Abstract
Human T-lymphotropic virus type 1 (HTLV-1)–associated myelopathy/tropical spastic paraparesis (HAM/TSP) is an inflammatory neurologic disease caused by HTLV-1 infection, in which HTLV-1–infected CD4+ T cells and HTLV-1–specific CD8+ T cells may play a role in the disease pathogenesis. Patients with HAM/TSP have high proviral loads despite vigorous virus-specific CD8+ T-cell responses; however, it is unknown whether the T cells are efficient in eliminating the virus in vivo. To define the dynamics of HTLV-1–specific CD8+T-cell responses, we investigated longitudinal alterations in HTLV-1 proviral load, amino acid changes in an immunodominant viral epitope, frequency of HTLV-1–specific T cells, and degeneracy of T-cell recognition in patients with HAM/TSP. We showed that the frequency and the degeneracy of the HTLV-1–specific CD8+ T cells correlated well with proviral load in the longitudinal study. The proviral load was much higher in a patient with low degeneracy of HTLV-1–specific T cells compared to that in a patient with comparable frequency but higher degeneracy of the T cells. Furthermore, in a larger number of patients divided into 2 groups by the proviral load, those with high proviral load had lower degeneracy of T-cell recognition than those with low proviral load. Sequencing analysis revealed that epitope mutations were remarkably increased in a patient when the frequency and the degeneracy were at the lowest. These data suggest that HTLV-1–specific CD8+ T cells with degenerate specificity are increased during viral replication and control the viral infection.
Introduction
Virus-specific CD8+ T cells recognize short peptide fragments bound to the cleft of major histocompatibility complex (MHC) class I molecules, which are endogenously processed within virus-infected cells.1,2 The CD8+ T cells play a pivotal role in controlling viral infection in both the acute and chronic phase. Human T-lymphotropic virus type 1 (HTLV-1) is a retrovirus that preferentially and persistently infects CD4+ lymphocytes in vivo.3 Even though HTLV-1 infection is lifelong, fewer than 1% of the individuals infected with HTLV-1 develop a neurologic disease termed HTLV-1–associated myelopathy/tropical spastic paraparesis (HAM/TSP) or a hematologic disease termed adult T-cell leukemia (ATL); the vast majority of individuals remain in the asymptomatic carrier state.4-6
HAM/TSP is an inflammatory disease in the spinal cord where inflammatory cells, predominantly CD8+ T cells, infiltrate the perivascular area.7 Patients with HAM/TSP show spastic paraparesis and sphincter dysfunction with mild sensory dysfunction, which are consistent with the pathologic changes in the spinal cord.8 It has been demonstrated that the HTLV-1 proviral load and the frequency of HTLV-1–specific CD8+ T cells are increased in the peripheral blood in patients with HAM/TSP compared with HTLV-1 carriers.9-11 In the cerebrospinal fluid in patients with HAM/TSP, HTLV-1–specific CD8+ T cells accumulate and HTLV-1–expressing CD4+ T cells are increased.11,12 Furthermore, HTLV-1–specific CD8+ T cells and the expression of HTLV-1 gene products have been demonstrated in the central nervous system.13,14Therefore, it has been suggested that these cells may play an important role in the pathogenesis of HAM/TSP.15
HTLV-1–specific T cells show strong killing activity toward HTLV-1–infected cells in vitro and the frequency is extraordinarily high in the circulation of patients with HAM/TSP,10reaching over 10% of the CD8+ cell population.16 The CD8+ T-cell response is preferentially directed against the HTLV-1 Tax protein. In HLA-A2+ patients especially, it has been shown that HTLV-1 Tax 11-19 is an immunodominant epitope and is one of the strongest binding peptides to the HLA-A2 molecule.17 Despite the vigorous T-cell response to the virus, patients with HAM/TSP show increased proviral load. Therefore, the question of whether the increased HTLV-1–specific CD8+ T cells are effective in killing virus-infected cells in vivo arises. To answer this question, a longitudinal study was conducted to examine the viral state and virus-specific CD8+ T-cell responses in patients with HAM/TSP, in which alterations in proviral load, amino acid changes in the epitope, HTLV-1–specific T-cell frequency, and degeneracy of antigen recognition of the T cells were investigated. The high frequency of the circulating HTLV-1–specific CD8+ T cells to the immunodominant Tax 11-19 and increased proviral load afford a unique opportunity to examine detailed virus-host immunity interactions focusing on a single epitope in bulk peripheral blood mononuclear cells (PBMCs) in patients with HAM/TSP.
Patients, materials, and methods
Patients
Fifty-three patients with HAM/TSP who had HLA-A*02 haplotype and were residing in Kagoshima, an endemic area in Japan, were included in this study. The diagnosis was based on the neurologic symptoms of the patients and seroreactivity to HTLV-1 according to the World Health Organization guidelines. These patients had not been treated with any antiretroviral drugs. PBMCs were separated by Ficoll gradient centrifugation from the heparinized blood of the patients, which was taken under informed consent and stored in liquid nitrogen until use. HLA-A*02 typing was carried out by polymerase chain reaction (PCR) sequence-specific primer reaction as previously described.18 In a longitudinal study, 3 patients were chosen for the precise detection of peptide-specific CD8+ T cells by flow cytometry. They had over 2% increase in HTLV-1–specific CD8+ T cells and had HLA-A*0201 haplotype after having their blood drawn several times. Approval was obtained from the Kagoshima University Ethics Committee for this study. Informed consent was provided according to the Declaration of Helsinki.
Peptides
The amino acid sequence of HTLV-1 Tax 11-19 is LLFGYPVYV.19l-Alanine–substituted peptides for Tax 11-19 at positions 4, 5, 6, and 8 (altered peptide ligand; APL) were synthesized using Fmoc solid-phase methodology (Kurabo, Osaka, Japan). Amino acid residues in these positions are expected to bind the T-cell receptor (TCR).20 The synthetic peptides were designated as G4A, Y5A, P6A, and Y8A, respectively. Influenza virus M1 peptide (GILGFVFTL) was used as a control peptide that binds to HLA-A*02.19HLA-A*0201-binding affinity of the peptides can be predicted via the Internet (http://bimas.dcrt.nih.gov/molbio/hla_bind/).21The estimated dissociation half-time is 2406 minutes by Tax 11-19, G4A, Y5A, and P6A and 437 minutes by Y8A and 550 minutes by M1 peptide. Purity of the peptides was over 90% by high-performance liquid chromatography (HPLC) analysis. The synthetic peptides were resolved in 50% dimethyl sulfoxide (DMSO) in phosphate-buffered saline at 1 mM.
Intracellular cytokine detection by flow cytometry
The assay was conducted by a modified protocol as previously described.16 Briefly, Hmy2.C1R cells transfected with HLA-A*0201 (Hmy-A2) were prepulsed with 1 μM Tax 11-19 or APL for 1 hour and washed. Cryopreserved PBMCs were quickly thawed and washed. Then 5 × 105 PBMCs were cocultivated with the same number of peptide-prepulsed Hmy-A2 cells for 6 hours. Brefeldin A (Sigma, Tokyo, Japan) was added to the cells at a final concentration of 10 μg/mL at the beginning of the culture to minimize the expression of HTLV-1 protein in the infected cells, which may lead to activation of HTLV-1–specific T cells.22 After culture, cells were harvested, washed, and stained with antihuman CD8 antibody conjugated with PC5 (Beckman-Coulter, Tokyo, Japan) at 4°C for 20 minutes. Cells were washed and fixed with 4% paraformaldehyde for 5 minutes, then washed again. The cells, resuspended in 50 μL permeabilization buffer containing 0.1% saponin (Sigma), were stained with antihuman interferon γ (IFN-γ) antibody conjugated with fluorescein isothiocyanate (FITC; Pharmingen, San Diego, CA) for 20 minutes. Epics-XL flow cytometer and SYSTEM II software were used for fluorescent signal detection and data analysis (Beckman-Coulter). Lymphocytes were readily distinguished from Hmy-A2 cells by size and were gated on forward and side scatter image. Ten thousand CD8+ cells were further gated and the proportion of IFN-γ+ cells in the CD8+ cell population was analyzed. The frequency of peptide-specific CD8+ T cells was obtained by subtracting the percentage of IFN-γ+cells without peptide from that with a peptide. The degeneracy of T-cell recognition is evaluated according to the degree by which the T cell recognizes altered peptides from a cognate peptide at the T-cell clone level. Therefore, the total degeneracy of virus-specific T-cell population in the PBMCs could be evaluated by relative T-cell recognition of an APL against the cognate peptide. The relative T-cell recognition of an APL against Tax 11-19 was given by the following formula: (frequency of APL-specific T cells)/(frequency of Tax 11-19–specific T cells) × 100. The functional T-cell avidity to the antigen was estimated as the peptide concentration required for half-maximum IFN-γ production.
Quantitative PCR for HTLV-1 proviral load
The method used has been previously described.9Briefly, gDNA was extracted from PBMCs by using Qiagen DNA extraction kit (Qiagen, Tokyo, Japan). Approximately 100 μg DNA was subjected to a real-time PCR using a TaqMan probe. The fluorescent signal was detected by an ABI PRISM 7700 sequence detector (Applied Biosystems, Chiba, Japan). The copy number of the target gene in the sample was estimated by the standard curves. HTLV-1 copy number per 104 PBMCs was calculated according to the following formula: (copy number of HTLV-I tax gene in the sample)/((copy number of β-globin gene in the sample)/2) × 10.4 The assay was conducted in triplicate.
Sequencing analysis of HTLV-1 tax gene
The method was previously described.18 Briefly, 100 ng DNA was amplified by 35 cycles of PCR using primers PXO1+: 5′-TCGAAACAGCCCTGCAGATA-3′ at position 7257-7276 and PX22−: 5′-TGGTGGGCAAACAGTCTTCG-3′ at position 7928-7947. One microliter of the first PCR products was further used for 20 cycles of nested PCR using internal primers PXI1+: 5′-ATACAAAGTTAACCATGCTT-3′ at position 7274-7293 and PXI1−: 5′-GGGTTCCATGTATCCATTTC-3′ at position 7644-7663. Amplified DNA products were purified using QIA quick purification kit (Qiagen). The purified tax gene was subcloned into pCR-Blunt II-TOPO cloning vector (Invitrogen, Burlingame, CA). The vector was linearized by EcoRI digestion, which does not cut thetax gene, and purified by the QIA quick purification kit. The tax gene was sequenced with PXI1+ or PXI1− primer using dye terminator DNA sequencing kit (Applied Biosystems) in an automatic sequencer (377 DNA Sequencer, Applied Biosystems). Approximately 50 clones were sequenced in each sample and mutations in thetax gene encoding Tax 11-19 peptide were confirmed by sequencing in the reverse direction.
Results
Frequency of HTLV-1–specific CD8+ T cells correlated with the proviral load
To optimize a concentration of peptide, which induced cytokine production in antigen-specific T cells, we conducted a peptide titration assay. IFN-γ production in bulk PBMCs in response to Tax 11-19 peptide appeared at 0.001 nM and reached the maximum over 1 nM, whereas weak responses to some analog peptides were observed at 1000 nM (data not shown). Therefore, we used peptides at 1 μM in the subsequent study. Figure 1 shows a representative IFN-γ production from CD8+ cells in PBMCs from patient no. 31.
Representative analysis of Tax 11-19– and APL-specific CD8+ T cells in PBMCs from patient no. 31 on 3/5/97 by flow cytometry.
PBMCs were cocultured with APCs prepulsed with either Tax 11-19, APL, or no peptide (NP) for 6 hours in the presence of brefeldin A, a reagent to accumulate synthesized proteins in the Golgi apparatus. The culture cells were stained with anti-CD8 and anti–IFN-γ antibodies. The lymphocytes were segregated from the APCs by size on the forward and side scatter and IFN-γ+ cells were gated as shown. The number in the gate indicates the frequency of IFN-γ+ cells in CD8+ cell population. Tax is the cognate Tax 11-19 peptide and APL was designated as G4A (glycine at position 4 of Tax 11-19 was substituted by alanine).
Representative analysis of Tax 11-19– and APL-specific CD8+ T cells in PBMCs from patient no. 31 on 3/5/97 by flow cytometry.
PBMCs were cocultured with APCs prepulsed with either Tax 11-19, APL, or no peptide (NP) for 6 hours in the presence of brefeldin A, a reagent to accumulate synthesized proteins in the Golgi apparatus. The culture cells were stained with anti-CD8 and anti–IFN-γ antibodies. The lymphocytes were segregated from the APCs by size on the forward and side scatter and IFN-γ+ cells were gated as shown. The number in the gate indicates the frequency of IFN-γ+ cells in CD8+ cell population. Tax is the cognate Tax 11-19 peptide and APL was designated as G4A (glycine at position 4 of Tax 11-19 was substituted by alanine).
A high frequency of Tax 11-19–specific CD8+ T cells, with a lesser extent to APLs, and a low frequency to antigen-presenting cells (APCs) without peptide (NP, background) were detected. The background was 0.42% ± 0.42% (mean ± SD) in 53 HLA-A2 HAM/TSP patients. Peptide-specific CD8+ T-cell frequencies were estimated by subtraction of percentage of IFN-γ+cells without peptide from that with a peptide. The Tax 11-19–specific CD8+ T-cell frequency, measured in 53 HAM/TSP patients, was 2.01% ± 3.97% (mean ± SD). We chose 3 patients who had high frequencies of over 2% and had their blood drawn at least 4 separate times for the longitudinal analysis.
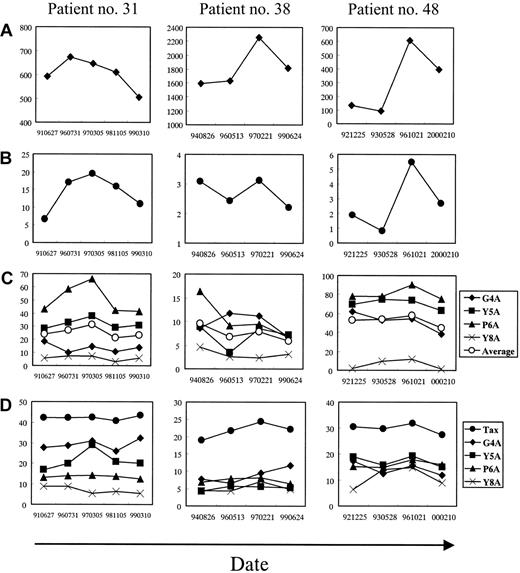
As shown in Figure 2A-B, in patient no. 31, the alterations in proviral load and frequency of HTLV-1 Tax 11-19–specific CD8+ T cells during 8 years ranged from 506 to 674 copies/104 PBMCs and from 6.7% to 19.5%, respectively. The CD8+ T cells were effectively increased in parallel with the slight increase in the proviral load. The alterations in patient no. 38 were from 1595 to 2225 copies/104 PBMCs and from 2.2% to 3.1%, respectively; the increase in virus-specific T-cell frequency was small. In patient no. 48, the alterations were from 93 to 605 copies/104 PBMCs and from 0.8% to 5.5%, respectively. The degree of the increase in virus-specific T-cell frequency to the increase of proviral load was different in each patient; however, HTLV-1 Tax 11-19–specific CD8+ T-cell frequency correlated well with the proviral load in each patient, suggesting that the HTLV-1 Tax 11-19–specific CD8+ T cells proliferate to control viral replication.
Degeneracy of T-cell recognition in HTLV-1–specific CD8+ T cells correlated with the proviral load
To test whether T-cell recognition of the antigen may be altered during the clinical course, especially when the proviral load was increased, a combination of intracellular cytokine detection and APL was used. With this method, the degeneracy of T-cell recognition in the HTLV-1–specific CD8+ T-cell population can be evaluated. For example, in Figure 1, the Tax 11-19–specific CD8+ T cells were relatively tolerated with the alanine substitution at position 6, and, to a lesser extent at position 5, whereas rarely at positions 4 and 8. The relative T-cell responses to the APL against those to the Tax 11-19 peptide were calculated according to the formula mentioned in “Patients, materials, and methods.” For example, the calculated value was 14.9% for G4A, 37.9% for Y5A, 66.2% for P6A, and 7.7% for Y8A from the data in Figure 1. The relative T-cell response to the APL was plotted according to the time course in the 3 patients (Figure 2C) and mean fluorescence intensity (MFI) of IFN-γ–FITC in the peptide-specific CD8+ T cells was graphed (Figure 2D). Although the relative T-cell response in patient no. 31 varied according to each APL, P6A was highly tolerated over 40%, and Y5A recognition was approximately 30%, but the recognitions for G4A and Y8A were low. In patient no. 38, the relative APL recognition was low compared to that in patient no. 31, in whom the responses to all the APLs were less than 17%. In patient no. 48, the T-cell response was relatively high to all the APLs except for Y8A, which reached up to 90% for P6A. This indicated that T-cell recognition of HTLV-1 Tax 11-19 peptide is strict in patient no. 38, but not in patient no. 48. These data suggest that not only the virus-specific T-cell frequency but also the extent of degeneracy in T-cell responses differ among the patients.
Longitudinal analysis in 3 HLA-A*0201+ patients with HAM/TSP.
The x-axis indicates the date when the PBMCs were obtained. (A) HTLV-1 proviral load (♦) measured by a quantitative real-time PCR (copy/104 PBMCs). (B) Frequency of HTLV-1 Tax 11-19–specific CD8+ T cells in CD8+ cell population (●), which were measured as shown in Figure 1 (%). (C) Degeneracy of T-cell recognition in HTLV-1 Tax 11-19–specific CD8+ T cells at each time point. Frequencies of T cells that recognized either cognate Tax 11-19 peptide or APL were measured as shown in Figure 1. The relative CD8+ T-cell frequency to APL against that to cognate Tax 11-19 was calculated. The y-axis indicates the relative percentage (%). (D) MFI of IFN-γ–FITC produced by T cells responding to cognate Tax 11-19 or APL (unit).
Longitudinal analysis in 3 HLA-A*0201+ patients with HAM/TSP.
The x-axis indicates the date when the PBMCs were obtained. (A) HTLV-1 proviral load (♦) measured by a quantitative real-time PCR (copy/104 PBMCs). (B) Frequency of HTLV-1 Tax 11-19–specific CD8+ T cells in CD8+ cell population (●), which were measured as shown in Figure 1 (%). (C) Degeneracy of T-cell recognition in HTLV-1 Tax 11-19–specific CD8+ T cells at each time point. Frequencies of T cells that recognized either cognate Tax 11-19 peptide or APL were measured as shown in Figure 1. The relative CD8+ T-cell frequency to APL against that to cognate Tax 11-19 was calculated. The y-axis indicates the relative percentage (%). (D) MFI of IFN-γ–FITC produced by T cells responding to cognate Tax 11-19 or APL (unit).
In the longitudinal study (Figure 2C), it is striking that the recognition for some APLs correlated with the frequency of the virus-specific T cells and the proviral load. This was particularly dramatic in patient no. 31, who showed a high frequency of HTLV-1 Tax 11-19–specific CD8+ T cells. For example, the T-cell recognitions of P6A and Y5A were well associated with the proviral load and the virus-specific T cell frequency (Figure 2A-C), whereas the recognition of G4A and Y8A was not changed. In patient no. 38, the relative T-cell responses to P6A and Y5A correlated with the proviral load; however, the G4A recognition correlated with neither the proviral load nor the T-cell frequency (Figure 2A-C). In patient no. 48, the curves of CD8+ T-cell recognition of P6A and G4A correlated with the curves of the proviral load and the frequency, but were not clear in the response to Y5A and Y8A (Figure 2A-C). Although alterations in MFI of IFN-γ–FITC were not apparent in the response to Tax, P6A and Y8A peptide in patient no. 31, the MFI to Y5A was increased when the virus-specific T-cell frequency peaked (Figure2B,D). In patient no. 38, it was hard to find any associations between the MFI and the proviral load or the T-cell frequency. In patient no. 48, all the MFI curves except for that to Y8A showed the same tendency as the curves for the proviral load and the virus-specific T-cell frequency (Figure 2A-B,D). These results suggest that, in some patients, when the proviral load was increased, the virus-specific T cells secrete cytokines more efficiently to some analog peptides compared to when the proviral load was decreased.
To compare the effect of HTLV-1–specific T cells on the proviral load among these 3 patients, we calculated the average of each factor during the time course (Table 1). Patient no. 38 had the highest proviral load with the lowest frequency and degeneracy of the virus-specific T cells. Patient no. 48 had the same frequency of the HTLV-1–specific T cells as patient no. 38, but increased degeneracy of 52.3% for the APLs and the lowest proviral load. Patient no. 31 had extremely high virus-specific T-cell frequency of 14.0%, but the proviral load was higher than that in patient no. 48. This patient showed a moderate extent of degeneracy in T-cell recognition among all the patients included.
Comparison of proviral load, frequency and degeneracy of HTLV-1 Tax 11-19-specific T cells, and epitope variant among 3 patients with HAM/TSP
| Patient no. . | Proviral load, copy/104 PBMCs . | Frequency, % in CD8+cells . | Degeneracy,* % . | Epitope variant,† % . |
|---|---|---|---|---|
| 31 | 606.0 ± 64.0‡ | 14.0 ± 5.1§ | 25.4 ± 18.61-155 | 3.4 ± 2.5 |
| 38 | 1823.0 ± 303.1¶ | 2.7 ± 0.5 | 7.5 ± 3.8# | 8.8 ± 10.5 |
| 48 | 306.5 ± 240.5 | 2.7 ± 2.0 | 52.3 ± 30.1 | 4.8 ± 2.3 |
| Patient no. . | Proviral load, copy/104 PBMCs . | Frequency, % in CD8+cells . | Degeneracy,* % . | Epitope variant,† % . |
|---|---|---|---|---|
| 31 | 606.0 ± 64.0‡ | 14.0 ± 5.1§ | 25.4 ± 18.61-155 | 3.4 ± 2.5 |
| 38 | 1823.0 ± 303.1¶ | 2.7 ± 0.5 | 7.5 ± 3.8# | 8.8 ± 10.5 |
| 48 | 306.5 ± 240.5 | 2.7 ± 2.0 | 52.3 ± 30.1 | 4.8 ± 2.3 |
The numbers indicate mean ± SD.
Degeneracy of HTLV-1 Tax 11-19-specific T cells is accessed by the average of relative T-cell responses to APLs during the time course.
Percentage of epitope variant is given by the division of the number of variants by the number of clones sequenced in each patient.
P, as estimated by the Mann-Whitney U test, is significantly different between patients no. 31 and no. 38 (‡P < .0001), no. 31 and no. 38 (§P< .0006), no. 31 and no. 48 (§P < .0006), no. 31 and no. 38 (
P < 0.0005), no. 31 and no. 48 (∥P < .0001), no. 38 and no. 48 (¶P < .0001), and no. 38 and no. 48 (#P < .0001).
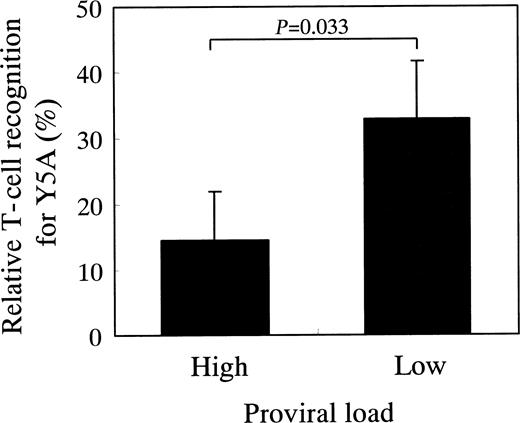
To investigate whether or not the degeneracy of T-cell recognition is associated with the proviral load as observed in patients no. 38 and no. 48, we measured degeneracy and proviral load in a series of 22 patients with HAM/TSP who had HLA-A*02 haplotype. The proviral load of these patients ranged from 2230 to 130 copies/104 PBMCs, with the median of 481 copies/104 PBMCs. We divided the patients into 2 groups according to the following: patients with proviral load more than the median were included in high proviral load group (n = 11), whereas patients with proviral load less than the median were included in low proviral load group (n = 11). As shown in Figure 3, the patients with low proviral load showed an increased degeneracy of HTLV-1 Tax 11-19–specific T cells as compared to the patients with high proviral load. This was observed when Y5A peptide was used, but not when the other peptides were used. These data suggest that virus-specific T cells with increased degeneracy have an advantage to control viral infection.
HAM/TSP patients with high proviral load have HTLV-1 Tax 11-19–specific T cells with low degeneracy of T-cell recognition.
Twenty-two patients with HAM/TSP who had HLA-A*02 were divided into 2 groups according to whether their proviral load is higher or lower than the median proviral load (481 copies/104 PBMCs). The high proviral load group (mean, 1190.0 copies/104 PBMCs; n = 11) had significantly higher proviral load than the low proviral load group (mean, 250.2 copies/104 PBMCs; n = 11;P < .0001, Mann-Whitney U test). The relative recognition of HTLV-1 Tax 11-19–specific T cells for Y5A was higher in the patient group with low proviral load than in the patient group with high proviral load (P = .033; Mann-Whitney Utest). There were no significant differences between the 2 groups for other APLs including G4A, P6A, and Y8A (data not shown). The column and vertical bar indicate the mean and SE, respectively.
HAM/TSP patients with high proviral load have HTLV-1 Tax 11-19–specific T cells with low degeneracy of T-cell recognition.
Twenty-two patients with HAM/TSP who had HLA-A*02 were divided into 2 groups according to whether their proviral load is higher or lower than the median proviral load (481 copies/104 PBMCs). The high proviral load group (mean, 1190.0 copies/104 PBMCs; n = 11) had significantly higher proviral load than the low proviral load group (mean, 250.2 copies/104 PBMCs; n = 11;P < .0001, Mann-Whitney U test). The relative recognition of HTLV-1 Tax 11-19–specific T cells for Y5A was higher in the patient group with low proviral load than in the patient group with high proviral load (P = .033; Mann-Whitney Utest). There were no significant differences between the 2 groups for other APLs including G4A, P6A, and Y8A (data not shown). The column and vertical bar indicate the mean and SE, respectively.
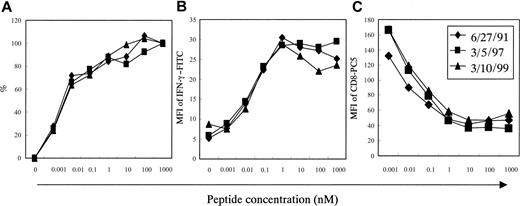
To test whether the increased degeneracy of T-cell recognition resulted from increased avidity of Tax 11-19–specific CD8+ T cells to the APCs, we conducted antigen titration assays using wild-type Tax 11-19 peptide at various concentrations ranging from 0.001 to 1000 nM.
As shown in Figure 4A, the antigen concentration required to reach the half-maximum number of IFN-γ+ cells were almost the same as in patient no. 31 (3.2 pM on June 27, 1991, 4.1 pM on March 5, 1997, and 4.5 pM on March 10, 1999). During the time course the curves of MFI for IFN-γ–FITC were almost the same (Figure 4B), suggesting that the amounts of IFN-γ production in the Tax 11-19–specific CD8+ T cells are the same among the samples. Additionally, the curves of MFI for CD8-PC5 were almost the same at different times (Figure 4C), suggesting that down-regulation of CD8 molecules after engaging with peptide/MHC may be almost the same. Similar titration curves were observed in patients no. 38 and no. 48 (data not shown). Collectively, it seems likely that the changes of degeneracy of T-cell recognition did not result from alterations in T-cell avidity.
Functional antigen avidity test in Tax 11-19–specific CD8+ T cells in PBMCs from patient no. 31.
Samples were chosen at 3 time points, when the HTLV-1 proviral load was either high or low as shown in Figure 2A. The x-axis indicates HTLV-1 Tax 11-19 peptide concentration. (A) Frequencies of the HTLV-1 Tax 11-19–specific CD8+ T cells in CD8+ cell population, which were measured as in Figure 1. The y-axis indicates relative percentage as standardized by the frequency at 1000 nM in each sample. The frequencies at 1000 nM were 6.3% on 6/27/91, 18.5% on 3/5/97, and 10.6% on 3/10/99. (B) The MFI of IFN-γ–FITC produced by the HTLV-1 Tax 11-19–specific CD8+ T cells. (C) The MFI of CD8-PC5 in the Tax 11-19–specific CD8+ T cells.
Functional antigen avidity test in Tax 11-19–specific CD8+ T cells in PBMCs from patient no. 31.
Samples were chosen at 3 time points, when the HTLV-1 proviral load was either high or low as shown in Figure 2A. The x-axis indicates HTLV-1 Tax 11-19 peptide concentration. (A) Frequencies of the HTLV-1 Tax 11-19–specific CD8+ T cells in CD8+ cell population, which were measured as in Figure 1. The y-axis indicates relative percentage as standardized by the frequency at 1000 nM in each sample. The frequencies at 1000 nM were 6.3% on 6/27/91, 18.5% on 3/5/97, and 10.6% on 3/10/99. (B) The MFI of IFN-γ–FITC produced by the HTLV-1 Tax 11-19–specific CD8+ T cells. (C) The MFI of CD8-PC5 in the Tax 11-19–specific CD8+ T cells.
HTLV-1–specific T-cell frequency was higher in patients with HAM/TSP than in asymptomatic HTLV-1 carriers
It would be of interest to know if degeneracy of T-cell recognition in HTLV-1–specific T cells differed between patients with HAM/TSP and asymptomatic HTLV-1 carriers. Therefore, we measured frequencies of HTLV-1 Tax 11-19–specific CD8+ T cells in CD8+ T-cell population both in 47 patients with HAM/TSP and 32 asymptomatic HTLV-1 carriers, who had HLA-A*02 haplotype. The frequency was 1.90% ± 2.68% (mean ± SD) and 0.25% ± 0.21%, respectively. The frequency of HTLV-1 Tax 11-19–specific CD8+ T cells was significantly higher in HAM/TSP patients than in the carriers (P < .0001; Figure5). Unfortunately, it was difficult to investigate degeneracy of HTLV-1 Tax 11-19–specific T cells in the carriers because the frequency of the T cells in the carriers was too small to evaluate T-cell degeneracy.
HTLV-1 Tax 11-19–specific T-cell frequency is higher in patients with HAM/TSP than in asymptomatic HTLV-1 carriers.
Frequency of HTLV-1 Tax 11-19–specific CD8+ T cells in CD8+ cell population was measured both in 47 patients with HAM/TSP and 32 asymptomatic HTLV-1 carriers (AC). The mean ± SD was 1.90% ± 2.68% and 0.25% ± 0.21%, respectively. The HAM/TSP patients had significantly higher frequency of HTLV-1 Tax 11-19–specific T cells than the carriers (P < .0001, Mann-Whitney U test).
HTLV-1 Tax 11-19–specific T-cell frequency is higher in patients with HAM/TSP than in asymptomatic HTLV-1 carriers.
Frequency of HTLV-1 Tax 11-19–specific CD8+ T cells in CD8+ cell population was measured both in 47 patients with HAM/TSP and 32 asymptomatic HTLV-1 carriers (AC). The mean ± SD was 1.90% ± 2.68% and 0.25% ± 0.21%, respectively. The HAM/TSP patients had significantly higher frequency of HTLV-1 Tax 11-19–specific T cells than the carriers (P < .0001, Mann-Whitney U test).
Variant epitopes were increased in a patient with low frequency and limited degeneracy in the virus-specific T cells
To determine if degeneracy or frequency of the HTLV-1–specific CD8+ T cells may have an effect on the viral epitope variations, the HTLV-1 tax gene containing the sequence coding Tax 11-19 peptide was sequenced at 3 time points in the 3 patients (Table 2).
Longitudinal analysis of variants in an HTLV-1 immunodominant epitope, Tax 11-19, in 3 patients with HAM/TSP
| Patient . | Date . | Proviral load* . | Frequency† . | Peptide sequence‡ . | Peptide frequency . | Percentage of variant epitope2-153 . | Predicted HLA-A2 binding affinity2-155 . |
|---|---|---|---|---|---|---|---|
| 31 | 6/27/91 | 594 | 6.67 | LLFGYPVYV | 48/49 | 2.0 | 2406.2 |
| LLFRYPVYV | 1/49 | 2406.2 | |||||
| 3/5/97 | 646 | 19.46 | LLFGYPVYV | 45/48 | 6.3 | 2406.2 | |
| PLFGYPVYV | 1/48 | 31.1 | |||||
| LLYGYPVYV | 1/48 | 2081.0 | |||||
| LLFRYPVYV | 1/48 | 2406.2 | |||||
| 3/10/99 | 506 | 11.00 | LLFGYPVYV | 48/49 | 2.0 | 2406.2 | |
| LLFGHPVYV | 1/49 | 2406.2 | |||||
| 38 | 5/13/96 | 1631 | 2.43 | LLFGYPVYV | 48/51 | 5.9 | 2406.2 |
| LLLGYPVYV | 1/51 | 2406.2 | |||||
| LLFGYPIYV | 2/51 | 2406.2 | |||||
| 2/21/97 | 2255 | 3.12 | LLFGYPVYV | 52/52 | 0.0 | 2406.2 | |
| 6/24/99 | 1810 | 2.20 | LLFGYPVYV | 39/49 | 20.4 | 2406.2 | |
| LPFGYPVYV | 1/49 | 15.7 | |||||
| LLLGYPVYV | 1/49 | 2406.2 | |||||
| LLFRYPVYV | 5/49 | 2406.2 | |||||
| LLFGNPVYV | 1/49 | 2406.2 | |||||
| LLFGHPVYV | 1/49 | 2406.2 | |||||
| LLFGYPVHV | 1/49 | 437.5 | |||||
| 48 | 5/28/93 | 93 | 0.83 | LLFGYPVYV | 45/48 | 6.3 | 2406.2 |
| LLFRYPAYV | 1/48 | 2406.2 | |||||
| LLFGFPVYV | 1/48 | 9143.4 | |||||
| LLFGYPVCV | 1/48 | 437.5 | |||||
| 10/21/96 | 606 | 5.47 | LLFGYPVYV | 48/51 | 5.9 | 2406.2 | |
| LLFRYPVYV | 1/51 | 2406.2 | |||||
| LLFGYLVYV | 1/51 | 5534.1 | |||||
| LLFGYPVYE | 1/51 | 0.5 | |||||
| 2/10/00 | 395 | 2.70 | LLFGYPVYV | 47/48 | 2.1 | 2406.2 | |
| PLFGYPVYV | 1/48 | 31.1 |
| Patient . | Date . | Proviral load* . | Frequency† . | Peptide sequence‡ . | Peptide frequency . | Percentage of variant epitope2-153 . | Predicted HLA-A2 binding affinity2-155 . |
|---|---|---|---|---|---|---|---|
| 31 | 6/27/91 | 594 | 6.67 | LLFGYPVYV | 48/49 | 2.0 | 2406.2 |
| LLFRYPVYV | 1/49 | 2406.2 | |||||
| 3/5/97 | 646 | 19.46 | LLFGYPVYV | 45/48 | 6.3 | 2406.2 | |
| PLFGYPVYV | 1/48 | 31.1 | |||||
| LLYGYPVYV | 1/48 | 2081.0 | |||||
| LLFRYPVYV | 1/48 | 2406.2 | |||||
| 3/10/99 | 506 | 11.00 | LLFGYPVYV | 48/49 | 2.0 | 2406.2 | |
| LLFGHPVYV | 1/49 | 2406.2 | |||||
| 38 | 5/13/96 | 1631 | 2.43 | LLFGYPVYV | 48/51 | 5.9 | 2406.2 |
| LLLGYPVYV | 1/51 | 2406.2 | |||||
| LLFGYPIYV | 2/51 | 2406.2 | |||||
| 2/21/97 | 2255 | 3.12 | LLFGYPVYV | 52/52 | 0.0 | 2406.2 | |
| 6/24/99 | 1810 | 2.20 | LLFGYPVYV | 39/49 | 20.4 | 2406.2 | |
| LPFGYPVYV | 1/49 | 15.7 | |||||
| LLLGYPVYV | 1/49 | 2406.2 | |||||
| LLFRYPVYV | 5/49 | 2406.2 | |||||
| LLFGNPVYV | 1/49 | 2406.2 | |||||
| LLFGHPVYV | 1/49 | 2406.2 | |||||
| LLFGYPVHV | 1/49 | 437.5 | |||||
| 48 | 5/28/93 | 93 | 0.83 | LLFGYPVYV | 45/48 | 6.3 | 2406.2 |
| LLFRYPAYV | 1/48 | 2406.2 | |||||
| LLFGFPVYV | 1/48 | 9143.4 | |||||
| LLFGYPVCV | 1/48 | 437.5 | |||||
| 10/21/96 | 606 | 5.47 | LLFGYPVYV | 48/51 | 5.9 | 2406.2 | |
| LLFRYPVYV | 1/51 | 2406.2 | |||||
| LLFGYLVYV | 1/51 | 5534.1 | |||||
| LLFGYPVYE | 1/51 | 0.5 | |||||
| 2/10/00 | 395 | 2.70 | LLFGYPVYV | 47/48 | 2.1 | 2406.2 | |
| PLFGYPVYV | 1/48 | 31.1 |
Total numbers of variants of Tax 11-19 are 2, 1, 3, 9, 4, 1, 3, 2, and 1, in the order of amino acid position, respectively.
HTLV-1 proviral load is shown as copies/104 PBMCs.
HTLV-1 Tax 11-19-specific T-cell frequency in CD8+ cell population is shown.
Amino acid sequence is presented by the single letter. Underlined amino acids indicate mutations.
Percentage of variant epitope in the sequenced clones is shown at each time point.
HLA-A2-binding affinity by dissociation half-time (minutes) of each peptide is predicted through the Internet (http://bimas.dcrt.nih.gov/moibio/hla_bind/).
For this experiment 2 DNA samples with low virus-specific T-cell frequencies and one sample with the highest frequency during the time course were chosen. In patient no. 31, the amino acid mutation rate in the Tax 11-19 ranged from 2.0% to 6.3%. In patient no. 48, the mutation rate was also not widely changed from 2.1% to 6.3%. However, in patient no. 38 who had relatively high proviral load and low frequency of the HTLV-1–specific T cells (Figure 2; Table 1), the variation rate reached 20.4% in the sample drawn on June 24, 1999. In this time point, the frequency and the degeneracy of the virus-specific T cells were the lowest in the time course (Figure 2B-C). The numbers of amino acid substitutions of Tax11-19 in the overall 445 clones sequenced were 2, 1, 3, 9, 4, 1, 3, 2, and 1 in the order of the amino acid position, respectively (Table 2). This indicates that the substitutions were frequently observed at positions 4 and 5. The substitution of glycine by arginine at position 4 was most frequently observed with 9 of 26 variants (34.6%). When we predicted the HLA-A2–binding affinity of the variants through the Internet, only 6 of 26 variants would make a lower HLA-A2–binding affinity than Tax 11-19 (Table 2). There was no accumulation of variant epitope during the time course as seen in HIV infection.23-25
Discussion
In this longitudinal study, we have shown that: (1) the HTLV-I Tax 11-19–specific CD8+ T-cell frequency correlated with the proviral load during the time course of the disease; (2) the T cells showed an increased degeneracy of T-cell recognition when the T-cell frequency and the proviral load were increased; (3) in 2 patients with comparable virus-specific T-cell frequency, the proviral load was much higher in the patient who had low degeneracy of the T cells as compared to the other patient who had high degeneracy and the patients with high proviral load had lower degeneracy than those with low proviral load; (4) the degeneracy of T-cell recognition was independent of the T-cell avidity; (5) the frequency of HTLV-1 Tax 11-19–specific T cells is significantly higher in HAM/TSP patients than in the asymptomatic HTLV-I carriers; (6) the epitope variants were remarkably increased in a patient when the frequency and the degeneracy of the T cells were the lowest in the time course; (7) the amino acid changes in the epitope predominantly occurred in the central positions of the epitope; and (8) there is no accumulation of any variant epitope during the time course of the disease.
When the proviral load was increased, HTLV-1–specific CD8+T cells were increased and the T-cell population that recognized APLs substituted at positions 5 and 6 was increased in patients no. 31 and no. 38 (Figure 2A-C). Then, according to the decrease in proviral load, both the frequency and the degeneracy of virus-specific T cells were decreased. This suggests that the fine specificity of HTLV-1–specific CD8+ T cells is altered according to the viral load in vivo and that the degenerate specificity may give virus-specific T cells an advantage to eliminate the virus. When we compared several factors including viral load, frequency and degeneracy of virus-specific T cells among the patients, patients no. 38 and no. 48 had comparable virus-specific T-cell frequencies (Table 1), and MFI curves of IFN-γ–FITC in the T cells were similar using an antigen titration assay (data not shown). These results suggest that the degree of virus-specific T-cell responses in the 2 patients were almost the same in the given experimental conditions. However, patient no. 38 with limited degeneracy showed 6 times higher proviral load than patient no. 48 who had high degeneracy. Furthermore, in the experiment in which a greater number of patients with HAM/TSP were examined, the patients with high proviral load had lower degeneracy of T-cell recognition than those with low proviral load. Again, it is possible that the increase in degeneracy of the HTLV-1–specific T-cell responses tend to reduce the proviral load in vivo. In chronic viral infections, the virus-specific T-cell repertoire is diversified in the acute phase, whereas in the persistent phase, the T cells show a narrow TCR repertoire, suggesting that memory T cells may have a strict antigen recognition.26,27 Other experiments demonstrate that naı̈ve cytotoxic T lymphocytes (CTLs) show heterogeneous diversity, whereas virus-specific memory CTLs show limited diversity.28-30 In the study presented, IFN-γ was detected as a marker of antigen-specific T cells in the culture condition, in which PBMCs were cocultivated with APCs for 6 hours; therefore, the IFN-γ+ cells may be memory or effector T cells.31 Furthermore, the functional antigen avidities were comparable in the HTLV-1 Tax11-19–specific CD8+ T cells during the time course (Figure 4A). Therefore, memory or effector T cells specific for HTLV-1 Tax 11-19 could show degenerate specificity of T-cell recognition during viral replication in chronic viral infections.
The increased frequency and degeneracy of HTLV-1–specific CD8+ T cells in response to the increased proviral load may have another biologic role. If virus-specific CD8+ T cells have limited degeneracy, they could eliminate wild-type viruses. However, some viruses with a mutation at the epitope would escape from the T cells, thereby increasing the proportion of the mutant viruses. The rate of epitope variants in patient no. 38 on June 24, 1999 was as high as 20.4%, whereas the rates were 0% or 5.9% at other time points (Table 2). However, the degree of IFN-γ production from the virus-specific T cells was similar throughout the time course (as shown in Figure 4B for patient no. 31). At the time point, the degeneracy of the T-cell population was the lowest, around an average of 6% during the course investigated (Figure 2C, June 24, 1999). The presented data support the hypothesis that the heterogeneous responses of virus-specific CD8+ T cells have an advantage to eliminate mutant viruses over homologous T-cell responses.26 32 Therefore, it seems likely that the degenerate specificity of virus-specific T-cell responses are defensive against mutant viruses in chronic viral infections.
The HTLV-1–specific T-cell frequency correlated well with the proviral load in each individual. Recently, it has clearly been shown that HTLV-1 mRNA load correlates with proviral DNA load and virus-specific CD8+ T-cell frequency by a quantitative method.33 If the increased proviral load during the clinical course in our patients suggests an elevated level of HTLV-1 mRNA load, then increased Tax protein expression would effectively stimulate the Tax 11-19–specific T cells in vivo (Figure 2A-B). In HTLV-1 infection, it has been suggested that HTLV-1 predominantly replicates as a provirus via mitotic proliferation of the infected cells in vivo.3 High virus-specific T-cell response observed in patients with HAM/TSP suggests that the immune system is continuously exposed to the viral antigens in vivo.10Recently, it has been shown that circulating CD4+ T cells infected with HTLV-1 rarely express viral antigens; however, they readily express the viral proteins in a 6-hour ex vivo culture and are subsequently killed by the CD8+ CTLs in vitro.34 Patient no. 31 showed a marked increase of HTLV-1–specific T-cell frequency (2.9 times elevation from 6.7% to 19.5%) corresponding to the increase of proviral load (1.3 times elevation from 506 to 674 copies/104 PBMCs) during the course (Figure 2A-B), suggesting that the T cells proliferated by a stimulation from HTLV-1. The proviral load was then reduced, implying that the T cells killed the virus-infected cells in vivo. However, the proviral load finally reached a final level of 506 copies/104 PBMCs despite continuous high virus-specific T-cell frequency on March 10, 1999. This suggests that silent HTLV-1–infected cells, which do not express the viral antigens, may survive in the body of the infected individual.
A marked increase in proviral load is a virologic hallmark in patients with HAM/TSP as compared to asymptomatic HTLV-1 carriers.9However, it is still controversial as to whether HTLV-1–specific T cells are increased in patients with HAM/TSP as compared to the carriers. It has been reported that circulating HTLV-1–specific T cells are significantly increased in patients with HAM/TSP as compared to asymptomatic HTLV-1 carriers with the use of CTL assay, limiting dilution assay, and intracellular cytokine assay.10,11,16However, it has also been reported that HTLV-1–specific T cells are increased both in HAM/TSP patients and asymptomatic HTLV-1 carriers.35 It was not clear what causes this discrepancy. One possibility may be that the numbers of subjects included were small in both studies. In this study with a larger number of subjects, HTLV-1 Tax 11-19–specific T-cell frequency was significantly increased in the patients with HAM/TSP as compared to the carriers. It has been shown that HTLV-1 Tax 11-19–specific T cells accumulate and are activated in cerebrospinal fluid in patients with HAM/TSP as compared to the periphery and that CD8+ T cells accumulate in the spinal cords of patients with HAM/TSP.7,11,13 Collectively, these data suggest that HTLV-1–specific CD8+ T cells may play an important roll in the pathogenesis of HAM/TSP.15
Crystal structure analysis of the HLA-A2/Tax 11-19/ TCR complex in a Tax 11-19–specific T-cell clone clearly shows that the amino acid at position 5 fits into the pocket formed by the TCR Vα and Vβ and makes numerous contact sites with the TCR,20 suggesting that the amino acid is a primary TCR contact residue. The sequence analysis of an immunodominant epitope Tax 11-19 revealed that amino acid mutations accumulated in positions 4 and 5 and were observed in 13 of 26 variant epitopes (50%; Table 2). However, the mutated variants at the HLA-A2 anchor positions 2 and 9 were observed in only 2 of 26 variants (7.7%), which resulted in reduction in the binding affinity to HLA-A2.21 In the patients with HAM/TSP, amino acid changes in the epitope preferentially occurred at TCR-binding positions rather than at HLA anchor positions. This is a marked contrast to HIV infection, where viral variants with a change at the HLA anchor positions become dominant during the time course because the majority of the variants readily escape from host immunity.23-25The reasons why amino acid substitutions preferentially occurred at the TCR-binding sites rather than at the HLA-binding sites in HTLV-1 infection are not clear. On the other hand, alterations in amino acids around position 5 of the epitope may provide a chance for the virus to escape from the HTLV-1–specific T cells. In the 3 patients, the T-cell recognition of APL substituted at positions 5 and 6 were increased when the proviral load was increased, although the increase was slight in patient no. 48 who had higher degeneracy of T-cell recognition than the others (Figure 2A-C). Therefore, the increased degeneracy of T-cell recognition preferentially directing amino acids at positions 5 and 6 may have an advantage to eliminate mutant viruses at these positions.
The naturally occurring variant epitope altered from glycine to arginine at position 4 of Tax 11-19 epitope, designated as G4R (LLFRYPVYV), is of interest, because the variant was predominantly investigated among the 3 patients (9 of 26 variants; Table 2). This mutation has previously been demonstrated in HLA-A*02+HAM/TSP patients and asymptomatic HTLV-1 carriers with different ethnic origins and in HLA-A*02+ ATL patients.18,36 In the present study, the variant did not become dominant among the patients during the time course of the disease. The predicted HLA-A2–binding affinity of G4R is comparable to that of cognate Tax 11-19 peptide (Table 2). If the variant peptide is endogenously processed and expressed efficiently with HLA on the infected cells, it is expected that G4R-specific CD8+ T cells might emerge and kill the cells infected with the mutant virus. Therefore, we conducted an experiment to investigate whether G4R-specific CD8+ T cells may emerge during the time course. However, CD8+ T cells in PBMCs did not recognize the G4R peptide during the course in the 3 patients (data not shown), suggesting that the nonpredominance of G4R did not result from an appearance of G4R-specific CD8+T cells. It has been demonstrated that Tax protein acts as atrans-activator on its long terminal repeat and activates a number of cellular genes such as interleukin 2 through nuclear transcription factor NF-κB.37 Thus, a mutation of Tax protein may give an advantage or a disadvantage to the infected cells for proliferation. A trans-activation assay using the mutant gene that causes G4R mutation reveals that thetrans-activation activity disappeared.36 Therefore, a possible reason for nonpredominance of G4R despite lack of G4R-specific CD8+ T cells is that the loss of function of Tax protein may lead to a decreased expression of the viral proteins and low replication rate of the mutant virus.
In conclusion, HTLV-1 persistently infects humans despite a vigorous virus-specific T-cell response, in part because the majority of the virus-infected cells rarely express viral antigens in vivo. During viral replication, HTLV-1–specific CD8+ T cells show increases in both frequency and degeneracy. Thereafter, the interaction between the T cells and the virus may transiently reach an equilibrium state. This high immune response to the virus may play a crucial role in the pathogenesis of HAM/TSP. The elucidation of the underlying mechanisms by which HTLV-1–infected cells rarely express the viral antigens in vivo will be beneficial in designing patient therapies.
Acknowledgments
We thank Ms Y. Nishino and T. Muramoto for the excellent technical assistance, and Samantha S. Soldan and Arlene R. Ng for the critical reading of the manuscript.
Prepublished online as Blood First Edition Paper, December 12, 2002; DOI 10.1182/blood-2002-08-2477.
Supported by the Program for Promotion of Fundamental Studies in Health Sciences of the Organization for Pharmaceutical Safety and Research (OPSR) in Japan.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Ryuji Kubota, Third Department of Internal Medicine, Faculty of Medicine, Kagoshima University, 8-35-1 Sakuragaoka, Kagoshima 890-8520, Japan; e-mail:kubotar@m2.kufm.kagoshima-u.ac.jp.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal