Glycoprotein (GP) Ib/V/IX complex–dependent platelet adhesion to von Willebrand factor (VWF) is supported by the 45-kd N-terminal extracellular domain of the GPIbα subunit. Recent results with an adhesion blocking antibody (RAM.1) against GPIbβ, which is disulfide linked to GPIbα, have suggested a novel function of this subunit in regulating VWF-mediated platelet adhesion, possibly involving its intracellular face. A putative cooperation between the GPIbα and GPIbβ cytoplasmic domains was investigated by measuring the adhesion under flow to immobilized VWF of K562 and Chinese hamster ovary (CHO) cells transfected with GPIb/(V)/IX containing mutations in this region. Adhesion of cells carrying a glycine substitution of the GPIbβ Ser166 phosphorylation site was 50% lower than normal and became insensitive to inhibition by RAM.1. In contrast, forskolin or PGE1 treatment increased both the phosphorylation of GPIbβ and adhesion of control cells, both effects being reversed by RAM.1, but had no influence on cells expressing the Ser166Gly mutation. A role of the GPIbα intracellular domain was also apparent as the VWF-dependent adhesion of cells containing deletions of the entire (Δ518-610) or portions (Δ535-568, Δ569-610) of the GPIbα cytoplasmic tail was insensitive to RAM.1 inhibition. Cells carrying progressive 11 amino acid deletions spanning the GPIbα 535-590 region were equally unresponsive to RAM.1, with the exception of those containing GPIbα Δ569-579, which behaved like control cells. These findings support a role of the GPIbβ intracellular domain in controlling the adhesive properties of the GPIb/V/IX complex through phosphorylation of GPIbβ Ser166 and point to the existence of cross-talk between the GPIbβ and GPIbα intracellular domains.
Introduction
Platelets play an essential role in hemostasis by adhering to injured blood vessels where they become activated and aggregate.1 The glycoprotein (GP) Ib/V/IX receptor mediates the initial adhesion of platelets by binding to von Willebrand factor (VWF) exposed on the subendothelium of the damaged vessel wall.2 This interaction is capable of withstanding high shear forces, which is necessary for its function of tethering platelets in rapidly flowing blood.3 4
The GPIb/V/IX receptor belongs to the leucine-rich repeat glycoproteins and is composed of GPIbα, disulfide linked to GPIbβ to form GPIb, which is noncovalently associated with GPIX and GPV on the platelet surface.5-7 The GPIbα (610 amino acids) adhesive subunit binds to VWF through its 45-kd globular extracellular domain,8,9 while its 96-residue cytoplasmic domain binds to filamin-1 (or ABP-280) through the 557-579 region10,11and to adaptor protein 14.3.3ζ through the 605-610 region.12,13 These interactions serve to anchor the receptor to the cytoskeleton, regulate VWF-dependent adhesion under flow, and transduce signals necessary for αIIbβ3 integrin activation.14-18 The roles of the other subunits are less well established apart from the requirement for GPIbβ and GPIX for correct processing and surface expression of the complex.19,20 These 2 subunits have similar extracellular sequences but differ in their intracellular domains of 34 and 6 residues, respectively. The GPIbβ intracellular serine at position 166 can be phosphorylated by a cyclic adenosine monophosphate (cAMP)–dependent kinase (PKA), a process thought to facilitate interaction with 14.3.3ζ.13,21,22 More recently, calmodulin has been proposed as a third intracellular partner of GPIb/V/IX with binding sites identified in GPIbβ and GPV.23
In a previous report, we described a monoclonal antibody (MoAb) RAM.1 against the GPIbβ extracellular domain that inhibited VWF-mediated adhesion of platelets and GPIb/V/IX-transfected K562 cells under flow.24 The mechanism controlling this effect of RAM.1 was unknown but suggested a role of GPIbβ in regulating GPIb/V/IX-dependent adhesion to VWF. In the present study, we tested the possibility that the inhibition of the GPIb–VWF interaction by RAM.1 was conveyed by the GPIb intracellular domain. Studies of adhesion to immobilized VWF in a flow system were performed using K562 and Chinese hamster ovary (CHO) cells transfected with GPIb/(V)/IX containing mutations and deletions in the intracellular domains of GPIbβ and GPIbα. The participation of cAMP-dependent phosphorylation of GPIbβ was also evaluated. Evidence was found that efficient cell tethering and sensitivity to inhibition by RAM.1 require an intact serine at position 166 of GPIbβ and are regulated by the level of phosphorylation. Moreover, deletions of the GPIbα intracellular region caused loss of the effect of RAM.1, suggesting a functional communication between the GPIbα and GPIbβ subunits.
Materials and methods
Materials
Cell culture reagents were from Life Technologies GIBCO BRL (Cergy-Pontoise, France) except for FuGene transfection reagent (Roche Diagnostic, Meylan, France), methotrexate (France Biochem, Meudon, France), and Zeocin (Invitrogen, San Diego, CA). Forskolin, prostaglandins E1 (PGE1) and I2 (PGI2), normal goat serum (NGS), fatty acid–free human serum albumin (HSA), bovine serum albumin (BSA), and protein G–sepharose were from Sigma Aldrich (St Louis, MO). Apyrase was purified from potatoes as previously described.25 Human VWF and bovine VWF were prepared according to published procedures.15,26 The cAMP(125I) assay kit and phosphorus-32 ([32P]PO4) were from Amersham Pharmacia Biotech (Uppsala, Sweden). Fluorescein isothiocyanate (FITC)–conjugated goat anti–mouse immunoglobulin G (IgG) and FITC-conjugated goat F(ab')2 fragments anti–rat IgG were from Jackson ImmunoResearch (West Grove, PA). Purified rat IgG1 was from Pharmingen (Le Pont de Claix, France) and the murine MoAb SZ2 against human GPIbα was from Immunotech (Marseille, France). Different MoAbs were produced in our laboratory as IgG1, κ isotype. Mouse MoAbs ALMA.12 and ALMA.19 are directed against human GPIbα, ALMA.16 against human GPIX, and V.1 against human GPV. RAM.1 is a rat MoAb directed against mouse and human GPIbβ.24 Complete protease inhibitor cocktail and calpain inhibitor 1 were from Roche Molecular Biochemicals (Mannheim, Germany).
Platelets and cell lines
Human platelets were isolated from acid-citrate-dextrose anticoagulated blood obtained from aspirin-free healthy volunteers and washed by sequential centrifugation in Tyrode buffer containing 5 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), pH 7.35, 0.35% HSA, and 0.5 μM PGI2.25 The platelets were finally resuspended at 3 × 105platelets/μL in the same buffer lacking PGI2 and containing 0.04 U/mL apyrase.
CHO cell lines expressing the GPIb/IX complex with deletions of the GPIbα intracellular domain have been reported previously.27,28 K562 cell lines expressing the GPIb/V/IX complex with deletions of the GPIbα intracellular domain (Δ518-610, Δ535-568, Δ569-610) were obtained by cotransfection of plasmids coding for wild-type GPIbβ, GPIX, and GPV essentially as described earlier.15,27 To obtain a cell line containing a Ser166Gly mutation of GPIbβ, K562 cells were transfected with a pSVZeo plasmid containing GPV cDNA and pDX expression plasmids individually containing cDNAs for GPIbα, GPIX, and mutated GPIbβ with glycine at position 166 using the U-DNA Mutagenesis kit (Boehringer Mannheim, Germany).29 GPIbβ primers1241CAGCGGGTCGGTCAGACCCAGCCGGGCTGC1212and1212GCAGCCCGGCTGGGTCTGACCGACCCGCTG1241containing the mutation (underlined) were each annealed with full-length GPIbβ inserted into the EcoRI sites of M13. M13 single- and second-strand DNA synthesis was performed using T4 ligase and polymerase. After sequencing, the mutated GPIbβ was reinserted into pDX. CHO-GPIb/IX cells were cultured in minimum essential medium (αMEM) supplemented with 10% fetal calf serum (FCS), PSG mix (100 U/mL penicillin, 100 μg/mL streptomycin, 2 mM glutamine), and 400 μg/mL G418. K562-GPIb/V/IX cells were cultured in RPMI 1640 medium containing 10% FCS, PSG mix, and 200 μg/mL Zeocin.
Measurement of intracellular cAMP
Transfected cells (1.5 × 105 in 0.5 mL) or platelets (9 × 107 in 0.5 mL) were incubated at 37°C with forskolin (10 μM or 50 μM) for 1 hour or with adrenaline (10 μM) or PGE1 (10 μM) for 5 minutes. Treatment was stopped by adding 50 μL of ice-cold 6 N perchloric acid and cAMP was isolated from the supernatant by extraction with a mixture of trioctylamine and freon (28/22 vol/vol). Following centrifugation at 12 000 rpm for 4 minutes at 4°C, the upper aqueous phase was recovered and lyophilized. The dry residue was dissolved and cAMP was quantified with a commercial cAMP(125I) assay kit.
32P phosphorylation studies
K562-GPIb/V/IX, K562-GPIb(βSer166Gly)/V/IX cells (106 cells/mL), and washed human platelets (109/mL) in Tyrode buffer lacking PO4 and PGI2 were labeled for either 15 minutes with 100 μCi/mL (3.7 MBq) [32P]PO4 (K562 cells) or one hour with 0.2 mCi/mL (7.4 MBq) [32P]PO4in the presence of 0.04 U/mL apyrase (platelets) at 37°C. After 2 washes, cells were first incubated with 10 μg/mL of RAM.1 or its isotypic control rat IgG1 for 30 minutes and then treated with 10 μM PGE1 or 10 μM forskolin for 10 minutes at 37°C. Following centrifugation, platelets were lysed by addition of 3 N perchloric acid and K562 cells by incubation with a ice-cold Triton X-100 lysis buffer containing protease inhibitors, 50 mM NaF, and 2.5 mM Na3VO4 for 20 minutes. After centrifugation at 15 000 rpm, proteins were immunoprecipitated by RAM.1 coupled to protein G–sepharose for 2 hours. Proteins were separated on a 7.5% to 15% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gel. The dried gel was exposed for autoradiography or analyzed in a phosphoimager system (BioRad, Hercules, CA).
Flow cytometry
K562 or CHO cells (2 × 105 in 100 μL) were incubated for 30 minutes at 4°C with purified IgG (10 μg/mL) in FMF buffer (RPMI medium, 5% NGS, 0.2% sodium azide). After centrifugation at 1200 rpm, the cells were resuspended in buffer containing a 100-fold dilution of FITC-conjugated goat F(ab′)2 anti–rat IgG or FITC-conjugated goat IgG anti–mouse IgG for 30 minutes at 4°C. Analyses were performed on 10 000 cells in a FACSCalibur flow cytometer (BD Biosciences, Rungis, France).
Adhesion assays in a flow system
Adhesion of cells under flow conditions was investigated according to Cranmer et al,15 using glass microcapillary tubes (Vitro Dynamics, Mountain Lakes, NJ) coated overnight at 4°C in a humid chamber with either 1% HSA or 25 μg/mL bovine or human VWF in phosphate buffered saline (PBS) and postcoated for one hour at room temperature with 1% HSA. Cells (1 × 106/mL) or platelets (3 × 108/mL) in Tyrode buffer containing 2 mM EDTA (ethylenediaminetetraacetic acid) were perfused through the capillaries at a shear rate of 150 s-1 for 10 minutes. The effects of MoAbs (10 μg/mL) were tested by preincubation with the cells for 10 minutes at room temperature. Cell adhesion was visualized at 10-fold magnification by video microscopy (Leica DMIRB; Leica Microsystems SA, Wetzlar, Germany). The images were recorded for subsequent analysis on an AV disc recorder WDR 200 (Matsushita Electric Industrial, Osaka, Japan). Under these conditions, cells tethered and rolled over the surface. The number of adherent rolling cells counted in fields of 1 mm2 progressively accumulated from zero to a maximum that the matrix concentration can support. EDTA was included as a standard condition for comparison between cell types, as it is problematic to specifically inhibit endogenous integrins in CHO cells due to lack of appropriate antibodies or antagonists. Adhesion of K562-GPIb/V/IX cells was identical in the presence or absence of EDTA, in keeping with the lack of endogenous integrins able to bind VWF. In some studies the microcapillary tube was perfused with cell-free buffer at the end of a 5-minute perfusion at 75 s-1 and the shear rate was increased incrementally to 150 s-1, 300 s-1, 750 s-1, 1500 s-1, 3000 s-1, and 6000 s-1. At each shear rate the field was taped for 2 minutes and the number of adherent cells was quantitated off-line.
Statistical analyses
The statistical significance of differences between means was evaluated using the Student t test for paired samples andP values of less than .05 were considered to be significant. Variations of means were calculated as the standard deviation.
Results
RAM.1 inhibits adhesion of platelets and GPIb/(V)/IX-transfected cells to bovine or human VWF
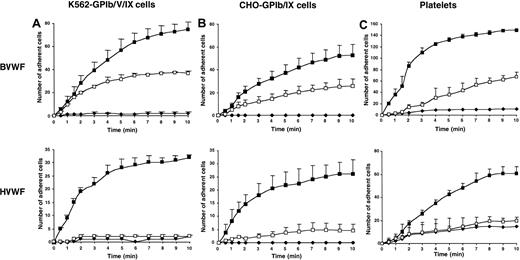
RAM.1 inhibited adhesion of K562-GPIb/V/IX cells to bovine VWF under flow (Figure 1A) and also decreased adhesion of platelets and CHO-GPIb/IX cells by 50% (Figure 1B-C). When experiments were performed with human VWF, fewer platelets and transfected cells were captured than on bovine VWF,15 and pretreatment with RAM.1 strongly inhibited or abolished adhesion. These results obtained with different cells and VWF matrixes supported our original proposal that RAM.1-occupied GPIbβ down-regulates GPIbα–VWF interactions.
Effects of RAM.1 on GPIb-dependent adhesion to bovine and human VWF under flow conditions.
K562-GPIb/V/IX cells (A), CHO-GPIb/IX cells (B), or human platelets (C) were perfused through microcapillaries coated with 25 μg/mL bovine VWF (BVWF, upper panels) or human VWF (HVWF, lower panels). Cells (1 × 106/mL) or platelets (4 × 108/mL) resuspended in Tyrode buffer were preincubated for 10 minutes with 10 μg/mL RAM.1 (■) or control rat IgG1 (▪) and perfused at a shear rate of 150 s-1 for 10 minutes. The number of adherent cells per field was counted off-line at the indicated times. (♦) indicates adhesion to albumin-coated control microcapillaries. The rate and extent of adhesion of transfected cells or platelets was decreased by RAM.1 treatment and this effect was more pronounced on human VWF (approximately 90%-100% inhibition) than on bovine VWF (50% inhibition). Results are expressed as the means ± SEM of 4 separate experiments performed in duplicate.
Effects of RAM.1 on GPIb-dependent adhesion to bovine and human VWF under flow conditions.
K562-GPIb/V/IX cells (A), CHO-GPIb/IX cells (B), or human platelets (C) were perfused through microcapillaries coated with 25 μg/mL bovine VWF (BVWF, upper panels) or human VWF (HVWF, lower panels). Cells (1 × 106/mL) or platelets (4 × 108/mL) resuspended in Tyrode buffer were preincubated for 10 minutes with 10 μg/mL RAM.1 (■) or control rat IgG1 (▪) and perfused at a shear rate of 150 s-1 for 10 minutes. The number of adherent cells per field was counted off-line at the indicated times. (♦) indicates adhesion to albumin-coated control microcapillaries. The rate and extent of adhesion of transfected cells or platelets was decreased by RAM.1 treatment and this effect was more pronounced on human VWF (approximately 90%-100% inhibition) than on bovine VWF (50% inhibition). Results are expressed as the means ± SEM of 4 separate experiments performed in duplicate.
GPIbβ Ser166 is required for efficient GPIb-dependent adhesion and the inhibitory effect of RAM.1
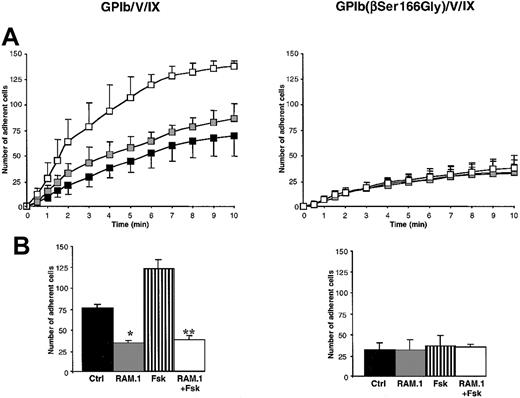
The role of the GPIbβ intracellular domain in mediating the effect of RAM.1 was assessed in experiments using cells expressing GPIb/V/IX with a GPIbβ(Ser166Gly) mutation (K562-GPIb(βSer166Gly)/V/IX cells). Ser166 was selected because of its reported phosphorylation by a cAMP-dependent kinase,21a process favoring 14.3.3ζ binding.22 Adhesion of K562-GPIb(βSer166Gly) cells to bovine or human VWF was approximately 50% less than that of cells containing the wild-type complex (P < .05) (Figure 2). Interestingly, adhesion of K562-GPIb(βSer166Gly)/V/IX cells was not modified by RAM.1 treatment. A similar decrease in adhesion was observed when the GPIbβ(Ser166Gly) mutation was introduced into CHO-GPIb/IX cells (data not shown).
Decreased adhesion to VWF and insensitivity to RAM.1 of GPIb/V/IX cells containing a GPIbβ(Ser166Gly) mutation.
K562 cells expressing the wild-type complex (GPIb/V/IX) or containing a Ser166Gly mutation of GPIbβ (GPIb(βSer166Gly)/V/IX) were perfused over a bovine (BVWF) or human VWF (HVWF) matrix at a shear rate of 150 s-1 as described in Figure 1. The cells were preincubated with 10 μg/mL RAM.1 (■) or control rat IgG1 (▪) for 10 minutes before perfusion through the VWF-coated capillaries. The number of adherent cells was counted off-line at 10 minutes. Adhesion to HSA was less than 5 cells/field. Cells containing the GPIb(βSer166Gly) mutation had approximately half the adhesive capacity of control cells on bovine or human VWF and this residual adhesion was unaffected by RAM.1 treatment. Results are expressed as the mean ± SEM of 3 separate experiments. *P < .05.
Decreased adhesion to VWF and insensitivity to RAM.1 of GPIb/V/IX cells containing a GPIbβ(Ser166Gly) mutation.
K562 cells expressing the wild-type complex (GPIb/V/IX) or containing a Ser166Gly mutation of GPIbβ (GPIb(βSer166Gly)/V/IX) were perfused over a bovine (BVWF) or human VWF (HVWF) matrix at a shear rate of 150 s-1 as described in Figure 1. The cells were preincubated with 10 μg/mL RAM.1 (■) or control rat IgG1 (▪) for 10 minutes before perfusion through the VWF-coated capillaries. The number of adherent cells was counted off-line at 10 minutes. Adhesion to HSA was less than 5 cells/field. Cells containing the GPIb(βSer166Gly) mutation had approximately half the adhesive capacity of control cells on bovine or human VWF and this residual adhesion was unaffected by RAM.1 treatment. Results are expressed as the mean ± SEM of 3 separate experiments. *P < .05.
To evaluate the effect of the mutation on adhesion at high shear, K562-GPIb/V/IX cells were preadhered at low shear (75 s-1) and then exposed to incremental increases in shear rates (Figure3). Cells with the GPIbβ(Ser166Gly) mutation were more easily detached than wild-type cells, such that approximately 55% of mutant cells were detached at 6000 s-1 compared with only 20% of wild-type cells. Defective adhesion under static or flow conditions was not due to lower levels of GPIbβ as the 2 cell lines displayed comparable surface expression of the 4 subunits (Figure 4A, upper panels).
Decreased resistance to detachment of GPIb/V/IX cells containing a GPIbβ(Ser166Gly) mutation.
K562-GPIb/V/IX (WT; ▪) or K562-GPIb(βSer166Gly)/V/IX (■) cells were perfused for 5 minutes at 75 s-1 over a bovine (BVWF) matrix followed by perfusion of buffer with incremental increases in shear rates up to 6000 s-1. At each shear rate the number of adherent cells was counted and expressed as percent of adherent cells relative to the number of adherent cells found at 75 s-1. Results are expressed as the mean ± SEM of 3 separate experiments.
Decreased resistance to detachment of GPIb/V/IX cells containing a GPIbβ(Ser166Gly) mutation.
K562-GPIb/V/IX (WT; ▪) or K562-GPIb(βSer166Gly)/V/IX (■) cells were perfused for 5 minutes at 75 s-1 over a bovine (BVWF) matrix followed by perfusion of buffer with incremental increases in shear rates up to 6000 s-1. At each shear rate the number of adherent cells was counted and expressed as percent of adherent cells relative to the number of adherent cells found at 75 s-1. Results are expressed as the mean ± SEM of 3 separate experiments.
Treatment with forskolin or PGE1 increases cAMP in K562-GPIb/V/IX cells without changing receptor surface expression.
(A) K562-GPIb/V/IX and K562-GPIb(βSer166Gly)/V/IX cells (1 × 106/mL) were treated (+Fsk) or not (-Fsk) with 50 μM forskolin. GPIb/V/IX cell surface expression was then analyzed by flow cytometry after incubation with 10 μg/mL of ALMA.12 against GPIbα (thick black line), RAM.1 against GPIbβ (gray line), ALMA.16 against GPIX (thin black line), V.1 against GPV (stippled line), or control MOPC21 (filled gray histogram). Histograms are representative of 2 separate experiments. (B) K562-GPIb/V/IX and K562-GPIb(βSer166Gly)/V/IX cells were treated with 10 μM (░) or 50 μM (▪) forskolin (Fsk) for one hour at 37°C, or with 10 μM PGE1 (PGE1) or 10 μM adrenaline (Adr) for 3 to 5 minutes at 37°C. Levels of cAMP were determined with a cAMP (125I) assay system and results are expressed as the mean ± SEM of 3 separate experiments. Surface expression of GPIb/V/IX was not affected by forskolin treatment, although forskolin and PGE1 increased cAMP levels in both cell lines.
Treatment with forskolin or PGE1 increases cAMP in K562-GPIb/V/IX cells without changing receptor surface expression.
(A) K562-GPIb/V/IX and K562-GPIb(βSer166Gly)/V/IX cells (1 × 106/mL) were treated (+Fsk) or not (-Fsk) with 50 μM forskolin. GPIb/V/IX cell surface expression was then analyzed by flow cytometry after incubation with 10 μg/mL of ALMA.12 against GPIbα (thick black line), RAM.1 against GPIbβ (gray line), ALMA.16 against GPIX (thin black line), V.1 against GPV (stippled line), or control MOPC21 (filled gray histogram). Histograms are representative of 2 separate experiments. (B) K562-GPIb/V/IX and K562-GPIb(βSer166Gly)/V/IX cells were treated with 10 μM (░) or 50 μM (▪) forskolin (Fsk) for one hour at 37°C, or with 10 μM PGE1 (PGE1) or 10 μM adrenaline (Adr) for 3 to 5 minutes at 37°C. Levels of cAMP were determined with a cAMP (125I) assay system and results are expressed as the mean ± SEM of 3 separate experiments. Surface expression of GPIb/V/IX was not affected by forskolin treatment, although forskolin and PGE1 increased cAMP levels in both cell lines.
GPIb-dependent adhesion to VWF is increased by raising cAMP levels
Since results for the GPIbβ(Ser166Gly) mutant cells indicated that lack of GPIbβ phosphorylation decreased adhesion efficiency, the consequences of increased phosphorylation were investigated by treating K562 cells expressing the wild-type or mutated complex with forskolin or PGE1. A 6-fold increase in cAMP levels using 50 μM forskolin and a 2-fold increase using 10 μM forskolin or PGE1 was observed in both cell lines (Figure 4B), whereas treatment with either reagent had no effect on surface expression of GPIb/V/IX as confirmed by fluorescence activated cell sorting (Figure 4A).
The level of GPIbβ phosphorylation was evaluated by 32P incorporation in platelets and K562-GPIb/V/IX cells. At a resting state, only a very low level of phosphorylation was observed in both platelets and cell lines (Figure 5). Treatment with forskolin or PGE1 increased phosphorylation of GPIbβ in platelets and cells expressing the wild-type complex and this effect was reversed by treatment with RAM.1. GPIbβ was not phosphorylated in cells expressing the Ser166Gly mutation.
Treatment with forskolin or PGE1 increases phosphorylation of GPIbβ in GPIb/V/IX-transfected cells and platelets, an effect reversed by RAM.1.
K562-GPIb/V/IX, K562-GPIb(βSer166Gly)/V/IX cells, or human platelets were labeled with [32P]PO4, then preincubated with 10 μg/mL RAM.1 (+) or rat control IgG (-) and treated with dimethyl sulfoxide (resting), 10 μM PGE1 (+PGE1), or 10 μM forskolin (+Fsk) for 10 minutes at 37°C. Following cell lysis, the GPIb/V/IX complex was immunoprecipitated by RAM.1 and proteins were separated on a 7.5%-15% SDS-PAGE gel. Gels were analyzed by autoradiography or using the phosphoimager system. Results are representative of 2 separate experiments. Forskolin or PGE1 treatment increased the GPIbβ phosphorylation on both platelets and K562-GPIb/V/IX cells whereas RAM.1 switched it off. In contrast, forskolin and PGE1 had no effect on phosphorylation of the GPIbβ(Ser166Gly) subunit.
Treatment with forskolin or PGE1 increases phosphorylation of GPIbβ in GPIb/V/IX-transfected cells and platelets, an effect reversed by RAM.1.
K562-GPIb/V/IX, K562-GPIb(βSer166Gly)/V/IX cells, or human platelets were labeled with [32P]PO4, then preincubated with 10 μg/mL RAM.1 (+) or rat control IgG (-) and treated with dimethyl sulfoxide (resting), 10 μM PGE1 (+PGE1), or 10 μM forskolin (+Fsk) for 10 minutes at 37°C. Following cell lysis, the GPIb/V/IX complex was immunoprecipitated by RAM.1 and proteins were separated on a 7.5%-15% SDS-PAGE gel. Gels were analyzed by autoradiography or using the phosphoimager system. Results are representative of 2 separate experiments. Forskolin or PGE1 treatment increased the GPIbβ phosphorylation on both platelets and K562-GPIb/V/IX cells whereas RAM.1 switched it off. In contrast, forskolin and PGE1 had no effect on phosphorylation of the GPIbβ(Ser166Gly) subunit.
Adhesion on VWF of platelets (Figure 6), and of cells transfected with the wild-type complex (Figures7 and 8), was increased following treatment with forskolin or PGE1 compared with untreated cells. Adhesion of platelets was increased to a similar extent with either forskolin or PGE1. Cells incubated with the higher concentration of forskolin adhered more efficiently (70%-100% increase,P < .01) than those incubated with the lower concentration of forskolin or with PGE1(P < .05). In contrast, forskolin or PGE1treatment did not influence adhesion of cells expressing the GPIbβ(Ser166Gly) mutation, despite identical rises in cAMP (Figures4B, 7, and 8). The effect of forskolin was completely abolished when K562-GPIb/V/IX cells were first treated with RAM.1 (P < .01) and adhesion levels were identical to those of cells treated with RAM.1 in the absence of forskolin (Figure7B).
Effect of treatment with forskolin or PGE1on platelet adhesion to VWF.
Washed human platelets (3 × 108/mL) were perfused for 5 minutes at a shear rate of 150 s-1 through microcapillaries coated with 1% HSA (⋄) or 25 μg/mL bovine VWF (▪, ■, ░). The cells were pretreated with 10 μM PGE1 (░), 10 μM forskolin (■), or buffer (▪). Adherent platelets were counted off-line at the indicated times and results are expressed as the mean ± SEM of 2 separate experiments.
Effect of treatment with forskolin or PGE1on platelet adhesion to VWF.
Washed human platelets (3 × 108/mL) were perfused for 5 minutes at a shear rate of 150 s-1 through microcapillaries coated with 1% HSA (⋄) or 25 μg/mL bovine VWF (▪, ■, ░). The cells were pretreated with 10 μM PGE1 (░), 10 μM forskolin (■), or buffer (▪). Adherent platelets were counted off-line at the indicated times and results are expressed as the mean ± SEM of 2 separate experiments.
Effect of forskolin on adhesion of K562-GPIb/V/IX cells to VWF under flow, sensitivity to RAM.1 treatment.
Adhesion of K562-GPIb/V/IX and K562-GPIb(βSer166Gly)/V/IX cells to microcapillaries coated with bovine VWF was followed for 10 minutes at a shear rate of 150 s-1. Adherent cells were counted off-line at the indicated times. (A) Cells were left untreated (■) or incubated for one hour at 37°C with 10 μM ( ) or 50 μM (□) forskolin (Fsk). (B) Cells incubated or not with 50 μM forskolin were further incubated for 10 minutes with 10 μg/mL RAM.1 or control rat IgG1 and perfused through bovine VWF-coated capillaries. Results are expressed as the mean ± SEM of 3 separate experiments; * P < .05, **P < .01. Treatment with forskolin significantly increased adhesion of cells transfected with the wild-type complex (P < .05), but had no effect on the adhesion of GPIb(βSer166Gly) mutant cells. In K562-GPIb/V/IX cells, RAM.1 treatment abolished the effect of forskolin.
) or 50 μM (□) forskolin (Fsk). (B) Cells incubated or not with 50 μM forskolin were further incubated for 10 minutes with 10 μg/mL RAM.1 or control rat IgG1 and perfused through bovine VWF-coated capillaries. Results are expressed as the mean ± SEM of 3 separate experiments; * P < .05, **P < .01. Treatment with forskolin significantly increased adhesion of cells transfected with the wild-type complex (P < .05), but had no effect on the adhesion of GPIb(βSer166Gly) mutant cells. In K562-GPIb/V/IX cells, RAM.1 treatment abolished the effect of forskolin.
Effect of forskolin on adhesion of K562-GPIb/V/IX cells to VWF under flow, sensitivity to RAM.1 treatment.
Adhesion of K562-GPIb/V/IX and K562-GPIb(βSer166Gly)/V/IX cells to microcapillaries coated with bovine VWF was followed for 10 minutes at a shear rate of 150 s-1. Adherent cells were counted off-line at the indicated times. (A) Cells were left untreated (■) or incubated for one hour at 37°C with 10 μM ( ) or 50 μM (□) forskolin (Fsk). (B) Cells incubated or not with 50 μM forskolin were further incubated for 10 minutes with 10 μg/mL RAM.1 or control rat IgG1 and perfused through bovine VWF-coated capillaries. Results are expressed as the mean ± SEM of 3 separate experiments; * P < .05, **P < .01. Treatment with forskolin significantly increased adhesion of cells transfected with the wild-type complex (P < .05), but had no effect on the adhesion of GPIb(βSer166Gly) mutant cells. In K562-GPIb/V/IX cells, RAM.1 treatment abolished the effect of forskolin.
) or 50 μM (□) forskolin (Fsk). (B) Cells incubated or not with 50 μM forskolin were further incubated for 10 minutes with 10 μg/mL RAM.1 or control rat IgG1 and perfused through bovine VWF-coated capillaries. Results are expressed as the mean ± SEM of 3 separate experiments; * P < .05, **P < .01. Treatment with forskolin significantly increased adhesion of cells transfected with the wild-type complex (P < .05), but had no effect on the adhesion of GPIb(βSer166Gly) mutant cells. In K562-GPIb/V/IX cells, RAM.1 treatment abolished the effect of forskolin.
Effect of PGE1 on adhesion of K562-GPIb/V/IX cells to VWF.
K562-GPIb/V/IX and K562-GPIb(βSer166Gly)/V/IX cells (1 × 106/mL) were perfused for 10 minutes at a shear rate of 150 s-1 through microcapillaries coated with 1% HSA (♦) or 25 μg/mL bovine VWF (▪,■,▵). The cells were pretreated with 10 μM PGE1 (■), 10 μM adrenaline (▵), or buffer (▪). Adherent cells were counted off-line at the indicated times and results are expressed as the mean ± SEM of 4 separate experiments. PGE1 treatment increased adhesion of K562 cells transfected with the wild-type complex but had no effect on the adhesion of cells expressing the GPIbβ Ser166Gly mutant.
Effect of PGE1 on adhesion of K562-GPIb/V/IX cells to VWF.
K562-GPIb/V/IX and K562-GPIb(βSer166Gly)/V/IX cells (1 × 106/mL) were perfused for 10 minutes at a shear rate of 150 s-1 through microcapillaries coated with 1% HSA (♦) or 25 μg/mL bovine VWF (▪,■,▵). The cells were pretreated with 10 μM PGE1 (■), 10 μM adrenaline (▵), or buffer (▪). Adherent cells were counted off-line at the indicated times and results are expressed as the mean ± SEM of 4 separate experiments. PGE1 treatment increased adhesion of K562 cells transfected with the wild-type complex but had no effect on the adhesion of cells expressing the GPIbβ Ser166Gly mutant.
The GPIbα intracellular domain is required to mediate the effect of RAM.1
The finding of a regulatory role of the GPIbβ intracellular domain and its close proximity to the GPIbα subunit suggested the possible involvement of the GPIbα intracellular domain in RAM.1 inhibition. Hence, flow studies were carried out using cells containing deletions of the entire (Δ518-610), central (Δ535-568), or C-terminal (Δ569-610) portion of the GPIbα intracellular domain. All 3 cell lines adhered with comparable efficiency and displayed kinetics similar to those of cells expressing the normal complex (Figure 9), in agreement with results for transfected CHO cells.15 Contrary to K562 cells expressing the wild-type GPIb/V/IX complex, the 3 cell lines containing GPIbα deletions did not exhibit any decrease in adhesion in the presence of RAM.1. This lack of sensitivity to RAM.1 was also observed in CHO-GPIb/IX cells expressing the same deletions (Figure10).
Effect of RAM.1 on adhesion of K562-GPIb/V/IX cells containing deletions in the intracellular domain of GPIbα.
Adhesion of K562 cells expressing GPIb/V/IX containing wild-type GPIbα (WT) or GPIbα with deletions of the entire intracellular domain (Δ518-610) or of residues 535-568 (Δ535-568) or 569-610 (Δ569-610) was followed in microcapillaries coated with 1% HSA (♦) or 25 μg/mL bovine VWF (▪,■). The cells (1 × 106/mL) were perfused through the capillaries for 10 minutes at a shear rate of 150 s-1 after preincubation for 10 minutes with 10 μg/mL RAM.1 (■) or control rat IgG1 (▪). Adherent cells were counted off-line at the indicated times and results are expressed as the mean ± SEM of 4 separate experiments. None of the deletions of the GPIbα intracellular region affected the kinetics and levels of adhesion as compared with cells containing the wild-type complex. However, all 3 mutants were resistant to treatment with RAM.1, unlike cells expressing the wild-type GPIbα sequence.
Effect of RAM.1 on adhesion of K562-GPIb/V/IX cells containing deletions in the intracellular domain of GPIbα.
Adhesion of K562 cells expressing GPIb/V/IX containing wild-type GPIbα (WT) or GPIbα with deletions of the entire intracellular domain (Δ518-610) or of residues 535-568 (Δ535-568) or 569-610 (Δ569-610) was followed in microcapillaries coated with 1% HSA (♦) or 25 μg/mL bovine VWF (▪,■). The cells (1 × 106/mL) were perfused through the capillaries for 10 minutes at a shear rate of 150 s-1 after preincubation for 10 minutes with 10 μg/mL RAM.1 (■) or control rat IgG1 (▪). Adherent cells were counted off-line at the indicated times and results are expressed as the mean ± SEM of 4 separate experiments. None of the deletions of the GPIbα intracellular region affected the kinetics and levels of adhesion as compared with cells containing the wild-type complex. However, all 3 mutants were resistant to treatment with RAM.1, unlike cells expressing the wild-type GPIbα sequence.
Effect of RAM.1 on adhesion of CHO-GPIb/IX cells containing progressive 11 amino acid deletions in the 535-590 intracellular domain of GPIbα.
CHO cells stably expressing GPIbβ/IX were transfected with GPIbα containing the deletions Δ535-568, Δ569-610, Δ535-545, Δ546-556, Δ557-568, Δ569-579, and Δ580-590. The cells were preincubated for 10 minutes with 10 μg/mL RAM.1 (■) or control rat IgG1 (▪) and perfused through microcapillaries coated with bovine VWF at a shear rate of 150 s-1 for 10 minutes. Adherent cells were counted off-line at 10 minutes and results are expressed as the mean ± SEM of 4 separate experiments; *P < .05. Cells carrying mutant GPIbα displayed comparable levels of adhesion in the presence or absence of RAM.1, except those containing Δ569-579, which behaved like control cells and exhibited approximately 50% lower adhesion in the presence of RAM.1.
Effect of RAM.1 on adhesion of CHO-GPIb/IX cells containing progressive 11 amino acid deletions in the 535-590 intracellular domain of GPIbα.
CHO cells stably expressing GPIbβ/IX were transfected with GPIbα containing the deletions Δ535-568, Δ569-610, Δ535-545, Δ546-556, Δ557-568, Δ569-579, and Δ580-590. The cells were preincubated for 10 minutes with 10 μg/mL RAM.1 (■) or control rat IgG1 (▪) and perfused through microcapillaries coated with bovine VWF at a shear rate of 150 s-1 for 10 minutes. Adherent cells were counted off-line at 10 minutes and results are expressed as the mean ± SEM of 4 separate experiments; *P < .05. Cells carrying mutant GPIbα displayed comparable levels of adhesion in the presence or absence of RAM.1, except those containing Δ569-579, which behaved like control cells and exhibited approximately 50% lower adhesion in the presence of RAM.1.
In an attempt to identify discrete residues required for transmission of the inhibitory effect of RAM.1, experiments were performed using cells containing progressive 11-12 amino acid deletions in the 535 to 590 region of GPIbα. As previously reported,11comparable levels of adhesion were observed for control cells and all cell lines expressing deletions (Figure 10). Pretreatment of these cells with RAM.1 did not significantly influence their kinetics and levels of adhesion, with the exception of the Δ569-579 clone, which displayed 50% lower adhesion and behaved similarly to cells containing the wild-type complex. These results indicate that a major portion of the intracellular domain of GPIbα is required to convey the inhibitory signal triggered by RAM.1.
Discussion
This study supports a functional role of GPIbβ and the involvement of its intracellular domain in regulating GPIbα-dependent adhesion to VWF. Experiments with the MoAb RAM.1 directed against the extracellular domain of GPIbβ and with cell lines expressing the GPIb/V/IX complex containing mutations in the intracellular domain of GPIbβ or GPIbα provided evidence for (1) a regulatory role of GPIbβ, which is dependent on the degree of phosphorylation of Ser166 in the intracellular domain and (2) cross-talk between GPIbβ and GPIbα, requiring an intact GPIbα intracellular domain.
Our original observation that RAM.1 inhibited platelet VWF binding, VWF-induced aggregation, and adhesion to a VWF matrix was unexpected because GPIbα was viewed as the only subunit capable of mediating platelet interactions with VWF. This study confirms and extends our previous results by demonstrating an effect of RAM.1 independent of the cell type, leading to inhibition of the adhesion of not only platelets but also K562-GPIb/V/IX– and CHO-GPIb/IX–transfected cells. A further observation is that RAM.1 was a more efficient inhibitor when experiments were carried out using a human VWF matrix (70%-80% inhibition) rather than bovine VWF (50% inhibition), in keeping with the lower capturing efficiency of the human matrix.15These results did not yet clarify the roles played by each subunit of the GPIb/V/IX complex and which domains were involved in conveying the negative signal of RAM.1.
Since GPIbβ has no direct VWF binding properties, we hypothesized that RAM.1 could act indirectly by modifying GPIbα-dependent adhesion. Intracellular regulation of GPIbα-dependent binding has been recently suggested in relation to its association with the cytoskeleton or adaptor proteins.15,28,31 A possible mechanism that was tested in this study was that GPIbβ could cooperate with GPIbα intracellularly. Reports of binding sites for the dimeric 14.3.3ζ adaptor protein on the GPIbα (amino acid [aa] 605-610) and GPIbβ (aa 160-175) intracellular domains suggested a putative link between the 2 subunits.12,13 The 14.3.3ζ binding site on GPIbβ includes a serine at position 166, a residue phosphorylated by a cAMP-dependent mechanism.21Interestingly, we found that blocking GPIbβ Ser166 phosphorylation with a glycine substitution decreased cell adhesion to VWF and the remaining adhesion was unaffected by treatment with RAM.1. This indicated that optimal adhesion and sensitivity to RAM.1 both required phosphorylation at position 166 of GPIbβ. It also suggested that a basal level of adhesion provided by GPIbα could be independent of its association with GPIbβ. Confirmation of the latter in a cellular system would require testing cells lacking the whole GPIbβ subunit. In the absence of GPIbβ, levels of GPIbα surface expression are, however, too low in our experience to allow sufficient cell attachment and a meaningful comparison.
One possible explanation for the decreased adhesion of the GPIb(βSer166Gly) mutant could relate to defective 14.3.3 binding. However, we observed normal GPIb/IX-14.3.3ζ coprecipitation in the GPIbβ(Ser166Gly) cell line (P.M., unpublished results, March 2002) and similar replacement of Ser166 by alanine only reduced interaction with 14.3.3ζ in a 2-hybrid system by 20% to 40%.30 Furthermore, several deletions of GPIbα that completely prevented 14.3.3 binding did not modify adhesion as compared with cells expressing the wild-type complex.31 On the other hand, increasing GPIbβ Ser166 phosphorylation has been reported to enhance binding of GPIbβ to 14.3.3ζ. In this study, treatment of platelets, CHO cells, or K562 cells expressing GPIb/(V)/IX with forskolin or PGE1 significantly increased phosphorylation of GPIbβ and adhesion to VWF, demonstrating the regulatory importance of this phosphorylated residue. RAM.1 treatment reversed these effects, suggesting that sensitivity to RAM.1 correlates with the degree of GPIbβ Ser166 phosphorylation. In these conditions, phosphorylation of GPIbβ seems to provide optimal initial interaction between GPIb/V/IX and VWF.
These results would appear to contradict an earlier report of decreased shear-induced aggregation of platelets treated with PGI2, another PKA activator,22 or a recently published report of decreased VWF-dependent adhesion of platelets and CHO-GPIb/IX treated with forskolin.32 Shear-induced aggregation results from both GPIb–VWF interaction and αIIbβ3 integrin activation, which is down-regulated by agents raising cAMP. We examined adhesion of GPIb/(V)/IX-transfected cells under conditions independent of integrin mobilization. In the more recent study, adhesion of platelets (but not that of GPIb/IX-transfected cells) was performed in the presence of integrin blockade. An important difference relates to the level of GPIbβ phosphorylation observed in platelets and GPIb/IX-transfected cells. Bodnar et al32 using a phosphopeptide-specific antibody concluded on a high level of GPIbβ phosphorylation in platelets and full phosphorylation in CHO-GPIb/IX cells at a resting state and proposed that this was responsible for the low adhesion efficiency of the CHO-GPIb/IX cells. It is our observation and that of other groups that GPIb/(V)/IX-transfected cells have acquired the ability to efficiently bind and/or adhere to VWF.14,15 33 In this study we observed, using direct measurement of 32P incorporation, only a very low level of GPIbβ phosphorylation in resting platelets, K562-GPIb/V/IX–transfected cells (Figure 5), and CHO-GPIb/IX cells (data not shown), and that all 3 cells have an intrinsic capacity to bind VWF and adhere under flow conditions, which is further enhanced by PGE1 or forskolin treatment.
The results obtained with RAM.1 and mutant GPIbβ suggested a model whereby direct or indirect interaction of the GPIbβ intracellular domain with GPIbα would modulate VWF-dependent adhesion. Studies of cells expressing complete or partial deletions of the GPIbα intracellular sequence showed that this domain was essential to sustain RAM.1 inhibition and pointed to the existence of cross-talk between the GPIbα and GPIbβ intracellular domains. This was indicated by the normal levels of adhesion of all cell lines containing mutant receptors, which became completely resistant to inhibition by RAM.1 despite normal binding of the MoAb. Only one construct behaved like the wild-type sequence, the GPIbαΔ569-579 deletion. Hence, no discrete subdomain could be pinpointed for the effect of RAM.1, which appears to require most of the intracellular sequence of GPIbα.
The normal adhesion and absence of sensitivity to RAM.1 of cells lacking the intracellular region of GPIbα suggest that this domain could negatively control VWF-dependent adhesion. According to this model, an increase in cAMP would relieve the control by phosphorylating GPIbβ Ser166. Another possibility is that cAMP could phosphorylate the GPIbα intracellular domain that contains 8 threonine and 10 serine residues. To date only Ser609 at the C-terminal end of GPIbα has been found to be phosphorylated in platelets under resting conditions and was proposed to bind 14.3.3ζ and regulate platelet adhesion.34 Several deletions of GPIbα that prevent RAM.1 inhibition such as GPIbαΔ557-568 and Δ535-568 have also been reported to prevent interaction with filamin-1,28suggesting that it could be involved in transmitting the effect of RAM.1. However, other deletions that preserve normal filamin-1 binding, such as GPIbαΔ591-610, Δ535-545, Δ546-556, and Δ580-590, also fail to exhibit inhibition by RAM.1. Moreover, the fact that filamin-1 interacts with the GPIbα intracellular region but not with GPIbβ does not support a role of filamin as a functional switch between the 2 subunits.
As stated earlier, 14.3.3ζ binds to and could bridge the GPIbα and GPIbβ subunits. However, mutations of GPIbβ Ser166 and deletions in the 535-568 region of GPIbα, both preserving 14.3.3ζ binding,31 were refractory to the effect of RAM.1, which does not favor involvement of the adaptor protein. Calmodulin has recently been identified as a third intracellular binding partner of the GPIb/V/IX complex,23 but its apparent lack of interaction with GPIbα would exclude a role in bridging to the GPIbβ subunit.
The exact mechanism by which RAM.1 regulates the adhesive properties of GPIbα is still not clear but could involve decreases in affinity or avidity for VWF. Gain-of-function mutations within the cysteine loops in platelet-type von Willebrand disease (GPIbαGly233Val, Met239Val) suggest that GPIbα could exist in at least 2 conformational states (low and high affinity).35 Furthermore, an intramolecular interaction between the 1-81 and 201-268 regions of resting GPIbα was recently identified and could be destroyed by addition of ristocetin or application of shear.36 The recently published crystal structure of the GPIbα N-terminal domain is compatible with regulated exposure of the VWF binding site by an unmasking mechanism.37,38 This raises the possibility of an inside-out regulation of the adhesive properties of GPIbα as has been documented for other adhesive receptors. For example, deletions within the intracellular domain of selectins affect cell rolling and attachment on their counterreceptors.39 Intracellular deletions in the α or β subunits of integrin heterodimers can negatively or positively influence their adhesive properties and binding affinity.40 Opposite effects of RAM.1 treatment or GPIbβ phosphorylation on VWF binding could potentially result in similar opening or closure of a GPIbα binding cavity.
The authors thank G. Kauffenstein for advice in measurement of intracellular cAMP and J. Mulvihill for correcting the English of the manuscript.
Prepublished online as Blood First Edition Paper, January 9, 2003; DOI 10.1182/blood-2002-06-1847.
Supported by the Association de Recherche et de développement en Médecine et Santé publique (ARMESA) (C.P. and P.M.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
François Lanza, INSERM U311, Etablissement Français du Sang-Alsace, 10 rue Spielmann, BP 36, 67065, Strasbourg Cedex, France; e-mail:francois.lanza@efs-alsace.fr.




![Fig. 5. Treatment with forskolin or PGE1 increases phosphorylation of GPIbβ in GPIb/V/IX-transfected cells and platelets, an effect reversed by RAM.1. / K562-GPIb/V/IX, K562-GPIb(βSer166Gly)/V/IX cells, or human platelets were labeled with [32P]PO4, then preincubated with 10 μg/mL RAM.1 (+) or rat control IgG (-) and treated with dimethyl sulfoxide (resting), 10 μM PGE1 (+PGE1), or 10 μM forskolin (+Fsk) for 10 minutes at 37°C. Following cell lysis, the GPIb/V/IX complex was immunoprecipitated by RAM.1 and proteins were separated on a 7.5%-15% SDS-PAGE gel. Gels were analyzed by autoradiography or using the phosphoimager system. Results are representative of 2 separate experiments. Forskolin or PGE1 treatment increased the GPIbβ phosphorylation on both platelets and K562-GPIb/V/IX cells whereas RAM.1 switched it off. In contrast, forskolin and PGE1 had no effect on phosphorylation of the GPIbβ(Ser166Gly) subunit.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/9/10.1182_blood-2002-06-1847/4/m_h80934215005.jpeg?Expires=1767798832&Signature=MwUiWsJWq~Mc~sfCgQd4rYHvEI25EVdhAkWn92LCTRGvUZZep3yhSeAS5025K5fAXNfrs4MyUPAWpbS4wMBTRXCX6FmHIPVl3Jc4a3Uz7FapyO6QwgYqe0Izu14~WuRtLFEMbwQSFPD9ynLwP~qeVDRF9y4rbrjDD3HLORyWQywOWsloPi1Kr3YxqmJO3cl4qxxm7fuNhE6mTwOlylFmKJ1sT2wGPar3yKHXEkXeqvpa0dZ6FtpjnCHrsMyF8F~ftt-NPcwGhiZGtjKSddz5rBNGW4L3Mg1s98DgCi2KioAWoF-PkcIpVyJ~HdVfp-oTm9a-08k8K9-~ZWAmM0jVPQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal