | NA |
| Patients . | Days to first positive PCR . | Maximum viral load, copies/mL . | Duration of infection, duration of positive PCR in days . | Days to CD4 from first positive PCR . | Days to CD8 from first positive PCR . | First antibody appearance, days from first positive PCR . | Clinical symptoms . | Start of antiviral therapy, days from transplantation . | Rejection episodes, number, days from transplantation . | Rejection therapy . |
|---|---|---|---|---|---|---|---|---|---|---|
| Asymptomatic patients | ||||||||||
| 1 | 26 | 22 000 | 28 | 10 | 21 | 14 | NA | NA | 0 | NA |
| 2 | 46 | 14 000 | 59 | 17 | 31 | 31 | NA | NA | 1, d 9 | MPNS |
| 3 | 18 | 22 000 | 42 | 14 | 28 | 21 | NA | NA | 0 | NA |
| 4 | 27 | 133 000 | 48 | 0 | 10 | 7 | NA | NA | 0 | NA |
| 5 | 26 | 32 000 | 66 | 10 | 24 | 17 | NA | NA | 1, d 19 | MPNS |
| Median | 26 | 22 000 | 48 | 10* | 24 | 17 | NA | NA | NA | NA |
| Symptomatic patients | ||||||||||
| 6 | 26 | 92 000 | 88 | 28 | 18 | 16 | Gastrointestinal | 33 | 0 | NA |
| 7 | 28 | 140 000 | 50 | 35 | 24 | 28 | Gastrointestinal | 49 | 2, d 57, 69 | MPNS, OKT 3 |
| 8 | 28 | 124 000 | 74 | 53 | 32 | 4 | Pancytopenia | 49 | 1, d 27 | MPNS |
| 9 | 22 | 140 000 | 35 | 42 | 14 | 14 | Gastrointestinal | 39 | 1, d 45 | MPNS |
| Median | 27 | 132 000 | 62 | 39* | 21 | 15 | NA | 44 | NA | NA |
NA indicates not applicable; MPNS, methylprednisolone.
Statistically significant, P < .05
Peptides
The HLA-A2–binding CMV pp65-derived peptide NLVPMVATV and the HLA-B7–binding CMV pp65-derived peptide TPRVTGGGAM were purchased from the Leids Universitair Medisch Centrum (LUMC) peptide synthesis library facility (Leiden, the Netherlands).
Generation of tetrameric complexes
Tetrameric complexes were generated essentially as described by Altman et al.26 In brief, purified HLA-A2.1 heavy chain or HLA-B7.2 heavy chain and β2-microglobulin were synthesized using a prokaryotic expression system (pET; Novagen, Milwaukee, WI). The heavy chain was modified by deletion of the transmembrane/cytosolic tail and COOH-terminal addition of a sequence containing the BirA enzymatic biotinylation site. The HLA-A2.1–binding CMV pp65-derived peptide NLVPMVATV and the HLA-B7.2–derived peptide TPRVTGGGAM were used for refolding. Monomeric complexes were concentrated, biotinylated by BirA (expressed, using the pET expression system, purified using Clontech cobalt beads; Palo Alto, CA) in the presence of biotin (Molecular Probes, Eugene, OR), adenosine triphosphate (ATP; Sigma Chemical, St Louis, MO), and MgC12. The biotinylated product was separated from free biotin by fast protein liquid chromatography (FPLC) using a Superdex 200 HR16/60 column (Amersham Pharmacia, Little Chalfont, United Kingdom). Streptavidin-allophycocyanin (APC) conjugate (Molecular Probes) was added in a 1:4 molar ratio and subsequently tetramers were purified by FPLC using the same column.
Immunofluorescent staining and flow cytometry
Thawed PBMCs were resuspended in RPMI medium, containing 10% fetal calf serum (FCS) and antibiotics. Then, 200 000 PBMCs were incubated with fluorescent-labeled conjugated mAbs (concentrations according to manufacturer's instructions) and an appropriate concentration of tetrameric complexes. Negative controls to validate specificity of the CMV-peptide-tetrameric complexes consisted of HLA-A2.1/HLAB7.2− CMV-seropositive or HLA-A2.1/HLA-B7.2+ CMV-seronegative healthy individuals and renal transplant recipients. Negative controls always showed tetramer staining of less than 0.01% of total lymphocytes (data not shown). For staining with the mouse antihuman CCR7 mAb, a 3-step staining protocol was performed consisting of incubation with the CCR7 antibody (Pharmingen, San Diego, CA), for 30 minutes, washing, incubation with biotinylated goat antimouse IgM (Pharmingen) for 30 minutes, incubation with 10% (vol/vol) normal mouse serum (CLB, Amsterdam, the Netherlands) followed by incubation with streptavidin-phycoerythrin (PE) and directly conjugated mAbs and tetrameric complexes for 30 minutes. Analyses consisted of APC-conjugated tetramers and CD8–peridinin chlorophyll protein (PerCP; Becton Dickinson, San Jose, CA) in combination with either CD45RA (Becton Dickinson) and CD27 (Becton Dickinson), CCR7 and CD45RA, CD27 and CD28 (Becton Dickinson), and CD45RA and CD45R0 (Becton Dickinson), all combinations in fluorescein isothiocyanate (FITC) and PE.
Intracellular Ki-67, granzyme B, and perforin staining was performed by incubating 0.5 million PBMCs with fluorescent-labeled conjugated mAbs to CD8 (Becton Dickinson) and CMV-tetrameric complexes, washed once, then fixed with 50 μL buffered formaldehyde acetone solution and subsequently permeabilized by washing with 0.1% saponin, 50 mMd- glucose. Cells were then incubated with anti–Ki-67 (Dako, Glostrup, Denmark), anti–granzyme B (CLB), and antiperforin antibodies (Hölzel Diagnostika, Köln, Germany) according to the manufacturer's instructions. Analysis of cells was performed using a FACSCalibur flow cytometer and CellQuest software (Becton Dickinson).
Determination of CMV-specific CD4+ and CD8+T cells by intracellular cytokine staining
CMV-specific CD4+ and CD8+ T-cell frequencies were determined essentially according to the method described by Waldrop et al27 and Kern et al,28 respectively. Briefly, 0.5 × 106freshly isolated PBMCs were incubated for 6 hours in the presence of either CMV antigen (60 μL/mL; Biowhittaker, Wokingham, United Kingdom), control antigen (60 μL/mL, negative control; Biowhittaker; determination of CMV-specific CD4+ T cells),Staphylococcus aureus enterotoxin B (SEB; 2 μg/mL, positive control; ICN/Fluka, Buchs SG, Switzerland), the HLA-A2–binding CMV peptide or the HLA-B7–binding CMV peptide or an irrelevant HLA-A2–binding HIV peptide (negative control; final concentration of 10 μg/mL, determination of CMV-specific CD8+ T cells). CD28 mAb (clone 15E8; CLB) and very late activation antigen 4 (VLA-4) mAb (Becton Dickinson) were added at 2 μg/mL (final concentration), respectively, 1 μg/mL (final concentration) in a final volume of 1 mL/tube RPMI 1640 (Gibco, Paisley, United Kingdom) containing 10% heat-inactivated FCS (Integro, Zaandam, the Netherlands), penicillin, and streptomycin. For the final 5 hours of culture, brefeldin A (Sigma) was added to the culture in a final concentration of 10 μg/mL. Cells were transferred to fluorescence-activated cell-sorting (FACS) tubes, fixed in 2 mL/tube FACS lysing solution (Becton Dickinson), permeabilized in 0.5 mL/tube FACS permeabilizing solution followed by (intracellular) staining with IFN-γ–FITC (Becton Dickinson) and CD69-PE (Becton Dickinson) and CD4-APC (Becton Dickinson) or CD8-APC (Coulter, Fullerton, CA). Cells were washed in PBA and refixed in Cellfix (Becton Dickinson) and flow cytometric analysis was performed on the following day, using a FACSCalibur equipped with a 488-nm argon ion laser and a 635-nm red diode laser. Data files containing 50 000 events positive for CD4-APC or CD8-APC fluorescence within a lymphocyte gate were saved. Frequencies of CD69+ IFN-γ+cells within the CD4+ or CD8+ lymphocyte gate were determined using Cellquest software (Becton Dickinson) and designated CMV-specific CD4+ or CD8+ T-cell frequencies, respectively. Negative controls showed less than 0.05% of CD69+ IFN-γ+ cells (data not shown).
CMV PCR
Quantitative PCR was performed in EDTA (ethylenediaminetetraacetic acid) whole blood samples as described for plasma or serum.29
Anti-CMV IgM and IgG
Anti-CMV IgM and IgG were determined in serum as described previously.25
Cytotoxicity assay
Ex vivo cytotoxicity was assessed by incubating51Cr-labeled (Amersham), peptide-pulsed, HLA-matched, EBV-transformed lymphoblastoid cell lines with PBMCs at effector- target (E/T) ratios of 1:1 to 1:4, calculated on absolute numbers of tetramer-positive CD8+ T cells present in the PBMC fraction, for 4 hours. Negative controls consisted of nonpeptide-pulsed target cells or HLA-mismatched target cells. Percentage specific lysis was calculated from the formula: percentage specific lysis = [(experimental counts−media control)/(detergent control−media control)] × 100%.
Statistical analysis
The 2-sided Mann-Whitney test was used for analysis of differences between groups; for correlations, the Spearman nonparametric correlation test was used. P values less than .05 were considered statistically significant.
Results
CD8+ T-cell responses in relation to viral load and CD4+ T-cell responses in primary, asymptomatic CMV infection
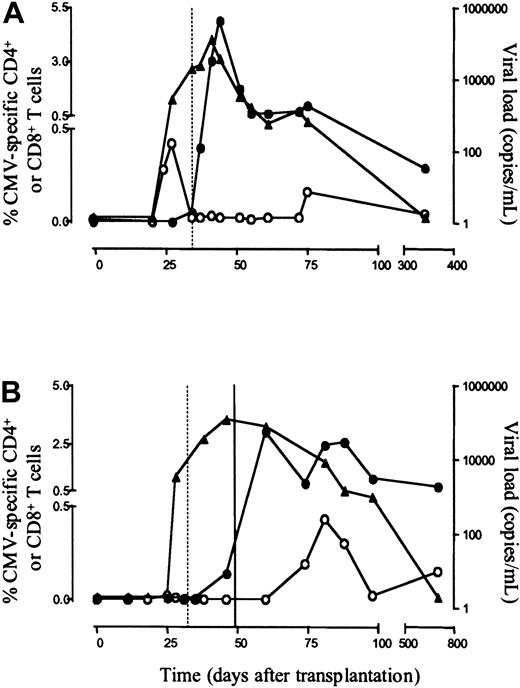
In accordance with our previous findings,23 peak frequencies of CMV-specific CD4+ T cells in 5 asymptomatic individuals, enumerated by IFN-γ production on specific stimulation, ranged from 0.42% to 2.5% of CD4+ T cells (median, 0.82%; absolute value, 0.38 × 107/mL; range, 0.17-1.1 × 107/mL) and were detected at a median of 10 days (range, 0-17 days) after first detection of CMV DNA. CMV-specific IgM and IgG antibodies were detected at a median of 7 days (range, 4-14 days) after first detection of CD4+ T cells (Table 1; Figure 1A).
Enumeration of CMV-specific CD4+ and CD8+ T cells in primary CMV infection.
Shown are the frequencies of CMV-specific CD4+ T cells, determined by intracellular staining for CD69 and IFN-γ on stimulation (○), CMV-specific CD8+ T cells, enumerated by tetramer binding (●), and first specific antibody appearance (dotted vertical line) in relation to viral load (CMV DNA, ▴) in one representative asymptomatic patient (patient 4, panel A) and one symptomatic patient (patient 8, panel B, closed vertical line, start of 14 days of ganciclovir therapy).
Enumeration of CMV-specific CD4+ and CD8+ T cells in primary CMV infection.
Shown are the frequencies of CMV-specific CD4+ T cells, determined by intracellular staining for CD69 and IFN-γ on stimulation (○), CMV-specific CD8+ T cells, enumerated by tetramer binding (●), and first specific antibody appearance (dotted vertical line) in relation to viral load (CMV DNA, ▴) in one representative asymptomatic patient (patient 4, panel A) and one symptomatic patient (patient 8, panel B, closed vertical line, start of 14 days of ganciclovir therapy).
CMV-specific CD8+ T cells were enumerated by HLA-A2 and HLA-B7 tetrameric complexes, folded with the pp65-derived peptides NLVPMVATV for HLA-A2 or TPRVTGGGAM for HLA-B7, both described as immunodominant in latent CMV infection. Peak frequencies of CMV-specific CD8+ T cells ranged from 0.55% to 4.97% (median, 2.21%; absolute value, 1.45 × 107/mL; range, 0.3-8.82 × 107/mL). In all asymptomatic patients CMV-specific CD4+ T cells preceded CMV-specific CD8+ T cells, which were detected at a median of 14 days (range, 10-14 days) after first detection of CMV-specific CD4+ T cells.
On antigenic encounter, naive cells will develop into effector cells, and after viral clearance, long-lived memory cells, with distinctly different proliferative and cytotoxic capacities.11 We analyzed these differentiation steps by extensively phenotyping CMV-specific CD8+ T cells with the differentiation markers CD28, CD27, and CCR7 and by determining CD45RA versus CD45R0 expression. Figure 2 shows the differentiation of the total and CMV-specific CD8+ T cells in one representative patient. Looking at expression of CD28 and CD27 in the course of infection, CMV-specific and total CD8+ T cells first lose CD28 and subsequently CD27 (Figure 2A). Loss of CD27, however, seems to occur only after viral replication has ceased, whereas loss of CD28 occurs early in acute infection. When analyzed, the percentage of CD28+CD27+virus-specific cells is correlated to high amounts of virus present (r = 0.48, P = .0018; data not shown) and the percentage of CD28−CD27− increases when viral load decreases (r = −0. 64, P = < .0001; Figure 2E).
Differentiation of CMV-specific and total CD8+ T cells in asymptomatic and symptomatic patients.
(A-D) Differentiation of CD8+ T cells in one asymptomatic individual representative of all patients (patient 2). Time defined as days after first positive PCR (day 0), all plots gated on CD8+ T cells. CMV-specific CD8+ T-cell frequencies (percent of total CD8+ T cells): day 31, 4.96%; day 59, 4.58%; day 76, 3.30%; day 279, 1.29%. CMV-specific CD8+ T cells as defined by specific tetramer staining are plotted in black, total CD8+ T cells are plotted in red. Quadrant percentages are depicted in black for CMV-specific CD8+ T cells and in red for total CD8+ T cells. (A) x-axis log fluorescence CD27-FITC, y-axis log fluorescence CD28-PE; (B) x-axis log fluorescence CD27-PE, y-axis log fluorescence CD45RA-FITC; (C) x-axis log fluorescence CCR7-PE, y-axis log fluorescence CD45RA-FITC; (D) x-axis log fluorescence CD45R0-PE, y-axis log fluorescence CD45RA-FITC. (E) Correlation of viral load and percentage of CD28−CD27− cells of CMV-specific CD8+ T cells, during all time points of infection, undetectable viral load was set at the cutoff point of the quantitative PCR at 80 copies/mL.
Differentiation of CMV-specific and total CD8+ T cells in asymptomatic and symptomatic patients.
(A-D) Differentiation of CD8+ T cells in one asymptomatic individual representative of all patients (patient 2). Time defined as days after first positive PCR (day 0), all plots gated on CD8+ T cells. CMV-specific CD8+ T-cell frequencies (percent of total CD8+ T cells): day 31, 4.96%; day 59, 4.58%; day 76, 3.30%; day 279, 1.29%. CMV-specific CD8+ T cells as defined by specific tetramer staining are plotted in black, total CD8+ T cells are plotted in red. Quadrant percentages are depicted in black for CMV-specific CD8+ T cells and in red for total CD8+ T cells. (A) x-axis log fluorescence CD27-FITC, y-axis log fluorescence CD28-PE; (B) x-axis log fluorescence CD27-PE, y-axis log fluorescence CD45RA-FITC; (C) x-axis log fluorescence CCR7-PE, y-axis log fluorescence CD45RA-FITC; (D) x-axis log fluorescence CD45R0-PE, y-axis log fluorescence CD45RA-FITC. (E) Correlation of viral load and percentage of CD28−CD27− cells of CMV-specific CD8+ T cells, during all time points of infection, undetectable viral load was set at the cutoff point of the quantitative PCR at 80 copies/mL.
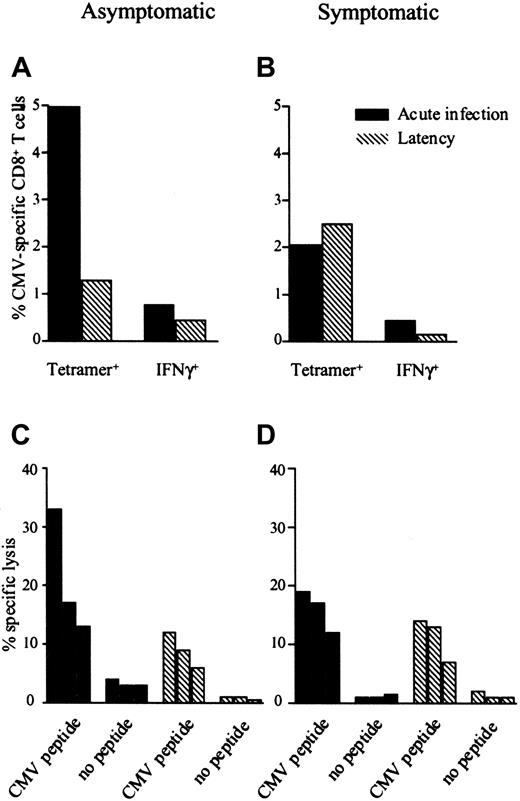
Before onset of infection, the majority of total CD8+T cells in all asymptomatic individuals is found in the naive CD27+CD45RA+ subset (median, 78.77%; range, 40.99%-83.86%). After the first emergence of CMV-specific CD8+ T cells, however, the CD45RA−CD27+ population of total CD8+ T cells is highly increased (median, 52.66%; range, 46.94%-72.39%) and most of the tetramer-positive cells are confined to this subset (Figure 2B). Analysis by CCR7 and CD45RA in the early days of infection shows in some patients a small population of CMV-specific CD8+CCR7+CD45RA−, that is, central memory cells,12 but in all patients most CMV-specific cells are devoid of CCR7 and thus apparently incapable of homing to secondary lymphoid organs (Figure 2C). Looking simultaneously at Ki-67 expression, it appears when in cell cycle, early in infection, these cells express CD45R0 abundantly (Figures 2D and 3A). When infection is resolved, CMV-specific CD8+ T cells acquire a CCR7−CD27−/dull CD45RA+phenotype. In all patients, CMV-specific CD8+ T cells express granzyme B and perforin from the first time point of detection (Figure 3B-C). Interestingly, a substantial portion of tetramer-negative CD8+ T cells gets activated as appears from expression of Ki-67, granzyme B, and perforin. Furthermore, most of the CD8+ T-cell compartment followed the same differentiation process as the tetramer-positive CD8+ T cells, implying that these cells represent CMV-specific cells to other epitopes of CMV. In direct ex vivo cytotoxicity assays, CMV-specific CD8+ T cells could lyse appropriately CMV peptide–loaded, HLA-matched target cells, and no difference was seen in percentage-specific lysis between CMV-specific CD8+ T cells with a CD45R0+CD27+CCR7−phenotype and a CD45RA+CD27−CCR7−phenotype (Figure 4). In all patients a proportion of CMV-specific CD8+ T cells, as enumerated by tetramer binding, was capable of production of IFN-γ on specific peptide stimulation (data not shown and Figure 4).
CMV-specific CD8+ T cells in primary CMV infection are cytotoxic irrespective of their phenotype.
All plots gated on total CD8+ T cells, time defined as days after first positive PCR (day 0); day 31, peak of CMV-specific CD8+ T cell frequency; day 279, time point during latency. (A) x-axis log fluorescence CMV-tetramer, y-axis log fluorescence Ki-67; (B) x-axis log fluorescence CMV-tetramer, y-axis log fluorescence granzyme B; (C) x-axis log fluorescence CMV-tetramer, y-axis log fluorescence perforin. Quadrant percentages depicted as percentages of total CD8+ cells.
CMV-specific CD8+ T cells in primary CMV infection are cytotoxic irrespective of their phenotype.
All plots gated on total CD8+ T cells, time defined as days after first positive PCR (day 0); day 31, peak of CMV-specific CD8+ T cell frequency; day 279, time point during latency. (A) x-axis log fluorescence CMV-tetramer, y-axis log fluorescence Ki-67; (B) x-axis log fluorescence CMV-tetramer, y-axis log fluorescence granzyme B; (C) x-axis log fluorescence CMV-tetramer, y-axis log fluorescence perforin. Quadrant percentages depicted as percentages of total CD8+ cells.
Both asymptomatic and symptomatic individuals show functional CMV-specific CD8+ T cells.
The percentage CMV-specific CD8+ T cells enumerated by tetramer binding, percentage CMV-specific IFN-γ–producing CD8+ T cells enumerated by intracellular cytokine staining on specific peptide stimulation and specific lysis (E/T ratios, 1:1, 1:2, and 1:4) in one asymptomatic patient (patient 2, panels A,C) and one symptomatic patient (patient 6, panels B,D).
Both asymptomatic and symptomatic individuals show functional CMV-specific CD8+ T cells.
The percentage CMV-specific CD8+ T cells enumerated by tetramer binding, percentage CMV-specific IFN-γ–producing CD8+ T cells enumerated by intracellular cytokine staining on specific peptide stimulation and specific lysis (E/T ratios, 1:1, 1:2, and 1:4) in one asymptomatic patient (patient 2, panels A,C) and one symptomatic patient (patient 6, panels B,D).
Effector-memory CD4+ T-cell responses are delayed in symptomatic patients
In 4 patients, primary CMV infection followed a complicated course. All patients suffered severe organ involvement and required antiviral therapy consisting of ganciclovir (Table 1). In these patients CMV DNA was detectable in peripheral blood at a median of 27 days after transplantation (range, 22-28 days; NS compared with asymptomatic patients), and whereas there was no statistical difference in maximum viral load between asymptomatic and symptomatic patients (P = .06), viral load tended to be higher in symptomatic patients. Although these patients experienced CMV disease and needed antiviral treatment to control viral replication, no differences were found with respect to the emergence of CMV-specific antibodies and CMV-specific CD8+ T cells nor differentiation pattern of CD8+ T cells between symptomatic and asymptomatic individuals. CMV-specific antibodies could be detected at a median of 15 days (range, 4-28 days; NS) and CMV-specific CD8+ T cells at a median of 21 days after first CMV DNA detection (range, 14-32 days; NS) with peak frequencies ranging from 0.33% to 3.04% (median, 1.92%; absolute value 0.84 × 107/mL; range, 0.31-1.3 × 107/mL; NS) of total CD8+T cells (Figure 1B). No differences could be detected in either cytotoxicity or specific IFN-γ production of CMV-specific CTLs between symptomatic and asymptomatic patients, implying that other parameters define successful clearance of CMV (Figure 4). Markedly, the time interval between first detection of CMV DNA and first detection of CMV-specific CD4+ T cells was significantly longer than in asymptomatic patients (28-53 days; median, 39 days;P = .01) and only after start of antiviral therapy could CMV-specific CD4 responses be measured. Both CMV-specific CD8+ T-cell responses and CMV-specific IgG antibody responses were detectable before emergence of CMV-specific CD4+ T cells in all symptomatic patients (Table 1) implying that CMV-specific CD4+ T cells were present in lymph nodes to provide help for B cells and CD8+ T cells. Peak frequencies of CMV-specific CD4+ T cells ranged from 0.36% to 1.42% of CD4+ T cells (median, 0.47%; absolute value, 0.17 × 107/mL; range, 0.07-0.27 × 107/mL) and, as in asymptomatic individuals, rapidly decreased to become undetectable. Possibly this short presence in the peripheral blood of CMV-specific effector CD4+ T cells reflects migration of these cells through the peripheral blood to their target site.
Discussion
Here we document the development of an adaptive primary antiviral immune response in humans. We show that in asymptomatic patients, CMV-specific CD4+ T cells emerge in the peripheral blood compartment preceding both CMV-specific antibodies and CD8+T cells. These coordinate responses lead to clearance of the virus. In contrast, in symptomatic patients, specific antibodies as well as specific CD8+ T cells appear in the peripheral blood compartment prior to IFN-γ–producing CMV-specific CD4+ T cells. Only after start of antiviral treatment do these latter cells emerge and the virus is cleared. Remarkably, no differences in either kinetics or functional differentiation/maturation of CMV-specific CD8+ T cells were detected between asymptomatic and symptomatic patients.
The different kinetics of CMV-specific CD4+ T cells in our study of asymptomatic and symptomatic individuals confirm that CD4+ T cells influence outcome of disease in primary infection.30,31 Recent studies show that CD4+T cells can be divided into 2 populations with distinct migratory capacities, one consisting of interleukin 2 (IL-2)–producing central-memory CD4+ T cells, able to recirculate through secondary lymphoid organs, and a second population of effector-memory CD4+ T cells whose main function is to secrete antimicrobial lymphokines, exerting their function in peripheral target organs and thus contributing directly to containment of viral replication.32,33 The emergence of specific IgG antibody responses in both asymptomatic and symptomatic individuals indicates that CMV-specific CD4+ help indeed is present in peripheral lymph nodes to support B-cell differentiation and IgM-IgG class switching4 as well as CD8+ T-cell differentiation.
In our study, impaired control of viral replication, leading to clinical disease symptoms, can be explained by lack of IFN-γ–secreting effector-memory CD4+ T cells at the site of infection.34 The absence of these cells in the peripheral blood compartment early in infection can be ascribed to several variables. Variations in virulence of individual virus strains and their ability to interfere with immune functions, such as antigen presentation and cytokine production, should be taken into account.35-37 One explanation would be impaired antigen priming of the effector-memory CD4+ T-cell subset by dendritic cells possibly due to an altered cytokine environment caused by immunosuppressive therapy38 or suppression of dendritic cell maturation by CMV itself as was recently described for murine and human CMV.39,40 The normal appearance of antibodies and CD8+ T effector cells indicates that specifically the Th1 CD4 response is impaired, suggesting altered IL-12 secretion by dendritic cells.39 40 That effector-memory CD4+ T cells become detectable in peripheral blood shortly after the start of antiviral therapy infers a direct effect of CMV on the immune system.
No difference was seen in the CMV-specific CD8+T-cell differentiation pattern between asymptomatic and symptomatic patients, although the administration of antiviral therapy leading to clearance of virus could account for this finding. During acute infection, CMV-specific cells show a CCR7−CD27+CD45RA−CD45R0+phenotype, previously designated as skewed and immature in chronic HIV infection.10 In our patients, however, this phenotype was displayed on the height of CMV-specific CD8+ T-cell frequencies; only when viral load dropped, did CMV-specific CD8+ T cells lose CD45R0, and a substantial number lost CD27. Regardless of their phenotype, all of these cells expressed granzyme B and perforin from the start of infection, implicating that either a CD27−CD45RA+ phenotype is reached when no antigen is present anymore or that cells of this phenotype cannot be obtained by peripheral blood sampling in replicative stages of viral infection. This would be in line with recent studies, where specific CD8+ T cells in persistent replicative viral infection show a CD45R0+CD27+ phenotype, whereas specific CD8+ T cells in persistent latent viral infection show a more differentiated CD45RA+CD27− phenotype.41 Based on CD28 and CD27 expression successive stages of T-cell differentiation can be depicted in early virus infection, where CD28+CD27+, CD28−CD27+, and CD28−CD27− subsets correspond to early, intermediate, and late phenotypes.17,41 We here show that the appearance of these distinct stages is related to viral load. That in persistent active viral infection, as in hepatitis C virus (HCV), HIV, and lytic EBV infection, CD8+ T cells specific for these viruses accumulate in the early and intermediate subsets is most likely a reflection of redistribution of cytotoxic CD28−CD27− cells to peripheral target sites of active infection, where these cells would exert their function, as was found in animal studies,42 rather than a direct immunomodulatory effect of different viruses themselves on the differentiation pathway of CD8+ T cells. The appearance of CD28−CD27− late effector cells in the course of CMV infection, when active infection is resolved and the virus enters its latent stage, could reflect emergence of these cells out of infected tissues and back into the peripheral blood. Recently, we could demonstrate that CCR7−CD27−CD45RA+ T cells, after stimulation by antigen in vitro, proliferate and revert from CD45RA+ to CD45R0+, suggesting the CCR7−CD45RA− phenotype is induced by presence of antigen.43 When analyzed for Ki-67, CMV-specific CD8+ T cells were found cell cycling early in infection, and when so, were uniformly CD45R0. The described skewed maturation CCR7−CD45RA− phenotype in HIV infection accordingly seems to be a consequence of the presence of antigen and not a cause of disease.44 Also, the difference in expression of CD45 isoforms on virus-specific memory cells during latency would reflect recent cell cycling of these cells, due to antigen exposure or homeostatic instruction.17 45
In our study, the difference between adequate viral clearance and viral persistence was determined by the absence or presence of effector-memory CD4 responses. Indeed, also in persistent HCV infection no CD4 responses can be detected in peripheral blood.46,47Furthermore, in HIV infection the clinical outcome is directly correlated to CD4+ T-cell numbers.48 The influence of these CD4+ T-cell responses on viral clearance in HIV and HCV combined with the phenotypes found in chronic infection with these viruses suggests a model of CD8+ T-cell differentiation where presence of antigen defines the maturation stage of CD8+ T cells detected in peripheral blood, where CD45R0 is a marker for recent replicative history of the antigen specific cell, and the presence of antigen-specific CD4+ T-effector cells defines successful viral clearance. Furthermore, the presence or absence of CD27− effector T cells would reflect redistribution of CTLs to peripheral target sites. It seems that, according to findings of us and others,18 memory CD8+ T cells can expand at any point in their differentiation pathway, even when displaying a CCR7−CD27−CD45RA+ phenotype, previously thought to have no proliferative potential at all.10 43 More insight into the distribution of human virus-specific cells in the different lymphoid compartments and peripheral target organs during active and latent infection will provide better understanding on the significance of T-cell subset findings in peripheral blood.
Taken together, our data, although obtained in a small number of patients awaiting further corroboration in separate cohorts, imply that functional CD8+ T cells cannot clear antigen without functional effector-memory CD4+ T cells. Furthermore, when antigen is present, CD8+ T cells display a so-called memory phenotype, formerly associated with poor cytotoxic function, but here shown to be cytotoxic indeed. These findings implicate that in designing vaccination strategies, both CD4+ and CD8+ effector immune responses should be triggered and sustained.
The authors thank the patients for their blood donations, Dr Sugianto Surachno for assistance in collecting patient material, Frank van Diepen and Dr Debbie van Baarle for invaluable assistance in the preparing of tetrameric complexes, technicians from the Department of Clinical Virology for performing CMV PCRs, and Drs Louis J. Picker, Ton N. M. Schumacher, and Rien H. J. van Oers for critical reading of the manuscript.
Prepublished online as Blood First Edition Paper, October 31, 2002; DOI 10.1182/blood-2002-08-2502.
Supported by grant C98-1724 from the Dutch Kidney Foundation (L.E.G. and E.B.M.R.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Laila E. Gamadia, Academic Medical Center, University of Amsterdam G1-106, PO Box 22700, 1100 DD Amsterdam, the Netherlands; e-mail: l.e.gamadia@amc.uva.nl.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal