Virus-specific CD8+ T-cell responses play a pivotal role in limiting viral replication. Alterations in these responses, such as decreased cytolytic function, inappropriate maturation, and limited proliferative ability could reduce their ability to control viral replication. Here, we report on the capacity of HIV-specific CD8+ T cells to secrete cytokines and proliferate in response to HIV antigen stimulation. We find that a large proportion of HIV-specific CD8+ T cells that produce cytokines in response to cognate antigen are unable to divide and die during a 48-hour in vitro culture. This lack of proliferative ability of HIV-specific CD8+ T cells is defined by surface expression of CD57 but not by absence of CD28 or CCR7. This inability to proliferate in response to antigen cannot be overcome by exogenous interleukin-2 (IL-2) or IL-15. Furthermore, CD57 expression on CD8+ T cells, CD4+ T cells, and NK cells is a general marker of proliferative inability, a history of more cell divisions, and short telomeres. We suggest, therefore, that the increase in CD57+ HIV-specific CD8+ T cells results from chronic antigen stimulation that is a hallmark of HIV infection. Thus, our studies define a phenotype associated with replicative senescence in HIV-specific CD8+ T cells, which may have broad implications to other conditions associated with chronic antigenic stimulation.
Introduction
CD8+ T cells are crucial to the recognition and clearance of virus-infected cells.1,2Fully functional CD8+ T cells have the ability to proliferate and mediate antiviral activity through cytokine and chemokine secretion, Fas/Fas ligand interactions, and/or perforin/granzyme-mediated cell lysis after recognition of cognate antigen. When naive CD8+ T cells are activated during a primary viral infection, they proliferate and become effector T cells that fulfill these functions.2 3 Following clearance of virus, the majority of the virus-specific CD8+ T cells die, and few memory CD8+ T cells remain to combat subsequent infections with further rounds of proliferation and elaboration of effector functions.
Studies have suggested that chronic stimulation of T cells, such as that which occurs with rheumatoid arthritis,4-6multiple myeloma,7 cytomegalovirus (CMV) and HIV infections,8,9 and following bone marrow transplantation10 can result in the development of CD8+ T cells that are capable of cytokine secretion yet incapable of cell division. Although the provenance and exact phenotype of such CD8+ T cells remain unclear,10-16 such a failure to proliferate is generally attributed to replicative senescence resulting from continual stimulation by antigen and/or cytokine. Indeed, it has been suggested that replicative senescence17-21 or “clonal exhaustion”22-24 of HIV-specific T cells may underlie the inability of T-cell immunity to suppress virus adequately. Other studies have suggested that deficiencies in HIV-specific CD8+ T-cell function may arise from insufficient CD4+ T-cell help25-28 or specific signaling and cytotoxic functional defects.25,29 30
The phenotypes associated with replicatively senescent CD8+T cells are not well defined31,32 but are generally attributed to lack of CD28 or expression of CD577,30,33-36and are thought to result in the inability of these CD8+ T cells to proliferate.37,38 These T cells commonly are found in individuals with chronic immune activation,4,6-10,39,40 and they increase in frequency with age.41 42 We examined the relationship between CD57 expression and 2 functional aspects of HIV-specific, CMV-specific, and other CD8+ T cells: their ability to produce cytokines and to proliferate in response to stimulation by cognate antigen. We found that CD57+ HIV-specific CD8+ T cells, irrespective of CD28 or CCR7 expression, produce IFN-γ but are unable to proliferate in response to cognate peptide with costimulation. This proliferative defect was found in all CD57-expressing CD4+and CD8+ T cells and NK cells and could not be overcome by the addition of exogenous interleukin-2 (IL-2) or IL-15. We also show that CD57+ lymphocytes have undergone more cell divisions compared with other memory cells of other phenotypes. Finally, expression of CD57 renders cells susceptible to activation-induced cell death by apoptosis. Notably, the phenotype we describe here is not predicted by lack of CD28 or CCR7 expression but is defined by expression of CD57.
Patients, materials, and methods
Study subjects
For this study, 30 HIV-1–infected subjects were recruited at the University of Texas Southwestern Medical Center and the National Institutes of Health. As detailed in Table1, the study subjects include those who have been treated with antiretroviral drugs and those who are treatment naive. Viral loads were determined using either the Roche Amplicor Monitor assay or the Roche Ultradirect assay (Basel, Switzerland). All subjects gave informed consent prior to entry into this study, and all studies were approved by the institution's institutional review board.
Study subjects
| Subject . | CD4 count, cells/μL . | Viral load, copies/mL . | Duration of treatment, y . | Duration of infection, y . |
|---|---|---|---|---|
| 1 | 693 | 3 996 | — | 15 |
| 2 | 636 | 1 800 | — | > 12 |
| 3 | 327 | 125 | — | > 12 |
| 4 | 875 | 10 152 | — | > 3 |
| 5 | 1311 | 12 226 | — | > 15 |
| 6 | 648 | 4 846 | — | 8 |
| 7 | 750 | 4 800 | — | > 6 |
| 8 | 255 | 22 950 | — | > 3 |
| 9 | 577 | 24 135 | — | > 2 |
| 10 | 335 | 110 950 | — | < 10 |
| 11 | 742 | 170 044 | — | < 1 |
| 12 | 369 | 6 401 | — | < 2 |
| 13 | 706 | 13 930 | — | < 1 |
| 14 | 1130 | 49 980 | — | < 1 |
| 15 | 64 | 65 000 | — | > 1 |
| 16 | 181 | 71 712 | — | > 6 |
| 17 | 227 | 446 000 | — | < 4 |
| 18 | 824 | 16 584 | — | < 2 |
| 19 | 718 | 44 389 | — | < 4 |
| 20 | 705 | 832 | 11 | > 11 |
| 21 | 190 | 1 005 | 6 | > 6 |
| 22 | 636 | 2 546 | 5 | > 5 |
| 23 | 588 | 5 936 | 8 | > 8 |
| 24 | 307 | 13 838 | 4 | > 4 |
| 25 | 869 | 18 049 | 5 | > 5 |
| 26 | 723 | 18 393 | 3 | > 3 |
| 27 | 462 | 18 480 | 10 | > 10 |
| 28 | 131 | 47 760 | 5 | > 5 |
| 29 | 412 | 84 093 | 4 | > 4 |
| 30 | 403 | 120 | 2 | > 2 |
| 31 | HIV− | HIV− | HIV− | HIV− |
| Subject . | CD4 count, cells/μL . | Viral load, copies/mL . | Duration of treatment, y . | Duration of infection, y . |
|---|---|---|---|---|
| 1 | 693 | 3 996 | — | 15 |
| 2 | 636 | 1 800 | — | > 12 |
| 3 | 327 | 125 | — | > 12 |
| 4 | 875 | 10 152 | — | > 3 |
| 5 | 1311 | 12 226 | — | > 15 |
| 6 | 648 | 4 846 | — | 8 |
| 7 | 750 | 4 800 | — | > 6 |
| 8 | 255 | 22 950 | — | > 3 |
| 9 | 577 | 24 135 | — | > 2 |
| 10 | 335 | 110 950 | — | < 10 |
| 11 | 742 | 170 044 | — | < 1 |
| 12 | 369 | 6 401 | — | < 2 |
| 13 | 706 | 13 930 | — | < 1 |
| 14 | 1130 | 49 980 | — | < 1 |
| 15 | 64 | 65 000 | — | > 1 |
| 16 | 181 | 71 712 | — | > 6 |
| 17 | 227 | 446 000 | — | < 4 |
| 18 | 824 | 16 584 | — | < 2 |
| 19 | 718 | 44 389 | — | < 4 |
| 20 | 705 | 832 | 11 | > 11 |
| 21 | 190 | 1 005 | 6 | > 6 |
| 22 | 636 | 2 546 | 5 | > 5 |
| 23 | 588 | 5 936 | 8 | > 8 |
| 24 | 307 | 13 838 | 4 | > 4 |
| 25 | 869 | 18 049 | 5 | > 5 |
| 26 | 723 | 18 393 | 3 | > 3 |
| 27 | 462 | 18 480 | 10 | > 10 |
| 28 | 131 | 47 760 | 5 | > 5 |
| 29 | 412 | 84 093 | 4 | > 4 |
| 30 | 403 | 120 | 2 | > 2 |
| 31 | HIV− | HIV− | HIV− | HIV− |
Clinical information for subject cohort. — indicates treatment naive.
Peptides
15-mer peptides overlapping by 11 amino acids and corresponding to sequences of clade B HXBc2/Bal R5 chimeric HIV strain (gag, pol, env, nef) were pooled into mixture groups by HIV proteins as previously described.43
Identification of HIV-specific CD8+ T cells (6-hour assay)
Peripheral blood mononuclear cells (PBMCs) were isolated and, in some instances, viably cryopreserved until later use. Stimulation was performed on fresh or frozen PBMCs as previously described.43,44 In every experiment a negative control (anti-CD28/CD49d) was included to control for spontaneous production of IFN-γ, as well as a positive control (Staphylococcusenterotoxin B (SEB) 1 μg/mL final, Sigma, St Louis, MO) to ensure that cells were responsive. Cultures were incubated for 1 hour at 37°C, followed by an additional 5 hours in the presence of Brefeldin-A (BFA) (1 μg/mL, Sigma). CD8+ T cells that produce effector cytokines following antigenic-specific stimulation are contained within and are thought to represent the tetramer-binding CD8+ T cells.43 45-47
Antigen-specific proliferation (48-hour assay)
PBMCs were initially stained with carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes, Eugene, OR) as previously described.48 These PBMCs were suspended in media supplemented with IL-2 (10 U/mL, Sigma) or IL-15 (100 ng/mL, R&D Systems, Minneapolis, MN) and were then stimulated with HIV peptide pools as described.43 44 Cells were then incubated for 36 hours at 37°C followed by an additional 12 hours in the presence of Brefeldin-A (10 μg/mL, Sigma). Cells were then stained for flow cytometric analysis. The percentage of HIV-specific CD8+ T cells was determined as in the previous paragraph. The proportion of CD8+ T cells that showed CFSE dilution (division) at 48 hours was divided by 2 (a cell division represents a 2-fold increase in frequency, hence the percentage of proliferated CD8+ T cells must be halved) and then divided by the 6 hours' response; for instance ([1/2 48 hour divided response]/6 hours response). This number represents the percentage of antigen-specific CD8+ T cells that had divided in response to antigenic stimulation.
Mitogen and superantigen stimulations
For superantigen stimulations SEB (Sigma) was used at 1 μg/mL. For mitogenic stimulations phytohemagglutinin (PHA) (Sigma) was used at 5 μg/mL. PBMCs stimulated with SEB were cultured for 4 days prior to immunofluorescent staining, and PHA-stimulated PBMCs were cultured for 2 days prior to propidium iodide staining. For apoptosis studies, SEB-stimulated PBMCs were cultured for 24 hours in the presence of activated caspase 3 binding peptide Z-VK(biotin)D(OMe)-FMK at 43 μg/mL (Enzyme Systems Products, Livermore, CA).
Immunofluorescence staining
Stimulated PBMCs were washed and then surface stained with directly conjugated antibodies to CD3 and other surface markers (Becton Dickinson Immunocytometry Systems (BDIS), San Jose, CA) for 20 minutes on ice. The cells were washed and fix/permeabilized (fixation/permeabilization solution [BDIS]), and stained with directly conjugated antibodies to IFN-γ or other intracellular molecules (as required for specific experiments), and resuspended in 1% paraformaldehyde in phosphate-buffered saline.
Flow cytometric analysis
Using a FACScalibur flow cytometer (BDIS), 6-parameter flow cytometric analysis was performed. Fluorescein isothiocyanate (FITC) or CFSE, PE, PerCP, and allophycocyanin (APCs) were used as the fluorophores. At least 100 000 live CD3+lymphocytes were collected. Twelve-parameter flow cytometric analysis was performed using a modified FACSDIVA flow cytometer (BDIS). Alexa 430, FITC, PE, Texas-Red PE, Cy5PE, Cy5.5PE, Cy7PE, APC, Cy7APC, and Alexa 594 were used as fluorophores. The list-mode data files were analyzed using PAINT-A-GATE software (BDIS) and FlowJo software (Tree Star, San Carlos, CA). CFSE distributions were analyzed using FlowJo software.
Cell sorting
Cell sorting was accomplished using a FACSDiva cell sorter (BDIS). FITC, PE, Cy5PE, and APC were used as the fluorophores. At least 10 000 cells were sorted for PCR analysis, and at least 106 cells were sorted for telomere length and cell-division analysis.
NK cells were magnetically sorted by a positive selection method with use of anti-CD16 antibodies conjugated to microbeads (Miltenyi Biotec, Auburn, CA).
T-cell receptor excision circle analysis
Telomere length analysis
Telomere length analysis was performed using peptide nucleic acids (Dako, Carpinteria, CA) as previously described.51 52 Analysis was accomplished by comparing mean fluorescence intensity of the telomere channel between different populations that were all gated for similar DNA content (propidium iodide fluorescence) between samples.
Statistical analysis
Correlations were performed by Spearman rank correlation, and statistical significances were performed by Wilcoxon matched pairs test using Prism 3.0 software (San Diego, CA).
Results
Proliferative defect in HIV-specific CD8+ T cells
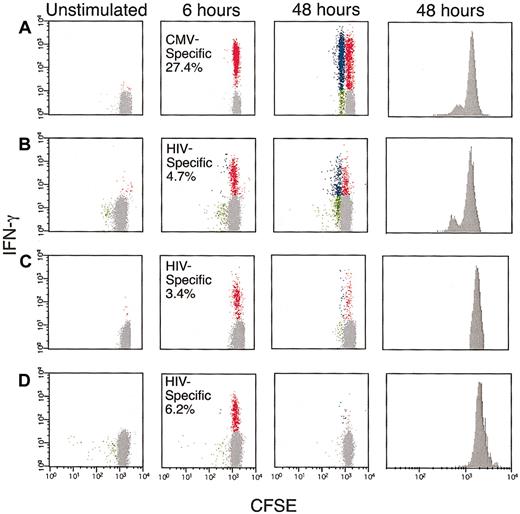
As functional defects of antigen-specific T cells in humans recently have been described in HIV infection, we analyzed antigen-specific CD8+ T-cell responses in healthy and HIV-infected subjects by IFN-γ secretion and proliferation by flow cytometry. Figure 1A illustrates this using PBMCs from a healthy HLA-A2+ individual (subject 31), which we labeled with CFSE and stimulated with the HLA-A2–restricted CMV pp65 peptide (NLVPMVATV). After 6 hours, 27% of CD8+ T cells produced IFN-γ but had not divided. By 48 hours, 13% of the CD8+ T cells had divided and continued to produce IFN-γ. The percentage of responding CD8+ T cells and their ability to proliferate were assessed also by tetramer analysis to assure that the IFN-γ–producing CD8+ T cells adequately represented the antigen-specific CD8+ T cells (data not shown). Despite this relatively high percentage of antigen-specific CD8+ T cells, this response clearly demonstrated that the described assay could be used to monitor proliferation in response to antigenic stimulation.
Antigen-specific proliferation of CMV and HIV-specific CD8+T cells.
PBMCs, obtained from subjects 31, 15, 12, and 17 (A-D, respectively), were CFSE labeled and incubated with either the HLA-A2–restricted CMV pp65 peptide (A) or overlapping peptides encompassing the HIV gag protein (B-D). The cells were cultured for either 6 or 48 hours and then labeled with anti–CD3 PerCP, anti–CD8 APC, and anti–IFN-γ-PE. The flow cytometric data represent gated CD3+CD8+ T cells and is demonstrated as follows: background (costimulatory antibodies alone, left), 6-hour stimulation (center, left), and 48-hour stimulation dot plots (center, right) and CFSE histogram (right). The Y-axes in the dot plots represent staining for IFN-γ. Red dots represent CD8+ T cells that produced IFN-γ but did not divide. Blue dots correspond to IFN-γ+ proliferating CD8+ T cells, and green dots correspond to IFN-γ−–proliferating CD8+ T cells. The numbers represent the total antigen-specific response at 6 hours. Gray dots represent CD8+ T cells that did not proliferate or produce IFN-γ.
Antigen-specific proliferation of CMV and HIV-specific CD8+T cells.
PBMCs, obtained from subjects 31, 15, 12, and 17 (A-D, respectively), were CFSE labeled and incubated with either the HLA-A2–restricted CMV pp65 peptide (A) or overlapping peptides encompassing the HIV gag protein (B-D). The cells were cultured for either 6 or 48 hours and then labeled with anti–CD3 PerCP, anti–CD8 APC, and anti–IFN-γ-PE. The flow cytometric data represent gated CD3+CD8+ T cells and is demonstrated as follows: background (costimulatory antibodies alone, left), 6-hour stimulation (center, left), and 48-hour stimulation dot plots (center, right) and CFSE histogram (right). The Y-axes in the dot plots represent staining for IFN-γ. Red dots represent CD8+ T cells that produced IFN-γ but did not divide. Blue dots correspond to IFN-γ+ proliferating CD8+ T cells, and green dots correspond to IFN-γ−–proliferating CD8+ T cells. The numbers represent the total antigen-specific response at 6 hours. Gray dots represent CD8+ T cells that did not proliferate or produce IFN-γ.
To examine cytokine secretion and proliferation in HIV-specific CD8+ T cells, we stimulated PBMCs from 11 HIV-infected subjects with a pool of overlapping 15-mer peptides encompassing HIV gag.43 45 In contrast to the CD8+ T-cell proliferation observed for CMV-specific CD8+ T cells from subject 31, a high proportion of HIV-specific CD8+ T cells did not proliferate (Table 2). Three patterns of responsiveness were apparent, as summarized in Figure 1B-D. HIV gag-specific CD8+ T cells from subject 15 were capable of proliferation and continued to produce IFN-γ after 48 hours, similar to the CMV-specific CD8+ T cells from subject 31 (Figure 1B). In contrast, HIV gag-specific CD8+ T cells from subject 12 continued, at least in part, to produce IFN-γ for 48 hours, yet failed to proliferate (Figure 1C). Finally, subject 17 showed strong (6%) but short-lived IFN-γ production in HIV gag-specific CD8+ T cells, and no subsequent proliferation (Figure 1D). These patterns of responsiveness were not restricted to CD8+ T cells specific for gag, as many subjects had detectable CD8+ T-cell populations specific for other HIV proteins that contained similarly deficient proliferative potential (data not shown).
Cell division of antigen-specific (HIV and CMV) CD8+ T cells
| Subject . | 6 hours . | 48 hours . | No division . | Division . | % CD57+ . | % CCR7+ . | % CD28+ . | % CD27+ . |
|---|---|---|---|---|---|---|---|---|
| 24 | 3.5 | 2.5 | 1.9 | 0.7 | 61 | 15 | 65 | 72 |
| 6 | 6.5 | 3.9 | 2.3 | 1.6 | 44 | 6 | 56 | 81 |
| 4 | 3.8 | 2.8 | 1.8 | 1.0 | 43 | 15 | 51 | 72 |
| 7 | 2.6 | 0.5 | 0.3 | 0.3 | 70 | 8.5 | 45 | 43 |
| 12 | 3.4 | 1.5 | 1.2 | 0.3 | 60 | 5 | 48 | 43 |
| 17 | 6.3 | 0.2 | 0.1 | 0.1 | 76 | 7 | 45 | 76 |
| 14 | 4.8 | 5.0 | 2.9 | 2.1 | 33 | 14 | 54 | 91 |
| 15 | 4.7 | 9.8 | 3.6 | 6.2 | 18 | 12 | 68 | 87 |
| 16 | 7.5 | 1.4 | 0.9 | 0.4 | 56 | 9.5 | 42 | 44 |
| 27 | 2.6 | 1.2 | 0.7 | 0.5 | 55 | 8 | 35 | 77 |
| 13 | 2.1 | 0.6 | 0.6 | 0 | 77 | 3 | 45 | 61 |
| 31 | 27.4 | 25 | 11.4 | 13.4 | 21 | 19 | 76 | 93 |
| R2 | — | — | — | — | 0.90 | 0.28 | 0.48 | 0.33 |
| Subject . | 6 hours . | 48 hours . | No division . | Division . | % CD57+ . | % CCR7+ . | % CD28+ . | % CD27+ . |
|---|---|---|---|---|---|---|---|---|
| 24 | 3.5 | 2.5 | 1.9 | 0.7 | 61 | 15 | 65 | 72 |
| 6 | 6.5 | 3.9 | 2.3 | 1.6 | 44 | 6 | 56 | 81 |
| 4 | 3.8 | 2.8 | 1.8 | 1.0 | 43 | 15 | 51 | 72 |
| 7 | 2.6 | 0.5 | 0.3 | 0.3 | 70 | 8.5 | 45 | 43 |
| 12 | 3.4 | 1.5 | 1.2 | 0.3 | 60 | 5 | 48 | 43 |
| 17 | 6.3 | 0.2 | 0.1 | 0.1 | 76 | 7 | 45 | 76 |
| 14 | 4.8 | 5.0 | 2.9 | 2.1 | 33 | 14 | 54 | 91 |
| 15 | 4.7 | 9.8 | 3.6 | 6.2 | 18 | 12 | 68 | 87 |
| 16 | 7.5 | 1.4 | 0.9 | 0.4 | 56 | 9.5 | 42 | 44 |
| 27 | 2.6 | 1.2 | 0.7 | 0.5 | 55 | 8 | 35 | 77 |
| 13 | 2.1 | 0.6 | 0.6 | 0 | 77 | 3 | 45 | 61 |
| 31 | 27.4 | 25 | 11.4 | 13.4 | 21 | 19 | 76 | 93 |
| R2 | — | — | — | — | 0.90 | 0.28 | 0.48 | 0.33 |
Values are listed as a percentage of total CD8+ T cells that produced IFN-γ after 6 hours and 48 hours, and IFN-γ–producing cells that had or had not divided after 48 hours (columns 2-5). Percentages of antigen-specific CD8+ T cells that express listed surface molecules are also shown (columns 6-9). R2 describes correlation based on linear regression between expression of individual surface molecules with the percentage of those CD8+ T cells that proliferated in response to antigenic stimulation (last row).
HIV-specific CD8+ T cells that do not proliferate are defined by CD57 expression
Having determined the proliferative potential of the HIV-specific CD8+ T cells (Figure 1; Table 2), we sought to define phenotypic markers that might differentiate proliferating from nonproliferating HIV-specific CD8+ T cells (Table2).
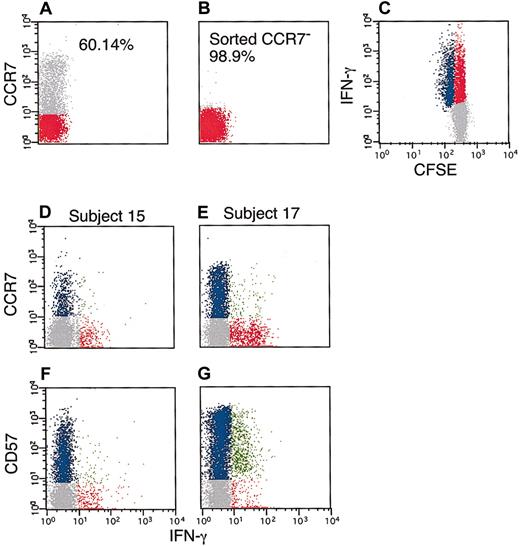
As shown in Table 2, CD57 was the only surface marker we found that alone defined the proliferative ability of HIV-specific CD8+ T cells (Figure 2; Table2). In addition to surface molecules listed in Table 2, we also studied expression patterns of CD152, CD11b, CD45RA, CD95, and CD45RO by HIV-specific CD8+ T cells but found that these molecules did not differ between T cells that proliferated and those that did not. A recent study suggested that CCR7− T cells were unable to proliferate.30 We sorted, by flow cytometry, CCR7− cells from subject 15. When we stimulated these CCR7− PBMCs (which contained the HIV-specific CD8+ subset) with peptides encompassing HIV gag, the T cells recognizing the HIV peptides were clearly capable of cell division (Figure 2). Thus, HIV-specific CD8+ T cells from one individual (subject 15) lacked CCR7 expression but maintained the ability to proliferate in response to antigenic stimulation, indicating that CCR7 expression was not a reliable predictor of proliferative potential of HIV-specific CD8+ T cells.
Antigen-specific proliferation of CCR7− HIV-specific CD8+ T cells.
PBMCs were obtained from subject 15 and labeled with anti–CCR7 PE antibody (A). CCR7− cells were sorted by flow cytometry (B), followed by CFSE labeling and 48 hours of HIV−peptide stimulation (C). Red and gray dots represent CCR7−and CCR7+ cells, respectively (A-B). Red, blue, and gray dots are as in Figure 1 (C-G). In a separate experiment, PBMCs were obtained from subject 15 (proliferating) and subject 17 (nonproliferating) followed by 6 hours of stimulation with HIV-peptides as described in “Patients, materials, and methods.” Cells were then labeled with anti–CD3 PerCp, anti–CD8 APC, anti-CCR7 (D-E), or anti–CD57 PE (F-G) and anti–IFN-γ FITC. The data are gated for CD3+CD8+ T cells. In panels D-G, blue dots represent CD8+ T cells that express the marker of interest but did not produce IFN-γ. Red dots correspond to CD8+ T cells that produced IFN-γ but did not express the marker of interest. Finally, green dots represent CD8+ T cells that expressed the marker of interest and produced IFN-γ. This experiment was repeated with PBMCs from the same subject to assure reproducibility.
Antigen-specific proliferation of CCR7− HIV-specific CD8+ T cells.
PBMCs were obtained from subject 15 and labeled with anti–CCR7 PE antibody (A). CCR7− cells were sorted by flow cytometry (B), followed by CFSE labeling and 48 hours of HIV−peptide stimulation (C). Red and gray dots represent CCR7−and CCR7+ cells, respectively (A-B). Red, blue, and gray dots are as in Figure 1 (C-G). In a separate experiment, PBMCs were obtained from subject 15 (proliferating) and subject 17 (nonproliferating) followed by 6 hours of stimulation with HIV-peptides as described in “Patients, materials, and methods.” Cells were then labeled with anti–CD3 PerCp, anti–CD8 APC, anti-CCR7 (D-E), or anti–CD57 PE (F-G) and anti–IFN-γ FITC. The data are gated for CD3+CD8+ T cells. In panels D-G, blue dots represent CD8+ T cells that express the marker of interest but did not produce IFN-γ. Red dots correspond to CD8+ T cells that produced IFN-γ but did not express the marker of interest. Finally, green dots represent CD8+ T cells that expressed the marker of interest and produced IFN-γ. This experiment was repeated with PBMCs from the same subject to assure reproducibility.
We further confirmed the association between CD57 expression and proliferative inability in 2 ways. First, in subject 15, whose CD8+ T cells proliferated in response to HIV gag, all the IFN-γ–producing cells were CD57− and CCR7−. However, in subject 17, whose CD8+ T cells did not proliferate in response to HIV gag, all the IFN-γ–producing cells were CD57+. Second, the percentage of CD57+ HIV gag-specific CD8+ T cells was inversely proportional to the percentage of gag-specific CD8+ T cells that had divided after 48 hours (R2 = 0.90, P < .0001) (Table 2). These data confirm that CD57 expression on HIV-specific CD8+ T cells defines an inability to divide in response to cognate antigen stimulation.
The presence of nondividing CD57+CD8+ T cells specific for HIV gag correlated with that of CD57+CD8+ T cells specific for HIV pol, env, and nef (R = 0.95, P < .0001), suggesting that the generation of such nondividing cells is related to the response to the whole pathogen rather than to individual antigens (Figure3A). In addition, the fraction of HIV-specific CD8+ T cells that are CD57+remained constant (the absolute number of CD57+HIV-specific CD8+ T cells decreased) following highly active antiretroviral therapy (data not shown). Figure 3B demonstrates that CD57 expression by CD8+ T cells specific for antigens other than HIV is only marginally correlated with expression of CD57 by HIV-specific CD8+ T cells (R = 0.58, P = .003). Whether this disparity is a result of differential cytokine expression by a significant proportion of HIV-specific CD8+ T cells or an underestimation of responses due to sequence differences or HIV accessory gene–specific CD8+ T cells remains unclear. However, it is clear that HIV-specific CD8+ T cells can contribute substantially (up to 80%) to the total CD57+CD8+ T-cell population in HIV-infected individuals.
Antigen-induced expression of CD57.
(A) Percentage of gag-specific CD8+ T cells that express CD57 was correlated with percentage nef, pol, or env-specific CD8+ T cells that express CD57, R = 0.95,P < .0001. (B). The percentage of gag-specific CD8+ T cells that express CD57 was correlated with the percentage of CD8+ T cells (specific for antigens other than HIV) that express CD57, R = 0.58, P = .003.
Antigen-induced expression of CD57.
(A) Percentage of gag-specific CD8+ T cells that express CD57 was correlated with percentage nef, pol, or env-specific CD8+ T cells that express CD57, R = 0.95,P < .0001. (B). The percentage of gag-specific CD8+ T cells that express CD57 was correlated with the percentage of CD8+ T cells (specific for antigens other than HIV) that express CD57, R = 0.58, P = .003.
We also investigated the effect of CD57 expression on all T-cell subsets by stimulating CFSE-labeled PBMCs from 2 HIV-infected individuals and 5 HIV-uninfected subjects with superantigen. No proliferation was observed within the CD57+CD8+or CD57+CD4+ T cells. Furthermore, we studied proliferation of natural killer (NK) cells following stimulation with IL-2 and similarly found no proliferation within the NK cells, which expressed CD57 (data not shown). Hence, lack of proliferation by cells expressing CD57 is not restricted to HIV-specific CD57+CD8+ T cells but is a property of all T cells and NK cells.
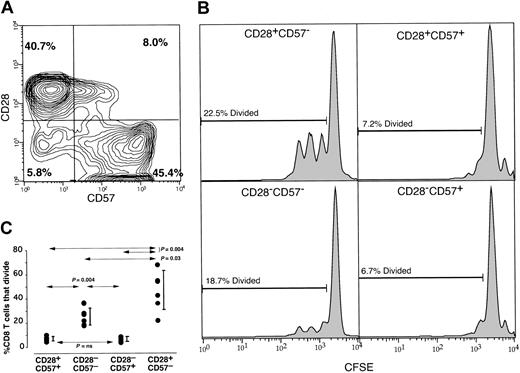
It has been described that CD57 and CD28 expression by CD8+T cells are mutually exclusive.7,33,36 53 However, by multiparameter flow cytometric analysis of memory CD8+ T cells from 6 healthy subjects, we found that 4 populations of CD8+ T cells were evident by CD28 and CD57 expression (Figure 4). In all subjects there were considerable levels of CD8+ T cells that were either CD57+CD28+ or CD57−CD28−. Indeed, up to 20% (mean, 14%) of the memory CD8+ T cells from a cohort of 6 healthy individuals were either CD57+CD28+ or CD57−CD28− (Figure 4, upper left). Furthermore, by stimulating CFSE-labeled PBMCs with the superantigen SEB, we found that virtually no cell division occurred in the CD57+ population regardless of CD28 expression (Figure 4, right histograms). Thus, CD28+ T cells that express CD57 are not capable of proliferation, whereas the CD28−CD57− population contained proliferation-competent T cells (Figure 4, left histograms). These data were confirmed in a total of 6 individuals by stimulating PBMCs with anti-CD3, SEB, or phytohemagglutinin (PHA) and the CD57−subset (regardless of CD28 expression) had statistically significantly more proliferation-competent CD8+ T cells than either CD57+ subset (Figure 4, bottom panel). These results demonstrate that lack of CD28 expression is neither the cause nor the predictor for lack of proliferative capacity.
Division of T-cell populations defined by their CD57 and CD28 expression patterns.
PBMCs from a healthy individual were stained with anti–CD57 FITC, anti–CD28 PE, anti–CD8 PerCp, and anti–CD45RA APC. Panel A shows the CD57 (x-axis) versus CD28 (y-axis) staining pattern of memory CD8+ T cells with CD45RA+CD28+events removed to examine the memory CD8+ T cells. The numbers represent the percentages of CD28+CD57−, CD28+CD57+, CD28−CD57+, and CD28−CD57− populations. (B) PBMCs were CFSE labeled and stimulated with SEB for 4 days. Following stimulation, PBMCs were stained with CD57 PE, CD8 PerCP, and CD28 APC. CFSE histograms are demonstrated representing the CD28+CD57− population (upper left histogram), CD28+CD57+ population (upper right histogram), CD28−CD57− population (lower left histogram), and CD28−CD57+ population (lower right histogram). The numbers indicate the percentages of each population that had divided (calculated using the proliferation platform of FlowJo). These data are representative of experiments conducted on PBMCs from 6 separate individuals and using SEB, anti-CD3, or phytohemagglutinin as the stimulus (C). Wilcoxon matched pairs test demonstrated that the CD28−CD57−CD8+ T-cell populations contain significantly more proliferation-competent T cells compared with either CD57+CD8+ T-cell population (P = .004). Vertical bars represent the 25th to 75th percentiles.
Division of T-cell populations defined by their CD57 and CD28 expression patterns.
PBMCs from a healthy individual were stained with anti–CD57 FITC, anti–CD28 PE, anti–CD8 PerCp, and anti–CD45RA APC. Panel A shows the CD57 (x-axis) versus CD28 (y-axis) staining pattern of memory CD8+ T cells with CD45RA+CD28+events removed to examine the memory CD8+ T cells. The numbers represent the percentages of CD28+CD57−, CD28+CD57+, CD28−CD57+, and CD28−CD57− populations. (B) PBMCs were CFSE labeled and stimulated with SEB for 4 days. Following stimulation, PBMCs were stained with CD57 PE, CD8 PerCP, and CD28 APC. CFSE histograms are demonstrated representing the CD28+CD57− population (upper left histogram), CD28+CD57+ population (upper right histogram), CD28−CD57− population (lower left histogram), and CD28−CD57+ population (lower right histogram). The numbers indicate the percentages of each population that had divided (calculated using the proliferation platform of FlowJo). These data are representative of experiments conducted on PBMCs from 6 separate individuals and using SEB, anti-CD3, or phytohemagglutinin as the stimulus (C). Wilcoxon matched pairs test demonstrated that the CD28−CD57−CD8+ T-cell populations contain significantly more proliferation-competent T cells compared with either CD57+CD8+ T-cell population (P = .004). Vertical bars represent the 25th to 75th percentiles.
It has been suggested that memory CD8+ T cells that “revert” to a CD45RA+ phenotype represent a population of terminally differentiated fully competent “effector memory” T cells.30,54 55 In order to define further the CD57+CD8+ T-cell population, we sought to examine the relationship between expression of the markers that have been used to define naive, “central memory,” and effector memory populations: CD57, CD27, CD28, CCR7, CD62L, CD45RO, and CD45RA. Figure5 shows how we performed this using 10-color flow cytometry. Figure 5A shows that most of the effector memory (CD45RA+CD27−) CD8+ T cells were CD57+. However, only half of the CD57+CD8+ T cells were CD45RA+CD27− (Figure 5D). Analysis of the CD57+CD8+ T cells (Figure 5E-J) revealed that the majority of this population (70%-80%), regardless of their CD45 expression, does not express CD62L, CD28, CD27, nor CCR7. However, a small percentage of the CD57+CD8+ T cells express one or more of these surface markers. This is especially evident when examining the CD45RO+CD57+CD8+ T-cell population, as 30% of this population express CD28 or CD27. Furthermore, when studying the ability of the CD57+CD8+ T-cell population to respond to TCR-mediated stimulation with anti-CD3, similar frequencies of the CD45RO+CD57+CD8+ and CD45RA+CD57+CD8+ T-cell populations produce IFN-γ. Taken together, these data demonstrate that although there is substantial overlap between classical effector memory T cells and the CD57+CD8+ T cells, the former population is only a subset of the CD57+CD8+T-cell population. Similar levels of overlap between expression of CD57, CD27, CD28, CCR7, CD62L, and CD45RA were observed in 5 other healthy individuals (Table 3). In addition, CD57+ T cells divided into smaller subsets are capable of rapid effector cytokine production in response to stimulation. Taken together, these data suggest that all CD57+ T cells (regardless of expression of other phenotypic markers examined here) are of the same functional status and T-cell populations classified as effector memory T cells may be better classified by CD57 expression as the classical CD45RA+CD27− effector memory phenotype does not include all CD57+CD8+ T cells.
Ten-color flow-cytometric analysis of CD57+CD8+T cells.
PBMCs from a healthy volunteer were stimulated with anti-CD3 in the presence of BFA for 5 hours. Following stimulation, the cells were surface stained with anti–CD3 Texas red PE (TRPE), anti–CD8 Cy7PE, anti–CD27 APC, anti–CD28 PE, anti–CD45RO Cy7APC, anti–CD45RA FITC, anti–CD57 Alx430, and anti–CD62L Cy5.5PE followed by intracellular staining for anti–IFN-γ Alx594. Plots were gated through CD3 and CD8 followed by additional gating for CD45RA and CD27 or for CD57 then CD45RO or CD45RA as shown by arrows. Consistent patterns of coexpression of these molecules were observed in 6 healthy individuals (Table 3).
Ten-color flow-cytometric analysis of CD57+CD8+T cells.
PBMCs from a healthy volunteer were stimulated with anti-CD3 in the presence of BFA for 5 hours. Following stimulation, the cells were surface stained with anti–CD3 Texas red PE (TRPE), anti–CD8 Cy7PE, anti–CD27 APC, anti–CD28 PE, anti–CD45RO Cy7APC, anti–CD45RA FITC, anti–CD57 Alx430, and anti–CD62L Cy5.5PE followed by intracellular staining for anti–IFN-γ Alx594. Plots were gated through CD3 and CD8 followed by additional gating for CD45RA and CD27 or for CD57 then CD45RO or CD45RA as shown by arrows. Consistent patterns of coexpression of these molecules were observed in 6 healthy individuals (Table 3).
Coexpression of CD57, CD27, CD28, CCR7, CD62L, and CD45RA by memory CD8+ T cells
| Memory subset . | Healthy donor . | % CD27+ . | % CD28+ . | % CCR7+ . | % CD62L+ . | % CD45RA+ . |
|---|---|---|---|---|---|---|
| CD57+ | 1 | 35 | 27 | 11 | 27 | 69 |
| 2 | 65 | 39 | 17 | 35 | 72 | |
| 3 | 41 | 29 | 22 | 43 | 68 | |
| 4 | 54 | 38 | 31 | 51 | 89 | |
| 5 | 31 | 40 | 41 | 47 | 81 | |
| 6 | 23 | 19 | 10 | 29 | 75 | |
| CD57− | 1 | 80 | 76 | 87 | 85 | 10 |
| 2 | 93 | 92 | 67 | 91 | 5 | |
| 3 | 75 | 79 | 59 | 81 | 8 | |
| 4 | 91 | 85 | 72 | 87 | 15 | |
| 5 | 84 | 72 | 81 | 68 | 19 | |
| 6 | 76 | 57 | 70 | 86 | 6 |
| Memory subset . | Healthy donor . | % CD27+ . | % CD28+ . | % CCR7+ . | % CD62L+ . | % CD45RA+ . |
|---|---|---|---|---|---|---|
| CD57+ | 1 | 35 | 27 | 11 | 27 | 69 |
| 2 | 65 | 39 | 17 | 35 | 72 | |
| 3 | 41 | 29 | 22 | 43 | 68 | |
| 4 | 54 | 38 | 31 | 51 | 89 | |
| 5 | 31 | 40 | 41 | 47 | 81 | |
| 6 | 23 | 19 | 10 | 29 | 75 | |
| CD57− | 1 | 80 | 76 | 87 | 85 | 10 |
| 2 | 93 | 92 | 67 | 91 | 5 | |
| 3 | 75 | 79 | 59 | 81 | 8 | |
| 4 | 91 | 85 | 72 | 87 | 15 | |
| 5 | 84 | 72 | 81 | 68 | 19 | |
| 6 | 76 | 57 | 70 | 86 | 6 |
Coexpression patterns of CD27, CD28, CCR7, CD62L, and CD45RA by memory CD57+ and memory CD57−CD8+T cells.
Percentage of CD57+ and memory CD57−CD8+ T cells that express individual surface markers are shown for 6 healthy, HIV-uninfected individuals.
CD57+ CD8+ T cells are characterized by replicative senescence
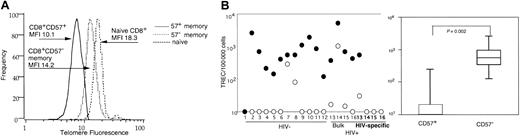
The lack of proliferation of CD57+ HIV-specific and other CD8+ T cells suggests that they may have reached replicative senescence. Indeed, it has been shown that CD28−CD8+ T cells, the majority of which we have shown are a subset of CD57+CD8+ T cells, have shortened telomeres.19,41 56 To address this possibility, we examined the telomere lengths of CD57+ and CD57− memory and naive CD8+ T cells in 3 healthy volunteers (representative plot in Figure6A). The CD57+CD8+ T cells had shorter telomeres than either CD57− memory or naive CD8+ T cells. Taken together, these results indicate that the CD57+CD8+ T-cell population, as a whole, had undergone more cell divisions than the CD57−CD8+ T-cell population. However, the observed differences in telomere length between each subset, although consistent from subject to subject, were small and did not reach statistical significance.
Replicative history of CD57+ and CD57− memory and HIV-specific CD8+ T cells.
PBMCs were collected from healthy subjects, and 1 million CD57+ and CD57−CD45RO+CD8+ T cells and CD45RA+CD62L+ naive CD8+ T cells were sorted by flow cytometry. Sorted populations were used for telomere measurements using telomere-specific peptide nucleic acid (PNA) followed by flow cytometry. Data were gate-based such that DNA content was consistent between naive and memory CD8+ T cells. Histograms of telomere channel fluorescence are depicted (A). The solid line corresponds to CD57+memory CD8+ T cells (mean fluorescent intensity, 10.1), the dotted line depicts CD57− memory CD8+ T cells (MFI 14.2), and the dashed line represents naive CD8+ T cells (MFI 18.3); MFI of the negative control was 7.6. This experiment was repeated on PBMCs from a total of 3 additional healthy volunteers to ensure reproducibility. Telomere length trends were identical in all 3 subjects. PBMCs then were collected from HIV−1-12 and HIV+subjects13-16 and sorted by flow cytometry for CD57+CD8+ T cells (○) and CD57−CD45RO+CD8+ T cells (●) (B). HIV-specific CD57+ (○, bold numbers) or, when possible, CD57− (●, bold numbers) CD8+T cells were additionally sorted from the 4 HIV+individuals based on production of IFN-γ following stimulation with HIV peptides13-16 (panel B, left). At least 10 000 sorted cells of interest were then assayed for TREC using quantitative real-time PCR. Ten is the lower limit of detection for the assay. CD57− memory CD8+ T cells have undergone statistically significantly fewer rounds of proliferation compared with CD57+CD8+ T cells (B, right panel). Vertical bars represent 25th to 75th percentiles.
Replicative history of CD57+ and CD57− memory and HIV-specific CD8+ T cells.
PBMCs were collected from healthy subjects, and 1 million CD57+ and CD57−CD45RO+CD8+ T cells and CD45RA+CD62L+ naive CD8+ T cells were sorted by flow cytometry. Sorted populations were used for telomere measurements using telomere-specific peptide nucleic acid (PNA) followed by flow cytometry. Data were gate-based such that DNA content was consistent between naive and memory CD8+ T cells. Histograms of telomere channel fluorescence are depicted (A). The solid line corresponds to CD57+memory CD8+ T cells (mean fluorescent intensity, 10.1), the dotted line depicts CD57− memory CD8+ T cells (MFI 14.2), and the dashed line represents naive CD8+ T cells (MFI 18.3); MFI of the negative control was 7.6. This experiment was repeated on PBMCs from a total of 3 additional healthy volunteers to ensure reproducibility. Telomere length trends were identical in all 3 subjects. PBMCs then were collected from HIV−1-12 and HIV+subjects13-16 and sorted by flow cytometry for CD57+CD8+ T cells (○) and CD57−CD45RO+CD8+ T cells (●) (B). HIV-specific CD57+ (○, bold numbers) or, when possible, CD57− (●, bold numbers) CD8+T cells were additionally sorted from the 4 HIV+individuals based on production of IFN-γ following stimulation with HIV peptides13-16 (panel B, left). At least 10 000 sorted cells of interest were then assayed for TREC using quantitative real-time PCR. Ten is the lower limit of detection for the assay. CD57− memory CD8+ T cells have undergone statistically significantly fewer rounds of proliferation compared with CD57+CD8+ T cells (B, right panel). Vertical bars represent 25th to 75th percentiles.
In order to quantify proliferation differences between CD57+ and memory CD57−CD8+ T cells more accurately, we used flow cytometry to sort HIV-specific CD57+CD8+ T cells that produced IFN-γ in response to stimulation with antigen, bulk CD57+CD8+ T cells, and CD57−CD45RO+ central memory CD8+ T cells. We then used TREC levels as a measure of proliferative history.49 57 The CD57+ memory CD8+ T-cell subset from healthy and HIV-infected individuals contained statistically significantly fewer TREC compared with the CD57− population (P < .002) (Figure 6B), indicating that the CD57+ population had undergone more rounds of proliferation. In all 4 individuals where we were able to sort HIV-specific CD8+ T cells, the CD57+ HIV-specific CD8+ T cells contained low TREC compared with either CD57− HIV-specific CD8+ T cells or bulk memory CD57−CD8+ T cells (Figure 6B, bold numbers), indicating that HIV-specific CD8+ T cells do not prematurely express CD57.
Previous data have suggested that replicatively senescent T cells can be defined by loss of CD28 expression.19,56 58 Because we found discordance in proliferative ability within populations defined by CD57 and CD28, we sought to determine the proliferative history of each population defined in Figure 4. Hence, we sorted by flow cytometry CD8+ T cells that were naive CD45RO−CD28+, memory CD57+CD28+, memory CD28−CD57−, memory CD28+CD57−, or memory CD28−CD57+, and examined the TREC levels within each population. We found that the TREC levels within these different CD8+ T-cell subsets were as follows: naive T cells > CD28−CD57− memory T cells ≥ CD28+CD57− > CD28+CD57+ ≈ CD28−CD57+. Sample TREC levels from 1 of 4 healthy subjects are as follows: naive CD8+ T cells 641 TREC/105 cells, memory CD28−CD57−CD8+ T cells 281 TREC/105 cells, memory CD28+CD57−CD8+ T cells 192 TREC/105 cells, memory CD28+CD57+CD8+ T cells 15 TREC/105 cells, and CD28−CD57+CD8+ T cells 2 TREC/105 cells. These data indicate that CD28+CD57+ T cells have undergone as many cell divisions as CD28−CD57+ T cells and that CD28−CD57− T cells have undergone fewer cell divisions than CD57+ T cells irrespective of CD28 expression. Hence, CD57 alone predicts replicative senescence of CD8+ T cells.
CD57+ CD8+ T cells are sensitive to induction of apoptosis
The observation that CD57+ HIV-specific CD8+ T cells fail to proliferate and can no longer be detected by IFN-γ after 48 hours in vitro stimulation suggests that these T cells might be undergoing activation-induced cell death. Alternatively, they may remain in culture but fail to produce IFN-γ at 48 hours. To address these possibilities, we used an HLA-A2 tetramer complexed with an HLA-A2–restricted HIV gag peptide (amino acid sequence SLYNTVATL) in an HLA-A2+ subject (subject 8) with a response to this epitope and found that 51% of tetramer-binding CD8+ T cells were CD57+. After stimulation with SLYNTVATL peptide followed by a 48-hour incubation, only 11% of the tetramer-binding CD8+ T cells were CD57+ (data not shown), suggesting that the CD57+ tetramer binding CD8+ T cells were no longer present in the culture.
To determine if CD57+CD8+ T cells were more prone to undergo activation-induced cell death, we sorted CD57+ and CD57−CD8+ T cells and stimulated them with mitogen. After 48 hours these cells were assayed for membrane permeability using propidium iodide. Approximately twice as many CD57+ T cells were positive for propidium iodide compared with CD57− T cells, confirming their increased susceptibility to activation-induced cell death (Figure7A). Uptake of propidium iodide by stimulated CD57+ T cells supports activation-induced cell death of this population but does not determine the mechanism for cell death. Therefore, we stimulated PBMCs from a healthy donor with PHA in the presence of a biotinylated activated caspase 3-binding peptide for 24 hours. Comparing stimulated (CD69+) CD8+ T cells that express CD57 with those that do not, we were able to show that 25 times more CD57+CD8+ T cells bound the activated caspase substrate, indicating that they were, indeed, undergoing apoptosis (Figure 7B).
Apoptosis of CD57+CD8+ T cells.
(A) CD8+CD57+ T cells, CD8+CD57− T cells, and CD8−CD57− lymphocytes were sorted by flow cytometry followed by PHA stimulation (5 μg/mL). Cells were stained for propidium iodide and analyzed 48 hours following stimulation. (B) PBMCs were stimulated for 12 hours using PHA (5 μg/mL) followed by addition of biotinylated substrate for caspase 3 (Z-VK [Biotin] D(OMe)-FMK, 43 μg/mL) for an additional 12 hours. PBMCs then were stained with anti–CD57 FITC, anti–CD69 APC, anti–CD8 PerCP, and strepavidin PE. Plots shown are gated on live CD8+CD69+ then discriminated based on CD57 expression. This experiment was performed on 3 individuals, and the data trends were identical. Percentages indicate fraction positive for propidium iodide or activated caspase inhibitor.
Apoptosis of CD57+CD8+ T cells.
(A) CD8+CD57+ T cells, CD8+CD57− T cells, and CD8−CD57− lymphocytes were sorted by flow cytometry followed by PHA stimulation (5 μg/mL). Cells were stained for propidium iodide and analyzed 48 hours following stimulation. (B) PBMCs were stimulated for 12 hours using PHA (5 μg/mL) followed by addition of biotinylated substrate for caspase 3 (Z-VK [Biotin] D(OMe)-FMK, 43 μg/mL) for an additional 12 hours. PBMCs then were stained with anti–CD57 FITC, anti–CD69 APC, anti–CD8 PerCP, and strepavidin PE. Plots shown are gated on live CD8+CD69+ then discriminated based on CD57 expression. This experiment was performed on 3 individuals, and the data trends were identical. Percentages indicate fraction positive for propidium iodide or activated caspase inhibitor.
The cytokine IL-15 has been shown to reduce apoptosis59,60and promote proliferation of HIV-specific CD8+ T cells.61 However, we found that although IL-15 dramatically increased the percentage of proliferating CD57−CD8+ T cells after SEB or HIV stimulation, there was no increased proliferation of the CD57+ population even at IL-15 concentrations up to 100 ng/mL (data not shown).
Discussion
We have shown that HIV-specific CD8+ T cells that express the surface molecule CD57 are incapable of proliferating after antigen-specific stimulation in vitro and undergo activation-induced apoptosis. Furthermore, this behavior is not associated with loss of CD28 expression. Some studies have suggested that CD57 and CD28 expression are mutually exclusive in memory T-cell populations.7,35,53,56 However, we found that CD57+CD28+ and CD57−CD28− T-cell subsets are clearly identifiable in peripheral blood. By measuring TREC content within different T-cell subsets based on CD57 and CD28 expression, we showed that both proliferative history and proliferative ability are better defined by expression of CD57 than CD28. Taken together, our data show that expression of CD57 rather than lack of CD28 expression defines replicative senescence. Previous interpretations suggesting that proliferative defects are caused by lack of appropriate CD28 costimulation are due to the relatively high (80%) but incomplete association between CD57 expression and loss of CD28.62 63
CD57 expression is not limited to HIV-specific CD8+ T cells but is associated with the same properties among all T cells and NK cells. These data indicate that cells of both the adaptive arm as well as the innate arm of the immune system are capable of reaching a state of replicative senescence.
From the very low TREC levels and the shortened telomere lengths in CD57+CD8+ T cells, it is clear that this population has undergone more rounds of replication in vivo than CD57− T cells, consistent with observations of others.7,19 36 We additionally show that HIV-specific CD57+CD8+ T cells contain very low TREC levels. Therefore, these T cells have undergone many rounds of cell division, and their inability to divide in response to HIV antigens is not a result of premature expression of CD57. Thus, the lack of proliferative response to HIV does not reflect a defect particular to the HIV-specific CD8+ T-cell population per se, but rather reflects the consequence of an extended replicative history.
While previous studies have suggested an inability of HIV-specific CD8+ T cells to mature to CD45RA+CCR7− phenotypes,30 we found that although most CD57+CD8+ T cells are CCR7−, CCR7 expression alone does not determine the ability to proliferate. Using antigen-specific proliferation assays, we showed that CCR7−CD8+ T cells in a single HIV-infected individual are capable of replicating in response to antigenic stimulation.
Furthermore, CD8+ T cells belonging to the effector memory population are defined as CD45RA+CCR7−CD62L−CD27−.30,54 55Although all effector memory CD8+ T cells express CD57, only a subset of the CD57+CD8+ T cells are of the effector memory phenotype. The CD57+CD8+ T cells include all effector memory as well as CD8+ T cells of other phenotypes. In addition, all CD57+CD8+T cells (either effector memory or otherwise) (1) are capable of rapid secretion of effector cytokine following antigenic stimulation; (2) cannot proliferate in response to antigen; (3) apoptose following stimulation; and (4) have reached replicative senescence. Hence, the effector memory CD8+ T-cell population may be better defined by CD57 alone rather than by various combinations of CD45RA, CCR7, CD28, CD27, and/or CD62L.
HIV-specific CD8+ T cells can contribute a large proportion (up to 80%) of the total CD57+ T cells, and there is a statistically significant correlation between the percentage of CD57+ HIV gag-specific CD8+ T cells and CD57+CD8+ T cells specific for other HIV antigens. Others have reported increased CD57 expression in persons with chronic CMV infection,9,36,64 even suggesting that all CD57+CD8+ T cells are specific for CMV antigens.65 Thus, the generation of replicative senescence of CD8+ T cells may occur as a result of repetitive antigenic stimulation in vivo by chronic persistent viruses such as CMV and HIV.
Studies aimed at correlating HIV-specific CD8+ T-cell responses with markers of HIV disease have resulted in different conclusions.27,45,66-68 Whereas there is a negative correlation between viral load and specific populations of bulk CD57+CD8+ and CD28−CD8+ T cells,69 we did not observe this correlation when measuring expression of CD57 by HIV-specific CD8+ T cells. Since our data support the hypothesis that generation of CD57+ HIV-specific CD8+ T cells is dependent on antigenic stimulation, one might expect to find a positive correlation between viral load and percentage of CD57+ HIV-specific CD8+ T cells. However, our observation that these cells are exquisitely sensitive to activation-induced apoptosis would likely mask a positive correlation at steady state. Furthermore, we do not know the lifespan of this population in vivo in absence of antigenic stimulation. Thus, during persistent antigenic stimulation we propose that HIV-specific CD57+ T cells are constantly being generated and subsequently induced to apoptose after further stimulation.
While the mechanism by which CD57 expression exerts these described phenotypes upon lymphocytes is unknown, and if CD57 is even, itself, involved in apoptosis and lack of proliferation is unknown, the proliferative defects and apoptotic nature observed within HIV-specific CD8+ T cells can be predicted by expression of CD57 on cells that have previously undergone multiple rounds of cell division. The presence of these cells, however, does not reflect a defect particular to the immune response to HIV or an effect limited to any particular virus, but simply reflects the normal consequence of persistent immune activation. Taken together, our data shed light on the functionality and provenance of CD57+ lymphocytes. Hence, in HIV disease, the presence of proliferation-incompetent HIV-specific CD8+ T cells is the result, not the cause, of uncontrolled viral replication. Although several conditions characterized by chronic antigenic stimulation may result in such an immune state, this underlying pathogenesis is a hallmark of untreated HIV infection.
Prepublished online as Blood First Edition Paper, November 14, 2002; DOI 10.1182/blood-2002-07-2103.
Supported by NIH grant AI49990 (N.J.K.) and RO1 AI47603 (N.J.K.), and the Distinguished Young Researcher Award from the UT Southwestern President's Research Council (N.J.K.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Richard A. Koup, Vaccine Research Center, National Institute of Allergy and Infectious Disease, National Institutes of Health, 40 Convent Dr, Bethesda, MD 20892; e-mail:rkoup@mail.nih.gov.



![Fig. 7. Apoptosis of CD57+CD8+ T cells. / (A) CD8+CD57+ T cells, CD8+CD57− T cells, and CD8−CD57− lymphocytes were sorted by flow cytometry followed by PHA stimulation (5 μg/mL). Cells were stained for propidium iodide and analyzed 48 hours following stimulation. (B) PBMCs were stimulated for 12 hours using PHA (5 μg/mL) followed by addition of biotinylated substrate for caspase 3 (Z-VK [Biotin] D(OMe)-FMK, 43 μg/mL) for an additional 12 hours. PBMCs then were stained with anti–CD57 FITC, anti–CD69 APC, anti–CD8 PerCP, and strepavidin PE. Plots shown are gated on live CD8+CD69+ then discriminated based on CD57 expression. This experiment was performed on 3 individuals, and the data trends were identical. Percentages indicate fraction positive for propidium iodide or activated caspase inhibitor.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/7/10.1182_blood-2002-07-2103/3/m_h80734099007.jpeg?Expires=1770371953&Signature=Lx6pUUsWvgQfiRvMM2uwOykWBAN05CXAaw9CVdOfLcWvH6jMSb0vnm-WKcbEhg5M36ymnF1sO3WRLxf8Wbmf2mo1gjKJF4x~FdYPQeuRAEX6I2h5PdB8WUCCavHnAo0bq3XVjD~YN2ln~7cEqQurx8BPAwq9VcjU9WE7l9kxzr8a6cArARwrnLrJVFKPp2x4vBiXPTYOaRHpr2DyFstN0q9A0b3eV-tg1d5VQUmQggmYHWuSPjgMumVjkONtA~M7vJbmHzMls9FouCTP9tQZcQ2golRurEg2EgWK-s5J3rg7yeSlRImJSwXbhUQ1LTM288ISmGhSH5BQBQmEsCAYCA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal