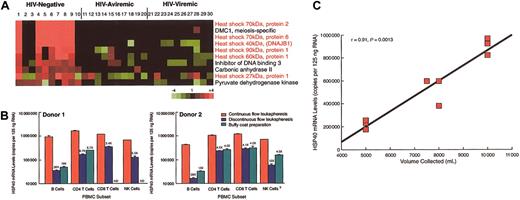
DNA microarray technology offers new means to investigate gene expression patterns in health and disease.1-3 We recently used DNA microarray technology to investigate gene expression in lymphocytes of 3 groups of individuals: HIV-infected patients with detectable plasma viremia (viremic) and undetectable plasma viremia (aviremic), and HIV-negative donors (S.M., C.E.B., A.S.F., unpublished observations, 2003). The pattern and nature of genes found to be up-regulated in the latter group relative to the other 2 groups (Figure 1A) suggested that the method used to collect the samples might have been responsible for the differences observed. Nearly two thirds of the genes in this cluster were heat shock proteins (HSPs), recently shown to be up-regulated by mechanical stress,6 and 3 of the HIV-negative donors (donors 2, 3, and 10) had profiles that appeared to be more closely related to the profiles of both groups of HIV-infected patients. A retrospective analysis revealed that the blood products collected from these 3 outliers were buffy coat (BC) preparations, whereas the blood products from the other 7 HIV-negative donors were obtained from continuous flow leukapheresis (CFL) using a Baxter Healthcare CS-3000 Plus device (Deerfield, IL). Considering that the cells obtained from HIV-infected patients were processed by a third procedure, namely discontinuous flow leukapheresis (DFL) using a Haemonetics MCS+ device (Braintree, MA), we evaluated the effect of blood collection procedures on HSP expression by subjecting 2 HIV-negative donors to all 3 procedures. HSP40 and HSP90, 2 representative genes from the microarray analyses, were chosen for validation by quantitative RT-PCR. Measurements were made on B cells, as well as in natural killer (NK) cells, and CD4+ and CD8+ T cells.
Gene expression profiles in lymphocytes. (A) cDNA microarray analyses4 on B cells isolated from peripheral blood lymphocytes. (B) Real-time reverse transcription-polymerase chain reaction (RT-PCR) analysis5 of HSP40 expression in lymphocytes obtained by CFL, DFL, and BC procedures. Data are mean ± SD of triplicates and numbers above the data bars represent fold differences relative to CFL values. ND indicates not done. (C) Correlation between copies of HSP40 mRNA measured in B cells of HIV-negative donors and volume of blood collected by CFL.
Gene expression profiles in lymphocytes. (A) cDNA microarray analyses4 on B cells isolated from peripheral blood lymphocytes. (B) Real-time reverse transcription-polymerase chain reaction (RT-PCR) analysis5 of HSP40 expression in lymphocytes obtained by CFL, DFL, and BC procedures. Data are mean ± SD of triplicates and numbers above the data bars represent fold differences relative to CFL values. ND indicates not done. (C) Correlation between copies of HSP40 mRNA measured in B cells of HIV-negative donors and volume of blood collected by CFL.
CFL induced a dramatic increase in mRNA levels of HSP40 in B cells compared with BC and DFL preparations (Figure 1B). While not as dramatic, similar trends were observed in all 3 other lymphocyte populations investigated. Levels of HSP90 mRNA were also found to be up-regulated in samples derived from CFL compared with BC and DFL procedures (data not shown). These data demonstrate that CFL is associated with sharp increases in HSP gene expression and that B cells are the most profoundly affected among the lymphocyte populations.
The main difference between the 3 methods of collecting blood products is centrifugation time; cells undergo 90 to 180 minutes of centrifugation by CFL compared with 10 minutes by DFL and 6 minutes by BC. A direct association between time of centrifugation and HSP gene induction was confirmed by showing a direct and highly significant correlation between volume of blood collected by CFL, processed at a rate of approximately 55 mL/min, and levels of HSP40 mRNA measured in B cells (Figure 1C).
Continuous flow devices are routinely used, especially when resources are a limiting factor, to provide adequate quantities of mononuclear cells for research or therapeutic use. However, these devices may lead to confounding results in microarray and real-time RT-PCR analyses, especially when CFL-derived samples are compared with samples derived from HIV-infected and cancer patient populations, where DFL and BC procedures are favored. In this setting, the optimal device for collecting larger numbers of cells may be a continuous flow cell separator in which the collected component does not remain within the centrifugal field for the entire duration of the procedure.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal