Abstract
Damage to the integrity of the vessel wall results in exposure of the subendothelial extracellular matrix (ECM), which triggers integrin-dependent adhesion and aggregation of platelets. The role of platelet β1 integrins in these processes remains mostly undefined. Here, we demonstrate by intravital fluorescence microscopy that platelet adhesion and thrombus growth on the exposed ECM of the injured carotid artery is not significantly altered in α2-null mice and even in mice with a Cre/loxP-mediated loss of all β1 integrins on their platelets. In contrast, inhibition of αIIbβ3 integrin on platelets in wild-type mice blocked aggregate formation and reduced platelet adhesion by 60.0%. Strikingly, αIIbβ3 inhibition had a comparable effect in α2-null mice, demonstrating that other receptors mediate shear-resistant adhesion in the absence of functional α2β1 and αIIbβ3. These were identified to be α5β1 and/or α6β1 as αIIbβ3 inhibition abrogated platelet adhesion in β1-null mice. We conclude that shear-resistant platelet adhesion on the injured vessel wall in vivo is a highly integrated process involving multiple integrin-ligand interactions, none of which by itself is essential. (Blood. 2003;102:4021-4027)
Introduction
At sites of vascular injury, the subendothelial extracellular matrix (ECM) is exposed to the flowing blood, which triggers adhesion and aggregation of platelets.1 This process is crucial to limit posttraumatic blood loss but may also lead to occlusion of diseased vessels and infarction of vital organs. Integrins are the major class of receptors that mediate firm adhesion and aggregation of platelets. Integrins are heterodimeric transmembrane receptors composed of an α and a β subunit.2,3 Platelets express integrins of the β1 and the β3 family that are present on the membrane in a low-affinity state. Once the platelets become activated the integrins shift to a high-affinity state and efficiently bind their ligands.2,4-6
The subendothelial ECM contains many different macromolecular constituents that are suitable substrates for integrin-mediated platelet adhesion including collagens, laminins, fibronectin, and von Willebrand factor (VWF). Collagen has a central role as it not only supports platelet adhesion but also strongly activates the cells.7,8 This activation is mediated by the low-affinity collagen receptor glycoprotein VI (GPVI).9 GPVI belongs to the immunoglobulin (Ig) superfamily10 and is noncovalently associated with the signal-transducing FcRγ chain.11,12 Under conditions of elevated shear, platelet adhesion on collagen strictly depends on the interaction of GPIb-V-IX with collagen-bound VWF13 and that of GPVI with collagen.5 During this process, ligation of GPVI5 (and GPIb14 ) leads to platelet activation and the shift of β1 and β3 integrins to a high-affinity state via “inside-out” signals, enabling the platelet to establish firm adhesion contacts that resist the shear forces in the bloodstream (shear-resistant adhesion) and subsequent thrombus growth. The importance of these initial processes in arterial thrombus formation has been established through the demonstration that platelet adhesion and thrombus formation on the injured arterial wall are largely inhibited in the absence of functional GPVI15,16 or GPIb.16,17 The molecular determinants of firm platelet adhesion on the ECM, however, have not been identified. This might be explained by the complexity of the ECM and the presence of multiple integrins on platelets that made it difficult to define the significance of individual integrins in this process.
The major platelet integrin, αIIbβ3, binds multiple ligands including fibrinogen, VWF, and fibronectin and is essential for platelet aggregation but also contributes to platelet adhesion on collagen via VWF5,13 and fibronectin18 in vitro. The mandatory role of αIIbβ3 in physiologic and pathophysiologic thrombus formation is well documented2,4,19 but its significance for the initial adhesion process on the ECM in vivo is not firmly established. The other β3 integrin on platelets, αvβ3, is expressed at low levels and its role in platelet physiology is ill defined.20
Platelets express 3 different β1 integrins, namely α2β1 (collagen receptor), α5β1 (fibronectin receptor), and α6β1 (laminin receptor). Among them, α2β1 has been most intensively studied but its significance in the hemostatic and thrombotic process has been controversially debated (for review see Nieswandt and Watson21 ). A role for α2β1 in cardiovascular disease has been discussed for many years based on the results of clinical studies assessing a possible association of allelic differences in the α2 gene and different levels of α2β1 on platelets22 with an increased risk of myocardial infarction, diabetic retinopathy, and stroke. However, while many clinical studies found such an association, approximately the same number of studies did not.23-27 Furthermore, the pathologic significance of the other 2 β1 integrins expressed on platelets, α5β1 and α6β1, with regard to hemostasis and thrombosis as well as their cellular regulation is poorly defined,19 although fibronectin and laminin are highly expressed in the vascular wall, suggesting an involvement of both integrins in platelet-vessel wall interactions.
Recently, several groups reported the generation of α2-integrin-deficient28,29 and conditional β1-integrin-deficient5 mice, the latter lacking α2β1, α5β1, and α6β1 on their platelets. Unexpectedly, these mice were found to have normal tail bleeding times, although their platelets display partial defects in their interaction with collagen in vitro. The mutant platelets can adhere and aggregate on fibrillar collagen under high-shear-flow conditions, presumably mediated by αIIbβ3/VWF, but the newly formed aggregates detach from the collagen surface at a higher frequency than wild-type aggregates.30 Thus, α2β1 plays an important but not essential role in platelet adhesion on collagen in vitro. The significance of this interaction in vivo, where multiple integrin-ligand interactions may contribute to shear-resistant platelet deposition at sites of injury, has not been assessed.
In this study we used intravital fluorescence microscopy to examine the dynamic process of platelet accumulation at sites of vascular injury in the carotid artery of α2- and β1-deficient mice. Surprisingly, we found that platelet adhesion and thrombus growth on the exposed extracellular matrix of the arterial wall are largely unaffected in the absence of α2β1orall β1 integrins on platelets. In contrast, inhibition of αIIbβ3 in wild-type platelets not only blocked aggregate formation but also reduced adhesion by 60.0%. Strikingly, the same effect was seen in α2-null mice, suggesting that receptors other than α2β1 and αIIbβ3 mediate the reduced adhesion. These were identified to be α5β1 and/or α6β1, both of which are in a low-affinity state on resting platelets but efficiently bind their ligands upon platelet activation through GPVI.
Materials and methods
Animals
Mutant mice deficient in the α2 integrin subunit were produced as described.28 Both mutant and wild-type control animals were of 129/Sv × C57BL/6 genetic background and used at the age of 10 to 16 weeks.
Generation of mice with β1-null platelets. To produce mice carrying the β1-null allele in megakaryocytes, β1(fl/fl) mice31 were crossed with transgenic mice carrying the Mx-cre transgene (mx-cre+).32 Deletion of the β1 gene was induced in 4- to 5-week-old (β1(fl/fl)/Mx-cre+) mice by 3 intraperitoneal injections of 250 μg polyinosinic-polycytidylic acid (pI-pC) at 2-day intervals. Control mice β1(fl/fl) received the same treatment and were derived from the same litters. For experiments, mice were used at least 2 weeks after pI-pC injection.
Generation of GPVI-deficient mice. To generate mice lacking GPVI, C57BL6/J mice were injected with 100 μg JAQ1 intraperitoneally. Animals were used for in vivo assessment of platelet adhesion/thrombus formation on day 5. As reported previously, GPVI was not detectable on the platelets of those mice by flow cytometry and Western blot analysis.33
Monoclonal antibodies and chemicals
Monoclonal antibodies (mAbs) against GPVI (JAQ1), integrin αIIbβ3 (JON/A), and GPIbα (p0p4) integrins α2 (LEN1) and α5 (BAR1) were generated as described.34-36 F(ab)2 fragments of JON/A were generated as described.33 Irrelevant control rat IgG and fluorescein isothiocyanate (FITC)-conjugated anti-β1 integrin and α6 were obtained from Pharmingen (Hamburg, Germany). Mouse laminin and bovine fibronectin were from Sigma (Deisenhofen, Germany). Human VWF and collagen-related peptide (CRP) were kindly provided by G. Dickneite (Marburg, Germany) and S. P. Watson (Oxford, United Kingdom), respectively.
Flow cytometry
Heparinized whole blood was diluted 1:30 with modified Tyrode-HEPES buffer (134 mM NaCl, 0.34 mM Na2HPO4, 2.9 mM KCl, 12 mM NaHCO3, 20 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], pH 7.0) containing 5 mM glucose, 0.35% bovine serum albumin (BSA), and 1 mM CaCl2. The samples were incubated with fluorophore-labeled antibodies for 10 minutes at room temperature and directly analyzed on a FACScalibur (Becton Dickinson, Heidelberg, Germany).
Preparation of platelets for intravital microscopy
Blood from wild-type or mutant mice was drawn from the retro-orbital plexus and collected in 1.5-mL polypropylene tubes containing 0.1-mL volume of 38 mM citric acid/75 mM trisodium citrate/100 mM dextrose. The blood was centrifuged at 250g for 10 minutes and platelet-rich plasma was gently transferred to a fresh tube and the centrifugation was repeated at 2000g for 10 minutes. The pellet was resuspended in modified Tyrode-HEPES buffer containing 0.35% BSA and 5 mM glucose. Isolated platelets were labeled with 5-carboxyfluorescein diacetate succinimidyl ester (DCF; 5 μg/mL for 2 minutes) and adjusted to a final concentration of 200 × 106 platelets/250 μL. Where indicated, platelets were preincubated with 50 μg/mL F(ab)2 fragments of anti-αIIbβ3 (JON/A)35 for 5 minutes before infusion. Flow cytometric analysis with FITC-conjugated JON/A confirmed that more than 95% of surface αIIbβ3 integrins were occupied under these conditions.
Intravital microscopy
Intravital microscopy of the injured carotid artery was performed essentially as described.16 Briefly, mice were anesthetized by intraperitoneal injection of ketamine/xylazine (ketamine 100 g/kg, Parke-Davis, Karlsruhe, Germany; xylazine 5 mg/kg, Bayer AG, Leverkusen, Germany). Polyethylene catheters (Portex, Hythe, England) were implanted into the right jugular vein and fluorescent platelets (200 × 106/250 μL) were infused intravenously. The right common carotid artery was dissected free and ligated vigorously near the carotid bifurcation for 30 seconds using a surgical filament to induce vascular injury. Prior to and following vascular injury, the fluorescent platelets were visualized in situ by in vivo video microscopy of the right common carotid artery using a Zeiss Axiotech microscope (× 20 water immersion objective; W × 20/0.5; Zeiss, Göttingen, Germany) with a 100-W mercury short arc photo optic lamp (HBO) for epi-illumination. Platelet adhesion was recorded for 5 minutes after the induction of injury and the videotaped images were evaluated using a computer-assisted image analysis program (Visitron, Munich, Germany). The number of adherent platelets was assessed by counting the cells that did not move or detach from the vascular wall for at least 10 seconds. In each mouse, 3 nonoverlapping fields (size, 100 μm × 100 μm) were analyzed for 30 seconds (2.5-3.0 minutes after injury) in a slow-motion modus. Clusters of 2 or more platelets were defined as microaggregates. The total number of adherent platelets or microaggregates at t = 3 minutes was calculated by the following formula that reflects concave shape of the vessel wall: vessel diameter (μm) ×π (circle constant) × 2 x sin-1 (amplitude of measured area, in μm) × length of measured area (μm) and is presented per mm2. All experiments performed on animals were approved by the German legislation on protection of animals.
Histology
For histologic examination, carotid arteries were perfusion-fixed in situ with 4% paraformaldehyde, pH 7.0. Thereafter, the vessels were excised, fixed in 0.1 M cacodylate-buffered Karnovsky solution (2.5% glutaraldehyde and 1% paraformaldehyde; overnight, room temperature) and then fixed in 1% osmium tetroxide (2 h) at pH 7.3. The samples were dehydrated in graded ethanols and embedded in the EmBed-812 epoxy resin (all reagents from Science Services, Munich, Germany). After 48 hours heat polymerization at 60°C, semithin (0.8 μm) sections were cut with a diamond knife (Diatome, Fort Washington, PA) on a Reichert Ultracut-S ultramicrotome (Leica-Reichert, Leica-Microsysteme, Vienna, Austria) and double stained with aqueous solutions of 1% toluidine blue and basic fuchsin (60°C, 1 minute).
Static adhesion
Static adhesion was performed with washed platelets in modified Tyrode buffer containing 0.35% BSA and Ca2+/Mg2+ (each at 1 mM) on 96-well plates (Nunc, Wiesbaden, Germany). The plates were coated with laminin (0.2 μg/well), fibronectin (0.2 μg/well), or VWF (0.4 μg/well) in phosphate-buffered saline (PBS) overnight at 4°C and then blocked with 5% BSA for 2 hours at 37°C. Resting or CRP-activated (0.2 μg/mL) platelets were allowed to adhere for 60 minutes and adhesion was quantitated colorimetrically as described.33 Where indicated, the experiments were performed in the presence of the function-blocking anti-αIIbβ3 antibody, JON/A (50 μg/mL).
Statistical evaluation
Statistical analysis was performed using the unpaired Student t test.
Results
Platelet adhesion and thrombus growth on the injured arterial wall is unaltered in α2-null mice
To directly test the biologic significance of α2β1 in arterial thrombus formation we assessed platelet-vessel wall interactions following arterial injury in α2-null mice. Wild-type and α2-null mice had comparable platelet counts. Flow cytometric analysis confirmed the absence of α2 on mutant platelets, whereas expression levels of α5 and α6 were slightly increased.28 The α2 deficiency had no effect on expression levels of all other receptors tested including integrin β3 and the GPIb-V-IX complex (Figure 1A). In parallel, experiments were performed in mice with an antibody-induced GPVI deficiency.33
Platelet adhesion and thrombus formation in α2-null mice. (A) Flow cytometric analysis of glycoprotein expression on wild-type (black line) and α2-null (shaded area) mice. (B) Platelet-vessel wall interactions after vascular injury were investigated in wild-type, α2-null, and GPVI-deficient mice by in vivo fluorescence microscopy of the common carotid artery in situ. The left and right graphs summarize platelet adhesion and aggregate formation, respectively, of 7 experiments per group. Results are shown as mean ± SEM. (C) The photomicrographs show representative in vivo fluorescence microscopy images 3 minutes after injury in wild-type, α2-null, and GPVI-deficient mice. (D) Representative histologic sections of carotid arteries 20 minutes after injury demonstrating large platelet-rich thrombi wild-type, α2-null, but not GPVI-deficient mice. Sections stained with toluidine blue/basic fuchsin; original magnification, × 5.
Platelet adhesion and thrombus formation in α2-null mice. (A) Flow cytometric analysis of glycoprotein expression on wild-type (black line) and α2-null (shaded area) mice. (B) Platelet-vessel wall interactions after vascular injury were investigated in wild-type, α2-null, and GPVI-deficient mice by in vivo fluorescence microscopy of the common carotid artery in situ. The left and right graphs summarize platelet adhesion and aggregate formation, respectively, of 7 experiments per group. Results are shown as mean ± SEM. (C) The photomicrographs show representative in vivo fluorescence microscopy images 3 minutes after injury in wild-type, α2-null, and GPVI-deficient mice. (D) Representative histologic sections of carotid arteries 20 minutes after injury demonstrating large platelet-rich thrombi wild-type, α2-null, but not GPVI-deficient mice. Sections stained with toluidine blue/basic fuchsin; original magnification, × 5.
Platelets were purified from donor mice, fluorescently labeled, and injected into recipient mice of the same genotype. Vascular injury was induced by vigorous ligation of the carotid artery, which consistently causes disruption of the endothelial layer and frequently breaching of the internal elastic lamina followed by rapid platelet adhesion and aggregate formation at the site of injury.16 Unexpectedly, in vivo fluorescence microscopy revealed that the extent of platelet adhesion was indistinguishable between α2-null and wild-type mice (t = 3 minutes; n = 7 per group). Furthermore, in wild-type and α2-null mice, firmly adherent platelets recruited additional platelets from the circulation, leading to the formation of microaggregates that were similar in number in both groups of mice (Figure 1B-C). In contrast, platelet adhesion and aggregate formation was virtually abolished in GPVI-deficient mice, confirming previous results.16 In agreement with these early events, large platelet-rich thrombi were consistently found in the injured arteries of wild-type and α2-deficient mice after 20 minutes, whereas no thrombus had formed in GPVI-deficient mice (Figure 1D; n = 8 per group). These results demonstrated that although collagen is a major trigger for platelet adhesion and thrombus formation on the subendothelial matrix under high-shear conditions in vivo, α2β1 is not required for these processes.
Inhibition of integrin αIIbβ3 reduces platelet adhesion on the ECM in wild-type and α2-null mice
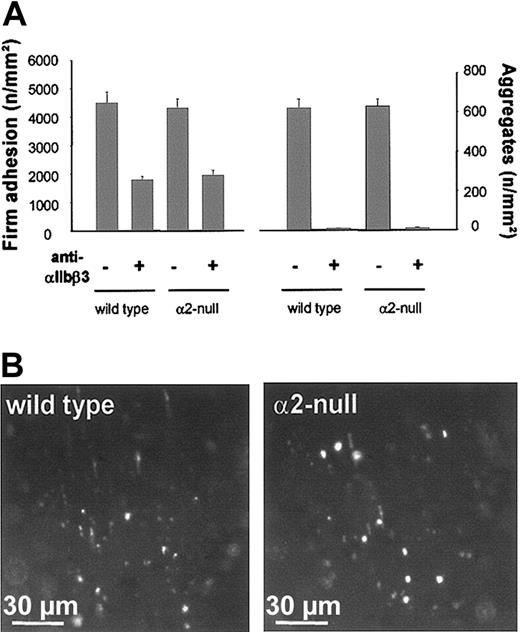
The experiments described above suggested that other receptors can mediate shear-resistant platelet adhesion on the injured arterial wall in the absence of α2β1. Integrin αIIbβ3, the major integrin on platelets, could mediate this adhesion as it binds to VWF and fibronectin, both of which are present in the ECM. To test the role of αIIbβ3, wild-type mice received fluorescently labeled platelets preincubated with saturating concentrations (50 μg/mL) of F(ab)2 fragments of the blocking anti-αIIbβ3 mAb JON/A35 prior to carotid injury. In vivo fluorescence microscopy revealed that platelet adhesion was reduced by 60.0% (1794 ± 118 vs 4495 ± 378/mm2; t = 3 minutes) upon αIIbβ3 inhibition in wild-type mice (Figure 2). Furthermore, aggregate formation was blocked under these conditions, confirming the essential function of αIIbβ3 in platelet aggregation in vivo. These results demonstrated that αIIbβ3 plays an important role for shear-resistant platelet adhesion on the injured arterial wall in vivo but that other receptors significantly contribute to this process. To test whether the residual adhesion of αIIbβ3-blocked platelets was mediated by α2β1, we inhibited αIIbβ3 in α2-null mice. Very unexpectedly, however, inhibition of αIIbβ3 resulted in a 56.7% reduction of platelet adhesion (1885 ± 176 vs 4355 ± 313/mm2; t = 3 minutes) and hence was similar to that observed in wild-type mice (Figure 2). As expected, aggregate formation was abolished under these conditions. These results demonstrated that platelets can firmly attach to the ECM under high-shear conditions in vivo independently of the 2 major integrins that mediate direct and indirect adhesion to collagen, α2β1 and αIIbβ3, respectively.
Effect of αIIbβ3 inhibition on platelet adhesion and aggregate formation in wild-type and α2-null mice. (A) Fluorescent wild-type or α2-null platelets were preincubated with 50 μg/mL anti-αIIbβ3 (JON/A F(ab)2 fragments) and injected into recipient mice of the same genotype. Platelet adhesion and aggregate formation following vascular injury were determined by intravital video fluorescence microscopy. The left and right graphs summarize platelet adhesion and aggregate formation, respectively, with and without αIIbβ3 inhibition. The results are presented as mean ± SEM of 7 experiments per group. (B) The photomicrographs show representative in vivo fluorescence microscopy images 3 minutes after injury, illustrating platelet adhesion in wild-type and α2-null mice in the presence of anti-αIIbβ3.
Effect of αIIbβ3 inhibition on platelet adhesion and aggregate formation in wild-type and α2-null mice. (A) Fluorescent wild-type or α2-null platelets were preincubated with 50 μg/mL anti-αIIbβ3 (JON/A F(ab)2 fragments) and injected into recipient mice of the same genotype. Platelet adhesion and aggregate formation following vascular injury were determined by intravital video fluorescence microscopy. The left and right graphs summarize platelet adhesion and aggregate formation, respectively, with and without αIIbβ3 inhibition. The results are presented as mean ± SEM of 7 experiments per group. (B) The photomicrographs show representative in vivo fluorescence microscopy images 3 minutes after injury, illustrating platelet adhesion in wild-type and α2-null mice in the presence of anti-αIIbβ3.
Integrins α5β1 and α6β1 are involved in shear-resistant platelet adhesion on the ECM in vivo
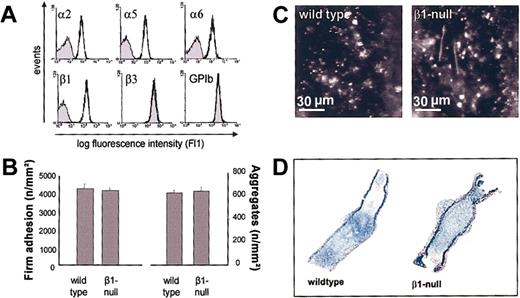
Platelets express 2 additional integrins of the β1 family, α5β1 and α6β1, both of which bind ligands that are present in the ECM and could therefore mediate the reduced adhesion observed in αIIbβ3-blocked α2-null platelets. This hypothesis was tested in mice with a Cre/loxP-mediated loss of β1 integrin on platelets.5 Flow cytometric analysis confirmed the absence of β1, α2, α5, and α6 integrin subunits on mutant platelets, whereas normal expression levels were found for all other tested receptors, including integrin β3 and the GPIb-V-IX complex (Figure 3A). Fluorescently labeled β1-null or wild-type platelets were injected into mice of the same genotype (β1(fl/fl) cre+ vs β1(fl/fl) cre-, respectively) and carotid injury was induced as described above. Intravital videomicroscopy revealed that similar numbers of platelets adhered at the site of injury in control and mutant mice after 3 minutes (Figure 3B-C). Also, the time course and extent of microaggregate formation was not significantly different between wild-type and mutant mice and, in agreement with this observation, large platelet-rich thrombi were found in both groups of mice 20 minutes after the induction of injury (Figure 3D). Thus, platelet adhesion and thrombus formation on the subendothelial matrix in vivo occurs in the absence of β1 integrins. This strongly suggested that αIIbβ3 alone is sufficient to mediate both platelet adhesion and thrombus growth in vivo in the absence of β1 integrins. This hypothesis was confirmed when αIIbβ3 was inhibited in β1-null mice with JON/A F(ab)2 (50 μg/mL). Under these conditions, platelet adhesion was almost completely blocked (Figure 4). As expected, aggregate formation was absent in these mice. Altogether, these results demonstrated that both αIIbβ3 and β1 integrins are independently able to mediate shear-resistant platelet adhesion on the subendothelial matrix under arterial flow conditions in vivo.
Platelet adhesion and thrombus formation in β1-null mice. (A) Flow cytometric analysis of glycoprotein expression on wild-type (black line) and β1-null (shaded area) mice. (B) Platelet-vessel wall interactions after vascular injury were investigated in wild-type (β1 fl/fl) and β1-null mice by in vivo fluorescence microscopy of the common carotid artery in situ. The left and right graphs summarize platelet adhesion and aggregate formation, respectively, of 7 experiments per group. Results are shown as mean ± SEM. (C) The photomicrographs show representative in vivo fluorescence microscopy images 3 minutes after injury in wild-type and β1-null mice. (D) Representative histologic sections of carotid arteries 20 minutes after injury demonstrating large platelet-rich thrombi in wild-type and β1-null mice.
Platelet adhesion and thrombus formation in β1-null mice. (A) Flow cytometric analysis of glycoprotein expression on wild-type (black line) and β1-null (shaded area) mice. (B) Platelet-vessel wall interactions after vascular injury were investigated in wild-type (β1 fl/fl) and β1-null mice by in vivo fluorescence microscopy of the common carotid artery in situ. The left and right graphs summarize platelet adhesion and aggregate formation, respectively, of 7 experiments per group. Results are shown as mean ± SEM. (C) The photomicrographs show representative in vivo fluorescence microscopy images 3 minutes after injury in wild-type and β1-null mice. (D) Representative histologic sections of carotid arteries 20 minutes after injury demonstrating large platelet-rich thrombi in wild-type and β1-null mice.
Inhibition of αIIbβ3 abrogates platelet adhesion and aggregate formation in β1-null mice. (A) Fluorescent wild-type or β1-null platelets were preincubated with 50 μg/mL anti-αIIbβ3 (JON/A F(ab)2 fragments) and injected into recipient mice of the same genotype. Platelet adhesion and aggregate formation following vascular injury were determined by intravital video fluorescence microscopy. The left and right graphs summarize platelet adhesion and aggregate formation, respectively, with and without αIIbβ3 inhibition. The results are presented as mean ± SEM of 7 experiments per group. (B) The photomicrographs show representative in vivo fluorescence microscopy images illustrating platelet adhesion 3 minutes after injury in wild-type and β1-null mice in the presence of anti-αIIbβ3.
Inhibition of αIIbβ3 abrogates platelet adhesion and aggregate formation in β1-null mice. (A) Fluorescent wild-type or β1-null platelets were preincubated with 50 μg/mL anti-αIIbβ3 (JON/A F(ab)2 fragments) and injected into recipient mice of the same genotype. Platelet adhesion and aggregate formation following vascular injury were determined by intravital video fluorescence microscopy. The left and right graphs summarize platelet adhesion and aggregate formation, respectively, with and without αIIbβ3 inhibition. The results are presented as mean ± SEM of 7 experiments per group. (B) The photomicrographs show representative in vivo fluorescence microscopy images illustrating platelet adhesion 3 minutes after injury in wild-type and β1-null mice in the presence of anti-αIIbβ3.
The affinity of platelet α5β1 and α6β1 for their ligands is regulated by GPVI
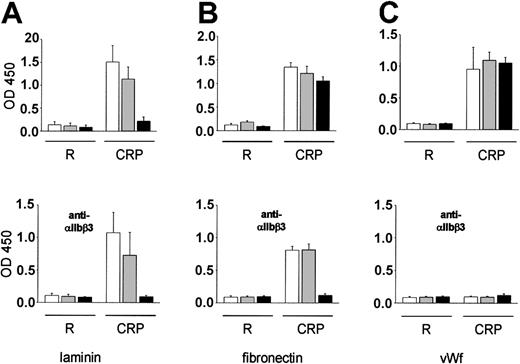
The finding that the αIIbβ3 blockade almost completely inhibited platelet adhesion in β1-null but not α2-null mice demonstrated for the first time that integrins α5β1 and/or α6β1 can mediate shear-resistant platelet adhesion under arterial flow conditions in vivo. We have previously shown that GPVI-mediated integrin activation is an essential prerequisite for platelet adhesion to collagen in vitro5 and that platelet adhesion to the subendothelial matrix in vivo is virtually abolished in the absence of GPVI16 (Figure 1). These observations suggested that the affinity of α5β1 and α6β1 for their ligands is also regulated by GPVI-dependent mechanisms. To test this hypothesis, adhesion of wild-type and mutant platelets was studied on laminin, fibronectin, and, as a control, human VWF (HVWF) in vitro. Virtually no adhesion of resting platelets on laminin was observed for up to 1 hour. In contrast, when platelets were activated with the GPVI-specific agonist collagen-related peptide (CRP, 0.2 μg/mL), robust adhesion of wild-type and α2-null but not β1-null platelets occurred (Figure 5A), suggesting that α6β1 is essential to mediate platelet adhesion to laminin. A slightly different picture emerged when adhesion was studied on fibronectin. Virtually no adhesion of unstimulated platelets to this ligand was observed for up to 1 hour, whereas robust adhesion of wild-type, α2-null, and β1-null platelets occurred upon stimulation with CRP (Figure 5B). However, when αIIbβ3 was blocked with JON/A (50 μg/mL), adhesion of wild-type and α2-null platelets was retained, whereas adhesion of β1-null platelets was almost completely abrogated. This finding suggests that the 2 major fibronectin-binding integrins on platelets are αIIbβ3 and α5β1 and that they can independently mediate adhesion to this ligand. Furthermore, these results demonstrate that the affinity of both integrins for fibronectin is up-regulated by GPVI-dependent signaling. The normal function of αIIbβ3 in wild-type and mutant platelets was confirmed when adhesion was studied on HVWF. It is important to note that hVWF does not efficiently interact with mouse GPIbα.37 Therefore, GPIb-dependent αIIbβ3 activation, which has been reported to induce adhesion of human platelets on HVWF,38 does not occur in this system. No adhesion of resting mouse platelets to this ligand was observed for up to 1 hour. However, when platelets were activated with CRP, strong adhesion of wild-type, α2-null, and β1-null platelets occurred. As expected, this adhesion was completely blocked in the presence of the anti-αIIbβ3 mAb JON/A (50 μg/mL; Figure 5C).
Platelet adhesion to laminin, fibronectin, and VWF under static conditions. Resting or CRP-activated washed wild-type (□), α2-null (▦), or β1-null (▪) platelets were allowed to adhere for 60 minutes under static conditions to laminin (A), fibronectin (B), or VWF (C) immobilized in microtiter plates. The experiments were performed in the presence of Mg2+/Ca2+ (1 mM each). Where indicated, platelets were preincubated with anti-αIIbβ3 (JON/A, 50 μg/mL). Adherent platelets were quantitated fluorimetrically. The data shown are from a single experiment representative of 6 identical experiments, and are expressed as the mean of quadruplicate reading ± SD for the indicated times.
Platelet adhesion to laminin, fibronectin, and VWF under static conditions. Resting or CRP-activated washed wild-type (□), α2-null (▦), or β1-null (▪) platelets were allowed to adhere for 60 minutes under static conditions to laminin (A), fibronectin (B), or VWF (C) immobilized in microtiter plates. The experiments were performed in the presence of Mg2+/Ca2+ (1 mM each). Where indicated, platelets were preincubated with anti-αIIbβ3 (JON/A, 50 μg/mL). Adherent platelets were quantitated fluorimetrically. The data shown are from a single experiment representative of 6 identical experiments, and are expressed as the mean of quadruplicate reading ± SD for the indicated times.
Discussion
In the current study we assessed the significance of α2β1 in the dynamic process of platelet adhesion and thrombus formation on the subendothelial matrix by intravital fluorescence microscopy of the injured carotid artery of α2-null mice. We found that both platelet adhesion and thrombus formation are not significantly altered in the mutant mice compared with wild-type controls. This finding demonstrates that α2β1 is not essential for arterial thrombus formation in mice but it does not exclude a supportive role of the integrin in this process. Although the data obtained in mice cannot be directly extrapolated to the situation in humans, our results indicate that platelet α2β1 may not play a major role in the onset of acute ischemic syndromes, such as myocardial infarction or stroke. However, our findings do not rule out a possible role of the integrin in the pathogenesis of chronic vascular diseases such as atherogenesis, which could explain the correlation between high-α2β1 levels and susceptibility to cardiovascular disease. A supportive rather than an essential role of α2β1 in thrombus formation in vivo is further suggested by the essentially normal tail bleeding times in α2-null mice,28,29 although this assay in mice has not been validated as a useful surrogate of normal hemostasis. Together, these observations show that α2β1 is dispensable for hemostasis and arterial thrombosis, at least in mice.
In addition to collagen, the subendothelial matrix contains multiple macromolecules that potentially provide an adhesive substrate for platelet integrins. Among these, VWF, immobilized on fibrillar collagen via its A3 domain, is thought to play a role in this process as it is a ligand for the dominant platelet integrin, αIIbβ3.13 Our studies with the αIIbβ3-blocking antibody, JON/A, demonstrate a major role of αIIbβ3 in platelet adhesion on the ECM in vivo (Figures 2 and 4). This finding is in agreement with in vitro studies showing that αIIbβ3-VWF interactions are sufficient to mediate shear-resistant platelet deposition on collagen in whole-blood perfusion experiments in the absence of functional α2β1.5,13 However, the newly formed aggregates are less stably attached to the collagen substrate compared with wild-type controls,30 suggesting that αIIbβ3-VWF interactions are unable to fully substitute for the lack of α2β1 in this system. In vivo, the situation appears to be different as αIIbβ3 not only binds to VWF but also to other ligands, including fibrinogen deposited on the surface of the damaged vessel and fibronectin, which is present in the ECM of the vessel wall. This may explain why no defect in adhesion and thrombus formation was detectable in α2-null mice. The pivotal role of αIIbβ3 was revealed in β1-null mice, where platelet adhesion and thrombus formation was not reduced compared with controls but abrogated on inhibition of αIIbβ3 (Figure 4). These results strongly suggest that αIIbβ3 is the major integrin that supports shear-resistant platelet adhesion on the injured vessel wall in vivo, presumably by interacting with multiple ligands in the ECM.
The surprising finding that platelet adhesion and aggregation on the ECM was not significantly altered in β1-null mice also excludes an essential role of α5β1 and α6β1 in arterial thrombus formation, which is in line with normal tail bleeding times in those mice.5 However, the observation that inhibition of αIIbβ3 abolished platelet adhesion in β1-null but not α2-null mice provides the first direct evidence that α5β1 and/or α6β1 can contribute to platelet attachment to the damaged vascular wall in vivo. We were unable to clarify whether α5β1, α6β1, or both were responsible for the observed adhesion in the absence of functional α2β1 and αIIbβ3. It appears likely, however, that both integrins are involved in this process as both fibronectin and laminin are highly expressed in the vessel wall and become accessible to the flowing blood at sites of injury. Laminins are a family of structurally related glycoproteins that are tightly assembled with collagen type IV through the action of nidogen39 in the basement membrane. Therefore, laminins are among the first constituents of the ECM that platelets get in touch with at sites of endothelial denudation and they are closely associated with collagen. Although collagen type IV is a relatively weak platelet agonist compared with collagen types I, III, and VI,40 it is known to activate αIIbβ3 through the GPVI/FcRγ-chain complex.41
Fibronectins are dimeric glycoproteins that are present in plasma (plasma fibronectin) and in tissue extracellular matrices (cellular fibronectin).42 At sites of vascular injury, platelets get in touch with both cellular and extravasated plasma fibronectin but the significance of either interaction is only partly understood. Initial studies in mice with a cre/loxP-mediated deletion of plasma fibronectin revealed no major hemostatic defect as shown by normal bleeding times, platelet aggregation, and clot retraction.43 However, recent studies with these mice in a model of arterial thrombosis demonstrated delayed thrombus formation and reduced thrombus stability, suggesting a role of plasma fibronectin in platelet-platelet interactions.44 Our results may extend the role of fibronectins also to the process of platelet adhesion and suggest that both fibronectin-binding integrins on platelets, αIIbβ3 and α5β1, play a significant role in this process. In the vascular wall, cellular fibronectin is closely associated with collagens including types I and III, both of which are strong platelet agonists on GPVI, suggesting that GPVI-collagen interactions may facilitate platelet adhesion on fibronectin.
Our adhesion studies (Figure 5) suggest that α5β1 and α6β1 are in a low-affinity state on resting mouse platelets unable to efficiently mediate adhesion to fibronectin or laminin, respectively, and that ligation of GPVI shifts these integrins to a high-affinity state. This finding is in line with previous studies demonstrating a similar regulation of α2β1 in mouse platelets5 and suggests that both integrins, together with α2β1, may contribute to shear-resistant platelet adhesion at sites of arterial injury in a GPVI-dependent manner. However, these results stand in contrast to reports that human platelets adhere to fibronectin18 and laminin45 under static conditions in the absence of cellular stimulation, suggesting that both integrins may be constitutively in a high-affinity conformation on human platelets. One possible explanation for this apparent discrepancy might be species-specific differences in the affinity regulation of both integrins. However, at least the third β1 integrin on platelets, α2β1, has been shown to be expressed in a low-affinity state on resting human6,46 and mouse5 platelets and to shift to a high-affinity state in response to cellular stimulation in both species. Based on the assumption that the different β1 integrins on platelets may be regulated by similar mechanisms, species-specific differences in the regulation of α5β1 and α6β1 appear unlikely. Another possible explanation for the discrepant results might be different protocols used for platelet preparation or washing of the adhesion plates or differences in the laminin/fibronectin preparations used in the individual studies, but this is difficult to assess. Therefore, further studies will be required to clarify whether or not α5β1 and α6β1 are differently regulated in human and mouse platelets.
The results of the present study suggest that the affinity of β1 and β3 integrins on platelets is regulated by similar mechanisms. At sites of arterial injury, GPVI-collagen interactions are a major trigger of this activation process.15,16 However, since GPVI-deficient humans9,47 and mice33,48 display no major bleeding phenotype, it appears that other agonist receptors/signaling pathways can substitute for GPVI in mediating integrin activation in normal hemostasis. The G-protein-coupled receptors for adenosine diphosphate (ADP), thromboxanes, or thrombin are likely to play major roles in this process, but further studies will be required to confirm this hypothesis.
In summary, we have shown that platelet attachment and thrombus formation at sites of vascular injury in mice can occur independently of α2β1 or even all β1 integrins on platelets. On the other hand, β1 integrins can mediate shear-resistant platelet adhesion independently of αIIbβ3, demonstrating that β1 and β3 integrins have largely redundant roles in this process. However, our findings do not rule out the possibility that individual integrins may have distinct functions that are of greater significance in other pathophysiologic processes. Studies with α2- and β1-null mice in models of systemic and local inflammation, tumor metastasis, and atherogenesis may help to answer this question. Besides mediating platelet adhesion and aggregation, platelet integrins trigger a variety of important functions like spreading, procoagulant activity, and clot retraction through “outside-in” signaling. This is best documented for αIIbβ3 (for review see Shattil49 ) but recent studies by Inoue et al50 suggest that α2β1 triggers similar functions in platelets as both integrins regulate a similar set of intracellular signaling molecules including Syk, SLP-76, FAK, and phospholipase Cγ2 (PLCγ2). These observations indicate that the signaling pathways triggered by αIIbβ3 and α2β1 in platelets are conserved and may also apply to α5β1, α6β1, and αvβ3. Together with the data reported here, this suggests that the cooperation of multiple integrin-ligand interactions ensures a high degree of functional redundancy enabling effective and well-controlled platelet adhesion, spreading, and thrombus formation on different compositions of the subendothelial matrix. These may vary significantly between different regions in the vascular system and may also depend on the type and severity of the lesion.
Prepublished online as Blood First Edition Paper, July 31, 2003; DOI 10.1182/blood-2003-05-1391.
Supported by grant Ni556/4-1 (B.N.) and SFB 589 (B.E., T.K.) from the Deutsche Forschungsgemeinschaft (DFG). B.N. and C.B. are Heisenberg fellows of the DFG.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to Reinhard Fässler for critically reading the manuscript and for helpful discussions. We thank J. Schröder for help with histology and Martina Koch and Stefanie Hartmann for excellent technical assistance.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal