Abstract
Induction of potent and sustained antiviral or antitumor immunity is dependent on the efficient activation of CD8+ and CD4+ T cells. While dendritic cells constitute a powerful platform for stimulating cellular immunity, presentation of endogenous antigens by dendritic cells transfected with nucleic acid-encoded antigens favors the stimulation of CD8+ T cells over that of CD4+ T cells. A short incubation of mRNA-transfected dendritic cells with antisense oligonucleotides directed against the invariant chain enhances the presentation of mRNA-encoded class II epitopes and activation of CD4+ T-cell responses in vitro and in vivo. Immunization of mice with the antisense oligonucleotide-treated dendritic cells stimulates a more potent and longer lasting CD8+ cytotoxic T-cell (CTL) response and enhances the antitumor efficacy of dendritic cell-based tumor vaccination protocols. Transient inhibition of invariant chain expression represents a simple and general method to enhance the stimulation of CD4+ T-cell responses from endogenous antigens. (Blood. 2003;102:4137-4142)
Introduction
Immunization using dendritic cells (DCs) loaded with tumor antigens is a potentially powerful method of inducing antitumor immunity.1 An effective way of loading DCs with tumor antigen is to transduce the DCs with recombinant viral vectors or to transfect with mRNA-encoding tumor antigens.2 CD8+ cytotoxic T cells (CTLs) are an important effector arm in the antitumor immune response, and the induction of potent CTL responses has been the main goal in developing immunotherapeutic strategies for cancer.3,4 Accumulating evidence, however, suggests that the CD4+ T-cell response also plays a key role in tumor immunity.5,6 CD4+ T cells provide important functions for the induction, expansion, and persistence of CD8+ CTLs.7 Secretion of effector cytokines such as interferon γ (IFNγ) by CD4+ T cells sensitizes tumor cells to CTL lysis via up-regulation of major histocompatibility complex (MHC) class I molecules, stimulates the innate arm of the immune system at the tumor site, and, as was recently suggested, inhibits local angiogenesis.8 The importance of the CD4+ T-cell response in tumor immunity was highlighted in murine studies showing that CD4+ T cells can eradicate tumor in the absence of CD8+ T cells9-11 or constitute the dominant effector arm in the antitumor response.12 Therefore, an optimal antitumor immune response will require the concomitant activation of both CD4+ and CD8+ T cells.
Endogenously expressed antigens, such as antigens expressed in DCs transfected with mRNA, will be channeled preferentially into the class I processing pathway to activate the CD8+ T-cell arm of the immune response.13 Antigens of cytoplasmic origin that access the endocytic/lysosomal compartments can generate peptides for loading class II molecules and stimulate, albeit weak, CD4+ T-cell responses.14 Wu et al have shown that it is possible to further enhance the class II presentation of endogenous antigens by appending a leader sequence to the amino end and a lysosomal sorting signal to the carboxyl end of the endogenously expressed antigen.15 Engineering leader sequences and/or lysosomal/endosomal targeting signals are, however, not applicable to immunization with tumor-derived antigenic mixtures such as tumor-derived mRNA. Furthermore, binding of endogenously derived peptides with nascent MHC class II molecules is inhibited by the invariant chain (below).
MHC class II-negative tumor cells transfected with MHC class II cDNA expression plasmids exhibit enhanced antitumor immunogenicity in mice.16 Coexpression of the invariant chain, however, abrogates the immunogenicity of the class II transfected tumor cells.17 This observation, and the findings that presentation of endogenous peptides is often, but not always, favored in cells expressing class II molecules in the absence of invariant chain,16,18-20 is illustrative of the natural role of invariant chain to prevent the association of endogenously derived class II-restricted peptides in the endosome or Golgi compartments with the nascent class II molecules.21,22 Transfection of tumor cells with patient-specific class II alleles is not a practical approach to stimulate antitumor CD4+ T-cell immunity in cancer patients. Recently, Qui et al have described a strategy whereby class II as well as invariant chain expression is induced in tumor cells, either by transfection with a CIITA expression plasmid or by treatment with IFNγ, followed by selective down-regulation of invariant chain expression using antisense oligonucleotides directed against invariant chain (Ii AS ODNs).23 This approach is, however, limited by tumor tissue availability and transfectability of primary human tumors or their responsiveness to IFNγ-mediated induction of class II expression.
In this study we tested the hypothesis that antisense ODN-mediated inhibition of invariant chain expression in mRNA-transfected DCs will lead to enhanced presentation of class II-restricted epitopes, enhanced induction of CD4+ T-cell and CD8+ CTL responses in mice, and improved tumor immunity. Since vaccination with tumor mRNA-transfected DCs does not require the identification of the effective tumor antigens in each patient with cancer and is not limited by tumor tissue availability, this approach could represent a broadly useful method to augment antitumor CD4+ T-cell immunity, alongside CD8+ T-cell immunity.
Materials and methods
Mice, cell lines, and reagents
C57BL/6 mice (H-2b; 6- to 8-weeks old) were obtained from the Jackson Laboratory, Bar Harbor, ME. Animal studies were approved by the Duke Institutional Animal Care and Use Committee (IACUC). Cell lines used were B16/F10.9 (C57BL/6, H-2b) melanoma tumor cells,24 B16/F10.9-OVA, chicken ovalbumin (OVA) cDNA-transfected B16/F10.9 cells,25 and RMA-S cells (C57BL/6, H-2b). OVA-specific class I- and class II-restricted RF33.70 and MF2.2D9 T-cell hybridomas, respectively, were a kind gift from Dr Kenneth Rock (University of Massachusetts, Amherst).
Peptides and oligonucleotides (ODNs)
OVA peptide (H-2Kb restricted, SIINFEKL, amino acids [aa's] 257-264),26 vesicular stomatitis virus (VSV) peptide (H-2Kb restricted, RGYVYGQL),27 OVA peptide (I-Ab restricted, IINFEKLTEWTSSNVMEER, aa's 258-276),28 and VSV II peptide (I-Ab restricted, SSKAQVFEHPHIQDAASQL)27 were purchased from Research Genetics (Huntsville, AL). The following phosphorothioate-modified ODNs were synthesized: AE40 (5′-TTGGTCATCCATGGCTCT-3′) and AE54 (5′-TGGTCATCCATGGCTCTA-3′) correspond to previously described invariant chain (Ii) antisense ODNs.23 SE40 (5′-TCTCGG TACCTACTGGTT-3′) is a scrambled sequence of AE40, and SE46 (5′-ATGGATGACCAACGCGAC-3′) and SE54 (5′-TAGAGCCATGGATGACCA-3′) correspond to the complementary (sense) strand of AE40 and AE54, respectively.
RNA preparation
Cellular RNA was extracted using the RNeasy kit (Qiagen, Valencia, CA). mRNA was isolated from total RNA using magnetic activated cell sorter (MACS) mRNA isolation kit (Miltenyi Biotec, Auburn, CA).The plasmids, pGEM4Z/OVA/A64, pGEM4Z/GFP/A64, and pGEM4Z/Flu/A64, used for in vitro transcription of OVA, green fluorescent protein (GFP), and influenza (Flu, M1) mRNA, respectively, were previously described.29 Murine tyrosinase-related protein 2 (TRP-2) mRNA and a truncated form of OVA from which the first 40 aa's were deleted were produced by in vitro transcription from a cDNA fragment amplified by reverse transcriptase-polymerase chain reaction (RT-PCR).
In vivo depletion of CD4+ or CD8+ T cells
At days -3, 0, +3, and +6 of tumor challenge, 150 μg anti-CD4+ ascites (GK1.5) or 200 μg anti-CD8+ (53-6.72) was injected into mice intraperitoneally. More than 98% and 94% of CD4+ or CD8+ T cells, respectively, were specifically depleted under those conditions. Nondepleted control mice received an injection of 200 μg mouse immunoglobulin G (IgG).
Tumor challenge
Mice were immunized twice weekly intraperitoneally with 2 to 4 × 105 electroporated DCs in 200 μL phosphate-buffered saline (PBS). After 10 days, the mice were challenged subcutaneously with 1 × 105 tumor cells in 200 μL PBS. For the treatment of pre-existing tumor, 3 × 104 B16/F10.9-OVA cells were injected subcutaneously at the right flank of C57BL/6 mice. The mice were then immunized intraperitoneally with 5 × 105 RNA-electroporated DCs at 3, 7, and 14 days after tumor inoculation. Site of tumor implantation was monitored daily for the appearance of palpable tumors. Tumor volume (smallest diameter2 × largest diameter) was measured every other day starting at days 13 to 15 after tumor challenge. Mice were killed when the diameter of the tumor reached 2 cm.
Results
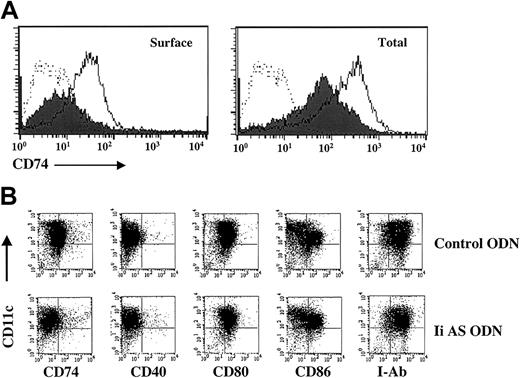
We used 2 phosphorothioate-modified antisense ODNs described by Qiu et al23 to test whether invariant chain expression can be inhibited in murine DCs. Surface expression of invariant chain (CD74) is significantly reduced in DCs treated with antisense (Ii AS), but not control, ODNs, whereas a partial inhibition was seen when both intracellular and cell surface expression were measured (Figure 1A). Similar effects were seen with a second antisense and control ODN (“Materials and methods,” data not shown). The specificity of the invariant chain antisense ODNs is demonstrated in Figure 1B. No inhibition of CD40, CD80, CD86, or MHC class II I-Ab expression was seen in DCs incubated with Ii AS ODNs. Invariant chain controls the proper folding and trafficking of the nascent MHC class II molecules and their appearance at the cell surface.21,22 However, this function is cell type-dependent, since in many instances class II molecules can traffic to the cell surface in the complete absence of invariant chain.32-35 Consistent with our observations, invariant chain-independent class II expression on the cell surface was seen in DCs and activated macrophages, but not in B cells.36,37
Inhibition of invariant chain expression (CD74) in DCs incubated with antisense oligonucleotides. (A) Day-7 DCs generated from the bone marrow of C57BL/6 mice30 were electroporated with OVA mRNA and 50 mM Ii AS ODNs (AE40) (filled histogram) or control ODNs (SE46) (open histogram).31 Cells were replated, cultured for 2 days, and stained with fluorescein isothiocyanate (FITC)-labeled antimouse CD74 (invariant chain) antibody (Ab). Dotted lines indicate DCs stained with FITC-labeled isotype control Ab. To measure total invariant chain expression, cells were permeabilized before Ab staining. (B) Shown is 2-color staining of DCs with phycoerythrin (PE)-labeled anti-CD11c and FITC-labeled anti-CD74, -CD40, -CD80, -CD86, or MHC class II (I-Ab) Ab (PharMingen, San Diego, CA). Data are representative of 5 experiments.
Inhibition of invariant chain expression (CD74) in DCs incubated with antisense oligonucleotides. (A) Day-7 DCs generated from the bone marrow of C57BL/6 mice30 were electroporated with OVA mRNA and 50 mM Ii AS ODNs (AE40) (filled histogram) or control ODNs (SE46) (open histogram).31 Cells were replated, cultured for 2 days, and stained with fluorescein isothiocyanate (FITC)-labeled antimouse CD74 (invariant chain) antibody (Ab). Dotted lines indicate DCs stained with FITC-labeled isotype control Ab. To measure total invariant chain expression, cells were permeabilized before Ab staining. (B) Shown is 2-color staining of DCs with phycoerythrin (PE)-labeled anti-CD11c and FITC-labeled anti-CD74, -CD40, -CD80, -CD86, or MHC class II (I-Ab) Ab (PharMingen, San Diego, CA). Data are representative of 5 experiments.
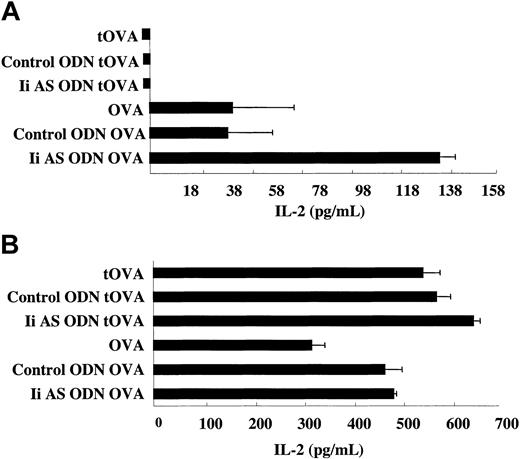
The presentation of MHC class I and class II chicken ovalbumin (OVA) epitopes is shown in Figure 2. C75BL/6 (H-2b) mice present an H-2Kb class I-restricted dominant epitope and an I-Ab-restricted class II dominant epitope. Processing and presentation of the class I and class II epitopes by the OVA mRNA-transfected DCs was determined using T-hybridomas specific to each epitope. No presentation of the class II epitope was seen with a truncated form of OVA (tOVA) from which the first 40 aa's containing the leader sequence were removed (Figure 2A). This is consistent with the observations that class II presentation of endogenous antigens is confined primarily to antigens that can access the endocytic compartments.14 In contrast, the native secreted form of OVA is capable of processing the class II epitope for presentation to the class II-restricted T-hybridoma. Incubation of the OVA mRNA-transfected DCs with an Ii AS, but not control, ODN enhanced the presentation of the class II OVA epitope. Presentation of the dominant OVA class I epitope was not significantly affected by the antisense or control ODN (Figure 2B). This experiment shows that transient and partial inhibition of invariant chain expression in cultured DCs enhances the presentation of class II, but not class I, epitopes from the endogenously expressed OVA antigen.
Inhibition of invariant chain synthesis enhances MHC class II presentation of OVA by DCs transfected with OVA mRNA. DCs were transfected with OVA mRNA or a truncated OVA mRNA (tOVA) from which sequences corresponding to the first 40 aa's of the OVA protein were deleted. As indicated, the OVA mRNA-transfected DCs were also treated with Ii AS (AE40) or control (SE40) ODNs. Presentation of the dominant MHC class II (A) and class I (B) OVA epitopes was determined by measuring IL-2 secretion from OVA class II and class I T-hybridomas, respectively.38 Measurements were done in triplicates. Variability is indicated by the error bars. Data are representative of 6 experiments using 2 Ii AS and control ODNs described in “Materials and methods.”
Inhibition of invariant chain synthesis enhances MHC class II presentation of OVA by DCs transfected with OVA mRNA. DCs were transfected with OVA mRNA or a truncated OVA mRNA (tOVA) from which sequences corresponding to the first 40 aa's of the OVA protein were deleted. As indicated, the OVA mRNA-transfected DCs were also treated with Ii AS (AE40) or control (SE40) ODNs. Presentation of the dominant MHC class II (A) and class I (B) OVA epitopes was determined by measuring IL-2 secretion from OVA class II and class I T-hybridomas, respectively.38 Measurements were done in triplicates. Variability is indicated by the error bars. Data are representative of 6 experiments using 2 Ii AS and control ODNs described in “Materials and methods.”
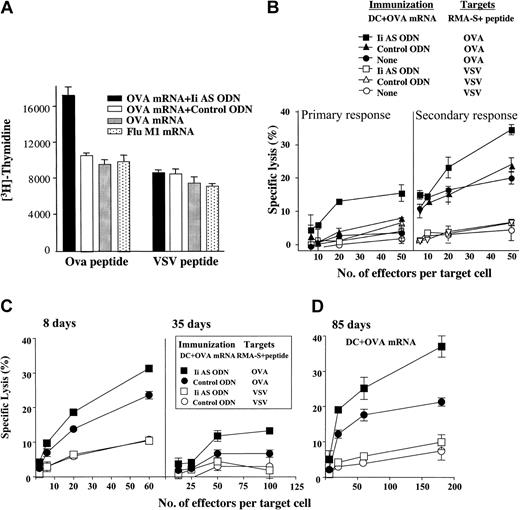
To determine whether the antisense ODN-mediated inhibition of invariant chain expression can lead to enhanced stimulation of OVA-specific CD4+ and CD8+ cytotoxic T-cell (CTL) responses in vivo, we immunized mice with OVA mRNA-transfected DCs treated with control or Ii AS ODNs and the induction of OVA-specific CD4+ T-cell and CTL responses was measured in the splenocytic population of the immunized mice. Only mice immunized with Ii AS ODN-treated DCs exhibited an OVA-specific CD4+ T-cell response, namely the CD4+ T cells proliferated against OVA, but not VSV, pulsed splenocytes (Figure 3A). Influenza matrix (Flu M1) mRNA-transfected DCs and OVA mRNA-transfected DCs alone or treated with a control ODN did not stimulate detectable levels of CD4+ T-cell responses above background. The high basal level of CD4+ T-cell proliferation seen in this experiment represents an anti-fetal calf serum (FCS) response commonly seen when immunizing with DCs cultured in the presence of FCS.39 Attempts to reduce background by growing the DCs or CD4+ T cells in syngeneic mouse serum or AIM-V media was unsuccessful. Nevertheless, the small difference in the proliferative capacity of the Ii AS ODN-treated mice against OVA targets shown in Figure 3 was seen reproducibly in 3 experiments. Following in vitro stimulation of splenocytes from mice immunized with OVA mRNA-transfected DCs, an enhanced CTL was seen if the DCs were treated with the Ii AS, but not with control, ODN (Figure 3B). When the immunizing DCs were treated with Ii AS ODN an OVA-specific CTL could be detected directly without ex vivo stimulation, thus underscoring the enhancing effect of invariant chain inhibition on CTL induction in vivo. When CTL analysis was performed 35 days after immunization the OVA-specific CTL activity was significantly diminished, yet in mice immunized with the Ii AS ODN-treated DCs the decrease in OVA CTL activity was less pronounced (Figure 3C). At 55 days after immunization, OVA-specific CTLs could not be detected in the in vitro stimulated splenocytes (data not shown). However, when mice were reimmunized with OVA mRNA-transfected DCs (not treated with Ii AS ODN) 85 days after the initial immunization, the mice that received Ii AS ODN-treated DCs demonstrated a superior CTL response (Figure 3D). These observations show that immunization with Ii AS ODN-treated DCs extends the persistence of CTL precursors in the spleen of the immunized mice (Figure 3C) and induces a larger pool of memory CTLs that can be reactivated by immunization (Figure 3D).
Inhibition of invariant chain synthesis enhances the generation of CD4+ T-cell responses and cytotoxic T-cell (CTL) responses in mice immunized with OVA mRNA-transfected DCs. (A) CD4+ T-cell proliferation assay. Mice were immunized intravenously with 2.5 × 105 DCs transfected with either OVA mRNA or influenza matrix (Flu M1) mRNA. Where indicated, the OVA mRNA-transfected DCs were also transfected with Ii AS (AE40) or control (SE40) ODNs. Splenocytes were harvested after 8 days and CD4+ T cells were isolated using StemSep Murine CD4+ Negative Isolation Column (StemCell Technologies, Vancouver, BC, Canada). CD4+ T cells were cocultured with I-Ab-restricted OVA or VSV peptide-pulsed DCs for 3 days. 3H-thymidine incorporation was measured for 17 hours prior to harvest. Data are representative of 3 experiments. (B) Cytotoxicity assay. Mice were immunized with 2.5 × 105 OVA mRNA-transfected DCs transfected with Ii AS (AE40) or control (SE40) ODNs, as indicated. Splenocytes isolated 8 days after immunization were either tested directly for OVA CTLs (primary response) or first incubated in vitro in the presence of OVA mRNA-transfected DCs and then tested for OVA CTLs (secondary response).29 RMA-S cells pulsed with the MHC class I-restricted OVA or VSV peptides were used as targets. Data are representative of 5 experiments. (C) CTL responses were measured 8 or 35 days after immunization. (D) At 85 days after immunization with Ii AS (AE40) or control ODN (SE40)-treated OVA mRNA-transfected DCs, mice were reimmunized with OVA mRNA-transfected DCs and OVA CTLs were measured. Measurements were done in triplicates. Variability is indicated by the error bars.
Inhibition of invariant chain synthesis enhances the generation of CD4+ T-cell responses and cytotoxic T-cell (CTL) responses in mice immunized with OVA mRNA-transfected DCs. (A) CD4+ T-cell proliferation assay. Mice were immunized intravenously with 2.5 × 105 DCs transfected with either OVA mRNA or influenza matrix (Flu M1) mRNA. Where indicated, the OVA mRNA-transfected DCs were also transfected with Ii AS (AE40) or control (SE40) ODNs. Splenocytes were harvested after 8 days and CD4+ T cells were isolated using StemSep Murine CD4+ Negative Isolation Column (StemCell Technologies, Vancouver, BC, Canada). CD4+ T cells were cocultured with I-Ab-restricted OVA or VSV peptide-pulsed DCs for 3 days. 3H-thymidine incorporation was measured for 17 hours prior to harvest. Data are representative of 3 experiments. (B) Cytotoxicity assay. Mice were immunized with 2.5 × 105 OVA mRNA-transfected DCs transfected with Ii AS (AE40) or control (SE40) ODNs, as indicated. Splenocytes isolated 8 days after immunization were either tested directly for OVA CTLs (primary response) or first incubated in vitro in the presence of OVA mRNA-transfected DCs and then tested for OVA CTLs (secondary response).29 RMA-S cells pulsed with the MHC class I-restricted OVA or VSV peptides were used as targets. Data are representative of 5 experiments. (C) CTL responses were measured 8 or 35 days after immunization. (D) At 85 days after immunization with Ii AS (AE40) or control ODN (SE40)-treated OVA mRNA-transfected DCs, mice were reimmunized with OVA mRNA-transfected DCs and OVA CTLs were measured. Measurements were done in triplicates. Variability is indicated by the error bars.
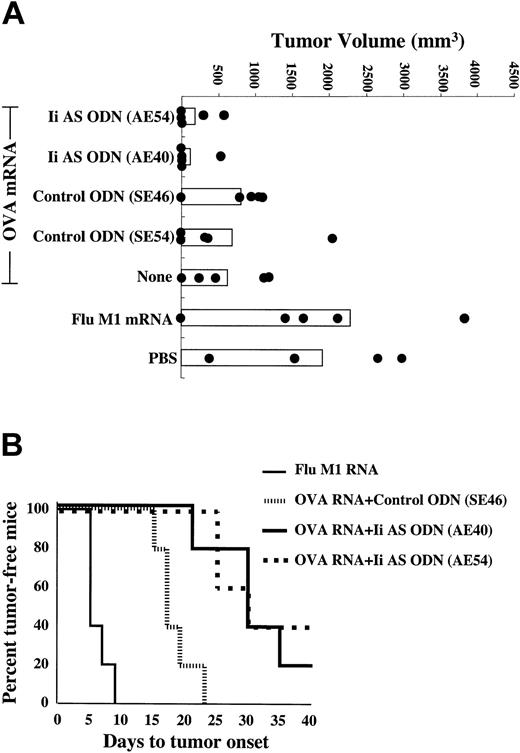
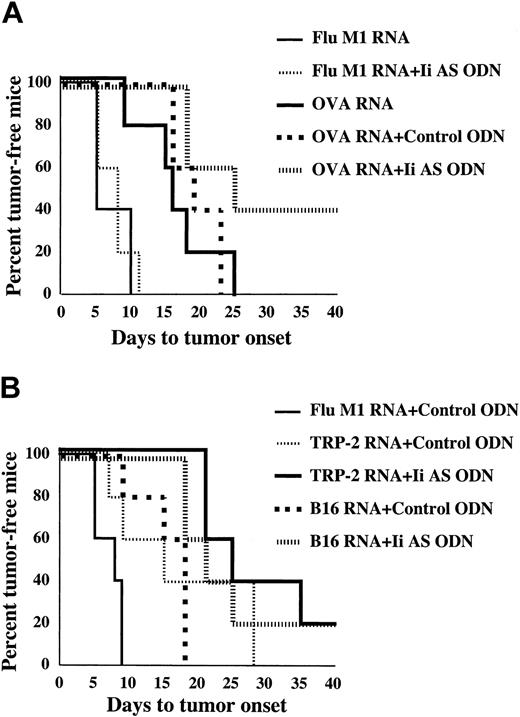
In the next series of experiments we tested whether antisense-mediated inhibition of invariant chain synthesis in DCs can enhance antitumor immunity. Mice (n = 5) were immunized with OVA mRNA-transfected DCs and challenged subcutaneously with OVA-expressing B16/F10.9 melanoma tumor cells. Figure 4A shows tumor sizes at day 21 after tumor challenge. Individual tumor measurements show a considerable intragroup variability. Nevertheless, significant differences were seen among the various treatment groups. Tumor growth was inhibited in mice immunized with OVA mRNA-transfected DCs compared with mice immunized with influenza M1 mRNA-transfected DCs or injected with PBS (P = .002). Treatment of the OVA mRNA-transfected DCs with either of 2 Ii AS ODNs significantly enhanced the antitumor effect, whereas neither of 2 control ODNs had any effect (P = .002). As shown in Figure 4B, treatment of OVA mRNA-transfected DCs with the Ii AS ODNs significantly delayed the appearance of palpable tumors compared with OVA mRNA-transfected DCs treated with control ODNs (P = .0001). Mice were monitored until day 42, at which time the experiment was terminated.
Enhancement of antitumor immunity in mice immunized with Ii AS ODN-treated DCs. Mice were immunized twice weekly with DCs transfected with either OVA or Flu M1 mRNA, or mock-immunized with PBS, treated with either of 2 Ii AS ODNs (AE54 or AE40) or 2 control ODNs (SE46 or SE54), as indicated, and challenged subcutaneously with B16/F10.9-OVA tumor cells 10 days after the second immunization (5 mice per group). (A) Day-21 tumor measurements. There was no statistical difference between the PBS group relative to the Flu M1 RNA mRNA group, among the 3 OVA mRNA groups not treated or treated with either of the 2 control ODNs, and between the 2 OVA mRNA groups treated with either of the 2 Ii AS ODNs. The P values for the PBS group relative to the OVA mRNA + control ODN groups and for the OVA mRNA + control ODN groups relative to the OVA mRNA + Ii AS ODN groups were less than .002. (B) Time to appearance of palpable tumors. Statistical significance was determined using the log-rank test. P values were .0015 for the OVA mRNA + control ODN group relative to the Flu M1 RNA group and .001 for the OVA mRNA + Ii AS ODN groups relative to the OVA mRNA + control ODN group. There was no statistical difference between the 2 OVA mRNA groups treated with either of 2 Ii AS ODNs. The median time to tumor onset was 9 days for the Flu M1 group, 17 days for the control OVA mRNA + ODN group, and 30 days for the OVA mRNA + Ii AS ODN-treated groups. Data are representative of 3 experiments.
Enhancement of antitumor immunity in mice immunized with Ii AS ODN-treated DCs. Mice were immunized twice weekly with DCs transfected with either OVA or Flu M1 mRNA, or mock-immunized with PBS, treated with either of 2 Ii AS ODNs (AE54 or AE40) or 2 control ODNs (SE46 or SE54), as indicated, and challenged subcutaneously with B16/F10.9-OVA tumor cells 10 days after the second immunization (5 mice per group). (A) Day-21 tumor measurements. There was no statistical difference between the PBS group relative to the Flu M1 RNA mRNA group, among the 3 OVA mRNA groups not treated or treated with either of the 2 control ODNs, and between the 2 OVA mRNA groups treated with either of the 2 Ii AS ODNs. The P values for the PBS group relative to the OVA mRNA + control ODN groups and for the OVA mRNA + control ODN groups relative to the OVA mRNA + Ii AS ODN groups were less than .002. (B) Time to appearance of palpable tumors. Statistical significance was determined using the log-rank test. P values were .0015 for the OVA mRNA + control ODN group relative to the Flu M1 RNA group and .001 for the OVA mRNA + Ii AS ODN groups relative to the OVA mRNA + control ODN group. There was no statistical difference between the 2 OVA mRNA groups treated with either of 2 Ii AS ODNs. The median time to tumor onset was 9 days for the Flu M1 group, 17 days for the control OVA mRNA + ODN group, and 30 days for the OVA mRNA + Ii AS ODN-treated groups. Data are representative of 3 experiments.
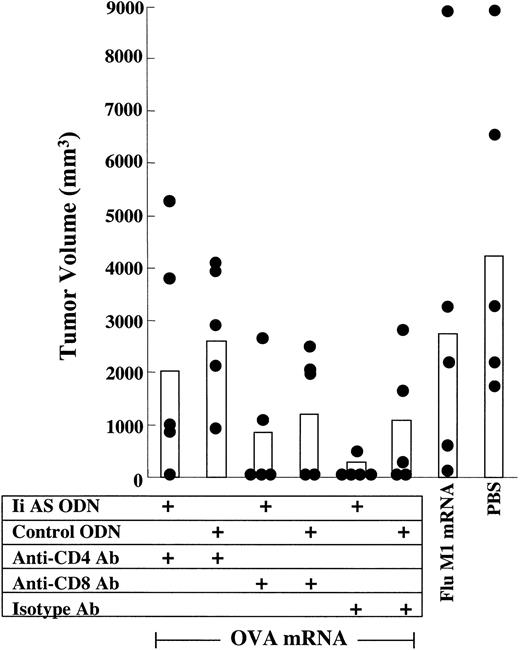
To examine the mechanism underlying the invariant chain inhibition-mediated enhancement of tumor immunity, mice immunized with OVA mRNA-transfected DCs were depleted of either CD4+ or CD8+ T cells prior to challenge with B16/F10.9-OVA tumor cells. Tumor growth in mice vaccinated with OVA mRNA-transfected DCs expressing normal levels of invariant chain (DCs treated with control ODNs) was enhanced in CD4+ T-cell-depleted mice, whereas depletion of CD8+ T cells had no effect (Figure 5). CD4+ T cells are, therefore, the primary effectors of tumor inhibition in this model as has been seen in other studies.9-11 Notably, depletion of CD8+ T cells abrogated the Ii AS ODN effect. This observation suggests that the antisense-mediated inhibition of invariant chain expression enhances tumor inhibition by inducing a CD4+ T-cell-dependent CD8+ T-cell response. Additional studies using several tumor models will be required to determine the generality of this observation. It will be important also to determine the role of CD4+ and CD8+ T cells at the induction phase, by depleting the T-cell subsets prior to immunization.
The role of CD4+ and CD8+ T-cell subsets in the antitumor response in mice immunized with DCs expressing reduced invariant chain levels. Role of CD4+ and CD8+ T-cell subsets. Mice were immunized with OVA mRNA-transfected DCs and challenged with B16/F10.9-OVA tumor cells as described in Figure 4A. Mice were depleted of CD4+- or CD8+-expressing cells before tumor challenge, as indicated. Mice immunized with Flu M1 RNA or treated with PBS also received isotype Ab. Black circles represent individual tumor measurements and white bars represent average tumor size of the treatment group. Day-25 measurements are shown (5 mice per group).
The role of CD4+ and CD8+ T-cell subsets in the antitumor response in mice immunized with DCs expressing reduced invariant chain levels. Role of CD4+ and CD8+ T-cell subsets. Mice were immunized with OVA mRNA-transfected DCs and challenged with B16/F10.9-OVA tumor cells as described in Figure 4A. Mice were depleted of CD4+- or CD8+-expressing cells before tumor challenge, as indicated. Mice immunized with Flu M1 RNA or treated with PBS also received isotype Ab. Black circles represent individual tumor measurements and white bars represent average tumor size of the treatment group. Day-25 measurements are shown (5 mice per group).
In the experiments shown in Figures 4 and 5 mice were immunized prior to challenge with tumor. To test whether invariant chain inhibition can also augment protective immunity in tumor-bearing mice, mice were first implanted with B16/F10.9-OVA tumor cells and immunized with OVA mRNA-transfected DCs starting 3 days following tumor implantation. Tumor growth was measured by monitoring the time to appearance of palpable tumors in the various treatment groups (Figure 6). Experiments were terminated after statistical significance was reached (days 41-43). A significant retardation in tumor growth was seen when mice were immunized with OVA mRNA-transfected DCs, alone or treated with a control ODN, compared with mice immunized with Flu M1 mRNA-transfected DCs (Figure 6A, P = .0015). Inhibition of tumor growth was further enhanced when the OVA mRNA-transfected DCs were treated with the Ii AS ODNs (P = .006). To test whether Ii inhibition can also potentiate antitumor immunity directed against natural tumor antigens, mice were immunized with TRP-2 mRNA or total B16/F10.9 tumor RNA. TRP-2 is a melanocyte-specific endoplasmic reticulum (ER)-resident dominant tumor antigen in the B16/F10.9 melanoma tumor.40 As shown in Figure 6B, the antitumor effect seen in tumor-bearing mice immunized with either TRP-2 mRNA (P = .0535) or B16/F10.9 tumor RNA-transfected DCs (P = .03) was enhanced when the DCs were treated with Ii AS, but not control, ODNs. Treatment of influenza matrix (Flu M1) mRNA-transfected DCs with Ii AS ODNs had no significant antitumor effect (Figure 6A, P = .9 and data not shown), suggesting that the effect of invariant chain inhibition was OVA antigen-specific. This, and the observation that Ii AS ODN treatment of DCs transfected with tOVA mRNA, which is not capable of presenting class II epitopes (Figure 2), does not enhance tumor immunity (data not shown), argues against the contribution of FCS-derived antigens used in the DC generation protocol to the observed antitumor effects.
Enhancement of tumor regression in mice immunized with Ii AS ODN-treated DCs. (A) B16/F10.9-OVA tumor cells were implanted subcutaneously in C57BL/6 mice followed 3 days later by treatment with OVA or influenza matrix (M1) mRNA-transfected DCs exposed to Ii AS (AE40) or control (SE40) ODNs as indicated (5 mice per group). Mice were monitored for the appearance of palpable tumors. Using the log-rank test, the P values were .006 for the Ii AS ODN group relative to the control ODN group, and .0015 for the control ODN group relative to the Flu M1 group. There was no statistical difference between the OVA mRNA relative to the OVA mRNA + control ODN groups, or the Flu M1 mRNA group relative to the Flu M1 mRNA + Ii AS ODN group (P = .9). The median time to tumor onset was 8 days for the Flu M1 with or without control ODN groups, 16 days for the OVA mRNA group, 16 days for the OVA mRNA + control ODN group, and 35 days for the OVA mRNA + Ii AS ODN group. (B) Experiment is the same as in panel A, except that B16/F10.9 tumor cells were used and mice were immunized with either TRP-2 mRNA or B16/F10.9 tumor RNA-transfected DCs. P values by the log-rank test were .0535 for the TRP-2 mRNA + Ii AS ODN group relative to the TRP-2 mRNA + control ODN group, .03 for the B16 RNA + Ii AS ODN group relative to the B16 RNA + control ODN group, .0563 for the TRP-2 mRNA + control ODN group relative to the Flu M1 + control ODN group, and .0079 for the B16 RNA + control ODN relative to the Flu M1 group. The median time of tumor onset was 8 days for the Flu M1 group, 18 days for the B16 RNA + control ODN group, 15 days for the TRP-2 mRNA + control ODN group, 21 days for the B16 RNA + Ii AS ODN group, and 25 days for the TRP-2 mRNA + Ii AS ODN group. Data are representative of 2 experiments.
Enhancement of tumor regression in mice immunized with Ii AS ODN-treated DCs. (A) B16/F10.9-OVA tumor cells were implanted subcutaneously in C57BL/6 mice followed 3 days later by treatment with OVA or influenza matrix (M1) mRNA-transfected DCs exposed to Ii AS (AE40) or control (SE40) ODNs as indicated (5 mice per group). Mice were monitored for the appearance of palpable tumors. Using the log-rank test, the P values were .006 for the Ii AS ODN group relative to the control ODN group, and .0015 for the control ODN group relative to the Flu M1 group. There was no statistical difference between the OVA mRNA relative to the OVA mRNA + control ODN groups, or the Flu M1 mRNA group relative to the Flu M1 mRNA + Ii AS ODN group (P = .9). The median time to tumor onset was 8 days for the Flu M1 with or without control ODN groups, 16 days for the OVA mRNA group, 16 days for the OVA mRNA + control ODN group, and 35 days for the OVA mRNA + Ii AS ODN group. (B) Experiment is the same as in panel A, except that B16/F10.9 tumor cells were used and mice were immunized with either TRP-2 mRNA or B16/F10.9 tumor RNA-transfected DCs. P values by the log-rank test were .0535 for the TRP-2 mRNA + Ii AS ODN group relative to the TRP-2 mRNA + control ODN group, .03 for the B16 RNA + Ii AS ODN group relative to the B16 RNA + control ODN group, .0563 for the TRP-2 mRNA + control ODN group relative to the Flu M1 + control ODN group, and .0079 for the B16 RNA + control ODN relative to the Flu M1 group. The median time of tumor onset was 8 days for the Flu M1 group, 18 days for the B16 RNA + control ODN group, 15 days for the TRP-2 mRNA + control ODN group, 21 days for the B16 RNA + Ii AS ODN group, and 25 days for the TRP-2 mRNA + Ii AS ODN group. Data are representative of 2 experiments.
Discussion
In this study we describe a simple and general method to enhance the stimulation of CD4+ T-cell responses by DCs presenting endogenous antigens. Using DCs transfected with mRNA encoding the chicken ovalbumin (OVA), we have shown that a partial inhibition of invariant chain expression mediated by a short treatment with antisense oligonucleotides leads to enhanced presentation of class II OVA epitopes in vitro and in vivo (Figures 2,3A).
CD4+ T cells are required for the persistence and proliferation of activated CD8+ T cells and the generation of long-lived memory CD8+ T cells.7 The CD4+ T-cell arm of the cellular immune response is also a critical component of an effective antitumor response.5,6 Here we show that enhanced activation of OVA-specific CD4+ T cells in mice immunized with DCs expressing reduced levels of invariant chain is accompanied by increased induction of CD8+ CTLs (Figure 3B), extended persistence of a measurable CTL response (Figure 3C), and a larger pool of memory CTLs that can be reactivated in vivo (Figure 3D). In addition, inhibition of invariant chain synthesis in DCs correlates with enhanced tumor immunity (Figures 4, 5, 6). This was seen reproducibly using DCs transfected with either mRNA encoding the strong model antigen OVA, the endogenous TRP-2 tumor antigen, or unfractionated tumor-derived antigenic mixtures, in both prophylactic (Figures 4-5) and therapeutic (Figure 6) immunization protocols. Overall, these experiments underscore the important role of the CD4+ T-cell arm in tumor immunity and suggest that induction of CD4+ T-cell responses by immunization with mRNA-transfected DCs is a limiting factor in stimulating antitumor immunity.
Invariant chain inhibition-mediated enhancement of class II presentation requires the translocation of endogenous antigens into the ER/endosomal compartment,14 which can be achieved by appending a lysosomal targeting signal to the carboxyl end of the antigen.15 Whereas the lysosomal targeting approach is limited to defined antigens that have to be modified individually with the lysosomal targeting signal, the invariant chain inhibition strategy described here will be applicable also when undefined cell (tumor)-derived antigenic mixtures are used, as illustrated in the case of immunotherapy with B16/F10.9 melanoma tumor RNA-transfected DCs (Figure 6). Conceivably, in this instance inhibition of invariant chain expression leads to enhanced stimulation of CD4+ T-cell responses against a subset of tumor antigens, including perhaps TRP-2, which can access the endosomal compartment.
The strategy to enhance CD4+ T-cell immunity described in this study is by no means limited to mRNA-transfected DCs and should be equally useful when used in conjunction with other antigen-loading techniques such as DC-tumor fusions, transduction of DCs with viral vectors, or incubation with apoptotic bodies or whole tumor cells.
Prepublished online as Blood First Edition Paper, August 14, 2003; DOI 10.1182/blood-2003-06-1867.
Supported by grants 1RO1 CA 85307 and 1RO1 CA 89536 from the National Institutes of Health and the National Cancer Institute.
E.G. has declared a financial interest in a company (MERIX Bioscience Inc.) whose potential product (mRNA-loaded DC) was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal