Abstract
At clinical presentation, multiple myeloma (MM) is already a well-established disease. The processes involved in earlier stages are, however, unknown. Here the 5T2MM murine model was used to analyze differentiation, proliferation, invasion, and apoptosis of MM cells during disease progression. Naive mice were injected with 5T2MM cells and from the onset of the experiment 3 mice were killed each week until the end stage. Myeloma cells were isolated from the bone marrow and selected by sequential gating of 5T2MM idiotype+ cells by flow cytometry. Microscopic analysis of these sorted 5T2MM idiotype+ cells confirmed their identity as true myeloma cells. Based on serum paraprotein concentration and bone marrow tumor load, 3 disease stages were distinguished: a quiescent stage, an intermediate stage, and an end stage, of slow, moderate, and accelerated tumor progression, respectively. In the quiescent stage, the majority of the myeloma cells were CD45+CD138−IL-6Rα+, corresponding to an immature, invasive, and apoptosis-resistant phenotype. In the end stage the majority of the myeloma cells had differentiated into CD45−CD138+IL-6Rα− cells, corresponding to a mature, less invasive, and apoptosis-sensitive phenotype. In the intermediate stage a gradual transition from the quiescent toward the end stage was observed. In line with these data, analysis of sorted 5T2MM cells demonstrated a significant decrease in invasive capacity and a significant increase in (dexamethasone-induced) apoptosis sensitivity and in proliferation during the disease progression. These data suggest that myeloma disease progression is a multistage and dynamic process of differentiation, proliferation, invasion, and apoptosis.
Introduction
Multiple myeloma (MM) is still an incurable plasma cell cancer, predominantly localized in the bone marrow (BM). Our group demonstrated that the in vivo homing of these malignant cells to the BM is a selective process of directed migration and selective survival in this organ.1 Once the cells have entered the BM, they have an intensive cross-talk with the BM stromal cells and induce a microenvironment that supports their survival and growth.2At time of diagnosis, MM is already a well-established disease. So far myeloma research has been focused on homing and other mechanisms involved in the clinical stage of the disease. The processes involved in the earlier, preclinical, stages are unknown. In this work we took a first step in illumination of the preclinical stages. Since such investigation requires an in vivo model, the 5T2MM experimental mouse model was used. Animals intravenously inoculated with MM cells were analyzed during the entire process of myeloma progression and monitored for 3 markers: CD45, CD138 (syndecan-1), and interleukin 6 receptor (IL-6R). CD45 is a transmembrane protein tyrosine phosphatase, expressed on all B-cells. During maturation toward plasma cells the CD45 expression is gradually down-regulated. Fully matured plasma cells are CD45−.3 In myeloma patients both CD45+ and CD45− MM cells have been observed.4-6 More recently, CD45 appeared to be a predictor of therapeutic response.7 Myeloma patients were grouped according to percentages of CD45bright, CD45low, and CD45− MM cells, and grouping was associated with clinical outcome: patients with a high percentage of CD45− MM cells had the poorest outcome. The significance of this CD45 heterogeneity, from a biologic point of view, is, however, not understood. In the present work CD45 was used as a differentiation marker together with CD138. CD138 is a transmembrane heparan sulfate proteoglycan expressed on sessile B cells. All pre-B cells are CD138+, but during circulation in the peripheral blood CD138 expression is lost and CD138 is re-expressed on mature plasma cells in the BM. Immature CD45+ MM cells are CD138−. CD138 is also proteolytically cleaved and shed from the cell surface, and CD138− MM cells appeared to possess a higher invasive capacity.8
IL-6R consists of 2 subunits: an α chain (gp80, CD126), which specifically binds IL-6, and a shared β chain (gp130, CD130), which is responsible for the signal transduction.9-11 IL-6 is a growth factor for MM cells,12,13 and, worthy of mention in this work, it is required in the early stages of plasma cells in the BM to escape apoptosis.14 We report herein a multistage process of myeloma disease progression based on the evolution of serum M component (paraprotein) and tumor load. Flow-cytometric analysis demonstrated a CD45+CD138−IL-6Rα+ phenotype of the majority of MM cells in the initial stage of tumor progression. Toward the end stage the cells gradually acquired a CD45−CD138+IL-6R− phenotype. Functional analysis of sorted MM cells from different stages of disease progression indicated an increased proliferative activity and apoptosis sensitivity and a decreased invasive capacity during the disease progression.
Materials and methods
5T2MM myeloma model
5T2MM myeloma cells originate from spontaneously developed myeloma in elderly C57BL/KaLwRijHsd mice.15,16 The model was initiated, and is continued, by intravenous injection of diseased BM cells into young (6- to 10-week-old) syngenic recipients (Harlan, Horst, Netherlands) as described previously.17
Study design
A group of 33 naive mice were intravenously injected with 2 × 106 5T2MM cells. From this time point 3 mice were killed each week until the remaining animals were terminally diseased. From each mouse, paraprotein level and tumor load was quantified and the MM cells in the BM were phenotyped. In the second part of the study the MM cells were sorted from animals in different disease stages for functional experiments.
Serum paraprotein quantification and isolation of BM cells
Mice were bled before being killed and serum paraprotein levels were quantified by standard electrophoretic techniques.18 BM cells were flushed out from femora and tibiae and bone marrow mononuclear cells (BMNCs) were isolated by centrifugation of the samples on Lympholyte M (Cedarlane Laboratories, Hornby, ON, Canada).
Flow cytometry
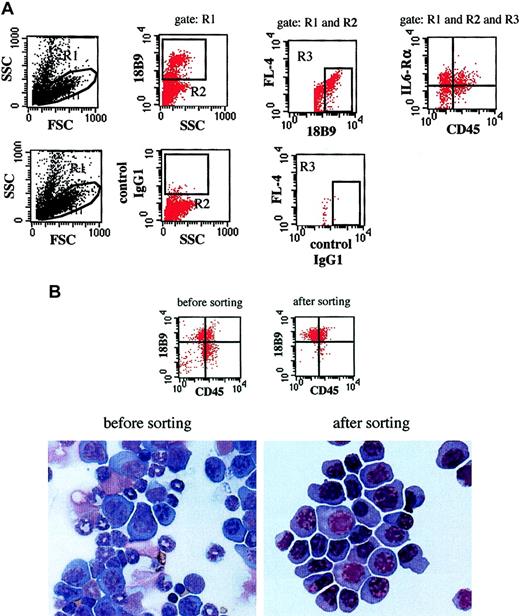
Tumor load in the BM was assessed by staining of the BMNCs with anti-5T2MM idiotype-specific antibodies (18B9, mouse IgG1).18 Rat antimouse IgG1-PerCP (Becton Dickinson, San Jose, CA) was used as a secondary reagent. To phenotype the 5T2MM cells, tricolor stainings were performed. Cells were stained with anti-5T2MM idiotype-specific antibodies as described above and with CD45–fluorescein isothiocyanate (FITC; clone AMS4508; Biosource International, Camarillo, CA), CD138-PE (clone 281-2, rat IgG2a; Becton Dickinson), or phycoerythrin (PE)–conjugated anti–IL-6 receptor α chain (IL-6Rα) monoclonal antibodies (clone D7715A7, rat IgG2b; Pharmingen, San Diego, CA). For all stainings, isotype-matched irrelevant antibodies were used as controls. All samples were analyzed on a FACSCalibur flow cytometer (Becton Dickinson). The first weeks after tumor injection, the frequency of myeloma cells in the BMNC population was low. Therefore all samples were analyzed according to the principle described for the phenotyping of rare hematopoietic stem cells in the BM.19 Cell debris was eliminated by setting a lymphoblastoid gate in a forward scatter/side scatter (FSC/SSC) dot plot. 5T2MM idiotype+ cells were defined within a live gate on a SSC/18B9 dot plot. The 18B9 is the anti-5T2MM monoclonal antibody and was labeled with rat antimouse IgG1-PerCP (FL-3) as secondary reagent. Autofluoresence was excluded in an 18B9/fluorescence channel 4 (FL-4) dot plot. Phenotypes of the 5T2MM cells were analyzed on a FL-1 (CD45-FITC)/FL-2 (CD138-PE or IL-6Rα-PE) dot plot (Figure 1A). In this way true 5T2MM idiotype+ cells were obtained, as confirmed by light microscopic examination of sorted cells (Figure1B). The data presented in Figure 1B also demonstrate that the CD45+5T2MM idiotype+ cells are true myeloma cells and not contaminating cells. All cell sortings were performed on a FACSvantage-SE flow cytometer (Becton Dickinson).
Gating, sorting, and microscopic examination of 5T2MM cells.
(A) Gating of 5T2MM cells. Cell debris was excluded by a lymphoblastoid gate (R1) on an FSC/SSC dot plot. 5T2MM idiotype (18B9)+ cells were defined within a live gate (R2) on an SSC/18B9 dot plot. Autofluoresence was excluded on an 18B9/FL-4 dot plot. No marker was used in the FL-4 channel. 5T2MM cell phenotype was then analyzed on an FL1/FL2 dot plot. Upper panels are dot plots from a bone marrow sample labeled with CD45-FITC, IL-6Rα–PE, and 18B9 with rat-antimouse-PerCP as a secondary reagent. Lower panels are from the same sample stained with isotype-matched control antibody. (B) Sorting and microscopic examination of 5T2MM cells. Dot plots illustrate expression pattern of 5T2MM idiotype (18B9) and CD45 on a bone marrow sample before and after sorting. Photographs below illustrate May-Grünwald-Giemsa–stained cytospin of same sample before and after sorting. These data were obtained from a mouse with 0.57 g/dL paraprotein concentration and 40% of its 5T2MM cells CD45+. After sorting, myeloma purity of 97% was obtained, as analyzed by flow cytometry. Microscopic examination of the cytospin indicated a purity of 99%. Original magnification × 400.
Gating, sorting, and microscopic examination of 5T2MM cells.
(A) Gating of 5T2MM cells. Cell debris was excluded by a lymphoblastoid gate (R1) on an FSC/SSC dot plot. 5T2MM idiotype (18B9)+ cells were defined within a live gate (R2) on an SSC/18B9 dot plot. Autofluoresence was excluded on an 18B9/FL-4 dot plot. No marker was used in the FL-4 channel. 5T2MM cell phenotype was then analyzed on an FL1/FL2 dot plot. Upper panels are dot plots from a bone marrow sample labeled with CD45-FITC, IL-6Rα–PE, and 18B9 with rat-antimouse-PerCP as a secondary reagent. Lower panels are from the same sample stained with isotype-matched control antibody. (B) Sorting and microscopic examination of 5T2MM cells. Dot plots illustrate expression pattern of 5T2MM idiotype (18B9) and CD45 on a bone marrow sample before and after sorting. Photographs below illustrate May-Grünwald-Giemsa–stained cytospin of same sample before and after sorting. These data were obtained from a mouse with 0.57 g/dL paraprotein concentration and 40% of its 5T2MM cells CD45+. After sorting, myeloma purity of 97% was obtained, as analyzed by flow cytometry. Microscopic examination of the cytospin indicated a purity of 99%. Original magnification × 400.
Proliferation, invasion, and apoptosis of 5T2MM cells
For functional analysis, flow cytometric–sorted 5T2MM cells were used. To assess the proliferation, 0.25 × 1065T2MM cells were incubated for 1 hour with 12.5 μg/mL bromodeoxyuridine (BrdU; Sigma, St Louis, MO) in Dulbecco modified essential medium (DMEM) supplemented with penicillin-streptomycin, glutamine, Minimum Essential Medium (MEM; Gibco BRL, Merelbeke, Belgium) and 10% bovine serum (Hyclone, Logan, UT). Cells incubated in medium without BrdU were used as controls. Subsequently the cells were stained for flow cytometric analysis with anti-BrdU monoclonal antibodies (clone BRD.3, mouse IgG1; Biosource International) according to the manufacturer's instructions. Rat antimouse IgG1-PE (Becton Dickinson) was used as a second step. Invasion of 5T2MM cells was analyzed by Matrigel invasion assay (Becton Dickinson) as described previously.20Apoptosis sensitivity of the myeloma cells was measured after coculturing 0.25 × 106 cells per well on confluent layers of normal BM stromal cells (BMSCs) in 24-well plates in 0.5 mL DMEM supplemented with penicillin-streptomycin, glutamine, MEM, and 10% bovine serum. Where indicated, 5μM dexamethasone (Sigma) was added to the cells. After overnight incubation, the percentage of apoptotic cells was analyzed by staining with annexin V and propidium iodide according to the manufacturer's instructions (Becton Dickinson).
Results
5T2MM myeloma disease progression is a multistage process
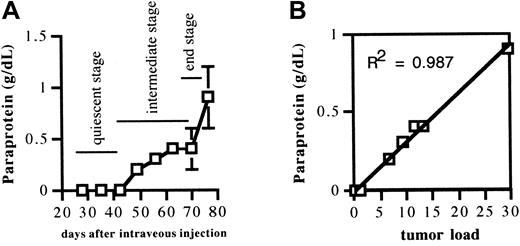
Naive mice were injected with 2 × 106 5T2MM cells. After injection, 3 mice were killed each week until the remaining animals were terminally diseased. Serum paraprotein concentration and BM tumor load were quantified and were detectable above the background 4 weeks after tumor injection. The serum paraprotein concentration, illustrated in Figure 2A, showed an increase during disease progression, as expected. There was a good correlation between the serum paraprotein concentration and tumor load in the BM (Figure 2B). These data demonstrate that the serum paraprotein concentration can be used as an independent indicator for disease progression. Based on the paraprotein levels, 3 stages were distinguished in the progression of the myeloma disease (indicated in Figure 2A): a quiescent stage of slow tumor progression (increase of paraprotein from 0 to 0.20 g/dL, during the 7 weeks after tumor injection), an intermediate stage of moderate tumor progression (increase of paraprotein from 0.30 to 0.57 g/dL, within a time interval of 3 weeks [7-10 weeks after tumor injection]), and an end stage of accelerated tumor progression (increase of paraprotein from 0.64 to 1.2 g/dL, within a time interval of 1 week [10-11 weeks after tumor injection]). These data clearly demonstrate that 5T2MM myeloma disease progression is a multistage process.
Evolution of serum paraprotein concentration during disease progression and correlation between serum paraprotein concentration and 5T2MM myeloma tumor load in bone marrow.
(A) Evolution of serum paraprotein concentration during disease progression. Mice injected with 5T2MM cells were bled before being killed at indicated time points. Paraprotein in serum was quantified by protein electrophoresis. Each point represents mean value ± SD of 3 animals. (B) Correlation between serum paraprotein concentration and 5T2MM myeloma tumor load in the bone marrow. Paraprotein concentrations were measured as described in Figure 2A legend. Bone marrow mononuclear cells from the same mice were analyzed for 5T2MM tumor load by flow-cytometric staining with anti-idiotype–specific monoclonal antibodies. Each point represents mean value of 3 mice ± SD.
Evolution of serum paraprotein concentration during disease progression and correlation between serum paraprotein concentration and 5T2MM myeloma tumor load in bone marrow.
(A) Evolution of serum paraprotein concentration during disease progression. Mice injected with 5T2MM cells were bled before being killed at indicated time points. Paraprotein in serum was quantified by protein electrophoresis. Each point represents mean value ± SD of 3 animals. (B) Correlation between serum paraprotein concentration and 5T2MM myeloma tumor load in the bone marrow. Paraprotein concentrations were measured as described in Figure 2A legend. Bone marrow mononuclear cells from the same mice were analyzed for 5T2MM tumor load by flow-cytometric staining with anti-idiotype–specific monoclonal antibodies. Each point represents mean value of 3 mice ± SD.
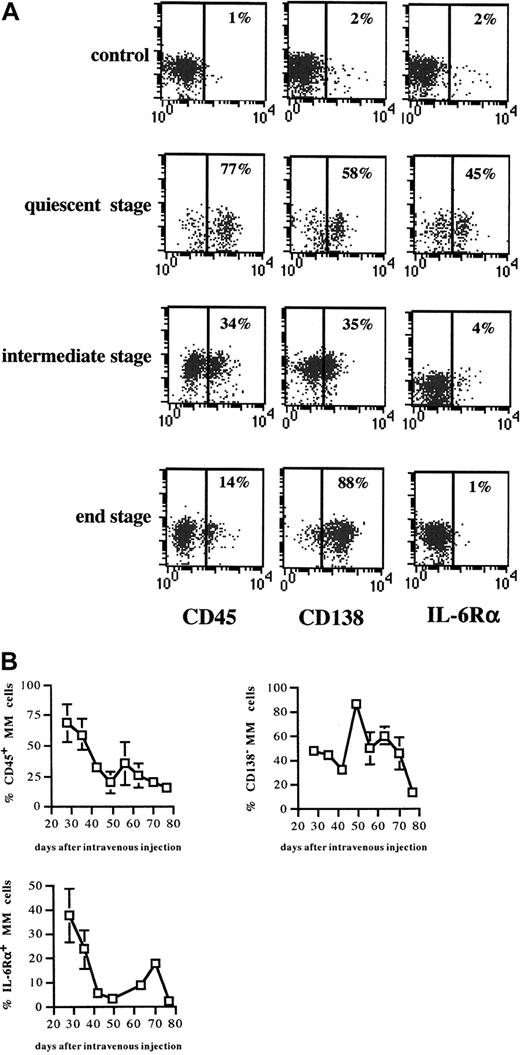
Phenotypical alterations in the expression of CD45, CD138, and IL-6Rα during 5T2MM disease progression
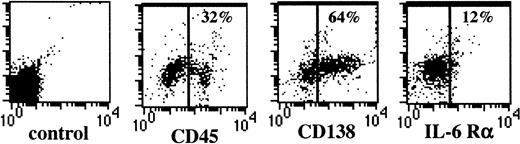
The phenotype of the 5T2MM cells before injection into the naive mice is illustrated in Figure 3. Three mice were killed each week until the end stage of the disease. The MM cells were phenotyped for CD45 expression as maturation markers and for CD138 and IL-6Rα as indicators of invasive capacity and apoptosis sensitivity, respectively. In Figure 4A, representative dot plots of the different tumor stages illustrate clear phenotypical alterations in the expression of CD45, CD138, and IL-6Rα on the 5T2MM cells. Figure 4B gives an overview of the alterations in subset composition during the entire process of disease progression. In the quiescent stage, the majority of the myeloma cells were CD45+CD138−IL-6Rα+, corresponding to an immature, invasive, and apoptosis-resistant phenotype. In the end stage the majority of the myeloma cells were CD45−CD138+IL-6Rα−, corresponding to a mature, less invasive, and apoptosis-resistant phenotype. In the intermediate stage there was a (gradual) transition from the quiescent phenotype toward the end-stage phenotype. These data indicate a differentiation of CD45+ myeloma cells into CD45−cells during the progression of myeloma. Moreover, the data suggest alterations in the invasive capacity and apoptosis sensitivity of the 5T2MM cells during disease progression.
Phenotype of 5T2MM cells before injection.
Bone marrow cells isolated from tumor-bearing 5T2MM cells were phenotyped by flow cytometry. 5T2MM myeloma cells were selected by staining with anti-5T2MM–specific antibodies as described in Figure1A. Expression of CD45, CD138, and IL-6Rα on gated 5T2MM is shown. Results are expressed as bivariate plots. x-axis: expression level of the indicated antigen; y-axis: autofluorescence; no marker was used in this channel (FL-4).
Phenotype of 5T2MM cells before injection.
Bone marrow cells isolated from tumor-bearing 5T2MM cells were phenotyped by flow cytometry. 5T2MM myeloma cells were selected by staining with anti-5T2MM–specific antibodies as described in Figure1A. Expression of CD45, CD138, and IL-6Rα on gated 5T2MM is shown. Results are expressed as bivariate plots. x-axis: expression level of the indicated antigen; y-axis: autofluorescence; no marker was used in this channel (FL-4).
Expression of CD45, CD138, and IL-6Rα during disease progression.
(A) 5T2MM cells were gated as explained in Figure 1A. For each tumor stage, plots from one mouse are illustrated. Each column of plots illustrates the expression of the antigen indicated below at the different tumor stages. Results are expressed as bivariate plots. x-axis: expression level of the indicated antigen; y-axis: autofluorescence; no marker was used in this channel (FL-4). Percentages of positive cells are indicated. (B) Alterations in CD45, CD138, and IL-6Rα expression during disease progression. Three mice were killed each week until the remaining animals were terminally diseased. Myeloma cells were phenotyped as described in Figure 1A legend. Percentages of CD45+, CD138−, and IL-6R+ MM cells are shown. Each point represents mean value of 3 mice ± SD.
Expression of CD45, CD138, and IL-6Rα during disease progression.
(A) 5T2MM cells were gated as explained in Figure 1A. For each tumor stage, plots from one mouse are illustrated. Each column of plots illustrates the expression of the antigen indicated below at the different tumor stages. Results are expressed as bivariate plots. x-axis: expression level of the indicated antigen; y-axis: autofluorescence; no marker was used in this channel (FL-4). Percentages of positive cells are indicated. (B) Alterations in CD45, CD138, and IL-6Rα expression during disease progression. Three mice were killed each week until the remaining animals were terminally diseased. Myeloma cells were phenotyped as described in Figure 1A legend. Percentages of CD45+, CD138−, and IL-6R+ MM cells are shown. Each point represents mean value of 3 mice ± SD.
Proliferation, invasion, and apoptosis of 5T2MM cells during disease progression
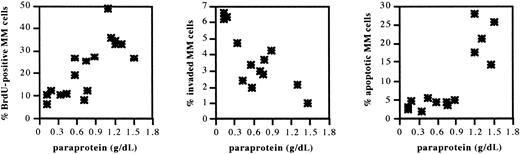
5T2MM cells sorted from BM of tumor-bearing mice in different disease stages were analyzed for proliferation, invasion, and apoptosis as described. Scatter plots in Figure 5illustrate the evolution during disease progression. As shown in Table1, there was a significant increase 5T2MM cell proliferation and apoptosis sensitivity and a decrease in invasive capacity. In the intermediate stage there was a gradual transition from the quiescent toward the end stage (Figure 5). These data are in line with the phenotype of the 5T2MM cells in the corresponding disease stage and demonstrate that 5T2 myeloma disease progression is a dynamic process of proliferation, invasion, and apoptosis.
Proliferation, invasion, and apoptosis of 5T2MM cells during disease progression.
5T2MM cells at different stages of disease progression were sorted as described in Figure 1. Proliferation was assessed by incubation of the cells with bromodeoxyuridine for 1 hour followed by flow-cytometric analysis of nuclei. Invasion was measured by assessing migration of the cells through Matrigel-coated Transwell filters overnight. Apoptosis was analyzed by staining of the cells with annexin V and propidium iodide after overnight incubation on bone marrow stromal cells. Data are illustrated as a function of the corresponding paraprotein concentration, which is an indicator of the tumor stage. Each point represents data from one mouse in an independent experiment.
Proliferation, invasion, and apoptosis of 5T2MM cells during disease progression.
5T2MM cells at different stages of disease progression were sorted as described in Figure 1. Proliferation was assessed by incubation of the cells with bromodeoxyuridine for 1 hour followed by flow-cytometric analysis of nuclei. Invasion was measured by assessing migration of the cells through Matrigel-coated Transwell filters overnight. Apoptosis was analyzed by staining of the cells with annexin V and propidium iodide after overnight incubation on bone marrow stromal cells. Data are illustrated as a function of the corresponding paraprotein concentration, which is an indicator of the tumor stage. Each point represents data from one mouse in an independent experiment.
Proliferation, invasion, and apoptosis at quiescent and end stages
| . | Quiescent stage (paraprotein 0-0.2 g/dL) . | End stage (paraprotein 0.64-1.2 g/dL) . | P . |
|---|---|---|---|
| Proliferation | 9.2 ± 2.3 | 30.9 ± 3.7 | <.0001 |
| Invasion | 5.8 ± 1.0 | 2.4 ± 1.2 | <.02 |
| Apoptosis | 2.4 ± 0.5 | 25.6 ± 2.7 | <.0001 |
| . | Quiescent stage (paraprotein 0-0.2 g/dL) . | End stage (paraprotein 0.64-1.2 g/dL) . | P . |
|---|---|---|---|
| Proliferation | 9.2 ± 2.3 | 30.9 ± 3.7 | <.0001 |
| Invasion | 5.8 ± 1.0 | 2.4 ± 1.2 | <.02 |
| Apoptosis | 2.4 ± 0.5 | 25.6 ± 2.7 | <.0001 |
Dexamethasone-induced apoptosis of 5T2MM cells during disease progression
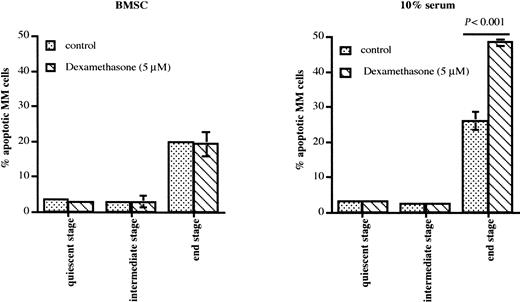
Dexamethasone is a principal agent for treatment of MM. Therefore, we analyzed the sensitivity to this drug of the 5T2MM cells sorted from different disease stages. 5T2MM cells isolated from the quiescent and intermediate stages were resistant to dexamethasone-induced apoptosis, both in the presence and in the absence of BMSCs (Figure6). Dexamethasone was not able to induce apoptosis of end-stage 5T2MM cells in coculture with BMSCs. However, when these MM cells were cultured without stromal cells, apoptosis of the end-stage 5T2MM cells increased and was further augmented by dexamethasone (Figure 6). These data indicate that end-stage 5T2MM cells, corresponding to human MM cells at clinical presentation, are dexamethasone sensitive but are protected by the BM microenvironment.
Dexamethasone-induced apoptosis of 5T2MM cells during disease progression.
5T2MM cells from different stages of disease progression were sorted as described. The MM cells were incubated with or without BMSCs and 5 μM dexamethasone was added where indicated. Apoptosis was analyzed by staining of the cells with annexin V and propidium iodide after overnight incubation. Data represent mean values ± SD of 3 mice (3 independent experiments) at each disease stage.
Dexamethasone-induced apoptosis of 5T2MM cells during disease progression.
5T2MM cells from different stages of disease progression were sorted as described. The MM cells were incubated with or without BMSCs and 5 μM dexamethasone was added where indicated. Apoptosis was analyzed by staining of the cells with annexin V and propidium iodide after overnight incubation. Data represent mean values ± SD of 3 mice (3 independent experiments) at each disease stage.
Discussion
According to current concepts there are 2 types of MM emerging from different oncogenetic transformation processes: MM secondary to pre-existing monoclonal gammopathy of undetermined significance (one third of new MM cases) and de novo MM, in which a normal plasma cell directly transforms into overt myeloma (two thirds of new MM cases).21 In the latter situation, at the time of diagnosis MM is already an established disease with clinical symptoms. The processes involved in the earlier, preclinical, stages of disease progression in this type of MM are unknown. The murine 5T2MM model originates from de novo myeloma22 with symptoms similar to the human variant.23 In this work we used this model to analyze the differentiation, proliferation, invasion, and apoptosis of the myeloma cells during disease progression. The MM cells were selected by stringent flow-cytometric sequential gating of anti-idiotype+ cells. May-Grünwald-Giemsa staining of sorted cells demonstrated that this method results in the selection of true 5T2 myeloma cells. Based on the evolution of paraprotein concentration and the tumor load, we distinguished 3 phases in the disease progression: a quiescent stage of slow tumor progression, an intermediate stage of moderate tumor progression, and an end stage with accelerated progression. In the quiescent stage the majority of the 5T2MM cells were CD45+. This expression was gradually lost during the disease progression and, in analogy to the human situation, in the end stage the majority of the cells were CD45−. This evolution in the CD45 expression pattern demonstrates a clear maturation process of the 5T2MM cells during the disease progression. Although the proportion of CD45+ 5T2MM cells was low (one third) in the population initially injected into the mice, the majority of the MM cells detected in the early stage expressed CD45. We reported previously that the BM homing of CD45− 5T2MM cells is impaired, compared with the CD45+ MM cells.24Moreover, like human CD45− MM cells,25,26 in the presence of IL-6, a portion of the CD45− 5T2MM cells dedifferentiate into CD45+ cells, both in vitro and in vivo.24 Apparently, it is advantageous for the 5T2MM cells to express CD45 in the early stage of the disease, when remodeling of the BM microenvironment is still in progress to make it more supportive for the MM cells. The CD45+ 5T2MM cells express high levels of IL-6R, and it is known that IL-6 has a crucial role in the onset of plasma cell tumors in vivo and prevents apoptosis in the early stages of plasma cells in the BM.14,27 In line with the latter finding, we observed that in vitro the 5T2MM cells are less sensitive to apoptosis in the early stage of the disease, when the expression of IL-6R on the cell surface is high. Moreover, these cells were resistant to dexamethasone-induced cell death, in contrast to end-stage 5T2MM cells. The latter cells were protected from the effects of dexamethasone when they were cocultured with BMSCs. This finding is in agreement with the human situation at clinical presentation. Protection of human MM cells (corresponding to end-stage 5T2MM cells in our model) from dexamethasone-induced apoptosis by BMSCs has been well documented.28,29 Remodeling of the extracellular matrix, to activate the local microenvironment, is an important process in cancer progression.30,31 Matrix metalloproteinases (MMPs), in particular MMP-9, play an essential role in this process.32 These proteases appear to be important in the early stage of cancer progression and may no longer be required once the tumor has been established.31 Our observation of higher invasive capacity of the 5T2MM cells in the quiescent stage than in the end stage is in agreement with these findings in solid tumors. Moreover, we have recently demonstrated that CD45+ 5T2MM cells (the major population in the quiescent stage) secrete high levels of MMP-9, in contrast to CD45−MM cells (the major population in the end stage).20 The absence of syndecan-1 (CD138) on the cell surface of the MM cells in the early stage is also in line with these cells' more invasive and CD45+ characteristics. The expression of CD45 and the expression of CD138 on MM cells are negatively correlated with each other,8 and CD45+ 5T2MM cells are also CD138− (K. A., unpublished observation, July 2001). In a recent report we demonstrated that the CD45+5T2MM cells are much more invasive than CD45− MM cells.20 Others have demonstrated that syndecan-1–transfected ARH-77 B-lymphoid cells are less invasive than the wild-type cells, which have no endogenous syndecan-1.33 Syndecan-1 is also constitutively shed from the cell surface,34,35 and ARH-77 cells engineered to produce a soluble form of this transmembrane heparan sulfate proteoglycan are hyperinvasive.36
The proliferation of the 5T2MM cells increased during the disease progression, parallel with their differentiation into CD45− cells. It is tempting to speculate that in early disease stages the BM microenvironment is remodeled by the 5T2MM cells to create a more supportive niche for their expansion in later tumor stages. Although the 5T2MM cells were more sensitive to apoptosis in vitro toward the end stage of the disease, the tumor load and paraprotein increased. This indicates that the net balance is in favor of proliferation. The proliferative capacity of CD45 subsets in MM is controversial. Some groups report that only a small subset of CD45+ MM cells form a proliferating population in MM6,26; another group recently reported that CD45+ and CD45− MM cells have equal proliferative capacities.37 In vivo studies in the severe combined immunodeficiency human (SCID-hu) mouse model38 and the 5TMM model24 demonstrated that purified CD45− MM cells are able to proliferate and can induce myeloma disease. Moreover, our group demonstrated a higher in vivo proliferation of CD45− 5T2MM cells than of CD45+ MM cells.24 These data are in line with the findings in this work. The signaling pathways by which CD45 affects the proliferation of MM cells are unclear. However, recent reports demonstrate that CD45 gene disruption leads to hyperactivation of Janus kinase–signal transducer and activator of transcription (JAK-STAT) and mitogen-activated protein kinase (MAPK) signaling pathways, resulting in enhanced cytokine-triggered proliferation of hematopoietic cells, including B cells.39,40 In addition, it has been well documented that JAK-STAT and MAPK signaling pathways are involved in the proliferation of MM cells.41
Together, the data presented in this work open new perspectives in myeloma biology. Our in vivo data obtained in the 5T2MM murine model demonstrate that myeloma disease progression is a multistage and dynamic process of differentiation, proliferation, invasion, and apoptosis. Most important, they form a new foundation for further investigations pointing toward novel therapeutic approaches.
The authors thank Willems Angelo for excellent technical assistance.
Prepublished online as Blood First Edition Paper, December 12, 2002; DOI 10.1182/blood-2002-10-3000.
Supported by the Belgian Federation Against Cancer, Onderzoeksraad-VUB, the Fund for Scientific Research–Flanders (FWO-Vlaanderen), and International Myeloma Foundation (IMF) Ashley Barit Research grant (K.A.). K.A. and K.V. are postdoctoral fellows of FWO-Vlaanderen.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Kewal Asosingh, Department of HEIM, Vrije Universiteit Brussel (VUB), Laarbeeklaan 103, B-1090, Brussels, Belgium; e-mail: kewal.asosingh@vub.ac.be.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal