Abstract
Costimulatory blockade using cytotoxic T lymphocyte–associated antigen 4 immunoglobulin (CTLA4Ig) efficiently down-regulates immune responses in animal models and is currently used in autoimmune and transplantation clinical trials, but the precise cellular and molecular mechanisms involved remain unclear. Rats that received allogeneic heart transplants and were treated with adenoviruses coding for CTLA4Ig show long-term allograft survival. The immune mechanisms regulating induction of long-term allograft acceptance were analyzed in splenocytes using mixed leukocyte reactions (MLRs). MLRs of splenocytes but not purified T cells from CTLA4Ig-treated rats showed higher than 75% inhibition compared with controls. Splenocytes from CTLA4Ig-treated rats inhibited proliferation of naive and allogeneically primed splenocytes or T cells. MLR suppression was dependent on soluble secreted product(s). Production of soluble inhibitory product(s) was triggered by a donor antigen-specific stimulation and inhibited proliferation in an antigen-nonspecific manner. CTLA4Ig levels in the culture supernatant were undetectable and neither interleukin-10 (IL-10), transforming growth factor β1 (TGFβ1), IL-4, nor IL-13 were responsible for suppression of MLRs. Inhibition of nitrous oxide (NO) production or addition of IL-2 could not restore proliferation independently, but the combined treatment synergistically induced proliferation comparable with controls. Stimulation of APCs using tumor necrosis factor (TNF)–related activation-induced cytokine (TRANCE) or CD40L and addition of IL-2 normalized MLRs of CTLA4Ig-treated splenocytes. Finally, dendritic cells (DCs), but not T cells, from CTLA4Ig-treated rats inhibited naive MLRs. Altogether, these results provide evidence that after in vivo CTLA4Ig treatment, splenocytes, and in particular DCs, can inhibit alloantigen-induced proliferative responses through secretion of inhibitory products, thus promoting alloantigen-specific tolerance in vivo.
Introduction
Therapies targeted to inhibit activation of recipient T cells can induce specific tolerance to donor allografts in rodent models.1 Depending on the model used, different mechanisms such as deletion, anergy, and suppression have been proposed to account for the induced tolerance,1 but the precise cellular and molecular pathways involved are not completely understood.
Activation of T cells specific for donor alloantigens is central to the development of allograft rejection. T cells become activated by a first set of T-cell receptor (TCR)–mediated stimulatory signals following recognition of peptides derived from donor alloantigens and by a second set of costimulatory signals.1 Both types of signals are originated from interactions of T cells with antigen-presenting cells (APCs). In transplantation, donor antigens are presented both by APCs from donor and recipient origin (by direct and indirect antigen presentation, respectively).2
Immature or mature dendritic cells (DCs) can act as initiators both of immune responses to foreign antigens and of tolerance to self-antigens.3 In allotransplantation, administration of immature DCs or CD8α+ mature DCs prolongs graft survival.4,5 Immature DCs can induce regulatory T cells in vitro6 and rapidly (< 7 days) induce tolerance.7,8 Recent studies suggest that T-cell differentiation into effector or regulatory T cells depends on the context (eg, type and level of costimulatory signals, cytokine milieu) in which T cells recognize antigens presented by DCs or other APCs.3,6,9-12 Recently, mature DCs5,13,14 or macrophages with suppressor activity after CD80/CD86-CD28 blockade15 have been shown to induce allogeneic T-cell tolerance. There is now clear evidence for the existence of regulatory T-APC interactions that can suppress transplant rejection by promoting generation of regulatory T cells.
Regulatory CD4+ T cells with immunosuppressive function constitute a heterogeneous group of cells for which the relationship to each other and their underlying molecular mechanisms of action still need to be clarified.16-24 Regulatory/suppressor CD8+ T cells have also been described.12 25
Suppressor CD8+ T cells12 or anergic CD4+ T cells19,26 can inhibit allogeneic immune responses through modulation of DC function indicating a mutual regulation between regulatory T cells and DCs.3,16,27 28
CD80/CD86 interaction with CD28 plays a central role in T-cell costimulation and development of immune responses.29Blocking this pathway has allowed dramatic improvement in transplantation and autoimmune experimental models and in clinical studies.29,30 The use of cytotoxic T lymphocyte–associated antigen 4 immunoglobulin (CTLA4Ig) or anti-CD80 or CD86 monoclonal antibody (MAb) as sole treatment has resulted in long-term allograft survival in many animal models, but evidence of immune tolerance has been described in only some of these models.31,32 Little is known about the mechanisms underlying immune tolerance induced by CTLA4Ig blockade of CD80/CD86-CD28 costimulation in vivo. Blockade of CD80/CD86-CD28 or CD40-CD40L interactions in vitro induces regulatory T cells15,21,22 and suppressive macrophages20against alloantigens.
We have previously reported that prolonged exposure to CTLA4Ig via gene transfer allowed long-term cardiac allograft survival in rats32 and that this survival was associated with acceptance of second cardiac grafts from first- but not third-party donors. The present study aimed to analyze in vitro the cellular and molecular mechanisms underlying the inductive phase of this in vivo tolerance.
Materials and methods
Animals, heart transplantation, and gene transfer with an adenovirus encoding the extracellular domain of mouse CTLA4 fused to the Fc portion of human IgG1 (AdCTLA4Ig)
Inbred male Lewis 1W (LEW.1W, haplotype RT1u) rats were used as donors, and inbred Lewis 1A (LEW.1A, haplotype RT1a) rats, as recipients (Centre d'elevage Réne Janvier, Le Genest Saint Isle, France).32Heterotopic cardiac allografts were placed into the abdomen. Graft survival was monitored daily by palpation; rejection (mean survival time [MST]) was defined as total cessation of cardiac beating.
Cell purification
Single-cell splenocyte suspensions were obtained and erythrocytes removed by hypotonic lysis. T cells were purified from total splenocytes after nylon wool adherence or depletion of 3.2.3 (CD161), OX42 (CD11b/c), and OX12 (Ig κ chain) MAb reactive cells using magnetic beads (Dynal, Compiègne, France). Culture medium was RPMI 1640 supplemented with 10% heat-inactivated fetal calf serum, 2 mM l-glutamine, 100 U/mL penicillin, 0.1 mg/mL streptomycin, 1 mM sodium pyruvate, 1% nonessential amino acids, 1% HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), and 5 × 10−5 M 2-βmercaptoethanol (all from Sigma, St Louis, MO).
APCs were enriched from spleen fragments digested with collagenase D as described.32,34 DCs were further purified (> 90% as assessed using MAbs specific for major histocompatibility complex [MHC] class II antigens, T cells, B cells, and macrophages) by positive selection using the OX62 MAb (αε-integrin expressed by 80% of splenic DCs)35 and magnetic bead separation (Miltenyi, Auburn, CA), as previously described.36 37 DCs were immediately used in mixed leukocyte reactions (MLRs) without further culture.
MLR cultures
LEW.1A responder cells from naive (did not receive transplant), CTLA4Ig-treated, or Addl324-treated (control) rats that underwent transplantation were seeded (105 cells/well) onto round-bottomed 96-well plates (Nunc, Roskilde, Denmark) in triplicate and evaluated for their proliferative response against γ-irradiated (35 Gy) APCs (5 × 104 cells/well) from LEW.1W, Brown Norway (BN, haplotype RT1n), or F1 hybrids (LEW.1W × LEW.1A).
For coculture MLRs, splenocytes, T cells, or DCs from CTLA4Ig-treated or control rats (5 × 104 cells) were added to splenocytes or T cells from naive LEW.1A rats (5 × 104cells) and irradiated LEW.1W or BN APCs (5 × 104 cells) in round-bottomed 96-well plates.
For transwell MLRs, the lower and upper compartments of 24-well plates were separated by a 0.45-μm pore size membrane (Becton Dickinson, Le Pont de Claix, France). The lower compartment contained splenocytes from CTLA4Ig-treated or control rats (6 × 105 cells) and irradiated APCs (3 × 105 cells). The upper compartment contained naive or primed splenocytes from untreated LEW.1A rats (6 × 105 cells) and irradiated APCs (3 × 105 cells).
Cells were cultured at 37°C in humidified air containing 5% CO2 for 3, 4, and 5 days, and, for the final 8 hours of culture, 1 μCi (0.037 MBq) and 5 μCi (0.185 MBq) [3H] thymidine deoxyribose ([3H]Tdr) were added to each 96- and 24-well plate, respectively. For experiments performed in 96-well plates, [3H]Tdr was directly assessed in the 96-well plates and results were expressed as the delta mean cpm ± SD after subtraction of proliferation in the presence of culture medium. For transwell cultures, [3H]Tdr was assessed after transferring cells into 96-well plates and results were expressed as counts per minute.
Modulation of MLRs by bioreagents
MLRs were cultured in the presence of the following mouse MAbs at 25 μg/mL: anti-rat interleukin (IL)–4 (OX81; ECACC, Wiltshire, United Kingdom), anti–transforming growth factor β1 (TGFβ1; clone 2G7),38 agonistic anti-CD28 (JJ31939; T. Hüning, University of Würzburg, Germany), anti-human IL-13 (Diaclone, Besançon, France), and an irrelevant control (3G8, anti-human CD16; American Type Culture Collection, Bethesda, MD). Rabbit neutralizing anti-rat IL-10 and rabbit IgG (both kindly provided by J. Khalife, Institut Pasteur, Lille, France) were used at 10 μg/mL. The extracellular domain of tumor necrosis factor (TNF)–related activation-induced cytokine (TRANCE) fused to the extracellular domain of human CD8α (TRANCE-CD8; kindly provided by Y. Choi, University of Pennsylvania, Philadelphia, PA) was used at 10 μg/mL. The supernatant of COS cells transfected with a coding plasmid (provided by Y. Choi)40was used as a source of the fusion molecule CD40L-CD8.NG-monomethyl-L-arginine (L-NMMA) and its inactive analog, NG-monomethyl-D-arginine (D-NMMA; Sigma) were diluted (5 mM) in culture medium. Human recombinant IL-2 was used as indicated in most experiments at 100 U/mL, or 500 U/mL in others.
Enzyme-linked immunosorbent assays (ELISAs)
CTLA4Ig was detected in culture supernatant using a sandwich ELISA (sensitivity, 1 ng/mL) as described.32 Cytokines were analyzed in duplicate serially diluted supernatants harvested on day 3 MLRs. IL-10 (PharMingen, Franklin Lakes, NJ), IL-13, IL-2, interferon γ (IFNγ) (Biosource, Camarillo, CA), and TGFβ1 (Promega, Madison, WI) were analyzed using ELISA kits according to manufacturers' instructions. The ELISA for IL-4 was performed using the OX81 and the biotinylated B11-3 (PharMingen) anti–IL-4 MAbs, and recombinant rat IL-4 (PharMingen) was used as a standard. The ELISA sensitivity was 0.015 ng/mL.
Flow cytometry
Cells were stained with the following biotin or fluorescein isothiocyanate (FITC)–conjugated MAbs: anti-CD3 (G4.18; PharMingen), anti-CD11b/c (OX42),41 anti-CD4 (W3/25), anti-CD8α chain (OX8), antimonomorphic class II MHC antigens (OX6), anti-CD25 (OX39), anti-CD8β chain (3.4.1), anti-CD161, and anti-CD161 (3.2.3) (ECACC); anti-CD4036 (PharMingen); a mixture of anti-CD80 (3H5) and anti-CD86 (24F) (PharMingen); anti-CD28 (JJ319); anti-CD40L (AH.F5; kindly provided by C. Benjamin, Biogen, Boston, MA); and an irrelevant mouse MAb (3G8, antihuman CD16). Binding of CTLA4Ig was revealed with a biotin-conjugated rat IgG-absorbed F(ab)′2 donkey anti–human IgG Fc antibody (Jackson Laboratories, West Grove, PA). Biotin or FITC-labeled mouse IgG1 was used as negative control (Immunotech, Marseille, France). Binding of biotin-labeled antibodies was detected by incubation with phycoerythrin (PE)–conjugated streptavidin (Immunotech). A FACScalibur cytofluorimeter was used and data were analyzed using CellQuest software (Becton Dickinson, Mountain View, CA).
Statistical analysis
Student t test was used for group comparisons;P values less than .05 were considered significant.
Results
Lack of allogeneic proliferation of splenocytes, but not T cells, from animals treated with AdCTLA4Ig
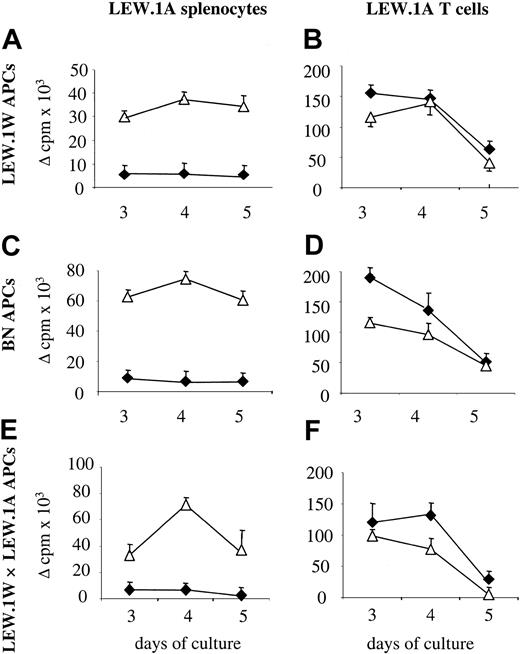
We have previously shown that treatment of LEW.1A rats with CTLA4Ig through gene transfer using recombinant adenoviruses results in long-term (> 150 days) survival of LEW.1W heart allografts, and that this was associated with a lack of MLR proliferation at day 3 of culture.32 The results in Figure1 confirm these previous results and show that MLRs using splenocytes of CTLA4Ig-treated rats were still profoundly decreased on days 4 and 5 of culture compared with that of splenocytes from control rats treated with a noncoding adenovirus. Lack of allogeneic proliferation was observed against first- and third-party alloantigens (Figure 1A,C).
Effect of in vivo CTLA4Ig treatment on MLRs of splenocytes and T cells.
Splenocytes were harvested on day 10 after transplantation from CTLA4Ig-treated (♦) or control (▵) LEW.1A rats that received LEW.1W heart transplants. Total splenocytes or T cells isolated from splenocytes of the same animal were assayed against donor (LEW.1W), third-party (BN), or F1 LEW.1W × LEW.1A APCs at the indicated time points. (A) Splenocyte proliferation against LEW.1W cells. (B) T-cell proliferation against BN cells. (C) Splenocyte proliferation against LEW.1W cells. (D) T-cell proliferation against BN cells. (E) Splenocyte proliferation against LEW.1W × LEW.1A cells. (F) T-cell proliferation against LEW.1W × LEW.1A cells. Results are expressed as delta (allogeneic − spontaneous) cpm ± SD. Results are representative of 3 experiments with identical results.
Effect of in vivo CTLA4Ig treatment on MLRs of splenocytes and T cells.
Splenocytes were harvested on day 10 after transplantation from CTLA4Ig-treated (♦) or control (▵) LEW.1A rats that received LEW.1W heart transplants. Total splenocytes or T cells isolated from splenocytes of the same animal were assayed against donor (LEW.1W), third-party (BN), or F1 LEW.1W × LEW.1A APCs at the indicated time points. (A) Splenocyte proliferation against LEW.1W cells. (B) T-cell proliferation against BN cells. (C) Splenocyte proliferation against LEW.1W cells. (D) T-cell proliferation against BN cells. (E) Splenocyte proliferation against LEW.1W × LEW.1A cells. (F) T-cell proliferation against LEW.1W × LEW.1A cells. Results are expressed as delta (allogeneic − spontaneous) cpm ± SD. Results are representative of 3 experiments with identical results.
Unlike total splenocytes, purified splenic T cells from the same CTLA4Ig-treated rats had similar proliferation against first- and third-party alloantigens compared with that of control animals (Figure 1B,D).
Allogeneic proliferative responses of splenocytes are triggered by alloantigens presented by both donor and recipient APCs (direct and indirect antigen presentation, respectively), whereas proliferation of purified splenic T cells is triggered by only donor APCs. Therefore, these results suggest that recipient APCs inhibited T-cell proliferation against alloantigens.
Lack of allogeneic proliferation of splenocytes from animals treated with AdCTLA4Ig is not due to indirect antigen presentation
The inhibition of MLRs using splenocytes from CTLA4Ig-treated rats may be due to either an inhibitory activity of recipient APCs or to the development of regulatory T cells by indirect alloantigen presentation by recipient APCs, as previously described.42
To evaluate this possibility, we performed MLRs using as stimulators APCs from F1 animals derived from crossing LEW.1W and LEW.1A rats, and T cells and splenocytes from LEW.1A CTLA4Ig-treated rats as responders. APCs from F1 rats express LEW.1W MHC molecules (direct alloantigen presentation) and display LEW.1W allopeptides in the context of LEW.1A MHC molecules (indirect alloantigen presentation). If indirect alloantigen presentation was responsible for activation of the recipient regulatory T cells, proliferation of T cells against F1 APCs should be inhibited. As expected, splenocytes from CTLA4Ig-treated rats did not proliferate in response to F1 APCs (Figure 1E). Importantly, T-cell proliferation against F1 APCs was not inhibited and was comparable with that of T cells from control rats (Figure 1F), thus ruling out the possibility that indirect alloantigen presentation was responsible for inhibition of proliferation.
We conclude that in vivo costimulatory blockade through treatment with CTLA4Ig resulted in a modification of APC–T-cell interactions and the suppression of T-cell proliferation by recipient APCs.
Inhibition of MLRs by soluble inhibitory products
Transwell culture experiments were performed to determine whether the lack of proliferation in response to alloantigens depends on production of soluble products or cell contact. Proliferation of naive MLR was assessed in the upper compartments of transwell plates in the presence, in the lower compartment, of splenocytes from control or CTLA4Ig-treated animals that underwent transplantation, cultured either with medium (nonstimulated) or irradiated donor or third-party APCs. These experiments showed that proliferation of naive MLRs was similar in the presence of unstimulated splenocytes from control or CTLA4Ig-treated animals (Figure 2A). In contrast, proliferation was inhibited in the presence of splenocytes from CTLA4Ig-treated animals stimulated with APCs from donor (Figure2B) but not from third-party origin (Figure 2C). The same transwell MLR experiments using naive purified T cells also showed decreased proliferation in the presence of MLRs from splenocytes of CTLA4Ig-treated rats (data not shown). Similarly, MLRs using naive splenocytes were inhibited by more than 90% upon addition of 50% cell-free culture supernatant of MLRs from CTLA4Ig-treated rats, in which CTLA4Ig was undetectable (data not shown). This suggests the presence of soluble inhibitory product(s) other than CTLA4Ig. Proliferation against third-party APCs in the upper compartment was inhibited in the presence of MLRs using splenocytes from CTLA4Ig-treated rats and donor APCs (Figure 2D).
Effect of transwell cultures on inhibition of MLRs.
Splenocytes from naive LEW.1A animals were cultured in the upper compartment of transwell plates with irradiated LEW.1W APCs, and proliferation was evaluated on days 3 and 5 of culture. The lower compartment contained splenocytes from 2 CTLA4Ig-treated or 1 control LEW.1A rat that received LEW.1W heart transplants harvested on day 10 after transplantation and cultured with (A) culture medium, (B) irradiated LEW.1W APCs, or (C) irradiated BN APCs. (D) Splenocytes from naive LEW.1A animals were cultured in the upper compartment of transwell plates with BN irradiated APCs in the presence, in the lower compartment, of splenocytes from CTLA4Ig-treated or control LEW.1A rats and irradiated LEW.1W APCs. [3H]Tdr incorporation was assessed in the upper compartment MLR on days 3 and 5 of culture. Results are expressed as delta (allogeneic − spontaneous) cpm ± SD and are representative of 2 identical experiments with a total of 4 animals in each group. Each curve represents the proliferation of cells from a single animal.
Effect of transwell cultures on inhibition of MLRs.
Splenocytes from naive LEW.1A animals were cultured in the upper compartment of transwell plates with irradiated LEW.1W APCs, and proliferation was evaluated on days 3 and 5 of culture. The lower compartment contained splenocytes from 2 CTLA4Ig-treated or 1 control LEW.1A rat that received LEW.1W heart transplants harvested on day 10 after transplantation and cultured with (A) culture medium, (B) irradiated LEW.1W APCs, or (C) irradiated BN APCs. (D) Splenocytes from naive LEW.1A animals were cultured in the upper compartment of transwell plates with BN irradiated APCs in the presence, in the lower compartment, of splenocytes from CTLA4Ig-treated or control LEW.1A rats and irradiated LEW.1W APCs. [3H]Tdr incorporation was assessed in the upper compartment MLR on days 3 and 5 of culture. Results are expressed as delta (allogeneic − spontaneous) cpm ± SD and are representative of 2 identical experiments with a total of 4 animals in each group. Each curve represents the proliferation of cells from a single animal.
Altogether these results show that stimulated splenocytes from CTLA4Ig-treated rats are able to inhibit the allogeneic proliferation of naive splenocytes and that this is due to the presence of inhibitory soluble products. The production of inhibitory products is induced by donor-specific alloantigens and has an antigen nonspecific bystander inhibitory activity.
Inhibition of naive MLRs is abrogated by splenocyte irradiation and is exerted on allogeneically primed splenocytes and T cells
To evaluate the effect of irradiation on the inhibitory function of splenocytes from CTLA4Ig-treated animals, MLRs using naive splenocytes were cocultured with irradiated (35 Gy) splenocytes from CTLA4Ig-treated or control rats. The inhibition of proliferation of naive MLRs by coculture with splenocytes from CTLA4Ig-treated animals (Figure 3A) was reverted by prior irradiation (Figure 3B).
Effect of irradiation on the inhibition of MLRs and of splenocytes from CTLA4Ig-treated rats on MLRs of allogeneically sensitized splenocytes and T cells.
Splenocytes were harvested on day 10 after transplantation from CTLA4Ig-treated (♦) or control (▵) LEW.1A rats that received LEW.1W heart transplants. These cells were either (A) untreated or (B) irradiated and cocultured (1:1 cell ratio) with naive LEW.1A splenocytes in the presence of irradiated LEW.1W APCs. Splenocytes from CTLA4Ig-treated (♦) or control (▵) rats were cocultured with irradiated LEW.1W APCs and either (C) allogenically primed LEW.1A splenocytes (1:1 cell ratio) or (D) allogenically primed LEW.1A T cells (1:1 cell ratio) harvested 10 days after heart transplantation. [3H]Tdr incorporation was assessed at the indicated time points. Results are expressed as delta (allogeneic − spontaneous) cpm ± SD and are representative of 3 identical experiments.
Effect of irradiation on the inhibition of MLRs and of splenocytes from CTLA4Ig-treated rats on MLRs of allogeneically sensitized splenocytes and T cells.
Splenocytes were harvested on day 10 after transplantation from CTLA4Ig-treated (♦) or control (▵) LEW.1A rats that received LEW.1W heart transplants. These cells were either (A) untreated or (B) irradiated and cocultured (1:1 cell ratio) with naive LEW.1A splenocytes in the presence of irradiated LEW.1W APCs. Splenocytes from CTLA4Ig-treated (♦) or control (▵) rats were cocultured with irradiated LEW.1W APCs and either (C) allogenically primed LEW.1A splenocytes (1:1 cell ratio) or (D) allogenically primed LEW.1A T cells (1:1 cell ratio) harvested 10 days after heart transplantation. [3H]Tdr incorporation was assessed at the indicated time points. Results are expressed as delta (allogeneic − spontaneous) cpm ± SD and are representative of 3 identical experiments.
We then asked whether inhibition of MLRs would also be exerted on in vivo allogeneically primed cells. Proliferation of splenocytes (Figure3C) and T cells (Figure 3D) against APCs from donor origin was inhibited when cocultured with splenocytes from CTLA4Ig-treated animals. Similarly, proliferation of allogeneically primed T cells against donor APCs was inhibited in transwell experiments (data not shown).
Thus, splenocytes from CTLA4Ig-treated animals are capable of inhibiting not only naive but also allogeneically primed T cells in coculture and transwell culture systems.
Inhibition of MLRs of splenocytes from CTLA4Ig-treated rats is dependent on NO production and reduced IL-2 synthesis
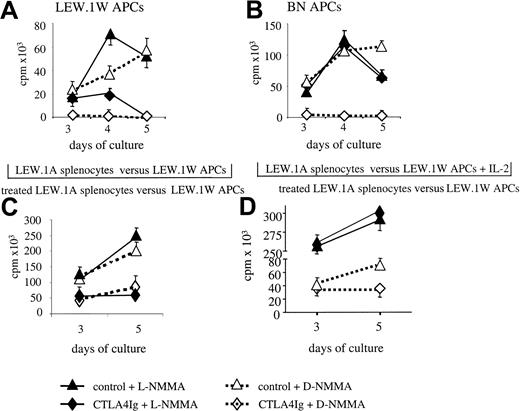
T-cell proliferative responses can be inhibited by NO.43 Addition of L-NMMA to MLRs against donor antigens performed in 96-well plates resulted in a small increase in proliferation of splenocytes from CTLA4Ig-treated rats on days 3 and 4 of culture. However, their responses were still lower than those of splenocytes from control rats treated with L-NMMA, by 75% and 95% on days 4 and 5 of culture, respectively (Figure4A).
Effect of NO blockade with or without added IL-2 on inhibited MLRs.
Splenocytes from CTLA4Ig-treated or control LEW.1A rats that received LEW.1W cardiac heart transplants were harvested on day 10 after transplantation and cultured in 96-well plates (A-B) or in the lower compartments of transwell plates (C-D) with irradiated APCs from donor origin. Cultures were performed with L-NMMA or D-NMMA (5 mM) in the absence (A-C) or presence (D) of IL-2 (100 U/mL). Results (D) for 96-well cultures are expressed as delta (allogeneic − spontaneous) cpm ± SD on days 3, 4, and 5 of culture and are representative of 2 experiments with a total of 4 animals. Results for transwell cultures indicate the proliferation in MLRs between naive LEW.1A splenocytes and irradiated LEW.1W APCs in the upper compartment on days 3 and 5 of culture. They are expressed as counts per minute (cpm) ± SD and are representative of 3 experiments with a total of 4 animals in each group.
Effect of NO blockade with or without added IL-2 on inhibited MLRs.
Splenocytes from CTLA4Ig-treated or control LEW.1A rats that received LEW.1W cardiac heart transplants were harvested on day 10 after transplantation and cultured in 96-well plates (A-B) or in the lower compartments of transwell plates (C-D) with irradiated APCs from donor origin. Cultures were performed with L-NMMA or D-NMMA (5 mM) in the absence (A-C) or presence (D) of IL-2 (100 U/mL). Results (D) for 96-well cultures are expressed as delta (allogeneic − spontaneous) cpm ± SD on days 3, 4, and 5 of culture and are representative of 2 experiments with a total of 4 animals. Results for transwell cultures indicate the proliferation in MLRs between naive LEW.1A splenocytes and irradiated LEW.1W APCs in the upper compartment on days 3 and 5 of culture. They are expressed as counts per minute (cpm) ± SD and are representative of 3 experiments with a total of 4 animals in each group.
The lack of proliferation of splenocytes from CTLA4Ig-treated animals against third-party APCs in the presence of D-NMMA was completely reverted in the presence of L-NMMA (Figure 4B).
Similar to the results obtained in 96-well plates, experiments in transwell cultures showed that the inhibition of naive MLRs by splenocytes from CTLA4Ig-treated rats was not reverted by addition of L-NMMA (Figure 4C).
These results indicate that in the absence of NO, inhibition of proliferation of splenocytes from CTLA4Ig-treated rats was LEW.1W antigen-specific and dependent on other soluble inhibitory products. Although NO played a role in the inhibition of MLRs when splenocytes from CTLA4Ig-treated rats and naive splenocytes were in close proximity (coculture), this was less prominent in transwell cultures (probably due to the short half-life and range of action of NO44), indicating that additional inhibitory soluble molecules were produced upon donor antigen-specific stimulation. The simultaneous production of NO and additional inhibitory soluble molecule(s) may also account for the apparent paradox whereby donor antigen-specific inhibition of MLRs was observed in transwell MLRs, but not in 96-well MLR experiments, in which both donor and third-party responses were inhibited.
We then asked whether concomitant production of NO and limited IL-2 production could account for lack of proliferation observed for splenocytes from CTLA4Ig-treated recipients. Simultaneous addition of IL-2 and L-NMMA to MLRs performed in transwell plates increased proliferation of both CTLA4Ig-treated and control splenocytes that showed comparable proliferation on days 3 and 5 of culture (Figure4D).
Compared with MLRs from control splenocytes (n = 7), supernatants harvested at day 3 of culture from MLRs performed with splenocytes from CTLA4Ig-treated rats (n = 7) showed significantly decreased levels of IL-2 (535 ± 62 pg/mL vs 287 ± 71, P = .022) and IFNγ (7147 ± 71 vs 1010 ± 606.5 pg/mL,P = .025).
Thus, production of NO and inhibition of IL-2 production by soluble inhibitory product(s), are responsible for the reduced donor-specific T-cell proliferative responses of CTLA4Ig-treated animals.
Supernatants harvested at day 3 of culture from MLRs performed with splenocytes from CTLA4Ig-treated rats (n = 4) or controls (n = 4) contained comparable levels of IL-13 (7-20 pg/mL), IL-10 (50-125 pg/mL), and TGFβ1 (< 31 pg/mL) and levels of IL-4 below the detection limit (< 15 pg/mL). Lack of allogeneic proliferation of splenocytes from CTLA4Ig-treated rats versus controls was unchanged in the presence of neutralizing anti–IL-4, anti–IL-10, anti–IL-13, or anti-TGFβ1 antibodies with or without added high IL-2 concentrations (100 U/mL or 500 U/mL) and L-NMMA (data not shown). Thus, inhibition of proliferation could not be explained by production of IL-4, IL-10, IL-13, or TGFβ1.
Phenotype of splenocytes from CTLA4Ig-treated rats
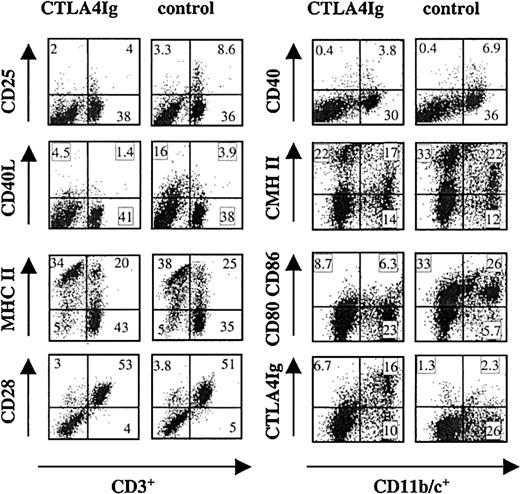
The percentages of CD3+, CD4+, CD8α+, B, and CD161+ natural killer (NK) cells among splenocytes from CTLA4Ig-treated rats were similar to those observed in control rats (data not shown). The phenotype of CD3+ T cells and CD11b/c+ cells (macrophages and DCs)41 was analyzed in 2-color immunofluorescence. Splenocytes from CTLA4Ig-treated rats contained fewer CD3+CD25+ cells compared with control splenocytes (Figure 5) and CD4+CD25+ cell population was similarly reduced (data not shown). CD3+CD40L+ and CD3+MHC-II+ cell populations were also reduced in splenocytes from CTLA4Ig-treated rats compared with control splenocytes (Figure 5). Expression of CD28 (Figure 5) or of CD45RC (data not shown) was identical among CD3+ cells from CTLA4Ig-treated rats and controls.
Phenotype of splenocytes from CTLA4Ig-treated or control rats.
Splenocytes were harvested on day 10 after transplantation from CTLA4Ig-treated or control LEW.1A rats that received LEW.1W heart transplants. Cells were double labeled with MAbs recognizing the indicated antigens. Numbers within each window represent the percentage of positive cells. Results are representative of 3 independent experiments with a total of 4 animals in each group.
Phenotype of splenocytes from CTLA4Ig-treated or control rats.
Splenocytes were harvested on day 10 after transplantation from CTLA4Ig-treated or control LEW.1A rats that received LEW.1W heart transplants. Cells were double labeled with MAbs recognizing the indicated antigens. Numbers within each window represent the percentage of positive cells. Results are representative of 3 independent experiments with a total of 4 animals in each group.
The percentages of CD11b/c+ cells were comparable between splenocytes from CTLA4Ig-treated rats and control animals. CD11b/c+ cells expressed lower levels of CD40, MHC class II, and B7s molecules (Figure 5).
Analysis of CTLA4Ig binding to different splenocyte populations showed that CD11b/c+ (Figure 5) but not CD3+ (data not shown) cells displayed CTLA4Ig on their surface.
Thus, both T cells and CD11 b/c+ cells from rats that underwent transplantation and were treated with CTLA4Ig expressed levels of activation molecules lower than those on the corresponding cells from control animals.
Activation of T cells and/or DCs restores allogeneic proliferation of splenocytes
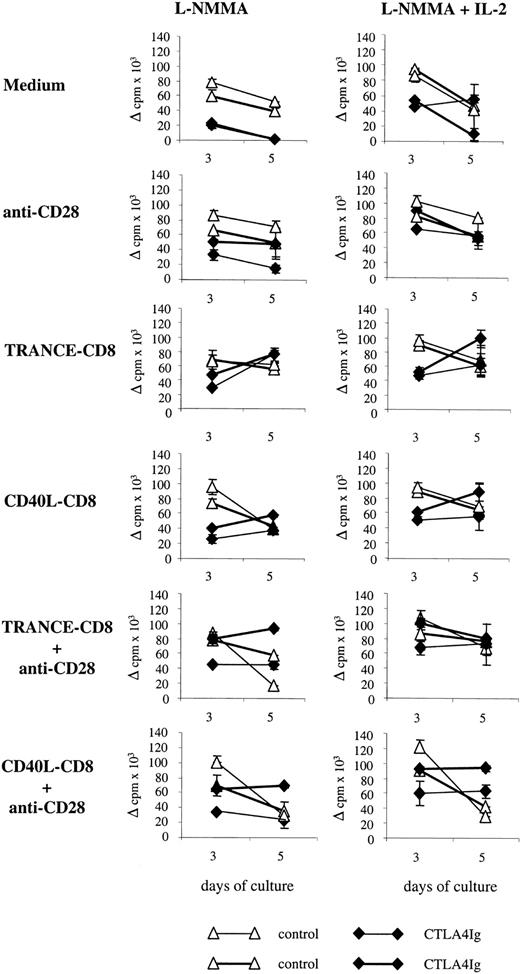
T cells and APCs from CTLA4Ig-treated rats showed a phenotype of resting cells, and we reasoned that inhibition of allogeneic proliferation could be reverted by T and/or APC activation. Therefore, we evaluated MLRs in the presence of L-NMMA to block NO production with or without added IL-2 and anti-CD28 agonistic antibodies to effectively stimulate T cells and/or TRANCE-CD8 or CD40L-CD8 to stimulate APCs.
Stimulation of T cells through CD28 enhanced proliferation of splenocytes from CTLA4Ig-treated animals at days 3 and 5 of culture, but this was 25% to 50% lower than that of control cells similarly treated. In the presence of anti-CD28 and IL-2 the proliferation of splenocytes from CTLA4Ig-treated animals was comparable with those of control cells in the same conditions (Figure6).
Effect of stimulation of T cells or APCs on the inhibition of MLRs.
Splenocytes were harvested on day 10 after transplantation from 2 CTLA4Ig-treated or 2 control LEW.1A rats that received LEW.1W heart transplants, and proliferation was assessed on days 3 and 5 of culture. MLRs were performed in the presence of L-NMMA (5 mM) and in the presence or absence of IL-2 (100 U/mL). Stimulation of T cells was performed using anti-CD28 agonistic antibodies. Stimulation of APCs was performed using TRANCE-CD8 or CD40L-CD8. Results are expressed as delta (allogeneic − spontaneous) cpm ± SD and are representative of 2 experiments with a total of 3 animals per group. Each curve represents the proliferation of cells from a single animal.
Effect of stimulation of T cells or APCs on the inhibition of MLRs.
Splenocytes were harvested on day 10 after transplantation from 2 CTLA4Ig-treated or 2 control LEW.1A rats that received LEW.1W heart transplants, and proliferation was assessed on days 3 and 5 of culture. MLRs were performed in the presence of L-NMMA (5 mM) and in the presence or absence of IL-2 (100 U/mL). Stimulation of T cells was performed using anti-CD28 agonistic antibodies. Stimulation of APCs was performed using TRANCE-CD8 or CD40L-CD8. Results are expressed as delta (allogeneic − spontaneous) cpm ± SD and are representative of 2 experiments with a total of 3 animals per group. Each curve represents the proliferation of cells from a single animal.
Stimulation of APCs using TRANCE-CD8 or CD40L-CD8 enhanced proliferation of splenocytes from CTLA4Ig-treated animals to levels comparable with those of control cells, but with increased proliferation at day 5 for CTLA4Ig versus day 3 for control cells. This increase of proliferation was enhanced in the presence of IL-2 and followed the same kinetics as observed in its absence (Figure6).
Simultaneous stimulation of T cells and APCs using anti-CD28 and TRANCE-CD8 or CD40L-CD8 resulted in proliferation of CTLA4Ig-treated cells comparable and higher at days 3 and 5 with that of control cells. Addition of IL-2 further enhanced this phenomenon (Figure 6).
These results indicate that inhibition of allogeneic proliferative responses against donor APCs by splenocytes from CTLA4Ig-treated rats is reverted by T-cell and/or APC costimulation.
DCs from CTLA4Ig-treated rats inhibit MLRs
To characterize the type of cells responsible for inhibition of MLRs of splenocytes from CTLA4Ig-treated rats we performed coculture experiments in which naive MLRs were evaluated in the presence of T cells or DCs purified from splenocytes of CTLA4Ig-treated rats. Proliferation of naive cells on day 5 of culture in the presence of L-NMMA was inhibited by more than 50% by coculture with total splenocytes from CTLA4Ig-treated animals, but not with cells from control animals that underwent transplantation (Figure7A). Importantly, coculture with DCs from CTLA4Ig-treated rats but not from control rats in the presence of L-NMMA resulted on day 5 in more than 70% inhibition of MLRs (Figure7B). DCs also inhibited proliferation of naive T cells from 2 of 3 animals (data not shown). T cells purified from splenocytes of CTLA4Ig-treated rats did not inhibit MLRs (Figure 7C).
Effect of T cells and DCs from CTLA4Ig-treated animals on naive MLRs.
Splenocytes from naive LEW.1A rats were cocultured with irradiated LEW.1W APCs and with (A) splenocytes, (B) DCs, or (C) T cells purified from spleens from the same animals harvested on day 10 after transplantation from 2 CTLA4Ig-treated or 2 control LEW.1A rats that received LEW.1W heart transplants. Cells were cocultured at 1:1 cell ratio in the presence of L-NMMA (5 mM) for 3 or 5 days. Results are expressed as delta (allogeneic − spontaneous) cpm ± SD. Results are representative of 3 experiments with a total of 5 animals in each group. Each curve represents the proliferation of cells from a single animal.
Effect of T cells and DCs from CTLA4Ig-treated animals on naive MLRs.
Splenocytes from naive LEW.1A rats were cocultured with irradiated LEW.1W APCs and with (A) splenocytes, (B) DCs, or (C) T cells purified from spleens from the same animals harvested on day 10 after transplantation from 2 CTLA4Ig-treated or 2 control LEW.1A rats that received LEW.1W heart transplants. Cells were cocultured at 1:1 cell ratio in the presence of L-NMMA (5 mM) for 3 or 5 days. Results are expressed as delta (allogeneic − spontaneous) cpm ± SD. Results are representative of 3 experiments with a total of 5 animals in each group. Each curve represents the proliferation of cells from a single animal.
Thus, DCs from CTLA4Ig-treated rats are capable of inhibiting naive T-cell proliferation in response to alloantigens.
Discussion
Our results indicate that in vivo treatment with CTLA4Ig in a transplantation model promotes development of donor-specific tolerogenic mechanisms. Immune tolerance is critical for the prevention of autoimmunity and maintenance of immune homeostasis. The mechanisms involved in immune tolerance could be exploited to induce tolerance in pathologic conditions such as graft rejection and autoimmune diseases.2 3
The in vitro activation of T cells in the absence of costimulation results in T-cell anergy,45 the development of regulatory T cells,20-22 and suppressive macrophages,15 but the tolerogenic effects of costimulatory blockade in vivo are still ill-defined. One potential mechanism is the development of cells capable of inhibiting immune responses. These cells can be T cells or APCs, as both cell types are capable of reciprocal regulation resulting in immunosuppression.2,10,16 To our knowledge, the results reported in this work represent the first detailed analysis of tolerogenic cells induced by CTLA4Ig treatment in vivo. Our observations suggest that in vivo blockade of the B7/CD28 pathway with CTLA4Ig can result in tolerance to alloantigens by at least 2 mutually nonexclusive mechanisms. First, binding of CTLA4Ig to DCs results in the generation of tolerogenic DCs. Second, CTLA4Ig disrupting normal APC-T cell interactions results in the development of regulatory T cells, which induce tolerogenic DCs. It has already been shown that in vitro blockade of B7/CD28 costimulation induces generation of alternatively activated macrophages through IL-10 production by T cells.15 Similarly, T cells can regulate DC function; anergic T cells19,26 and T CD8+CD28− suppressor cells12 induce tolerogenic DCs, and regulatory CD4+ T cells protect against diabetes through bystander suppression acting on APCs.28
The generation of regulatory T cells or APCs inducing tolerance and not pathogenic immune responses depends on factors influencing APCs-T cells interactions, such as the type and level of costimulatory signals or cytokines expressed by each cell type and presented antigenic determinants.3,9-11,26 The development of tolerogenic DCs has been described after in vitro treatment with IL-10, corticoids, vitamin D3, low doses of granulocyte-macrophage colony-stimulating factor, TNFα, or IL-3.2-4,10,14,46-48 Genetically engineered DCs expressing FasL,49 viral IL-10,50 TGFβ,51or indoleamine 2′3-dioxygenase (IDO)52 inhibit allogeneic proliferative responses. Allogeneic stimulation of T cells by immature DCs6 enables the development of suppressive T cells in vitro. Furthermore, prolongation of allograft survival has been obtained by the administration of immature DCs,4 of DCs expressing FasL,49 of DCs cultured in the presence of IL-3 and CD40 stimulation,48 or even of mature CD8+DCs.5
Central thymic tolerance is mediated by DCs, and it has been suggested that certain forms of peripheral tolerance, either by deletion, anergy, or suppression, are mediated by them.3,13,53 The prevailing view is that steady-state or immature DCs expressing lower amounts of surface MHC and costimulatory molecules tolerize T cells, whereas activated or mature DCs, expressing higher levels of surface MHC and costimulatory molecules induce immune response.2-6,8 However, recent evidence shows that mature DCs can also generate tolerance and that the overall response (tolerance vs immune response) depends on the balance between yet unidentified tolerogenic signals and CD40-CD40L signaling.5,13,14 Tolerogenic DCs with NK characteristics generated in vivo after blockade of CD40-CD40L signals have been described only very recently in a diabetes autoimmune model.54 Tolerogenic macrophages or DCs are functionally characterized by increased expression of scavenger receptors, anti-inflammatory mediators (PGE2 and IL-10),3,9,10,48,55immunoglobulin-like transcript 3 and 4 (ILT3 and ILT4)12and IDO,56 reduced expression of IL-1β and IL-12,13 and induction of T-cell apoptosis.3,13,53 T cells from CTLA4Ig-treated rats showed reduced expression of CD40L, and APCs displayed reduced expression of CD80, CD86, and MHC class II antigens. Stimulation of APCs using TRANCE or CD40L restored proliferation. These results suggest that in vivo blockade of T-cell activation by CTLA4Ig resulted in a lack of DC activation and generated tolerogenic DCs. These tolerogenic DCs acted on the direct pathway of antigen presentation, since MLRs using purified T cells were inhibited and this is the predominant form of alloantigen presentation during acute allograft rejection in vivo and of MLRs in vitro. This inhibition could have been exerted on the recipient T cells or on the donor APCs. Experiments using purified T cells activated in a DC-free system (anti-CD3 and anti-CD28) are needed to clarify this point. Several DC subpopulations displaying different functions have been described in mice and humans.57 In rats, 2 subpopulations of DCs, based on the expression of CD4, have been recently described,35-37 and their role in the inhibition of MLRs after CTLA4Ig treatment is under investigation. Rat CD4− DCs have NK-like cytotoxic activity and myeloid morphology; they produce high levels of IL-12 and induce CD4+ but not (or much less) CD8+ T cells to proliferate and produce Th1 but not Th2 cytokines.36,37 Rat CD4+ DCs do not display cytotoxic activity; have heterogeneous lymphoid and myeloid morphology; produce low levels of IL-12; and induce strong proliferation of CD4+ and CD8+ T cells as well as production of Th1 and Th2 cytokines.36 37
Regulatory T cells have been isolated in vivo from naturally occurring CD4+CD25+ cells16 or after treatment with oral tolerance protocols (Th3),24anti-CD4,1,16,58 or donor-specific blood transfusion.25 In vitro development of regulatory T cells has been obtained using costimulation blockers20-22; soluble antigen or immobilized antibody to CD317-19; allogeneic immature DCs6; IL-10–treated DCs46; or IL-10–treated T cells (Tr1).23The fact that inhibition of proliferation by cells from CTLA4Ig-treated rats was triggered in a donor alloantigen-dependent manner argues either for regulatory T cells being implicated or for the generation of tolerogenic DCs of donor origin, but further work is needed to explore these possibilities. IL-10–producing T cells are implicated in the generation of suppressive macrophages after in vitro blockade of MLRs by CTLA4Ig.15
Despite the fact that addition of exogenous IL-2 was needed to abrogate inhibition of splenocyte proliferation, suggesting the induction of anergy, T cells from CTLA4Ig-treated rats proliferated in response to alloantigen rechallenge when isolated. The existence of other tolerogenic mechanisms is possible. Proliferation may proceed normally through the first rounds of cell division and effector mechanisms may be gradually reduced by an increase in cell apoptosis, as recently demonstrated in other in vitro13 and in vivo53 tolerance models induced by DCs. Alternatively, other CD4+ or CD8+ T cell functions, such as cytokine production or cytotoxic activity, may be down-regulated in CTLA4Ig-treated rats.
Previous publications have shown that the activity of regulatory T cells is thwarted by cell irradiation in certain21 but not all19,20,26 models. Irradiation (35 Gy) abrogated the inhibitory activity of splenocytes from CTLA4Ig-treated rats. This irradiation dose can result in not only blockade of proliferation but also in cell death.59 Therefore, inhibition of MLRs by splenocytes from CTLA4Ig-treated rats is dependent on at least cell metabolic activity and/or proliferation but not on passive release of preformed molecules. These results raise the question of whether the inhibitory activity on naive MLRs could be due to culture overcrowding by splenocytes primed in vivo by donor antigens. The fact that splenocytes from control animals that underwent transplantation did not show this effect, that inhibition was cell-contact independent, and that the suppressive effect of splenocytes from CTLA4Ig-treated rat versus responding naive splenocytes was observed with ratios as low as 1:5 (data not shown) argue against this hypothesis.
Bystander inhibition of immune responses was observed as splenocytes stimulated with donor cells inhibited proliferative responses against third-party antigens. This phenomenon is in keeping with the observation that inhibition depended on the secretion of soluble products. Inhibition of immune responses by secreted products has been described in vitro for Tr1 cells and Th3 cells (producers of IL-10 and TGFβ, respectively) as well as for IL-10 producing T cells after in vitro costimulatory blockade.15,21CD4+CD25+ regulatory T cells16 or anergic T cells generated in vitro18,19,46 act through cell contact. Nevertheless, the role of soluble- versus cell contact–dependent signals for CD4+CD25+ is controversial since their immunosuppressive action in vivo depends on IL-10.16 Whether tolerogenic DCs46,54 or alternatively activated macrophages15 mediate suppression through production of soluble products or cell-to-cell contact has not been clearly established.
Inhibition of NO enabled partial and complete restoration of proliferation against donor and third-party alloantigens, respectively. NO is produced by macrophages and DCs,44,55 and its production is decreased in alternatively activated macrophages generated by B7/CD28 blockade.15 NO inhibits T-cell proliferation by interfering with the IL-2R intracellular signaling pathway without affecting expression levels of IL-2 or IL-2R.43,44 Our results show that nearly complete restoration of proliferation was obtained upon addition of NO inhibitors and IL-2, thus suggesting that IL-2 production was inhibited by secreted product(s). This inhibition of IL-2 production was not mediated by known cytokines with immunosuppressive activities, such as IL-4, IL-10, TGFβ, or IL-13. The nature of the inhibitory molecule(s) provided by splenocytes and DCs from CTLA4Ig-treated animals is the subject of ongoing investigations. Potential inhibitory candidate molecules not requiring cell contact include not only secreted molecules but also intracellular enzymes that modify the extracellular milieu, such as IDO,52 which is expressed by a subset of DCs,56 and heme oxygenase-1.60 Similar to our results, addition of IL-2 reversed the inhibition of proliferation by CD4+CD25+ cells61 and by suppressor cells generated by either in vitro costimulatory blockade20,21,62 or anti-CD4 in vivo treatment.58
Therefore, CTLA4Ig in vivo treatment promotes T-cell unresponsiveness not only by blocking T-cell activation through CD28 but also by generating tolerogenic DCs.
We are grateful to Claire Usal, Helga Smit, and Emmanuel Merieau for heart transplantation; to all researchers who contributed reagents; and to Bice Perussia and Robert Lechler for critically reading the manuscript. We thank the Vector Core of the University Hospital of Nantes, supported by the Association Française contre les Myopathies (AFM), for producing the adenoviral vectors used in this study.
Prepublished online as Blood First Edition Paper, December 19, 2002; DOI 10.1182/blood-2002-07-2076 .
Supported in part by Fondation Transvie and EEC grant Biomed2 BMM-CT98-3277.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Ignacio Anegon, INSERM U437, 30 boulevard Jean Monnet, 44093 Nantes, France; e-mail:ianegon@nantes.inserm.fr.

![Fig. 2. Effect of transwell cultures on inhibition of MLRs. / Splenocytes from naive LEW.1A animals were cultured in the upper compartment of transwell plates with irradiated LEW.1W APCs, and proliferation was evaluated on days 3 and 5 of culture. The lower compartment contained splenocytes from 2 CTLA4Ig-treated or 1 control LEW.1A rat that received LEW.1W heart transplants harvested on day 10 after transplantation and cultured with (A) culture medium, (B) irradiated LEW.1W APCs, or (C) irradiated BN APCs. (D) Splenocytes from naive LEW.1A animals were cultured in the upper compartment of transwell plates with BN irradiated APCs in the presence, in the lower compartment, of splenocytes from CTLA4Ig-treated or control LEW.1A rats and irradiated LEW.1W APCs. [3H]Tdr incorporation was assessed in the upper compartment MLR on days 3 and 5 of culture. Results are expressed as delta (allogeneic − spontaneous) cpm ± SD and are representative of 2 identical experiments with a total of 4 animals in each group. Each curve represents the proliferation of cells from a single animal.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/8/10.1182_blood-2002-07-2076/4/m_h80834180002.jpeg?Expires=1764987079&Signature=OxK4XEeY7NAPSmcC~pRwrmgxtBcUGWrQK8lp1cO7GOmsE4gFiS~Q7ZuCtq2G9m8DpG3bB9ihYowvBx8P5Osp1gXqNpdOc7i2ilthCA8WO0WEpkCw6wlwwgwtEvoG3~cHg-uRmIBk~le8M8XWYjrbmn15hN0izCXQ9iOIJkmIGRv44XzUPvof16KB0P46DKE4W~lO0YQusdiLOoTm67pyvvzQVl13WJz~IVAHp1NpGxnDAsLySCMU3Uv3ENgkMlwnm6LZHrvyzddR8mDLqqQqhqmDZxphtnfqJqutF~rAIGe0u-ih07pvn-dzUWuHoWSnCZ7VtQBazPbVFhuztgguuA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Effect of irradiation on the inhibition of MLRs and of splenocytes from CTLA4Ig-treated rats on MLRs of allogeneically sensitized splenocytes and T cells. / Splenocytes were harvested on day 10 after transplantation from CTLA4Ig-treated (♦) or control (▵) LEW.1A rats that received LEW.1W heart transplants. These cells were either (A) untreated or (B) irradiated and cocultured (1:1 cell ratio) with naive LEW.1A splenocytes in the presence of irradiated LEW.1W APCs. Splenocytes from CTLA4Ig-treated (♦) or control (▵) rats were cocultured with irradiated LEW.1W APCs and either (C) allogenically primed LEW.1A splenocytes (1:1 cell ratio) or (D) allogenically primed LEW.1A T cells (1:1 cell ratio) harvested 10 days after heart transplantation. [3H]Tdr incorporation was assessed at the indicated time points. Results are expressed as delta (allogeneic − spontaneous) cpm ± SD and are representative of 3 identical experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/8/10.1182_blood-2002-07-2076/4/m_h80834180003.jpeg?Expires=1764987079&Signature=wxEypmQif0yYs5MWtgFvr18o0oY9UrH7h~o-zek8S30017fVQG~wCE8ivTS9l3chs~0C6rpRsKNEcYaY4aYL5QIm-wIA1xNBfDKgtxnUtHpn6dfZnA8E45uD9tv0qgzdwOJEzqe~p4gX4Z6LQYNCS0KQHgk~9dZHHanpXc1gKWIrCj0Dnrr9XIWohkDwCB2d8ZdK7IEV-aTiiNMTmtYeuPOF-mTZkR8r6XC4Cm3cXFR3FpMD2GEEZGQGBn174Tq05JXOT7lW~WC2dKcpFNIuJ~JNgNXP6a7jbt6G73u0DtH~ityHavVvPpuFN8T1RCY-QBW7VYcqTS2JYiUaQXPH5Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal