Abstract
Sarco–endoplasmic reticulum calcium ATPase (SERCA) enzymes control calcium-induced cellular activation by accumulating calcium from the cytosol into the endoplasmic reticulum (ER). To better understand the role of SERCA proteins and cellular calcium homeostasis in all-trans retinoic acid (ATRA)–induced differentiation, we investigated the effect of pharmacologic inhibition of SERCA-dependent calcium uptake into the ER on ATRA-induced differentiation of the HL-60 myelogenous and the NB4 promyelocytic cell lines. SERCA inhibitors di-tert-butyl-benzohydroquinone (tBHQ), thapsigargin, and cyclopiazonic acid significantly enhanced the induction of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity and CD11b marker expression induced by suboptimal concentrations of ATRA (50 nM) in both cell lines. Analysis of cellular calcium homeostasis revealed that a 60% mobilization of the total SERCA-dependent intracellular calcium pool was necessary to obtain enhancement of ATRA-dependent differentiation by tBHQ. Moreover, after 3 days of ATRA treatment in combination with tBHQ, NB4 cells showed a significantly decreased calcium mobilization compared with treatments with tBHQ or ATRA alone, suggesting that enhanced differentiation and calcium mobilization are causally related. Interestingly, several ATRA-resistant NB4-derived cell lines were partially responsive to the differentiation-inducing effect of the combination of the 2 drugs. In addition, we found that retinoic acid receptor α (RARα) and PML-RARα proteins are protected from ATRA-induced proteolytic degradation by SERCA inhibition, indicating that cellular calcium homeostasis may interact with signaling systems involved in the control of ATRA-dependent transcriptional activity. By linking calcium to ATRA-dependent signaling, our data open new avenues in the understanding of the mechanisms of differentiation-induction therapy of leukemia.
Introduction
In vitro, the HL-60 myelogenous and the NB4 promyelocytic cell lines can be induced toward neutrophil granulocytic differentiation exhibiting increased nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity, growth arrest, and induction of CD11b expression by exposure to all-trans retinoic acid (ATRA), dimethyl sulfoxide (DMSO), or adenosine 3′:5′-cyclic monophosphate (cAMP) analogs.1-5 The highly pleiotropic effects of retinoic acid are mediated by 2 families of ligand-dependent transcriptional regulators: the retinoic acid receptors (RARα, β, and γ) and the retinoid X receptors (RXRα, β, and γ), which as homo- or heterodimers recognize specific DNA sequences and regulate target gene expression.6-8ATRA-induced differentiation of HL-60 cells is essentially mediated by RARα,1 whereas in the NB4 cell line, which has been derived from a patient affected by acute promyelocytic leukemia (APL),2 differentiation may be mediated by both RARα and the PML-RARα oncogenic protein.9 Recently, it has been reported that the magnitude and duration of the effects of retinoid signals can be regulated by the ligand-dependent degradation of RARs by the ubiquitin-proteasome pathway.10,11 These findings are clinically relevant because retinoid-induced differentiation therapy represents a very successful approach for the treatment of APL in vivo.12,13 However, ATRA monotherapy may be complicated by the emergence of a differentiation-resistant malignant cell population.14 15
Calcium accumulation into intracellular calcium storage organelles or calcium pools is accomplished by sarco–endoplasmic reticulum calcium ATPase (SERCA)–type calcium pumps that transport calcium from the cytosol into the endoplasmic reticulum (ER) using ATP as a source of energy.16,17 In response to external signals, second messenger–induced calcium mobilization from the ER into the cytosol is a key component of intracellular signaling networks controlling cell activation,18 leading subsequently to calcium influx across the plasma membrane.19 The depletion of intracellular calcium storage in the ER can be pharmacologically achieved by the direct inhibition of SERCAs by permeable agents such as di-tert-butyl-benzohydroquinone (tBHQ),20thapsigargin (TG),21 or cyclopiazonic acid (CPA).22 These agents release calcium into the cytosol, mimicking second messenger–induced calcium mobilization, and activate capacitative calcium influx from the extracellular medium.23 Depending on the cell type, the direct and specific inhibition of SERCAs can induce cell activation leading to differentiation,24,25 growth arrest,26apoptosis,27,28 or enhanced HIV production,29indicating that SERCAs play a key role in the control of cell activation. By modulating the spatio-temporal characteristics of calcium transients, SERCAs participate in the modulation of calcium-dependent cell activation and permit specific and selective activation of target enzymes or transcription factors.30 31
We previously observed that HL-60 and NB4 cells, as well as fresh leukemia cells isolated from APL patients, coexpress simultaneously several isotypes of SERCA (SERCA2b and SERCA3) and that upon ATRA-induced differentiation of cells, the expression of SERCA3 is selectively induced approximately 4-fold.32 These results raise the question concerning the involvement of the modulation of SERCA function in leukemia cell differentiation and suggest that a relation may exist between calcium homeostasis and retinoic acid–induced differentiation.
In the present work, we studied the effect of pharmacologic inhibition of SERCA activity on ATRA-induced differentiation of HL-60 and NB4 cells and of their differentiation-resistant sublines. We quantitated intracellular calcium mobilization and ensuing calcium influx required for cell differentiation induced by SERCA inhibitors. We also measured the levels of intracellular calcium concentration in response to SERCA inhibitors in NB4 and ATRA-resistant NB4-derived cell lines. Then, we examined the stability of RARα and PML-RARα proteins during treatment of ATRA-sensitive and ATRA-resistant cells by ATRA in the presence of SERCA inhibitors. Our data show an enhancement of ATRA-dependent differentiation via the pharmacologic modulation of intracellular calcium homeostasis and indicate that a cross-talk may operate between RARα and calcium signaling that may modulate cell differentiation by altering RARα and PML-RARα protein stability.
Materials and methods
Cell culture and induction of differentiation
HL-60 cells33 were obtained from American Type Culture Collection (Manassas, VA). The ATRA-resistant HL-60RES variant34 was a generous gift of Dr Robert Gallagher (Montefiore Medical Center, Bronx, NY). The HL-60–derived Ast4 cell line35 was purchased from the European Collection of Cell Culture (Salisbury, United Kingdom). NB4 cells, and the NB4-R1 and NB4-R2 variants2,36 were kindly provided by Dr Michel Lanotte (INSERM U496, Hôpital Saint Louis, Paris, France). NB4-30637 and NB4-007/6 cell lines38 were a generous gift of Dr Carlo Passerini-Gambacorti (Istituto Nazionale dei Tumori di Milano, Milano, Italy). All cell lines were grown in RPMI-1640/Glutamax I medium (Gibco/Invitrogen, Paisley, United Kingdom) containing 2 mM glutamine and 10% heat-inactivated fetal calf serum.
Prior to experiments, exponentially growing cells were harvested and resuspended in complete medium at a density of 2 × 105cells/mL. The cyclic AMP analog 8-(4-chlorophenylthio)-adenosine 3′:5′-cyclic monophosphate (8-CPT-cAMP) and ritodrine were purchased from Sigma (St Louis, MO). ATRA, tBHQ, TG, or CPA (Sigma) were added to the cells from concentrated stock solutions in DMSO (Sigma). The amount of DMSO vehicle added to the cells did not exceed 0.1%, was included in control experiments, and did not interfere with the assays. To prevent oxidation, tBHQ stock solutions were stored in the vapor phase over liquid nitrogen. NB4-R1 cells were pretreated for 6 days with 10−6 M ATRA prior to experiments (primed cells) as described previously.36
Cell differentiation was assessed by (1) measuring NADPH oxidase activity using nitroblue-tetrazolium (NBT; Sigma) as substrate as described previously,32 39 (2) detecting growth arrest by trypan blue exclusion and cell counting, and (3) detecting surface CD11b antigen expression determined by flow cytometry analysis on a fluorescence-activated cell-sorter (FACS) EPICSXL-MCL (Beckman/Coulter, Paris, France) using fluorescein isothiocyanate–conjugated monoclonal antibody Bear-1 directed against CD11b (integrin αM subunit) (Immunotech/Coulter, Marseille, France) and fluorescein isothiocyanate–conjugated isotype γ1/γ1 matched control (Becton Dickinson Immunocytometry Systems, San Jose, CA), according to the instructions of the manufacturer. In control experiments we determined that the treatment of cells with tBHQ or ATRA for one hour prior to performing the NBT assay did not modify or induce the NBT-reducing activity of the cells. Similar results were obtained with cells that have been pretreated for 3 days with a single drug (ATRA or tBHQ). These data indicate that tBHQ or ATRA did not directly affect cellular NBT-reducing activity in an acute setting.
Cell lysates and Western blot analysis
Following treatments, cell counts and viabilities were determined, and cell lysates were prepared as described previously.32 Samples containing 70 μg total cellular protein per well were run on a Läemmli-type 8% sodium dodecyl sulfate–polyacrylamide gel and proteins were electroblotted onto nitrocellulose membranes. Blocking of nonspecific protein binding to nitrocellulose was performed by incubating the membranes for 30 minutes with Tris-buffered saline (TBS)–0.1% Tween-5% milk.32 The polyclonal RPαF′ anti-RARα antibody that also recognizes the PML-RARα fusion protein10 was kindly provided by Prof Pierre Chambon (Institut de Génétique et de Biologie Moléculaire et Cellulaire, Strasbourg, France). The C-20 anti-RARα polyclonal antibody and the anti–β-actin goat antiserum were from Santa Cruz Biotechnologies (Santa Cruz, CA). Staining was performed by incubating the blots overnight with the respective antibodies diluted 1000-fold in TBS-Tween-milk followed by incubation with a peroxidase-conjugated secondary antirabbit or antigoat antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA).10 Antibody binding was detected using the SuperSignal chemiluminescence reagent (Pierce, Rockford, IL) according to the instructions of the manufacturer.
Measurement of intracellular calcium concentration
Intracellular calcium concentration [Ca2+]i was measured as the change in Fura-2 fluorescence. After treatment with differentiation-inducing agents, cells (2 × 106/mL) were loaded with 1 μM Fura-2 acetoxymethyl ester in phenol red–free RPMI-1640 medium (Gibco/Invitrogen) at 37°C in the dark for 30 minutes, washed twice, and resuspended in a solution consisting of 10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH = 7.4), 120 mM NaCl, 5 mM KCl, 0.4 mM MgCl2, 40 μM CaCl2, 10 mM NaHCO3, 10 mM glucose, and 5 mM Na2HPO4, at 106 cells/mL, and then used for the experiments. Dual excitation, alternating at 340 and 380 nm, was provided by spectrofluorophotometers (models LS-50B [Perkin-Elmer, Milano, Italy] or RF-1501 [Shimadzu, Darmstadt, Germany]) equipped with 2 excitation monochromators. To eliminate extracellular calcium, 0.5 mM EGTA (ethylene glycol tetraacetic acid) was added to each sample before the addition of SERCA inhibitors. The fluorescence signals were calibrated as described previously40 using the following equation: [Ca2+]i = Kd[(F−Fmin)/(Fmax−F)]b, in which F is the measured fluorescence; Fmin and Fmax are the values of the F at [Ca2+]i less than 0.1 nM and more than 1 mM, respectively; and b is the ratio of the emission intensities (at 480 nm with the LS-50B model and 510 nm with the RF-1501) with excitation at 380 nm when the [Ca2+]i is less than 0.1 nM and more than 1 mM. Kd for Fura-2 was taken as 224 nM. The temperature was fixed at 37 ± 1°C. Triton X-100 and 50 mM EGTA were used to obtain the maximal and minimal Ca2+ levels, respectively. Maximal calcium release and subsequent capacitative influx values were determined following addition of tBHQ at supramaximal concentrations depending on the cell type (12 μM for HL-60 cells and 40 μM for NB4 cells). As determined in control experiments, ATRA did not induce any appreciable [Ca2+]i increase in acute experimental settings in HL-60 or NB4 cells (not shown).
Experiments shown in this work were performed 3 or more times (specified in figure legends). Data are presented as mean ± standard error of the mean (SEM).
Results
Enhancement of differentiation in the presence of ATRA and the SERCA inhibitor tBHQ in HL-60 cells
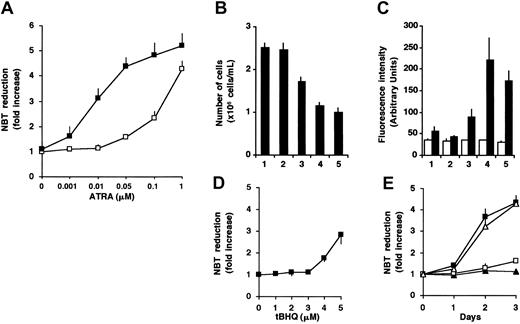
To investigate the effect of Ca2+ on ATRA-induced granulocytic differentiation, we treated HL-60 cells with various concentrations of ATRA in the absence or presence of tBHQ, a drug known to specifically inhibit SERCA enzymes.20,41 In accordance with data in the literature,3 1 μM ATRA induced terminal granulocytic differentiation of HL-60 cells, as reflected by the acquisition of NADPH oxidase activity measured by NBT reduction (4.3-fold increase; Figure 1A), growth arrest (Figure 1B), and an approximately 3-fold induction of CD11b expression (Figure 1C). A concentration of 50 nM ATRA alone did not have a strong differentiation-inducing effect. When 3 μM tBHQ was added to cells in the presence of 50 nM ATRA, a marked enhancement of the differentiation markers, without affecting viability (not shown), was observed (Figure 1A-C). This treatment resulted in the induction of NADPH oxidase activity (4.4-fold), growth arrest, and a 4-fold induction of CD11b expression similar to that obtained with 1 μM ATRA alone. These data indicate that cotreatment of HL-60 cells by ATRA and 3 μM tBHQ resulted in an approximately 20-fold decrease of the maximally effective ATRA concentration (50 nM versus 1 μM). At concentrations in which ATRA is already maximally effective alone (1 μM), the cotreatment with tBHQ did not enhance the NADPH oxidase activity (Figure 1A). A concentration of 2 μM tBHQ had no effect on ATRA-induced NADPH oxidase activity (not shown), whereas at higher concentrations (more than 4 μM), tBHQ alone induced an increase of NADPH oxidase activity (Figure 1D). The enhancement of the differentiation-inducing effect of 50 nM ATRA by 3 μM tBHQ was also studied in time-course experiments. Whereas 50 nM ATRA or 3 μM tBHQ was ineffective when applied alone, their combination resulted in the induction of differentiation of the cells with a rate comparable to 1 μM ATRA alone (Figure 1E). These results strongly suggest a role of SERCA inhibition in the ATRA-induced differentiation of HL-60 cells.
Enhancement of differentiation of HL-60 cells in the presence of ATRA and the SERCA inhibitor tBHQ.
(A) Cells were treated for 3 days with various concentrations of ATRA (0.001-1 μM) in the presence (▪) or in the absence (■) of 3 μM tBHQ, and cellular NADPH oxidase activity, as measured by NBT reduction, was determined. Data presented are the mean ± SEM (n = 3). (B-C) HL-60 cells were treated for 3 days with vehicle (lane 1), 3 μM tBHQ (lane 2), 50 nM ATRA (lane 3), 3 μM tBHQ plus 50 nM ATRA in combination (lane 4), and 1 μM ATRA (lane 5). (B) The inhibition of cell proliferation was determined by counting the number of cells after 3 days of the various treatments (mean ± SEM; n = 10). (C) Expression of CD11b was determined by FACS analysis and reported as fluorescence intensity in arbitrary units; control Igγ1 (■) and CD11b (▪). Mean ± SEM (n = 3). (D) HL-60 cells were treated for 3 days with various concentrations of tBHQ (1-5 μM), and cellular NADPH oxidase activity measured by NBT reduction was determined. Mean ± SEM (n = 3). (E) HL-60 cells were treated for 3 days with 3 μM tBHQ (▴), 50 nM ATRA (■), 3 μM tBHQ plus 50 nM ATRA in combination (▪), and 1 μM ATRA (▵); and cellular NADPH oxidase activity was determined by NBT reduction. Mean ± SEM (n = 3).
Enhancement of differentiation of HL-60 cells in the presence of ATRA and the SERCA inhibitor tBHQ.
(A) Cells were treated for 3 days with various concentrations of ATRA (0.001-1 μM) in the presence (▪) or in the absence (■) of 3 μM tBHQ, and cellular NADPH oxidase activity, as measured by NBT reduction, was determined. Data presented are the mean ± SEM (n = 3). (B-C) HL-60 cells were treated for 3 days with vehicle (lane 1), 3 μM tBHQ (lane 2), 50 nM ATRA (lane 3), 3 μM tBHQ plus 50 nM ATRA in combination (lane 4), and 1 μM ATRA (lane 5). (B) The inhibition of cell proliferation was determined by counting the number of cells after 3 days of the various treatments (mean ± SEM; n = 10). (C) Expression of CD11b was determined by FACS analysis and reported as fluorescence intensity in arbitrary units; control Igγ1 (■) and CD11b (▪). Mean ± SEM (n = 3). (D) HL-60 cells were treated for 3 days with various concentrations of tBHQ (1-5 μM), and cellular NADPH oxidase activity measured by NBT reduction was determined. Mean ± SEM (n = 3). (E) HL-60 cells were treated for 3 days with 3 μM tBHQ (▴), 50 nM ATRA (■), 3 μM tBHQ plus 50 nM ATRA in combination (▪), and 1 μM ATRA (▵); and cellular NADPH oxidase activity was determined by NBT reduction. Mean ± SEM (n = 3).
Enhancement of ATRA-induced differentiation of HL-60 cells in the presence of other SERCA inhibitors
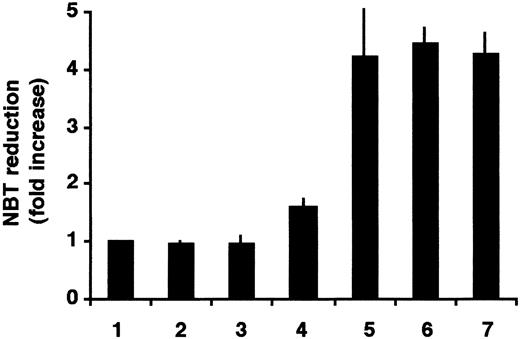
To study the specificity of the SERCA-inhibitory activity on the enhancement of the ATRA-induced differentiation, 2 other structurally unrelated SERCA inhibitors, CPA and TG, were used. HL-60 cells were treated with ATRA, CPA alone, TG alone, and 50 nM ATRA together with CPA or TG. As a positive control, cells treated with 1 μM ATRA were used. NBT assays were performed after 3 days of treatment (Figure2).
Enhancement of ATRA-induced differentiation of HL-60 cells by thapsigargin or cyclopiazonic acid.
Cells were treated for 3 days with vehicle (lane 1), 1.5 μM CPA (lane 2), 0.75 nM TG (lane 3), 50 nM ATRA (lane 4), 1.5 μM CPA plus 50 nM ATRA in combination (lane 5), 0.75 nM TG plus 50 nM ATRA in combination (lane 6), and 1 μM ATRA (lane 7); and cellular NADPH oxidase activity was determined by NBT reduction. Mean ± SEM (n = 3).
Enhancement of ATRA-induced differentiation of HL-60 cells by thapsigargin or cyclopiazonic acid.
Cells were treated for 3 days with vehicle (lane 1), 1.5 μM CPA (lane 2), 0.75 nM TG (lane 3), 50 nM ATRA (lane 4), 1.5 μM CPA plus 50 nM ATRA in combination (lane 5), 0.75 nM TG plus 50 nM ATRA in combination (lane 6), and 1 μM ATRA (lane 7); and cellular NADPH oxidase activity was determined by NBT reduction. Mean ± SEM (n = 3).
SERCA inhibitors when applied alone at low concentrations did not change the state of differentiation of HL-60 cells. However, when the SERCA inhibitors TG or CPA were applied in combination with 50 nM ATRA, induction of differentiation of HL-60 cells was as effective as the differentiation with 1 μM ATRA as a single drug, confirming the above results and indicating that the differentiation-enhancing effect is due to the specific SERCA-inhibitory activity of the drugs. We also tested ritodrine, a β-adrenergic agonist used in clinical practice for the treatment of preterm labor.42 Ritodrine has also been reported to possess an inhibitory effect on SERCA.43 Ritodrine induced an appreciable differentiation of HL-60 cells as measured by NBT reduction when applied alone, and a strong enhancement of differentiation was observed when the cells were treated with ritodrine at concentrations greater than 0.25 mM in combination with 50 nM ATRA for 3 days (not shown), suggesting that ritodrine may have a differentiation-inducing potential on leukemia cells.
Analysis of the calcium homeostasis of HL-60 and NB4 cell lines treated with SERCA enzyme inhibitors
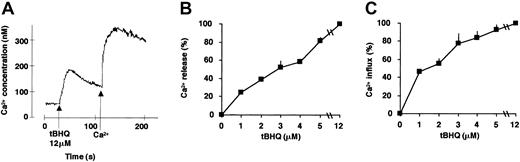
SERCA inhibition leads to Ca2+ mobilization from the ER Ca2+ pools and a subsequent capacitative Ca2+ influx across the plasma membrane. To gain insight into the functional consequences of SERCA inhibition on calcium homeostasis during ATRA-induced differentiation, we investigated cytosolic Ca2+ concentration after SERCA inhibition. tBHQ induced a dose-dependent calcium release and ensuing calcium influx in HL-60 cells (the effect of 12 μM tBHQ is shown in Figure 3A). A concentration of 3 μM tBHQ, which exhibits an optimal potentializing effect on ATRA-dependent differentiation (Figure 1), induced approximately 60% of Ca2+ release of the total SERCA-dependent intracellular calcium pool (Figure 3B), and this was followed by a capacitative calcium influx corresponding to 70% of the value obtained by complete SERCA inhibition (Figure 3C).
Measurement of Ca2+ release and Ca2+ influx in HL-60 cells treated with tBHQ.
SERCA enzyme inhibition-induced Ca2+ mobilization and ensuing capacitative calcium influx were measured spectrofluorimetrically using Fura-2–loaded HL-60 cells. (A) Cells were treated as described in “Materials and methods,” and Ca2+ release from sensitive intracellular pools was measured in cells suspended in Ca2+-free extracellular medium immediately after addition of 12 μM tBHQ (first peak). Thereafter, following addition of Ca2+, capacitative Ca2+ influx was recorded (second peak). To compare the magnitude of calcium release or influx obtained at various concentrations of tBHQ, the area under the calcium peaks was determined using the NIH Image 1.62b7 software (National Institutes of Health, Bethesda, MD) and is expressed as percentage of the maximal value (100%) obtained using the maximally effective concentration of tBHQ (12 μM), which mobilized the entire SERCA-sensitive ER calcium pool. (B) Ca2+ release measured in HL-60 cells treated with various concentrations of tBHQ (1-12 μM). (C) Ca2+ influx measured in HL-60 cells treated with various concentrations of tBHQ (1-12 μM). Mean ± SEM (n = 3).
Measurement of Ca2+ release and Ca2+ influx in HL-60 cells treated with tBHQ.
SERCA enzyme inhibition-induced Ca2+ mobilization and ensuing capacitative calcium influx were measured spectrofluorimetrically using Fura-2–loaded HL-60 cells. (A) Cells were treated as described in “Materials and methods,” and Ca2+ release from sensitive intracellular pools was measured in cells suspended in Ca2+-free extracellular medium immediately after addition of 12 μM tBHQ (first peak). Thereafter, following addition of Ca2+, capacitative Ca2+ influx was recorded (second peak). To compare the magnitude of calcium release or influx obtained at various concentrations of tBHQ, the area under the calcium peaks was determined using the NIH Image 1.62b7 software (National Institutes of Health, Bethesda, MD) and is expressed as percentage of the maximal value (100%) obtained using the maximally effective concentration of tBHQ (12 μM), which mobilized the entire SERCA-sensitive ER calcium pool. (B) Ca2+ release measured in HL-60 cells treated with various concentrations of tBHQ (1-12 μM). (C) Ca2+ influx measured in HL-60 cells treated with various concentrations of tBHQ (1-12 μM). Mean ± SEM (n = 3).
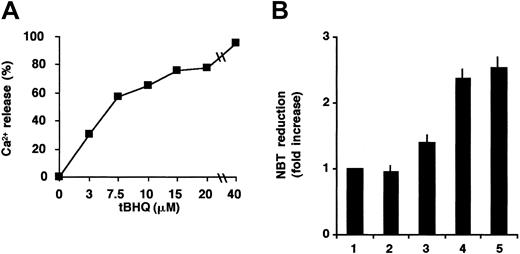
The effect of tBHQ on the differentiation induced by 50 nM ATRA in NB4 cells was then investigated. In contrast with HL-60 cells, no significant differentiation-enhancing effect was detectable in NB4 cells at 3 μM tBHQ (not shown). However, when measured by calcium fluorimetry (Figure 4A), this concentration of tBHQ mobilized approximately only 30% of the calcium stored in SERCA-dependent intracellular pools in NB4 cells, and 60% mobilization could be achieved by only a higher concentration (8 μM) of tBHQ. This result may be explained by the observation that the level of total SERCA protein expression was higher in NB4 than in HL-60 cells (not shown), suggesting that the SERCA-dependent calcium pool is larger in NB4 cells. This is in accordance with the observation that a higher concentration of tBHQ is needed to have a comparable level of Ca2+ release in NB4 than in HL-60 cells. When NB4 cells were treated with 50 nM ATRA in combination with 8 μM tBHQ, a significant enhancement of ATRA-induced differentiation was observed with a level comparable with that of the maximally active ATRA concentration (1 μM; Figure 4B). Taken together, these results suggest that the release of approximately 60% of Ca2+ from ER Ca2+ pools is necessary and sufficient to enhance ATRA-induced differentiation in HL-60, as well as in NB4 cells. This corresponds to different absolute levels of SERCA inhibition, presumably due to differences in SERCA expression or activity levels and ER calcium pool sizes in the 2 cell lines.
Potentialization of ATRA-induced differentiation of NB4 cells by tBHQ.
(A) Ca2+ release from intracellular pools was measured spectrofluorimetrically in NB4 cells immediately after addition of various concentrations of tBHQ (3-40 μM). Ca2+ release values were compared with the maximal release obtained using 40 μM tBHQ. Sixty percent of Ca2+ mobilization was obtained at approximately 8 μM tBHQ. (B) Cells were treated with vehicle (lane 1), 8 μM tBHQ (lane 2), 50 nM ATRA (lane 3), 8 μM tBHQ in combination with 50 nM ATRA (lane 4), and 1 μM ATRA (lane 5); and cellular NADPH oxidase activity was determined by NBT reduction. Mean ± SEM (n = 3).
Potentialization of ATRA-induced differentiation of NB4 cells by tBHQ.
(A) Ca2+ release from intracellular pools was measured spectrofluorimetrically in NB4 cells immediately after addition of various concentrations of tBHQ (3-40 μM). Ca2+ release values were compared with the maximal release obtained using 40 μM tBHQ. Sixty percent of Ca2+ mobilization was obtained at approximately 8 μM tBHQ. (B) Cells were treated with vehicle (lane 1), 8 μM tBHQ (lane 2), 50 nM ATRA (lane 3), 8 μM tBHQ in combination with 50 nM ATRA (lane 4), and 1 μM ATRA (lane 5); and cellular NADPH oxidase activity was determined by NBT reduction. Mean ± SEM (n = 3).
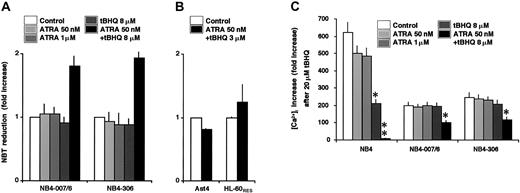
Interaction between tBHQ and ATRA partially reverts ATRA resistance of the NB4-007/6 and NB4-306 cell lines
On the basis of the data obtained on HL-60 and NB4 cells, indicating that the ATRA-induced differentiation can be enhanced by the presence of tBHQ, we examined, in a clinical interest, whether SERCA inhibition could contribute to rescue the resistance to ATRA of differentiation-resistant cells. We used 2 ATRA-resistant derivatives of NB4, the NB4-007/6 and the NB4-306 cell lines. In accordance with previous data,38 both cell lines were refractory to the cyto-differentiating action of 50 nM to 1 μM ATRA, and, as shown in Figure 5A, 8 μM tBHQ alone was ineffective as well. However, when cells were treated simultaneously with 50 nM ATRA and 8 μM tBHQ, a significant increase in NBT-reducing activity was observed. This result indicates that the addition of a SERCA inhibitor to ATRA partially reverted the ATRA-resistant character of the NB4-007/6 and the NB4-306 cell lines. This is an encouraging observation considering that NB4 and primary APL cells behave very similarly. This effect, however, is restricted to NB4-derived cells, as ATRA-resistant derivatives of HL-60 cells, Ast4 and HL-60RES, were refractory to treatments by ATRA in combination with tBHQ (Figure 5B). The resistance of HL-60 derivatives to the cotreatment by ATRA plus tBHQ in contrast to NB4 variants may be explained by the conditions of the Ca2+ pools, as suggested above, and/or by the different mechanisms of the resistance. Indeed, whereas in these NB4-derived cells ATRA resistance may be due to enhanced proteolytic degradation of PML-RARα,44 ATRA resistance of HL-60RES cells is due to an invalidating mutation in the RARα gene.34
Effect of tBHQ on ATRA-induced differentiation and calcium homeostasis in ATRA-sensitive and -resistant NB4 variants.
(A) NB4-007/6 and NB4-306 cells were treated for 3 days with vehicle, 50 nM ATRA, 1 μM ATRA, 8 μM tBHQ, and 50 nM ATRA in combination with 8 μM tBHQ (as indicated in the figure); and NBT-reducing activity was examined. Mean ± SEM (n = 3). (B) Ast4 and HL-60RES cells were treated for 3 days with vehicle (■) or with 3 μM tBHQ plus 50 nM ATRA in combination (▪), and cell differentiation was detected by NBT reduction. Mean ± SEM (n = 3). (C) NB4, NB4-007/6, and NB4-306 cells were treated for 3 days with vehicle, 50 nM ATRA, 1 μM ATRA, 8 μM tBHQ, and 50 nM ATRA in combination with 8 μM tBHQ (as indicated in the figure); and, after addition of 0.5 mM EGTA, the increase of cytosolic Ca2+concentration was recorded immediately after addition of 20 μM tBHQ, as described in “Materials and methods.” The increased intensities of peaks were measured for each treatment, and the results are expressed as percentage of cytosolic Ca2+ concentration increase compared with the basal cytosolic Ca2+concentration before addition of tBHQ. Mean ± SEM (n = 3). **P ≤ .01 and *P ≤ .05 according to the Duncan test for multiple comparisons.
Effect of tBHQ on ATRA-induced differentiation and calcium homeostasis in ATRA-sensitive and -resistant NB4 variants.
(A) NB4-007/6 and NB4-306 cells were treated for 3 days with vehicle, 50 nM ATRA, 1 μM ATRA, 8 μM tBHQ, and 50 nM ATRA in combination with 8 μM tBHQ (as indicated in the figure); and NBT-reducing activity was examined. Mean ± SEM (n = 3). (B) Ast4 and HL-60RES cells were treated for 3 days with vehicle (■) or with 3 μM tBHQ plus 50 nM ATRA in combination (▪), and cell differentiation was detected by NBT reduction. Mean ± SEM (n = 3). (C) NB4, NB4-007/6, and NB4-306 cells were treated for 3 days with vehicle, 50 nM ATRA, 1 μM ATRA, 8 μM tBHQ, and 50 nM ATRA in combination with 8 μM tBHQ (as indicated in the figure); and, after addition of 0.5 mM EGTA, the increase of cytosolic Ca2+concentration was recorded immediately after addition of 20 μM tBHQ, as described in “Materials and methods.” The increased intensities of peaks were measured for each treatment, and the results are expressed as percentage of cytosolic Ca2+ concentration increase compared with the basal cytosolic Ca2+concentration before addition of tBHQ. Mean ± SEM (n = 3). **P ≤ .01 and *P ≤ .05 according to the Duncan test for multiple comparisons.
To gain insight into the functional consequences of SERCA inhibition on calcium homeostasis upon ATRA with or without tBHQ treatment in ATRA-resistant NB4 cell sublines, we examined the increase of Ca2+ concentration in the cytosol [Ca2+]i acutely induced by 20 μM tBHQ in NB4, NB4-007/6, and NB4-306 cell lines in the absence and in the presence of a 3-day- long pretreatment by ATRA with or without 8 μM tBHQ (Figure 5C). [Ca2+]i increase induced by 20 μM tBHQ, corresponding to Ca2+ mobilization from SERCA-dependent Ca2+ pools, was lower (212% increase compared with the basal Ca2+ concentration) in NB4 cells treated for 3 days with tBHQ compared with untreated (620% increase) or ATRA-treated NB4 cells (500%), confirming that Ca2+pools were not filled completely as a partial quantity of SERCA was inhibited for 3 days. When NB4 cells were treated for 3 days by ATRA in combination with tBHQ, [Ca2+]i increase induced by 20 μM tBHQ was severely reduced (9%). These results suggest that a cross-talk between Ca2+ homeostasis and ATRA signaling may control the availability of Ca2+ from the SERCA-dependent Ca2+ pools.
In untreated NB4-007/6 and NB4-306 cell lines, the SERCA-dependent [Ca2+]i increase by an acute treatment with 20 μM tBHQ was much lower (200% and 248%, respectively) than in untreated wild-type NB4 cells (620%), suggesting that Ca2+accumulation by SERCA is less effective in ATRA-resistant cells (Figure5C). In contrast with NB4 cells treated with 8 μM tBHQ, [Ca2+]i increase after 20 μM tBHQ was not significantly modified in ATRA-resistant NB4 sublines treated with 8 μM tBHQ, suggesting again a difference in the filling of SERCA-dependent Ca2+ pools between the wild-type and the ATRA-resistant NB4 cells. However, a little, but significant modification of [Ca2+]i increase could be observed in NB4-007/6 (102%) and in NB4-306 (118%) cell lines following cotreatment by ATRA plus tBHQ, confirming that the partial enhancement of the differentiation observed on NBT reduction (Figure5A) paralleled the modifications of Ca2+ homeostasis. These results also indicate that the resistance of ATRA-resistant NB4 cell sublines may be partially reverted by SERCA inhibition.
Enhancement of differentiation induction by tBHQ is ATRA-specific
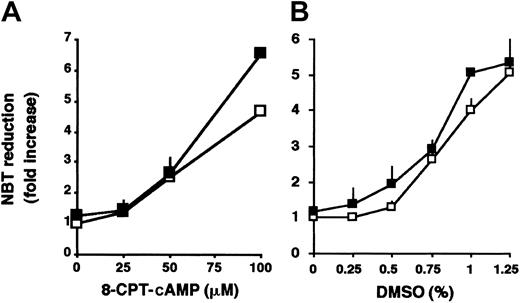
In addition to ATRA, the granulocytic differentiation of HL-60 cells can also be induced by other agents such as cAMP analogs5 or by high concentrations of DMSO.4To determine whether the enhancement by SERCA inhibitors is specific to ATRA-induced differentiation, HL-60 cells were treated with various concentrations of 8-CPT-cAMP (Figure 6A) or DMSO (Figure 6B) in the absence or presence of tBHQ. No significant enhancement of differentiation of HL-60 cells was observed when the differentiation of the cells was induced by 8-CPT-cAMP or DMSO in the presence of tBHQ. These results suggest that the potentializing effect of SERCA inhibition is specific to ATRA-induced differentiation.
Effect of tBHQ in differentiation induction by nonretinoid agents.
HL-60 cells were treated for 3 days with various concentrations of (A) 8-CPT-cAMP or (B) DMSO, in the absence (■) or presence (▪) of 3 μM tBHQ, and cellular NADPH oxidase activity measured by NBT reduction was determined. Mean ± SEM (n = 5).
Effect of tBHQ in differentiation induction by nonretinoid agents.
HL-60 cells were treated for 3 days with various concentrations of (A) 8-CPT-cAMP or (B) DMSO, in the absence (■) or presence (▪) of 3 μM tBHQ, and cellular NADPH oxidase activity measured by NBT reduction was determined. Mean ± SEM (n = 5).
Protection of RARα and of PML-RARα from proteolytic degradation by SERCA inhibition
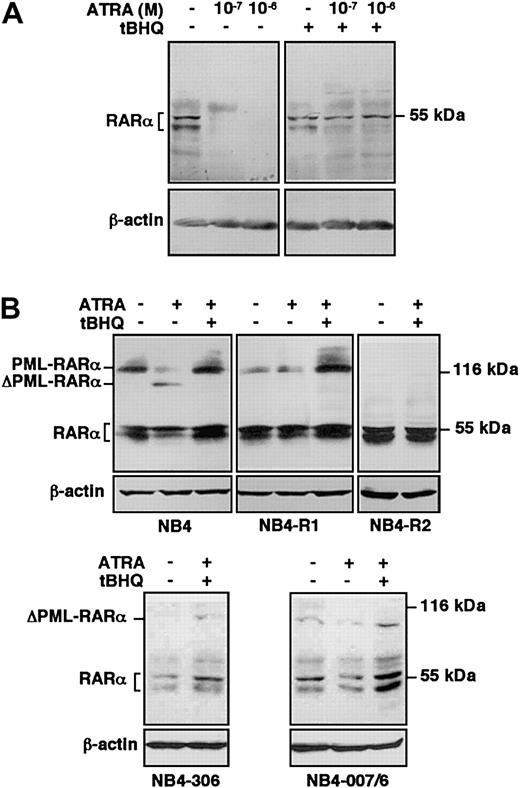
To better characterize the influence of SERCA inhibition on ATRA-dependent signaling pathways, we examined the effect of ATRA treatment in combination with tBHQ on protein levels of RARα in HL-60 cells and on RARα and PML-RARα in ATRA-sensitive and ATRA-resistant NB4 variants. HL-60 cells were treated with ATRA in the presence or absence of tBHQ, and RARα protein was detected by immunoblotting. In accordance with previous data,45 RARα has been detected as a doublet corresponding to phosphorylated and nonphosphorylated receptor proteins. Although ATRA alone induced an almost complete degradation of RARα at day one (Figure 7A, left panel), in accordance with previous reports on ligand-activated degradation of receptors by the ubiquitin-proteasome system,10,11 when HL-60 cells were treated with ATRA in the presence of tBHQ, RARα persisted at a level comparable with that observed in untreated cells, whereas tBHQ alone had no significant effect (Figure 7A, right panel). Protection from ATRA-induced degradation of RARα by tBHQ was also observed in NB4 cells. ATRA-sensitive wild-type NB4 cells were treated for one day with ATRA in the presence or absence of tBHQ, and RARα as well as PML-RARα protein were detected by immunoblotting (Figure 7B, top left panel).10 When NB4 cells were treated with ATRA alone, PML-RARα as well as RARα protein levels were decreased.10 11 Treatment with tBHQ alone had no significant effect on the stability of either RARα or PML-RARα (not shown). However, when NB4 cells were treated with ATRA in the presence of tBHQ, the PML-RARα protein, as well as RARα, persisted in the cells. These results show that treatment by SERCA inhibitors in combination with ATRA leads to the protection of RARα and PML-RARα proteins from degradation in ATRA-sensitive cells, suggesting that SERCA inhibition increases the stability of RARα and PML-RARα proteins in the presence of ATRA, leading to enhanced differentiation.
Protection of RARα and PML-RARα from ATRA-induced degradation by SERCA inhibition in ATRA-sensitive and ATRA-resistant cells.
(A) ATRA-sensitive wild-type HL-60 cells were treated for one day with vehicle, ATRA alone at concentrations indicated on the top of the figure, or with 3 μM tBHQ alone or in combination with ATRA; and RARα protein was detected by immunoblotting using the C-20 antibody. Immunoblotting of the same samples for β-actin was used as an internal control. (B) ATRA-sensitive wild-type NB4, as well as ATRA-resistant primed NB4-R1, NB4-R2, NB4-306, and NB4-007/6 cells were treated for one day with vehicle, 10−7 M ATRA (not shown for NB4-R2 and NB4-306 cells), or with a combination of 8 μM tBHQ and 10−7 M ATRA; and RARα, as well as PML-RARα protein was detected by immunoblotting using the RPαF′ antibody. Immunoblotting of the same samples for β-actin was used as an internal control. ΔPML-RARα corresponds to the degradation products of PML-RARα. Numbers on the right side correspond to the apparent molecular weight of the proteins in kDa. Western blots were performed 3 times.
Protection of RARα and PML-RARα from ATRA-induced degradation by SERCA inhibition in ATRA-sensitive and ATRA-resistant cells.
(A) ATRA-sensitive wild-type HL-60 cells were treated for one day with vehicle, ATRA alone at concentrations indicated on the top of the figure, or with 3 μM tBHQ alone or in combination with ATRA; and RARα protein was detected by immunoblotting using the C-20 antibody. Immunoblotting of the same samples for β-actin was used as an internal control. (B) ATRA-sensitive wild-type NB4, as well as ATRA-resistant primed NB4-R1, NB4-R2, NB4-306, and NB4-007/6 cells were treated for one day with vehicle, 10−7 M ATRA (not shown for NB4-R2 and NB4-306 cells), or with a combination of 8 μM tBHQ and 10−7 M ATRA; and RARα, as well as PML-RARα protein was detected by immunoblotting using the RPαF′ antibody. Immunoblotting of the same samples for β-actin was used as an internal control. ΔPML-RARα corresponds to the degradation products of PML-RARα. Numbers on the right side correspond to the apparent molecular weight of the proteins in kDa. Western blots were performed 3 times.
To further investigate the effect of SERCA inhibitors on the protection of PML-RARα and RARα proteins from proteolytic degradation and ensuing differentiation, we treated NB4-306 and NB4-007/6 cells and 2 other ATRA-resistant NB4-derived cell lines, NB4-R1 and NB4-R2, with ATRA in combination with tBHQ and examined the stability of PML-RARα and RARα proteins by immunoblotting. NB4-R1 cells, which do not display any known molecular defect(s),46 express a lower level of PML-RARα protein than wild-type NB4 (Figure 7B, top left and middle panels), probably due to instability of the protein in these cells.36 SERCA inhibition combined with ATRA treatment protected PML-RARα protein from degradation (Figure 7B, top middle panel) and partially increased the NBT-reducing activity in NB4-R1 cells (2.29- ± 0.08-fold increase; not shown), while no significant increase of RARα protein expression was observed. Taken together, these results suggest that the rescue of a response to ATRA may be essentially linked to the stabilization of PML-RARα protein as suggested previously in these cells.44 The NB4-R2 cell line carries a mutation at Gln to Stop at codon 411 of RARα, and at codon 913 in PML-RARα.46 Thus, in these cells PML-RARα is truncated and constitutively degraded by proteolysis, whereas RARα is truncated of 52 amino acids and inactive, although it remains detectable by immunoblotting. The combination of the 2 drugs was not effective in stabilizing PML-RARα protein in this cell line (Figure7B, top right panel), did not change the expression level of RARα protein, and did not induce any NBT-reducing activity (1.04 ± 0.14 fold-increase; not shown). Similar to the HL-60RES cell line, which carries the same mutation in RARα as NB4-R2,46 the combination of tBHQ and ATRA did not rescue the differentiation in NB4-R2 cells, probably because both PML-RARα and RARα are inactive and degraded in this cell line. NB4-306 and NB4-007/6 cells both express a normal RARα but no detectable PML-RARα protein due to constitutive proteolytic degradation, whereas corresponding mRNA levels are normal.37,38 44 The combination of the 2 drugs did not stabilize PML-RARα protein in either cell line (Figure 7B, bottom panels). However, like in wild-type HL-60 cells, an increase of the expression level of the RARα protein could be observed in the presence of tBHQ plus ATRA. These results may explain the partial responsiveness of both NB4-306 and NB4-007/6 cell lines in NBT-reducing activity induced by ATRA combined with SERCA inhibitors.
As summarized in Table 1, the partial differentiation by SERCA inhibition combined with ATRA treatment observed in NB4-306 and NB4-007/6 cells may be due to the stabilization mainly of RARα, whereas in NB4-R1 cells differentiation-induction may be due to the stabilization mainly of the PML-RARα protein. NB4-R2 and HL-60RES cells are refractory to the effect of double treatments because they carry an inactivating mutation in their RARα sequences leading to no functional RARα and/or PML-RARα protein. These data taken together suggest that in wild-type NB4 cells both RARα and PML-RARα proteins may contribute to full differentiation induction.
Involvement of RARα and of PML-RARα in the differentiation response of ATRA-resistant cells upon treatment by SERCA inhibitors in combination with ATRA
| . | NB4-306 . | NB4-007/6 . | NB4-R1 . | NB4-R2 . |
|---|---|---|---|---|
| Description | ||||
| PML-RARα | mRNA, no protein | mRNA, no protein | Less protein | Inactivating mutation |
| RARα | Normal | Normal | Normal | Inactivating mutation |
| Differentiation (NBT) | Partially rescued | Partially rescued | Partially rescued | No rescue |
| Stabilization | ||||
| PML-RARα | = | = | + | = |
| RARα | + | + | = | = |
| . | NB4-306 . | NB4-007/6 . | NB4-R1 . | NB4-R2 . |
|---|---|---|---|---|
| Description | ||||
| PML-RARα | mRNA, no protein | mRNA, no protein | Less protein | Inactivating mutation |
| RARα | Normal | Normal | Normal | Inactivating mutation |
| Differentiation (NBT) | Partially rescued | Partially rescued | Partially rescued | No rescue |
| Stabilization | ||||
| PML-RARα | = | = | + | = |
| RARα | + | + | = | = |
Description of defects in PML-RARα and RARα in ATRA-resistant NB4-derived cell lines and results of treatments by ATRA in combination with tBHQ on (1) levels of differentiation determined by NBT-reducing activity and (2) protection of PML-RARα and RARα proteins from proteolytic degradation as examined by immunoblotting. = indicates not modified; and +, protected.
Discussion
Treatment of the HL-60 and NB4 leukemic cell lines and of fresh APL cells with ATRA results in a 4-fold overexpression of the SERCA3 gene products, indicating that SERCA enzymes are involved in the acquisition of the mature granulocytic phenotype.32 On the other hand, the direct and specific inhibition of SERCA enzymes has been shown to lead to differentiation in other experimental systems.24 25 The data presented in this report show that a cross-talk may operate between cellular calcium homeostasis modulated by pharmacologic SERCA inhibitors and the ATRA-dependent signaling pathway during ATRA-induced differentiation, and that Ca2+homeostasis may be involved in the regulation of the ATRA-induced degradation of the RARα and PML-RARα proteins. In addition, in ATRA-resistant NB4-derived cell lines, which possess either a functional RARα or a functional PML-RARα protein, SERCA inhibition in the presence of ATRA can partially overcome resistance.
SERCA enzymes are directly involved in the control of cytosolic and intra–endoplasmic reticulum calcium levels and can modulate transplasma membrane calcium influx indirectly, because of capacitative calcium influx, a mechanism controlled by the filling state of the ER. Capacitative calcium influx can also be activated by the depletion of ER calcium content by SERCA inhibitors.23 Since an increase of the cytosolic calcium concentration and calcium influx47,48 can enhance myeloid differentiation as suggested in earlier works using calcium ionophores,49 50it was of interest to determine whether calcium pumping into the ER exerts a functional effect on the differentiation process. Our results indicate that a partial inhibition of calcium pumping into the ER significantly enhances ATRA-induced differentiation of cells that are sensitive to the effect of ATRA, suggesting that SERCA activity may be able to fine-tune the sensitivity of the cell to the differentiation-inducing activity of ATRA. An approximately 60% Ca2+ release of the total SERCA-dependent calcium pools, which leads to an approximately 70% capacitative calcium influx, was required for the potentialization of the differentiation-inducing effect of ATRA. Although potentialization of differentiation could be obtained in a relatively narrow SERCA inhibitor concentration range, cell type–dependent differences of inhibitor concentration were observed (3 and 8 μM tBHQ required to enhance differentiation of HL-60 and NB4, respectively). This is in agreement with the observation that NB4 cells are larger than HL-60 and express approximately 2 times more SERCA proteins (not shown).
As shown previously,32 ATRA-induced granulocytic differentiation is accompanied by the induction of SERCA3 expression, whereas, as shown in the present study, inhibition of SERCA activity enhances differentiation. This paradox can be reconciled by hypothesizing that SERCA activity exerts a negative feedback control on the differentiation process, presumably by decreasing the stability of retinoic acid receptors, as shown in the present work. However, the calcium affinity of SERCA3 is weaker than that of SERCA2b,51 which is also expressed in myeloid cells. Therefore, induction of SERCA3 expression, and a parallel decrease of SERCA2b levels, reported previously during granulocytic differentiation32 may lead to facilitated second messenger–induced calcium release, which, in turn would increase retinoic acid receptor half-lives in intact cells, similar to that seen upon SERCA inhibition presented in the present paper. In addition, data in the literature show that the size of the intracellular calcium pool that can be mobilized by second messengers such as inositol-1,4,5-trisphosphate (IP3), as well as the magnitude of calcium transients that can be elicited in the cells by extracellular stimuli, increase during myeloid differentiation.47,52,53 Moreover, SERCA3 is specifically associated with the IP3-sensitive intracellular calcium pool.54 Induction of SERCA3 expression during granulocytic differentiation may therefore reflect the formation of an excitable intracellular calcium compartment in the cell, involved in the control of the differentiation process, as well as in the responsiveness of the mature cell to extracellular stimuli.
In this work, we found that SERCA inhibition enhanced ATRA differentiation specifically through the ATRA-dependent signaling system, since the effect of other differentiation inducers such as cAMP analog or DMSO was not potentialized by calcium pump inhibition. The lack of potentialization of the differentiation-inducing effect of DMSO by SERCA inhibitors is compatible with the notion that they may share the same target (ie, calcium homeostasis). Indeed, previous observations show that DMSO induces a transient increase of the cytosolic calcium concentration of HL-60 cells55 and that DMSO enhances ATRA-dependent differentiation.3 56 These data suggest that calcium mobilization by DMSO may be involved in the differentiation-inducing activity of the molecule.
In acute promyelocytic leukemia, the t(15;17) translocation fuses the RARα gene to the gene of the PML nuclear matrix protein, blocking neutrophil differentiation and leading to leukemia.57 ATRA promotes the in vivo differentiation of primary APL cells as well as of the APL-derived NB4 cell line in vitro through PML-RARα, as well as RARα by activating ATRA-inducible genes. In HL-60, which is devoid of PML-RARα, differentiation is induced through RARα. The induction by ATRA of the degradation of PML-RARα or RARα proteins by the ubiquitin-proteasome pathway has also been reported.10 These findings provide a direct link between a positive effect of ATRA-dependent transcriptional activation leading to differentiation and a negative feedback control by RARα catabolism. We show in this report that specific SERCA inhibition protects PML-RARα and RARα from ATRA-induced degradation in ATRA-sensitive cells and suggests that calcium homeostasis may be involved in the modulation of the degradation of these receptors. It has been shown that calcium fluxes might activate the proteasome, a concept that is based on evidence that calcium induces assembly of the proteasome58 and regulates its activity.59Here, we demonstrate an unknown role for calcium fluxes that could protect RARα and PML-RARα from proteasome-mediated degradation. However, the mechanism how calcium can protect the degradation of RARα and PML-RARα is currently unknown. It has been reported that an increased activity of protein kinase C alpha (PKCα), which can be modulated by phosphatidylserine in a Ca2+-dependent manner,60 can also influence ATRA signaling by altering the stability of RARα protein.61 Taken together, these findings suggest that the stability of RARα and presumably of PML-RARα induced by the cotreatment ATRA plus tBHQ might involve specific modifications of the receptor (for example phosphorylation) mediated by Ca2+-sensitive effectors like PKC. Such a mechanism has recently been demonstrated for RARγ2.62One of our aims will be to clarify which signaling pathway is involved in this synergistic effect.
In ATRA-sensitive wild-type NB4 cells, granulocytic differentiation induced by ATRA in combination with the tyrosine kinase inhibitor ST1571 results in a decrease in ATRA-induced degradation of RARα and of the PML-RARα fusion protein.63 With the same treatment, a significant induction of NBT-reducing activity can be obtained in the NB4-derived, NB4-007/6, NB4-306, and NB4-R1 cell lines, showing that the resistant character of these cells can be partially rescued. Here, we show that treatment by ATRA in combination with SERCA inhibitors also decreases ATRA-induced degradation of RARα and of the PML-RARα fusion protein in ATRA-sensitive wild-type NB4 cells, and can partially relieve resistance in several NB4 derivatives, as shown by an increase of NBT-reducing activity, and this is associated with the protection of either RARα or PML-RARα proteins. Thus, the mechanism by which calcium can protect RARα and PML-RARα from degradation may be active also in ATRA-resistant NB4-derived cells, since the stabilization of RARα and of PML-RARα has been shown in NB4-007/6 and NB4-306 sublines and in NB4-R1 cells, respectively. However, the constitutive degradation of PML-RARα, which has been demonstrated to be important for the resistant phenotype,44 is not rescued by calcium in NB4-007/6 and NB4-306 sublines, nor RARα in NB4-R1 cells. The protection of some, but not all, proteins involved in ATRA-induced differentiation may account for the reduced differentiation response of these cells, as the complete differentiation response that occurs in NB4 cells is probably due to the protection of both RARα and PML-RARα proteins. Although further experiments are required to shed light on the biochemical events that determine which RARα or PML-RARα variants can be protected from degradation in a calcium-dependent manner, this work shows that calcium homeostasis can modulate the response of the cells to differentiation-inducing stimuli. Moreover, data presented in this work indicating that the ER calcium pool that can be mobilized by SERCA inhibition is smaller in the resistant NB4 variants (NB4-007/6 and NB4-306) than in wild-type NB4 cells, suggest that calcium homeostasis may be involved in the establishment and/or maintenance of a differentiation-resistant phenotype.
In conclusion, although the precise mechanisms involved in the enhancement of leukemia cell differentiation by SERCA inhibitors remain to be determined, because the concentration of ATRA required for differentiation induction could be reduced in HL-60 cells and APL-derived NB4 cells and since ATRA resistance could be partially relieved in NB4 variants by SERCA inhibitors, the modulation of calcium homeostasis of the malignant cell, as shown in this work, represents an interesting new approach in the search for new therapeutic modalities for leukemia based on the induction of cell differentiation.
We are indebted to Dr Robert Gallagher and Dr Michel Lanotte for the ATRA-resistant HL-60RES and the NB4, NB4-R1, and NB4-R2 cell lines; and to Dr Carlo Passerini-Gambacorti for the NB4-007/6 and NB4-306 cell lines. We would like to thank Dr Pierre Chambon for the gift of the RPαF′ anti-RARα antibody. The support of Dr Sylviane Lévy-Tolédano (Hôpital Lariboisière, Paris, France) and Prof Christine Chomienne (Hôpital Saint Louis, Paris, France) is gratefully acknowledged.
Prepublished online as Blood First Edition Paper, December 19, 2002; DOI 10.1182/blood-2002-09-2730.
Supported by the Association pour la Recherche sur le Cancer, the Ministère de l'Enseignement Supérieur et de la Recherche et de la Technologie, and the Cancer Research United Kingdom.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Sophie Launay, INSERM 517, IFR 100, Faculté de Médecine, 7 Boulevard Jeanne d'Arc, 21000 Dijon, France; e-mail:sophie.launay@u-bourgogne.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal