Intravenous immunoglobulin (IVIG) is used to treat immune thrombocytopenia resulting from a variety of autoimmune and nonautoimmune diseases such as idiopathic thrombocytopenic purpura (ITP), heparin-induced thrombocytopenia, and posttransfusion purpura. IVIG is a limited resource and although considered safe, may nevertheless carry some risk of transferring disease. Its high cost makes monoclonal antibodies, capable of mimicking the clinical effects of IVIG, highly desirable. We show here, using a murine model of ITP, that selected monoclonal antibodies can protect against thrombocytopenia. SCID mice were pretreated with 1 of 21 monoclonal antibodies before induction of thrombocytopenia by antiplatelet antibody. Four antibodies reacted with the CD24 antigen on erythrocytes. Two antibodies were of the IgM class, and although one IgM antibody caused a minimal degree of anemia (P < .05), neither antibody ameliorated immune thrombocytopenia. One of 2 anti-CD24 antibodies of the IgG class ameliorated immune thrombocytopenia and blocked reticuloendothelial system function at the same doses that protected against thrombocytopenia. Some antibodies reactive with other circulating cell types also protected against immune-mediated thrombocytopenia while no antibody without a distinct target antigen in the mice was protective. Protective monoclonal antibodies significantly prevented thrombocytopenia at down to a 1000-fold lower dose (200 μg/kg) as compared with standard IVIG treatment (2 g/kg). It is concluded that monoclonal IgG with specificity for a circulating cellular target antigen may provide an alternative therapeutic approach to treating immune thrombocytopenia.
Introduction
Intravenous immunoglobulin (IVIG) is prepared from large pools of human plasma from more than 5000 normal healthy donors. Most preparations consist of IgG with low levels of “contaminants” such as IgM. The IgG is present in predominantly monomeric form, with a subclass distribution characteristic of normal serum. IVIG has been used successfully to treat idiopathic thrombocytopenic purpura (ITP) since 1981, where it was initially reported that high doses of IVIG promoted fast recovery of ITP in children.1 Despite its extensive clinical use, the mechanism of action of IVIG remains unclear.
In addition to IVIG, intravenous preparations of human immunoglobulin with specificity for erythrocytes have also been demonstrated to effectively inhibit immune thrombocytopenia. In particular, polyclonal anti-D can reverse thrombocytopenia in patients with ITP who express the D antigen.2-11 In one study, 3 D-negative but c antigen–positive patients were successfully treated with anti-c.5 Thus, an antibody reactive with an Rh antigen on erythrocytes (whether to the D or c antigen or possibly any other appropriate antigen) can potentially reverse immune thrombocytopenia. Although the mechanism of action of IVIG is controversial, one of the major proposed mechanisms of action for both IVIG and anti-D is via reticuloendothelial system (RES) blockade by immune complexes. This theory has been strengthened by studies showing that anti-D appears to be ineffective in most patients who are Rh D antigen–negative,3,5,12 which suggests a requirement for antibody/antigen binding. A small prospective study to test a single human (IgG1) monoclonal anti-D in 7 D-positive patients with chronic ITP was unsuccessful.13 The ability of this monoclonal anti-D antibody to block RES function in the treated patients was not evaluated; however, the failure of this monoclonal antibody to ameliorate ITP, and even the concept of testing monoclonal antibodies in ITP therapy, has generated much criticism.14-17 Because of the failed trial with this one antibody and the resultant controversy,13-17 it would not be considered ethical to test other such antibodies in humans. In this report, we revisit and challenge the hypothesis that monoclonal antibodies are not useful in treating immune forms of thrombocytopenia. We clearly demonstrate that some, but not all, monoclonal IgGs that bind cell surface–associated antigens can protect against immune thrombocytopenia in a well-described murine model of ITP.18-20 These monoclonal antibodies that mimic the effect of IVIG may offer an alternative therapeutic approach to polyclonal IVIG or anti-D preparations.
Materials and methods
Reagents
The antimouse CD24 (IgM, clone IOTHSA) was purchased from Beckman Coulter (Mississauga, ON, Canada). Monoclonal antirat κ and λ light chains (clones RT-39 and RL-6) and the PKH26 red fluorescent cell linker kit were purchased from Sigma (Oakville, ON, Canada). The monoclonal antibody specific for integrin αIIb(antiglycoprotein IIb, clone MWReg30; rat IgG1, κ), the antibody against integrin β3 (antiglycoprotein IIIa, clone 2C9.G2; hamster IgG, group 1, κ), and all other antimouse monoclonal antibodies (Table 1) were purchased from BD PharMingen (Mississauga, ON). The IVIG used (Gamimune, 5%) was from Bayer (Elkhart, IN). Human serum albumin was from Miles Laboratories Canada (Etobicoke, ON).
Characteristics of monoclonal antibodies tested in the amelioration of murine ITP
| Tissue distribution of antigen in SCID mice . | Efficacy in ITP* . | Monoclonal antibodies . | Isotype . | Clone . | ||
|---|---|---|---|---|---|---|
| 50 μg . | 5 μg . | 0.5 μg . | ||||
| None | − | − | − | IgG1isotype control | IgG1, λ | A110-1 |
| None | − | − | − | IgG2a isotype control | IgG2a, κ | A110-2 |
| None | − | − | − | IgG2b isotype control | IgG2b, κ | A95-1 |
| None | − | − | − | IgG2c isotype control | IgG2c, κ | A23-1 |
| None | − | − | − | Anti–IL-2 | IgG2a | JES6-1A12 |
| None | − | − | − | Anti-IgG2a/2b | IgG1, κ | R2-40 |
| None | − | − | − | Anti-IgA | IgG1, κ | C10-1 |
| None | − | − | − | Anti-IgG1 | IgG2, κ | A85-1 |
| None | − | − | − | Anti-IgD | IgG2a, κ | 11-26c.2a |
| RBC | + | ± | − | Anti–TER-119 | IgG2b | TER-119 |
| RBC, PMN, Mφ | + | + | − | Anti-CD24 | IgG2b, κ | M1/69 |
| RBC, PMN, Mφ | − | − | − | Anti-CD24 | IgG2c, κ | 30-F1 |
| RBC, PMN, Mφ | − | − | − | Anti-CD24 | IgM, κ | J11d |
| RBC, PMN, Mφ | − | − | − | Anti-CD24 | IgM | IOTHSA |
| All cells† | + | + | − | Anti-CD44 | IgG1, κ | KM114 |
| All cells† | − | − | − | Anti-CD44 | IgG2b, κ | IM7 |
| All cells | − | − | − | Anti-CD47 | IgG2a, κ | Miap301 |
| NK, Mφ, PMN | + | − | − | Anti-CD16/32 | IgG2b, κ | 2.4G2 |
| RBC, PMN, Mφ | − | − | − | Anti-CD126 | IgG2b, κ | D7715A7 |
| PMN, Mφ, NK | − | − | − | Anti-CD11b | IgG2b, κ | M1/70 |
| Mφ, PMN | − | − | − | Anti-CD21/35 | IgG2b, κ | 7G6 |
| Tissue distribution of antigen in SCID mice . | Efficacy in ITP* . | Monoclonal antibodies . | Isotype . | Clone . | ||
|---|---|---|---|---|---|---|
| 50 μg . | 5 μg . | 0.5 μg . | ||||
| None | − | − | − | IgG1isotype control | IgG1, λ | A110-1 |
| None | − | − | − | IgG2a isotype control | IgG2a, κ | A110-2 |
| None | − | − | − | IgG2b isotype control | IgG2b, κ | A95-1 |
| None | − | − | − | IgG2c isotype control | IgG2c, κ | A23-1 |
| None | − | − | − | Anti–IL-2 | IgG2a | JES6-1A12 |
| None | − | − | − | Anti-IgG2a/2b | IgG1, κ | R2-40 |
| None | − | − | − | Anti-IgA | IgG1, κ | C10-1 |
| None | − | − | − | Anti-IgG1 | IgG2, κ | A85-1 |
| None | − | − | − | Anti-IgD | IgG2a, κ | 11-26c.2a |
| RBC | + | ± | − | Anti–TER-119 | IgG2b | TER-119 |
| RBC, PMN, Mφ | + | + | − | Anti-CD24 | IgG2b, κ | M1/69 |
| RBC, PMN, Mφ | − | − | − | Anti-CD24 | IgG2c, κ | 30-F1 |
| RBC, PMN, Mφ | − | − | − | Anti-CD24 | IgM, κ | J11d |
| RBC, PMN, Mφ | − | − | − | Anti-CD24 | IgM | IOTHSA |
| All cells† | + | + | − | Anti-CD44 | IgG1, κ | KM114 |
| All cells† | − | − | − | Anti-CD44 | IgG2b, κ | IM7 |
| All cells | − | − | − | Anti-CD47 | IgG2a, κ | Miap301 |
| NK, Mφ, PMN | + | − | − | Anti-CD16/32 | IgG2b, κ | 2.4G2 |
| RBC, PMN, Mφ | − | − | − | Anti-CD126 | IgG2b, κ | D7715A7 |
| PMN, Mφ, NK | − | − | − | Anti-CD11b | IgG2b, κ | M1/70 |
| Mφ, PMN | − | − | − | Anti-CD21/35 | IgG2b, κ | 7G6 |
+ denotes that the antibody effected a significant amelioration of thrombocytopenia at that dose; ±, the antibody effected a slight amelioration of thrombocytopenia at that dose; −, the antibody did not ameliorate thrombocytopenia at that dose; RBC, red blood cell; PMN, polymorphonuclear leukocyte; Mφ, monocyte/macrophage; and NK, natural killer cell.
ITP was induced in SCID mice by intraperitoneal injection of anti-αIIb antibody.
Except RBC and endothelial cells.
Mice
Severe combined immunodeficient (SCID) virgin female mice (6 to 8 weeks of age) were purchased from Charles River Laboratories (Montreal, QC, Canada), and female BALB/c mice (8 to 12 weeks of age) were from the Jackson Laboratory (Bar Harbor, ME). All mice were housed in the St Michael's Hospital Research Vivarium, and SCID mice were housed under gnotobiotic conditions.
Induction and treatment of passive-immune thrombocytopenia
As previously described,18 ITP was induced in mice by intraperitoneal injection of antiplatelet antibody (2 μg MWReg30 or 10 μg 2C9.G2) in 200 μL phosphate-buffered saline [PBS], pH 7.2; 24 hours later, 100 μL whole blood was collected via the tail vein into microvette tubes (Sarstedt, Montreal, QC, Canada) preloaded with 10 μL of 1% EDTA (ethylenediaminetetraacetic acid) in PBS. Then, 5 μL of this mouse blood was diluted into 100 μL of 1% EDTA/PBS. The blood was then further diluted in PBS to a final dilution of 1:12 000. The samples were acquired for 2 minutes on a flow rate–calibrated FACScan flow cytometer (Becton Dickinson, San Jose, CA) using forward scatter (FSC) versus side scatter (SSC) to gate and count the platelets as previously detailed.18Reference samples were incubated with fluorescein isothiocyanate (FITC)–conjugated antimouse platelet antibody to identify the platelet population. For IVIG pretreatment, mice were injected intraperitoneally with 1 mL of 5% IVIG (about 2 g/kg) 24 hours prior to induction of ITP. Initial experiments demonstrated that the protective effect of IVIG was equally successful whether the intraperitoneal or intravenous route (3 injections of 333 μL over an 8-hour period) was used (data not shown); we have employed the intraperitoneal route for IVIG itself due to the large volume (1 mL per mouse) of IVIG injected. For pretreatment with monoclonal antibodies, mice were injected intravenously with the indicated amount of monoclonal antibody in a volume of 200 μL 24 hours prior to induction of ITP.
Antibody binding to erythrocytes
Antibody binding was assessed by flow cytometry. For the in vitro binding, washed erythrocytes (10 μL) were incubated with 2 μL of the indicated concentration of monoclonal antibodies in 96-well round-bottomed microtiter plates and gently swirled at 22°C for 30 minutes. The cells were then washed twice with 250 μL PBS and the pellets incubated with 10 μg/mL FITC-labeled antirat IgG (H+L) in a volume of 100 μL for 30 minutes at 22°C. The cells were washed a further 3 times, transferred to tubes, and acquired on FACScan flow cytometer.
To assess in vivo binding, the indicated antierythrocyte monoclonal antibody was intravenously administered (50 μg per mouse) to SCID mice. Twenty-four hours later, 50 μL blood was withdrawn from the tail vein into 5 μL of 1% EDTA, and 2 μL of this blood was incubated with 1 μg FITC-labeled antirat IgG (H+L) for 30 minutes in a volume of 100 μL at 22°C. Cells were washed 3 times with PBS prior to acquisition on a FACScan flow cytometer.
RES blockade
Whole blood (2 mL, diluted with 1:5 volume 1% EDTA in PBS) from unmanipulated SCID mice was pooled and centrifuged at 200gfor 15 minutes to obtain 1 mL packed erythrocytes. These packed erythrocytes were resuspended in 4 mL PBS and incubated with 10 μg anti–TER-119 antibody (Table 1) at 22°C for 30 minutes. The resulting opsonized erythrocytes were then washed twice with PBS and labeled with a fluorescent marker (PKH26 Kit, Sigma, St Louis, MO) according to the manufacturer's directions. Briefly, the opsonized erythrocytes were resuspended in 3 mL PKH26 diluent C and mixed with another 4 mL diluent C containing 10 μL of the “PKH26 linker.” After a 5-minute incubation with constant swirling, the mixture was incubated for 5 minutes with an equal volume of PBS containing 1% bovine serum albumin. The erythrocytes were washed 5 times and resuspended in 2 mL PBS. Mice were then injected via the tail vein with 200 μL of these labeled cells. All mice were bled via the tail vein at 3 minutes, 10 minutes, 30 minutes, 120 minutes, and 960 minutes after injection, and the total number of erythrocytes, as well as the percent of PKH26-fluorescent erythrocytes, were counted by flow cytometry. The percentage of fluorescent erythrocytes at the 3-minute time point was considered to be 100%.
Statistical analysis
Unless otherwise indicated, data are expressed as mean ± SEM. The Student t test was used to evaluate the significance of observed differences between the groups. The significance level was set at P < .05.
Results
Monoclonal antibodies with specificity for erythrocytes can inhibit ITP
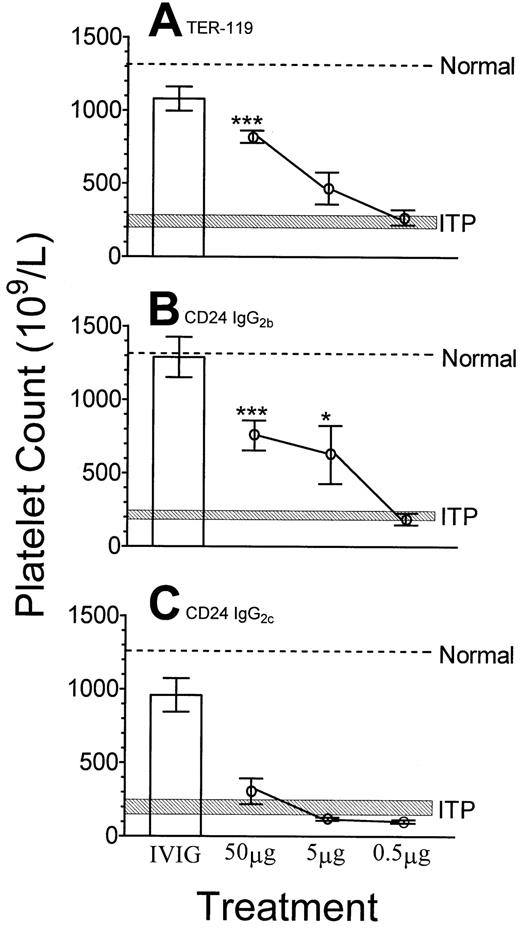
As shown in Figure 1, SCID mice injected with a rat anti-αIIb antibody became thrombocytopenic by 24 hours (Figure 1, shaded area), compared with unmanipulated control mice (Figure 1, dashed line). However, SCID mice pretreated with 50 mg IVIG per mouse (about 2 g/kg body weight) 24 hours prior to administration of the anti-αIIb antibody were significantly protected from thrombocytopenia (Figure 1, column 1 for all panels). SCID mice pretreated with 50 μg anti–TER-119 monoclonal antibody were significantly protected from ITP, compared with untreated mice (Figure 1A). SCID mice pretreated with an anti-CD24 monoclonal antibody (IgG2b) were also significantly protected against ITP at dosages of 50 μg per mouse and 5 μg per mouse (Figure 1B). In contrast, as demonstrated in Figure 1C, SCID mice treated with a different anti-CD24 antibody (IgG2c) or with 2 IgM anti-CD24 antibodies (Table 1) were not protected from thrombocytopenia at any dosage. Nine monoclonal antibodies that do not have specificity for a corresponding target antigen in SCID mice failed to prevent ITP in dosages up to 50 μg per mouse (about 2 mg/kg body weight) (Table 1).
Administration of monoclonal antibodies with specificity for erythrocytes can inhibit immune thrombocytopenia in SCID mice.
The indicated dose of monoclonal antibody or 50 mg IVIG (■) was administered to SCID mice followed by anti-αIIb antibody at 24 hours. After a further 24 hours, platelet counts were performed. (A) Anti–TER-119; (B) anti-CD24 (IgG2b); (C) anti-CD24 (IgG2c). Normal, the dotted line indicates the mean platelet count of unmanipulated mice; ITP, the hatched rectangle indicates the range of the mean platelet count (± 1 SEM) of mice treated with antiplatelet antibody only. The x-axis denotes the treatments given to mice (50 mg IVIG or the indicated dose of monoclonal antibody); the y-axis denotes the platelet count; n = 9 for each data point. *P < .05; ***P < .001 as compared with ITP mice. Error bars indicate SEM.
Administration of monoclonal antibodies with specificity for erythrocytes can inhibit immune thrombocytopenia in SCID mice.
The indicated dose of monoclonal antibody or 50 mg IVIG (■) was administered to SCID mice followed by anti-αIIb antibody at 24 hours. After a further 24 hours, platelet counts were performed. (A) Anti–TER-119; (B) anti-CD24 (IgG2b); (C) anti-CD24 (IgG2c). Normal, the dotted line indicates the mean platelet count of unmanipulated mice; ITP, the hatched rectangle indicates the range of the mean platelet count (± 1 SEM) of mice treated with antiplatelet antibody only. The x-axis denotes the treatments given to mice (50 mg IVIG or the indicated dose of monoclonal antibody); the y-axis denotes the platelet count; n = 9 for each data point. *P < .05; ***P < .001 as compared with ITP mice. Error bars indicate SEM.
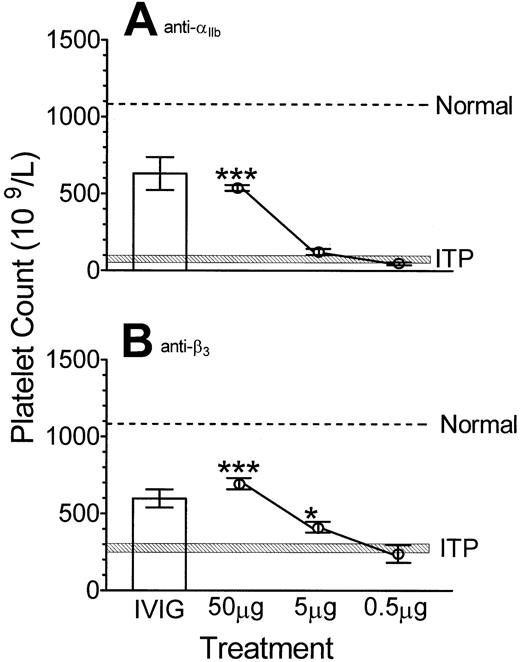
The ability of monoclonal antibodies to ameliorate thrombocytopenia in normal mice induced by 2 different antiplatelet antibodies was also examined. BALB/c mice pretreated with 50 μg anti–TER-119 monoclonal antibody were significantly protected from anti-αIIb–induced thrombocytopenia (Figure2A). In addition, 50 μg anti-CD24 (IgG2b) antibody inhibited thrombocytopenia in both BALB/c mice as well as CD-1 mice (data not shown). BALB/c mice pretreated with 50 μg as well as 5 μg anti–TER-119 monoclonal antibody were significantly protected from anti-β3–induced thrombocytopenia (Figure 2B).
Monoclonal antibodies can inhibit immune thrombocytopenia in normal mice induced by either anti-αIIb or anti-β3 antibody.
The indicated dose of monoclonal antibody anti–TER-119 or 50 mg IVIG (■) was administered to BALB/c mice followed by (A) anti-αIIb or (B) anti-β3 antibody at 24 hours. After a further 24 hours, platelet counts were performed. Normal, ITP, x-axis and y-axis are as described in Figure 1; n = 7 for each data point. *P < .05; ***P < .001 as compared with ITP mice. Error bars indicate SEM.
Monoclonal antibodies can inhibit immune thrombocytopenia in normal mice induced by either anti-αIIb or anti-β3 antibody.
The indicated dose of monoclonal antibody anti–TER-119 or 50 mg IVIG (■) was administered to BALB/c mice followed by (A) anti-αIIb or (B) anti-β3 antibody at 24 hours. After a further 24 hours, platelet counts were performed. Normal, ITP, x-axis and y-axis are as described in Figure 1; n = 7 for each data point. *P < .05; ***P < .001 as compared with ITP mice. Error bars indicate SEM.
The above therapeutic antibodies were evaluated for their ability to bind to erythrocytes, to induce anemia or, in the case of the IgG antibodies, to block the RES. The rank order of the ability of antibodies to bind to erythrocytes as assessed by flow cytometry was as follows (mean log fluorescence intensity ± the SEM): TER-119 (484.2 ± 37.9) more than CD24 (IgG2b) (353.1 ± 40.7), more than CD24 (IgG2c) (96.7 ± 36.0), more than CD24 (IgM, clone IOTHSA) (26.5 ± 7.5), more than CD24 (IgM, clone J11d) (3.1 ± 0.2), and more than IgG control (1.8 ± 0.06). In vivo, the TER-119 antibody also bound erythrocytes with the highest avidity (18.3 ± 3.1) followed by anti-CD24 IgG2b(11.8 ± 1.5), about the same as anti-CD24 IgG2c (11.1 ± 2.1), and more than IgG control (2.1 ± 0.2). Thus, the anti–TER-119 antibody and both anti-CD24 antibodies (IgG2b and IgG2c) successfully sensitized erythrocytes under in vivo therapeutic conditions.
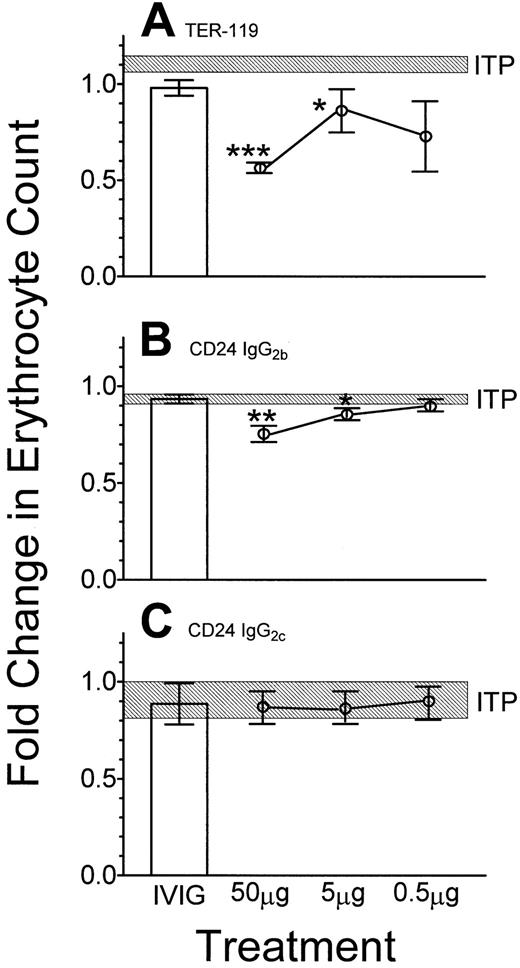
Because treatment of ITP with anti-D may be associated with anemia, mice were evaluated for the number of circulating erythrocytes 24 hours following injection of the antierythrocyte antibodies. Anti–TER-119 (Figure 3A) and anti-CD24 (IgG2b) (Figure 3B) induced significant decreases (50% and 25%, respectively) in the number of circulating erythrocytes. The IgM anti-CD24, clone IOTHSA, (not shown) also induced a significant decrease in erythrocyte numbers at 5 μg per mouse. In contrast, this was not seen with the IgG2c (Figure 3C) or the J11d IgM clone of anti-CD24 (not shown).
Effect of erythrocyte-reactive monoclonal antibodies on the number of circulating erythrocytes.
Fifty μg of the indicated monoclonal antibody or 50 mg IVIG (■) was administered intravenously 24 hours prior to injection of antiplatelet antibody. Twenty-four hours later, blood was sampled and the erythrocyte count was assessed by flow cytometry. (A) Anti–TER-119; (B) anti-CD24 (IgG2b); (C) anti-CD24 (IgG2c). The data are presented as fold change over the untreated group. The hatched rectangle indicates the range of the mean fold change of erythrocytes (± 1 SEM) of mice treated with antiplatelet antibody only; n = 9 for each data point. *P < .05; **P < .01; ***P < .001 as compared with ITP mice. Error bars indicate SEM.
Effect of erythrocyte-reactive monoclonal antibodies on the number of circulating erythrocytes.
Fifty μg of the indicated monoclonal antibody or 50 mg IVIG (■) was administered intravenously 24 hours prior to injection of antiplatelet antibody. Twenty-four hours later, blood was sampled and the erythrocyte count was assessed by flow cytometry. (A) Anti–TER-119; (B) anti-CD24 (IgG2b); (C) anti-CD24 (IgG2c). The data are presented as fold change over the untreated group. The hatched rectangle indicates the range of the mean fold change of erythrocytes (± 1 SEM) of mice treated with antiplatelet antibody only; n = 9 for each data point. *P < .05; **P < .01; ***P < .001 as compared with ITP mice. Error bars indicate SEM.
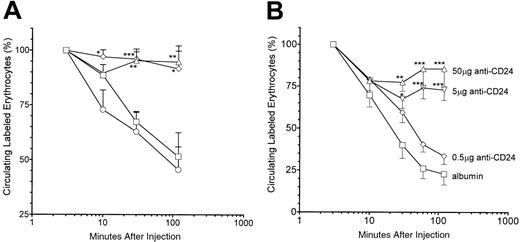
The IgG2b and IgG2c anti-CD24 antibodies were tested for their ability to inhibit RES function as assessed by clearance of TER-119 antibody–sensitized erythrocytes. Both IVIG and the IgG2b anti-CD24 significantly blocked RES function as reflected by the impaired ability of anti-CD24–treated mice to clear sensitized erythrocytes as compared with albumin-treated mice (Figure4A). In contrast, the IgG2canti-CD24 had no measurable effect on RES function (Figure 4A). The TER-119 antibody also inhibited RES function as assessed by clearance of sensitized erythrocytes (not shown). Anti-CD24 (IgG2b) blocked the RES at the 50 and 5 μg doses but not at the 0.5 μg dose (Figure 4B).
RES inhibition by antierythrocyte monoclonal antibodies.
(A) SCID mice were injected intravenously with 50 μg anti-CD24 (IgG2b, ⋄) or anti-CD24 (IgG2c, ○) or intraperitoneally with 50 mg IVIG (▵) or albumin (■) 24 hours prior to intravenous injection of 200 μL PKH26-labeled, TER-119 antibody–sensitized SCID mouse erythrocytes. Blood was sampled at the specified time points, and the percentage of fluorescent erythrocytes was assessed by flow cytometry. The percentage of fluorescent erythrocytes at the 3-minute time point was considered to be 100%. (B) SCID mice were injected with the indicated reagent and assessed as in panel A; n = 9 for each group. *P < .05; **P < .01; ***P < .001 as compared with albumin mice. Error bars indicate SEM.
RES inhibition by antierythrocyte monoclonal antibodies.
(A) SCID mice were injected intravenously with 50 μg anti-CD24 (IgG2b, ⋄) or anti-CD24 (IgG2c, ○) or intraperitoneally with 50 mg IVIG (▵) or albumin (■) 24 hours prior to intravenous injection of 200 μL PKH26-labeled, TER-119 antibody–sensitized SCID mouse erythrocytes. Blood was sampled at the specified time points, and the percentage of fluorescent erythrocytes was assessed by flow cytometry. The percentage of fluorescent erythrocytes at the 3-minute time point was considered to be 100%. (B) SCID mice were injected with the indicated reagent and assessed as in panel A; n = 9 for each group. *P < .05; **P < .01; ***P < .001 as compared with albumin mice. Error bars indicate SEM.
Monoclonal antibodies directed against nonerythrocyte antigens can also inhibit ITP
The ability of monoclonal antibodies reactive with white blood cell antigens to inhibit ITP was also assessed. Two of 7 antibodies tested protected against immune thrombocytopenia: an anti-CD16/32 at a dosage of 50 μg per mouse (Table 1) and an IgG1 anti-CD44 at dosages of 50 and 5 μg per mouse (Table 1). In contrast, other antileukocyte antibodies (anti-CD21/35, anti-CD11, anti-CD126, anti-CD44 (IgG2b), anti-CD47) failed to ameliorate thrombocytopenia at the doses tested (Table 1).
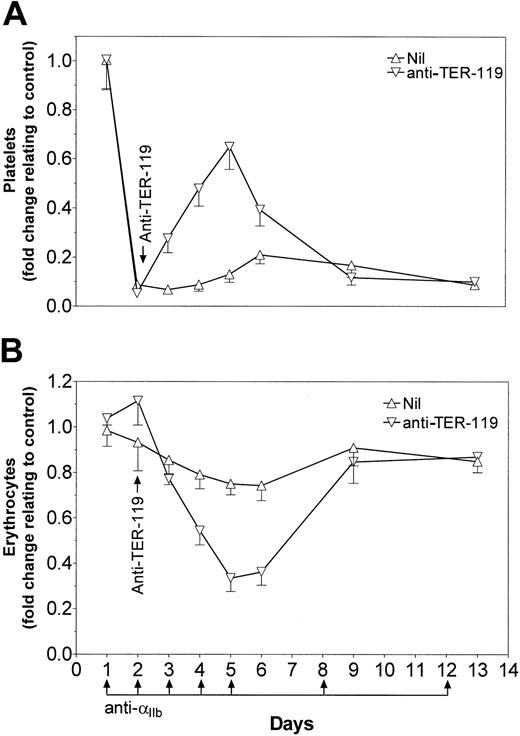
Monoclonal anti–TER-119 antibody demonstrates an enduring therapeutic effect on ITP
SCID mice were rendered thrombocytopenic 1 day before treatment with 50 μg anti–TER-119 monoclonal antibody (Figure5A). Continued injection of antiplatelet antibody induced thrombocytopenia in otherwise untreated mice for the duration of the experiment. Mice that were exposed to a single injection of anti–TER-119 on day 2 underwent a reversal of thrombocytopenia, peaking at day 5, and returning to pre–anti-TER-119 treatment levels by day 9 of the experiment (Figure 5A). The increase in platelet numbers induced by the anti–TER-119 antibody correlated inversely with the number of circulating erythrocytes in the mice (Figure 5B).
Anti–TER-119 demonstrates prolonged protection from thrombocytopenia.
SCID mice were injected with 2 μg anti-αIIb antibody on days 1 to 5, day 8, and day 12. On day 2, thrombocytopenic mice were treated with 50 μg anti–TER-119. Mice were bled at the indicated time points. Platelet counts (A) and erythrocyte counts (B) are presented as fold change over untreated control mice; n = 6 for each data point. Nil indicates the mice were not treated with anti–TER-119. Error bars indicate SEM.
Anti–TER-119 demonstrates prolonged protection from thrombocytopenia.
SCID mice were injected with 2 μg anti-αIIb antibody on days 1 to 5, day 8, and day 12. On day 2, thrombocytopenic mice were treated with 50 μg anti–TER-119. Mice were bled at the indicated time points. Platelet counts (A) and erythrocyte counts (B) are presented as fold change over untreated control mice; n = 6 for each data point. Nil indicates the mice were not treated with anti–TER-119. Error bars indicate SEM.
Discussion
IVIG has been widely utilized in the treatment of both autoimmune and inflammatory diseases such as immune thrombocytopenic purpura (ITP).1,2,8,21-29 IVIG is inherently a preparation of polyclonal antibodies representing a multitude of specificities. Preparations of human IgG containing high titers of polyclonal anti-D have been used as an alternative for IVIG in D-positive patients with ITP. One monoclonal anti-D has been tested in patients with chronic ITP, but it failed to ameliorate the thrombocytopenia.13The failure of this monoclonal anti-D has generated much controversy14-17 as well as a hypothesis that the polyclonality of IVIG, as well as that of anti-D, is critical to its effectiveness in ITP.16
We have recently demonstrated that some of the attributes of polyclonal immunoglobulins, such as anti-idiotypic antibodies, may play a role in mediating the amelioration of some disease states, such as alloimmunization to platelet transfusion.30 However, we have also provided evidence that in murine passive immune thrombocytopenia, anti-idiotype antibodies are not at all required for the acute therapeutic effects of IVIG.18 We have hypothesized that the protective effect of IVIG and polyclonal anti-D may not, in fact, be directly due to their polyclonal nature but rather due to their ability to inhibit RES function; it is thus feasible to speculate that administration of monoclonal antierythrocyte antibodies could inhibit RES function and mimic the acute actions of IVIG.
We demonstrate here that 2 monoclonal antierythrocyte antibodies, anti–TER-119 and IgG2b anti-CD24, can significantly inhibit antibody-induced thrombocytopenia in SCID mice at down to a 4 log-fold lower dose than standard IVIG treatment. Both these monoclonal antibodies, as well as an IgM anti-CD24 antibody (clone IOTHSA), caused a decrease in the number of circulating erythrocytes. The anti-CD24 antibody of the IgG2c isotype did not ameliorate ITP; although this antibody did bind to erythrocytes at a moderate level in vitro, it bound to erythrocytes to virtually the same extent as the therapeutically successful anti-CD24 (IgG2b) antibody under in vivo conditions. This apparent contradiction remains unexplained, but the inability of the anti-CD24 IgG2c to inhibit the RES may be due to the antibody having a poor interaction with Fcγ receptors (FcγRs) or a low affinity for FcγRs expressed on the monocytes in the RES. If the Fc portion of this anti-CD24 (IgG2c) binds poorly to murine FcγRs, the anti-CD24–erythrocyte complexes will not be cleared and can remain in circulation. This possibility is supported by the direct observation that the anti-CD24 (IgG2c) did not block RES function.
Salama and coworkers initially postulated that the success of IVIG in treating ITP was due to competitive inhibition of the RES by sensitized erythrocytes.2,3 In agreement with this, Fehr et al demonstrated that infusion of IVIG in 4 patients with ITP prolonged the in vivo clearance of radiolabeled antibody-sensitized erythrocytes.31 This has been confirmed by others, using both erythrocytes and platelets as target cells.27,28,32Several studies comparing intact IVIG to F(ab′)2 fragments from IVIG have clearly indicated that the intact preparations are more efficacious in reversing the thrombocytopenia,33,34 and our work has clearly demonstrated that F(ab′)2 fragments of IVIG lack the ability to inhibit immune thrombocytopenia.18 To address whether antierythrocyte antibodies inhibit thrombocytopenia due to inhibition of the RES, RES function after exposure to monoclonal antibody was assessed. Pretreatment of mice with anti–TER-119 and anti-CD24 (IgG2b) but not with anti-CD24 (IgG2c) resulted in prolonged clearance of antibody-sensitized erythrocytes, suggesting a relationship between RES inhibition and the therapeutic effect of monoclonal antibody in preventing thrombocytopenia.
Modulation of complement is one of the several documented immunomodulatory properties of IVIG that may contribute to its clinical effects.35-37 However, in a murine model of ITP very similar to the model of ITP employed in the current studies, IVIG was shown to increase platelet counts in complement–knock-out mice exposed to an antiplatelet antibody.19 In addition, IgM is a potent complement activator via the classical cascade,38but our data show that a monoclonal anti-CD24 antibody of IgM class, which resulted in a significant decline in erythrocyte counts, did not exert any inhibitory effect on thrombocytopenia induced in SCID mice. Finally, anti-CD21/35, an antibody against complement receptor CR2/CR1,39 did not prevent thrombocytopenia. It therefore appears that in this murine model of ITP, complement may not play a role in the amelioration of thrombocytopenia by IVIG or monoclonal antibodies.
Infusion of a murine-derived monoclonal anti-FcγR can reverse thrombocytopenia in refractory ITP.40 We found that monoclonal anti-CD16/32, an antibody against FcγRII/RIII,41 42 resulted in a significant inhibition of platelet destruction. In addition, an anti-CD44 antibody was also able to prevent thrombocytopenia. These data indicate that monoclonal antibodies need not be directed only to erythrocytes but that monoclonal antibodies directed to leukocyte antigens may also be able to mimic the effects of IVIG.
In the treatment of ITP, it has been observed that the ability of IVIG to ameliorate thrombocytopenia can continue for several days after treatment.43 44 Analysis of platelet counts in anti–TER-119 antibody–treated thrombocytopenic mice indicated that the monoclonal antibody protected against thrombocytopenia for at least 4 days after treatment.
An elegant model to study murine ITP would be based upon mice that are transgenic and functional for the human FcγRIIA.45However, in this study, we have specifically employed SCID mice because we wished to avoid an immune response against the human IVIG as well as to the monoclonal antibodies. Even a minimal antibody response against these IgGs could result in the formation of IgG dimers or multimers, which could have an independent effect on the thrombocytopenia. To ensure that the SCID environment is not required in the efficacy of the tested monoclonal antibodies, the anti–TER-119 and anti-CD24 (IgG2b) antibodies were also evaluated in normal inbred mice (BALB/c) as well as outbred mice (CD-1) and found to have virtually the same ability to inhibit thrombocytopenia as compared with SCID mice.
In conclusion, monoclonal antibodies are capable of substituting for IVIG in inhibiting immune thrombocytopenia and may provide an effective alternative to IVIG in the treatment of ITP; it may also be less expensive to produce, in more abundant supply, and less susceptible to transmission of viral diseases than the human-derived product.
We thank Mr Hoang Le-Tien, Ms Alison Starkey, and Mr Davor Brinc for assistance and helpful discussion and the St Michael's Hospital Research Vivarium staff.
Prepublished online as Blood First Edition Paper, December 27, 2002; DOI 10.1182/blood-2002-10-3078.
Supported by a grant from Bayer-Canadian Blood Services-Hema Quebec Partnership Fund.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Alan H. Lazarus, Transfusion Medicine Research, St Michael's Hospital, 30 Bond St, Toronto, ON, Canada M5B 1W8; e-mail: lazarusa@smh.toronto.on.ca.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal